Abstract
A DNA microarray-based global transcript profiling of Escherichia coli in response to cold shock showed that in addition to the known cold shock-inducible genes, new genes such as the flagellar operon, those encoding proteins involved in sugar transport and metabolism, and remarkably, genes encoding certain heat shock proteins are induced by cold shock. In the light of strong reduction in metabolic activity of the cell after temperature downshift, the induction of sugar metabolism machinery is unexpected. The deletion of four csps (cspA, cspB, cspG, and cspE) affected cold shock induction of mostly those genes that are transiently induced in the acclimation phase, emphasizing that CspA homologues are essential in the acclimation phase. Relevance of these findings with respect to the known RNA chaperone function of CspA homologues is discussed.
The cold shock response is a physiological response of living cells to temperature downshift (12) and has been studied in detail using Escherichia coli and Bacillus subtilis as model systems (for a review, see references 5, 21, 26, and 32). When an exponentially growing culture of E. coli is shifted from 37 to 15°C, an acclimation phase (lag period of cell growth) characterized by transient dramatic induction of cold shock proteins against a severe inhibition of general protein synthesis precedes the resumption of growth. Out of the nine CspA homologues of E. coli, only CspA, CspB, CspG, and CspI are cold shock inducible (6, 17, 20, 31). Interestingly, double or triple deletions of genes encoding cold shock-inducible CspA homologues do not result in cold sensitivity. In a triple-deletion strain, the ΔcspA ΔcspB ΔcspG strain, CspE that is normally produced at 37°C is overproduced at low temperatures (33). This observation suggests that the functions of the CspA family members may overlap and they are able to substitute for each other during cold acclimation. Indeed a quadruple-deletion strain (the ΔcspA ΔcspB ΔcspG ΔcspE strain) of E. coli exhibits cold sensitivity at 15°C, which can be complemented by overproduction of any one of the CspA homologues except CspD (33).
In spite of a wealth of knowledge accumulated in recent years, the cold shock response is not fully elucidated. The proteomic approaches that so far have been extremely useful in identification of many cold shock-induced proteins do have certain limitations: (i) not all the proteins can be resolved well on two-dimensional gel electrophoresis, and (ii) identification of proteins from the gel may sometimes be cumbersome. To overcome these shortcomings, in the present study, we carried out analysis of global cold shock gene expression profiles of an E. coli wild-type and cold-sensitive quadruple-deletion strain. Our main objectives were (i) to identify the E. coli open reading frames that exhibit significant increase or decrease in mRNA abundance caused by the temperature downshift and (ii) to explore the effect of deletion of four csp genes that leads to cold sensitivity. In brief, the E. coli JM83 strain [F− araΔ(lac-proAB) rpsL(Strr)] (35) (considered the wild-type strain in this study) was grown in Luria broth (LB). The cells grown overnight in LB medium at 37°C were diluted into fresh LB medium. Cells were grown at 37°C to exponential phase (optical density at 600 nm [OD600] of 0.8), and part of the cell culture was harvested and used as a control. Aliquots of the cells were transferred to a prechilled LB medium at 15°C, and the cells were harvested after 1 and 5 h of cold shock. The OD600 did not increase after 1 h of cold shock, while after 5 h of cold shock, it was 1.2. The 37°C controls were of corresponding OD600 values. For studies involving the quadruple-deletion strain, the wild-type and the deletion strain were grown at 37°C and subsequently cold shocked for 1 h as described above. Note that in the studies involving the quadruple-deletion strain, both the wild-type and the deletion cells are cold shocked at 15°C for 1 h and compared with each other. This enables us to directly single out the genes that were differentially expressed as a result of csp deletion upon cold shock. The RNA extraction and hybridization and DNA array analysis were carried out as described previously (24). The cell density of all samples used was the same; thus, the changes seen in the microarray were not substantially influenced by the difference in cell densities. Genes whose expression levels differed by a ratio of at least 4 after cold shock were considered. From replicates, we estimate that the chance random fluctuations giving rise to a fourfold up- or down-modulation is less than 0.14%, corresponding to a confidence interval of 99.86%. Thus, the chosen fourfold cutoff value is rather stringent and the modulation of expression beyond the cutoff is highly statistically significant. In some cases, genes belonging to the same operon or category were considered even if the ratios did not adhere to these specified values. Ratios above 1 indicate induction and below 1 indicate repression. Ratios averaged from three independent of sets of experiments are shown with standard deviation values.
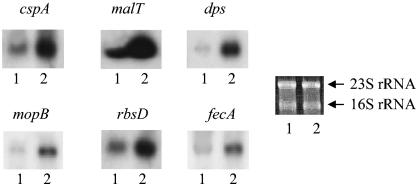
The result of the microarray analysis was confirmed by Northern blot analysis. The genes chosen for these experiments represent the important groups changed by the cold shock treatment, such as cspA and those encoding proteins involved in sugar metabolism, molecular chaperones, and iron metabolism. The deoxyoligonucleotides used for detection of cspA and dps were described previously (22, 24, 34). The deoxyoligonucleotides used for detection of malT (4) (accession number M13585), mopB (19), rbsD (3), and fecA (29) correspond to the region from codons 13 to 6 of malT and mopB, 21 to 14 of rbsD, and 13 to 8 of fecA. The Northern blot analysis was carried out as described previously (30). The results are shown in Fig. 1. These results are consistent with the microarray data.
FIG. 1.
Effect of cold shock on the levels of mRNAs. Total RNA was extracted by the hot phenol method as described in the text, and Northern blot analysis was carried out with deoxyoligonucleotides corresponding to cspA, malT, dps, mopB, rbsD, and fecA. Lanes 1 and 2 in each case except fecA represent mRNAs isolated from control (37°C) and cold-shocked (1 h) wild-type cells, respectively. In the case of fecA, lanes 1 and 2 represent mRNAs isolated from cold-shocked (1 h) wild-type and quadruple-deletion cells, respectively. The positions of the transcripts were determined using as reference ribosomal RNAs. Bands corresponding to 23S and 16S rRNAs were visualized by ethidium bromide staining of the gel.
The reliability of the present data was confirmed by (i) reproducible values obtained in multiple, independent experiments, (ii) induction of many known cold shock-inducible genes, and (iii) confirmation of levels of some of the significantly affected genes by Northern blot analysis. Our data did not show mRNA abundance of certain genes known to be cold shock-inducible, for example, genes belonging to the nusA-pnp operon, such as nusA, infB, rbfA, and pnp. The reason for this is not known at present; however, this observation is similar to that from a recent report by Polissi et al. (27), in which the discrepancy was attributed to differential stability of the 3′ end and the entire mRNA transcripts.
The genes affected by cold shock are grouped as those that (i) are transiently induced immediately following the cold shock in the acclimation phase (Table 1), (ii) show transient repression upon cold shock (Table 2), (iii) show prolonged induction beyond the acclimation phase (Table 3), and (iv) show prolonged repression upon cold shock.
TABLE 1.
Genes transiently induced upon cold shock
| Gene name and role | Gene product and/or function | Wild-type 15°C/37°C ratio at 1 h | Wild-type 15°C/37°C ratio at 5 h | Mutant/wild-type ratio (15°C, 1 h) |
|---|---|---|---|---|
| Genes involved in membrane synthesis/function | ||||
| aer | Aerotaxis receptor | 9.8 ± 0.14 | 4.8 ± 0.4 | |
| atoE | Short-chain fatty acid transporter | 6.5 ± 0.5 | 3 ± 0.5 | 0.22 ± 0.08 |
| dctA | DctA protein | 7.8 ± 1.07 | 1.75 ± 0.6 | 0.1 ± 0 |
| fabB | 3-Oxoacyl-[acyl-carrier protein] synthase I | 13 ± 1.1 | 2.6 ± 0.35 | |
| glnH | Glutamine-binding protein precursor | 6.1 ± 0.73 | 1 ± 0.1 | |
| malE, -F, -K, -M | Maltose transport proteins | 9.0-10 ± 0.5 | 0.5-2.7 ± 0.1 | 0.03-0.08 ± 0.01 |
| manY | Phosphotransferase system enzyme II | 8.9 ± 0.28 | 1.9 ± 0.2 | 0.13 ± 0.02 |
| manZ | PTS system, mannose-specific IID component | 6.9 ± 0.39 | 0.3 ± 0.01 | 0.14 ± 0.03 |
| nupC | Nucleoside permease NupC | 9.3 ± 0.015 | 1.2 ± 0.09 | |
| rbsA-D | Ribose transport proteins | 5.0-10 ± 0.8 | 1.5-2 ± 0.2 | 0.05-0.09 ± 0.02 |
| sanA | SanA protein | 4.3 ± 0.28 | 2.1 ± 0.5 | |
| trg | Methyl-accepting chemotaxis protein III | 9.2 ± 0.8 | 4.1 ± 0.6 | |
| xylF | d-Xylose-binding periplasmic protein precursor | 20 ± 2.1 | 3.5 ± 0.4 | 0.24 ± 0.03 |
| ybeJ | Amino acid ABC transporter binding protein | 6.8 ± 0.08 | 1.8 ± 0.5 | |
| Genes involved in cell metabolism | ||||
| adhE | Alcohol dehydrogenase | 18.8 ± 0.48 | 3.2 ± 0.45 | |
| agp | Glucose-1-phosphatase precursor | 16 ± 2 | 2.9 ± 0.3 | 0.21 ± 0.06 |
| aldA | Aldehyde dehydrogenase | 15 ± 1.5 | 0.15 ± 0.01 | 0.15 ± 0.01 |
| aphA | Acid phosphatase | 16.2 ± 0.44 | 2.1 ± 26 | 0.12 ± 0.005 |
| aspA | Aspartate ammonia-lyase | 20 ± 3 | 7.3 ± 0.8 | 0.13 ± 0.005 |
| bfr | Bacterioferritin | 5 ± 1 | 1 ± 0.1 | |
| carA | Carbamoyl-phosphate synthase small chain | 8.5 ± 0.15 | 0.9 ± 0.1 | |
| cfa | Cyclopropane fatty acid synthase | 8.2 ± 1.16 | 3.7 ± 0.9 | |
| cpdB | Cyclic nucleotide 2'phosphodiesterase | 10 ± 2 | 1.6 ± 0.15 | 0.12 ± 0.03 |
| cysK | Cysteine synthase | 7 ± 1.27 | 0.6 ± 0.025 | |
| deoA | Thymidine phosphorylase | 21.8 ± 1.02 | 3.6 ± 0.33 | |
| fpr | Ferredoxin-NADP+ reductase | 4.5 ± 0.45 | 1.6 ± 0.31 | |
| fruB | PTS system, fructose-specific IIA/FPR component | 7 ± 0.16 | 0.8 ± 0.2 | 0.16 ± 0.03 |
| fruK | 1-Phosphofructokinase (fructose 1-phosphate kinase) | 6.5 ± 0.75 | 0.7 ± 0.2 | 0.18 ± 0.015 |
| fucU | Fucose operon FucU protein | 6.3 ± 0.87 | 2.7 ± 0.22 | |
| fumA | Fumarate hydratase | 9.3 ± 0.79 | 1 ± 0.08 | |
| gapA | Glyceraldehyde-3-phosphate dehydrogenase | 13.5 ± 1.2 | 2.3 ± 0.3 | 0.2 ± 0.01 |
| glpK | Glycerol kinase | 12.8 ± 0.55 | 1 ± 0.02 | |
| lipA | Lipoic acid synthetase (lip-syn) | 4.2 ± 0.02 | 1.7 ± 0.25 | |
| malP | Maltodextrin phosphorylase | 20 ± 2.5 | 4 ± 0.75 | |
| malQ | 4-Alpha-glucanotransferase | 6.3 ± 0.46 | 3 ± 0.075 | |
| malT | MalT regulatory protein | 12 ± 0.72 | 4.2 ± 0.22 | |
| manX | Phosphotransferase system enzyme II | 11.4 ± 0.7 | 2.4 ± 0.03 | 0.13 ± 0.03 |
| mdh | Malate dehydrogenase | 3.2 ± 0.3 | 0.28 ± 0 | 0.24 ± 0.05 |
| nrdD | Oxygen-sensitive ribonucleoside-triphosphate reductase | 15.2 ± 1.58 | 1.8 ± 0.22 | |
| otsA | Tehalose-6-phosphate synthase | 4.3 ± 0.12 | 1 ± 0.29 | |
| otsB | Trehalose-phosphatase | 2.7 ± 0.19 | 0.7 ± 0.15 | |
| pgi | Glucose-6-phosphate isomerase | 6.1 ± 0.15 | 1.7 ± 0.15 | 0.19 ± 0.025 |
| poxB | Pyruvate oxidase | 3.6 ± 0.6 | 0.18 ± 0.02 | 0.25 ± 0.04 |
| pykA | Pyruvate kinase | 7.4 ± 0.14 | 0.75 ± 0.16 | 0.16 ± 0 |
| rbsK | Ribokinase | 5.1 ± 0.34 | 2.9 ± 0.37 | 0.19 ± 0.04 |
| srlA | Phosphoenolpyruvate-carbohydrate phosphotransferase system, glucitol/sorbitol-specific IIBC component | 9.3 ± 1.3 | 2.7 ± 0 | |
| srlB | Phosphotransferase system enzyme II | 4.4 ± 0.22 | 1.5 ± 0.3 | |
| srlD | Sorbitol-6-phosphate 2-dehydrogenase | 5.1 ± 0.23 | 1.5 ± 0.02 | |
| srlR | Glucitol operon repressor | 5.5 ± 0.06 | 2.6 ± 0.4 | |
| treB | Phosphotransferase system trehalose permease | 6 ± 1 | 0.7 ± 0.01 | |
| treC | Trehalose-6-phosphate hydrolase | 6 ± 1.1 | 0.45 ± 0.05 | |
| udp | Uridine phosphorylase | 10 ± 1.5 | 0.62 ± 0.02 | 0.08 ± 0 |
| ybeK | Pyrimidine-specific nucleoside hydrolase | 10.2 ± 0.55 | 1.6 ± 0.2 | |
| Genes encoding proteins with diverse functions | ||||
| cspA | CspA | 4 ± 0.3 | 1 ± 0.1 | |
| cspB | CspB | 9 ± 0.4 | 1 ± 0.1 | |
| cspG | CspG | 6 ± 0.5 | 0.8 ± 0.07 | |
| cspl | Cspl | 2 ± 0.03 | 0.7 ± 0.05 | |
| dps | DNA-binding protein Dps | 10 ± 1.3 | 0.9 ± 0.015 | 0.05 ± 0.01 |
| grxB | Glutaredoxin 2 | 4.7 ± 0.4 | 1 ± 0.06 | |
| hns | DNA-binding protein H-NS | 4.8 ± 0.8 | 2.4 ± 0.5 | 0.5 ± 0.1 |
| hobH | DNA binding protein, replication-origin specific | 10 ± 2 | 2 ± 0.35 | 0.15 ± 0.07 |
| htpG | Heat shock protein C62.5 | 4.5 ± 0.5 | 1.6 ± 0.4 | 0.27 ± 0.005 |
| kbl | 2-Amino-3-ketobutyrate coenzyme A ligase | 4.8 ± 0.24 | 1.8 ± 0.2 | |
| mdaA | Modulator of drug activity A | 11.6 ± 0.06 | 4 ± 0.9 | |
| mlc | Making large colonies protein | 6.8 ± 0.29 | 2.5 ± 0.67 | |
| mopA | GroEL protein | 9.3 ± 0.88 | 1.3 ± 0.21 | 0.13 ± 0.01 |
| mopB | GroES protein | 8 ± 1 | 0.9 ± 0.02 | 0.18 ± 0.04 |
| ppiA | Peptidyl-prolyl-cis-trans-isomerase A precursor | 4.6 ± 0.51 | 1.7 ± 0.24 | 0.22 ± 0.06 |
| rimJ | Ribosomal-protein-alanine acetyltransferase | 6.5 ± 0.74 | 2.7 ± 0.03 | |
| sseA | Putative thiosulfate sulfurtransferase | 7.6 ± 1.2 | 1.8 ± 0.15 |
TABLE 2.
Genes showing transient repression upon cold shock in the wild-type strain
| Gene name and role | Gene product and/or function | 15°C/37°C ratio at 1 h | 15°C/37°C ratio at 5 h |
|---|---|---|---|
| Genes involved in membrane synthesis/function | |||
| fecA | Iron(III) dicitrate transport protein FecA precursor | 0.02 ± 0.005 | 0.22 ± 0.001 |
| fecB | Iron(III) dicitrate-binding periplasmic protein precursor | 0.04 ± 0.01 | 0.3 ± 0.08 |
| fecC | FecC protein | 0.02 ± 0 | 0.9 ± 0.2 |
| fecE | Membrane-bound iron (III) dicitrate transport protein | 0.01 ± 0.005 | 0.9 ± 0.07 |
| fepC | Ferric enterobactin transport protein FepC | 0.14 ± 0.03 | 0.7 ± 0.06 |
| fimD | FimD protein | 0.29 ± 0.04 | 1.9 ± 0.13 |
| kgtP | Alpha-ketoglutarate permease | 0.3 ± 0.02 | 0.45 ± 0.01 |
| lgt | Prolipoprotein diacylglyceryl transferase | 0.25 ± 0.005 | 0.54 ± 0.12 |
| lolA | Outer membrane lipoproteins carrier protein precursor | 0.27 ± 0.005 | 0.6 ± 0.04 |
| msbA | MsbA protein | 0.26 ± 0.015 | 0.6 ± 0.02 |
| nlpD | Lipoprotein D precursor | 0.7 ± 0.02 | 0.26 ± 0.02 |
| oppB | Oligopeptide transport system permease protein | 0.31 ± 0 | 1.1 ± 0.1 |
| oppC | Oligopeptide permease membrane protein | 0.27 ± 0.01 | 1.5 ± 0.2 |
| plsX | PlsX protein | 0.16 ± 0.03 | 1 ± 0.005 |
| potA | Spermidine/putrescine transport protein A | 0.22 ± 0.01 | 0.45 ± 0.08 |
| potB | Spermidine/putrescine transport system permease protein PotB | 0.12 ± 0.01 | 0.31 ± 0.045 |
| potC | Spermidine/putrescine transport system permease protein PotC | 0.19 ± 0.025 | 0.31 ± 0.015 |
| proP | Proline/betaine transport protein | 0.17 ± 0.01 | 0.48 ± 0.01 |
| proV | Glycine betaine/I-proline transport ATP-binding protein ProV | 0.01 ± 0.005 | 0.06 ± 0.005 |
| proW | Glycine betaine/proline transport system protein prow | 0.04 ± 0 | 0.07 ± 0.015 |
| proX | Glycine betaine-binding periplasmic protein precursor | 0.08 ± 0.01 | 0.11 ± 0.01 |
| secG | P12 cytoplasmic membrane protein | 0.3 ± 0.005 | 0.57 ± 0.05 |
| tolA | TolA protein | 0.29 ± 0.025 | 1.2 ± 0.2 |
| trkH | TrkH protein | 0.33 ± 0 | 1.3 ± 0.2 |
| wzxE | Lipopolysaccharide biosynthesis protein | 0.19 ± 0 | 0.88 ± 0.03 |
| Genes involved in cell metabolism | |||
| aceB | Malate synthase A (Msa) | 0.04 ± 0 | 0.1 ± 0.005 |
| adhC | Formaldehyde dehydrogenase (glutathione) | 0.26 ± 0.035 | 0.45 ± 0.045 |
| argB | Acetylglutamate kinase | 0.18 ± 0.01 | 0.75 ± 0.01 |
| argC | N-acetyl-gamma-glutamyl-phosphate reductase | 0.12 ± 0.04 | 3 ± 0.4 |
| argD | Acetylomithine aminotransferase | 0.14 ± 0.02 | 0.3 ± 0.1 |
| argG | Argininosuccinate synthase | 0.15 ± 0.04 | 1.3 ± 0.15 |
| argH | Argininosuccinate lyase | 0.25 ± 0.08 | 0.4 ± 0.05 |
| aroA | 3-Phosphoshikimate 1-carboxyvinyltransferase | 0.2 ± 0.01 | 0.45 ± 0.07 |
| aroB | 3-Dehydroquinate synthase | 0.3 ± 0.015 | 1.2 ± 0.24 |
| cmk | Cytidylate kinase (cytidine monophosphate kinase) | 0.24 ± 0.02 | 0.4 ± 0.01 |
| ddg | Ddg protein | 0.1 ± 0.01 | 1.4 ± 0.01 |
| fdhE | FdhE protein | 0.27 ± 0 | 0.7 ± 0.6 |
| folP | Dihydropteroate synthase | 0.29 ± 0.03 | 0.5 ± 0.02 |
| glcF | Glycolate oxidase iron-sulfur subunit | 0.07 ± 0.005 | 1.7 ± 0.27 |
| gltB | Glutamate synthase (NADPH) large chain precursor | 0.12 ± 0.02 | 0.5 ± 0.005 |
| hisF | Cyclase HisF | 0.3 ± 0.02 | 0.8 ± 0.2 |
| iclR | Repressor protein IclR | 0.08 ± 0.01 | 0.56 ± 0.005 |
| phoH | PhoH protein | 0.5 ± 0.07 | 0.08 ± 0.005 |
| psd | Phosphatidylserine decarboxylase precursor | 0.32 ± 0 | 0.43 ± 0.01 |
| rffH | Glucose-1-phosphate thymidylyltransferase | 0.2 ± 0.01 | 0.55 ± 0.05 |
| thil | Thiamin biosynthesis protein | 0.27 ± 0.09 | 1.7 ± 0.5 |
| thyA | Thymidylate synthase | 0.28 ± 0 | 0.45 ± 0.02 |
| truA | Pseudouridylate synthase I | 0.31 ± 0.01 | 0.7 ± 0.08 |
| Genes encoding proteins with diverse functions | |||
| dedA | DedA protein | 0.29 ± 0.02 | 0.45 ± 0.07 |
| dedE | DedE protein | 0.3 ± 0.025 | 0.5 ± 0.09 |
| dinG | Probable ATP-dependent helicase DinG | 0.24 ± 0.003 | 1.4 ± 0.13 |
| dnaG | DNA primase | 0.2 ± 0.015 | 1.1 ± 0.13 |
| fkpA | FkpA protein | 0.29 ± 0.005 | 0.47 ± 0.005 |
| fldB | Flavodoxin | 0.32 ± 0.005 | 1.26 ± 0 |
| ftsH | Cell division protein FtsH, protease | 0.25 ± 0.01 | 0.9 ± 0.06 |
| ftsJ/rrmJ | Cell division protein, 23S rRNA methyltransferase | 0.29 ± 0.015 | 1.2 ± 0.12 |
| ftsK | Cell division protein FtsK | 0.2 ± 0.01 | 0.9 ± 0.07 |
| gidA | GidA protein | 0.24 ± 0.01 | 0.8 ± 0.09 |
| hflC | HflC protein | 0.22 ± 0.02 | 0.54 ± 0 |
| hflK | HflK protein | 0.25 ± 0.01 | 0.7 ± 0.06 |
| holA | DNA-directed DNA polymerase III delta chain | 0.31 ± 0.005 | 0.7 ± 0.12 |
| hscA | Heat shock cognate protein 66 | 0.09 ± 0.01 | 0.7 ± 0.13 |
| ksgA | Dimethyladenosine transferase | 0.24 ± 0.04 | 0.75 ± 0.07 |
| lepA | GTP-binding protein LepA | 0.28 ± 0.02 | 0.5 ± 0.03 |
| mrcA | Penicillin-binding protein 1a (pbp-1a) | 0.28 ± 0.02 | 1.3 ± 0.01 |
| mrdB | Rod shape-determining protein MrdB | 0.18 ± 0.02 | 0.74 ± 0.02 |
| pepB | Peptidase B | 0.17 ± 0 | 0.3 ± 0.06 |
| priA | Primosomal replication factor Y | 0.27 ± 0.015 | 1.1 ± 0.3 |
| recB | Exodeoxyribonuclease V 135-kDa polypeptide | 0.22 ± 0.01 | 1.2 ± 0.03 |
| recC | Exodeoxyribonuclease V 125-kDa polypeptide | 0.32 ± 0.01 | 0.9 ± 0.06 |
| recJ | Single-stranded-DNA-specific exonuclease | 0.3 ± 0.02 | 1.2 ± 0.17 |
| rimM | 16S rRNA processing protein | 0.16 ± 0.005 | 0.47 ± 0 |
| mhA | Ribonuclease HI | 0.26 ± 0.02 | 0.85 ± 0.15 |
| rpoA | DNA-directed RNA polymerase alpha chain | 0.23 ± 0.03 | 0.6 ± 0.03 |
| rpoB | DNA-directed RNA polymerase beta chain | 0.18 ± 0.005 | 0.4 ± 0.02 |
| rrmA | rRNA (guanine-N1)-methyltransferase | 0.25 ± 0.045 | 0.15 ± 0.025 |
| rtn | Rtn protein | 0.28 ± 0.01 | 1.65 ± 0.13 |
| sdiA | SdiA regulatory protein | 0.26 ± 0.025 | 0.44 ± 0.005 |
| sodA | Superoxide dismutase | 0.26 ± 0.01 | 0.07 ± 0.01 |
| tnpR | Resolvase | 0.08 ± 0.005 | 0.4 ± 0.1 |
| topA | DNA topoisomerase I | 0.23 ± 0.025 | 0.35 ± 0.04 |
| trmD | tRNA (guanine-n1)-methyltransferase | 0.17 ± 0.005 | 0.42 ± 0.02 |
TABLE 3.
Genes showing prolonged induction upon cold shock
| Gene name and role | Gene product and/or function | Wild-type 15°C/37°C ratio at 1 h | Wild-type 15°C/37°C ratio at 5 h | Mutant/ wild-type ratio (15°C, 1 h) |
|---|---|---|---|---|
| Genes involved in membrane synthesis/function | ||||
| cheW | Chemotaxis protein CheW, adapter protein | 3.5 ± 0.05 | 5.4 ± 0.01 | |
| cheY | Chemotaxis protein CheY | 4.3 ± 0.09 | 3.6 ± 0.51 | |
| dcuA | Anaerobic C4-dicarboxylate transporter DcuA | 7.4 ± 0.36 | 7.3 ± 0.7 | |
| dmsC | Dimethylsulfoxide reductase chain C | 4.3 ± 0.09 | 7 ± 1 | |
| flg and fli | Flagellar proteins | 2.0-12 ± 0.5 | 4.0-13 ± 1 | 0.01-0.1 ± 0 |
| frdA | Fumarate reductase flavoprotein subunit | 20 ± 0.5 | 15 ± 1 | 0.2 ± 0.02 |
| frdB | Fumarate reductase iron-sulfur protein | 17 ± 0.9 | 11 ± 0.9 | |
| frdD | Fumarate reductase, 13-kDa membrane anchor protein | 6 ± 0.55 | 3.5 ± 0.7 | |
| glpQ | Glycerophosphodiester phosphodiesterase | 4.3 ± 0.13 | 5.5 ± 1 | |
| glpT | Glycerol-3-phosphate transport protein | 7.8 ± 0.33 | 6.1 ± 0.36 | |
| hybA | HybA protein | 8.4 ± 0.92 | 8 ± 1 | |
| hybB | HybB protein | 4.6 ± 0.23 | 11 ± 1.5 | |
| hybC | HybC protein | 4.5 ± 0.13 | 6 ± 0.6 | |
| hypB | Hydrogenase isoenzymes formation protein | 17.8 ± 1.37 | 17 ± 1.5 | |
| hypE | HypE protein | 5.3 ± 0.12 | 13 ± 1.3 | |
| nupG | Nucleoside-transporting protein NupG | 18.6 ± 2.28 | 15 ± 1.4 | |
| ompC | Outer membrane protein C precursor | 5 ± 0.1 | 4 ± 0.08 | 0.24 ± 0.02 |
| ompT | Proteinase VII precursor | 19.7 ± 1.25 | 57 ± 6 | |
| tap | Methyl-accepting chemotaxis protein II | 4.2 ± 0.12 | 4.8 ± 0.4 | |
| tar | Methyl-accepting chemotaxis protein II | 11.1 ± 0.85 | 7.8 ± 1 | |
| Genes involved in cell metabolism | ||||
| ackA | Acetate kinase | 4.2 ± 0 | 5.5 ± 0.02 | |
| ansB | Asparaginase | 10.8 ± 0.16 | 25.3 ± 3 | |
| asnB | Asparagine synthase | 5.1 ± 0.34 | 6 ± 2 | |
| cydA | Cytochrome d ubiquinol oxidase subunit I | 3.8 ± 0.35 | 5.7 ± 0.9 | |
| ftn | Ferritin | 13 ± 1 | 13 ± 0.9 | |
| fumB | Fumarate hydratase | 9.6 ± 1.34 | 9.6 ± 1.5 | |
| glpB | Glycerol-3-phosphate dehydrogenase | 20.3 ± 1.43 | 14.8 ± 0.28 | |
| glpC | Glycerol-3-phosphate dehydrogenase | 27.4 ± 3.7 | 14.1 ± 2.5 | |
| pflB | Formate C-acetyltransferase | 5.6 ± 0.46 | 5.5 ± 1.5 | |
| wrbA | Trp repressor binding protein | 15 ± 2 | 8 ± 1 | |
| Genes encoding proteins with diverse functions | ||||
| clpB | ClpB protein (heat shock protein) | 10.4 ± 0.4 | 9.4 ± 0.39 | |
| gst | Glutathione transferase | 7.4 ± 0.36 | 5.1 ± 1 | |
| hsdR | Type I restriction enzyme EcoKI R protein | 9 ± 1 | 9.7 ± 0.92 | |
| katG | Catalase HPI | 17 ± 0.06 | 12.3 ± 1 | |
| speG | Spermidine n1-acetyltransferase | 3 ± 0.25 | 5 ± 0.38 |
Genes transiently induced and repressed in the acclimation phase upon cold shock.
The present analysis showed transient induction of a number of known cold shock-inducible genes, for example, cspA, cspB, cspG, cspI, otsA, otsB, and ppiA (Table 1). Other known genes such as gyrA (twofold), infA (twofold), infC (2.8-fold), and recA (threefold) were also induced. New genes shown by the present analysis to be cold shock-inducible in the acclimation phase include the following: (i) transport or metabolism of sugars (fructose, glucose, glycerol, maltose, mannose, ribose, and xylose) and (ii) molecular chaperones (mopA and mopB, encoding GroEL and GroES, respectively, htpG, and ppiA). Deletion of four csp genes led to repression of cold shock induction of all these genes (Table 1). Although cold shock response is characterized by strong repression of the major metabolic activity of the cell, the present study showed induction of several new genes after the temperature downshift. Transport and metabolism systems for sugars deserve special mention in this aspect. Cold shock caused induction of otsA (trehalose-6-phosphate synthase) and otsB (trehalose-6-phosphate phosphatase) (Table 1), consistent with the previously reported possible protective effect of this sugar upon cold shock (14). However, such a protective effect is not known for sugars such as ribose or mannose that were induced in the present system, and this induction could simply be a manifestation of the cell gearing up for the low-temperature-adapted metabolism. It should be noted that recently, cold stress accumulation and protective effect of maltose in plants was reported (16). It is interesting that cold shock induction of mannose and maltose transport systems was prominently repressed in the quadruple deletion that has significantly prolonged (4 h) lag period as opposed to the 1-h lag period of the wild-type strain (33) after the temperature downshift. This suggests that cold shock induction of these genes is indeed relevant for the cold acclimation of the cells.
As the cold shock response of the quadruple-deletion strain was severely affected, many genes repressed in the wild type upon cold shock were further repressed in the deletion strain. In addition to the genes listed in Table 1, the genes repressed in the quadruple-deletion strain included those involved in transport (ATP synthase, DctA protein, DsdX permease, fatty acid transport protein, maltoporin precursor, OmpF, OmpX, thiamine-binding protein precursor, and tryptophan permease) and a number of genes involved in cellular metabolism (especially amino acids and sugars).
On the other hand, a few genes were transiently induced in the quadruple-deletion strain compared with the wild-type strain, and these prominently constitute the genes encoding proteins involved in transport of iron, such as exbD, fecA to fecD, fepC, and fhuA and fhuF (Table 4). It is not clear why deletion of csp genes should result in induction of iron transport. It is noteworthy that these genes were repressed in the wild-type cells upon temperature downshift. In fact, judging from Table 4, a very divergent group of genes was induced by deletion of the four csp genes.
TABLE 4.
Genes induced by cold shock in the quadruple-deletion strain
| Gene name and role | Gene product and/or function | Mutant/wild type ratio (15°C, 1 h) |
|---|---|---|
| Genes involved in membrane synthesis/function | ||
| exbD | ExbD protein | 4.4 ± 0.2 |
| fecA | Iron(III) dicitrate transport protein FecA precursor | 4 ± 0.21 |
| fecB | Iron(III) dicitrate-binding periplasmic protein precursor | 12.5 ± 1.1 |
| fecC | FecC protein | 4 ± 0.2 |
| fecD | Iron(III) dicitrate transport system permease protein | 5.2 ± 0.18 |
| fecE | Membrane-bound iron(III) dicitrate transport protein | 5.8 ± 0.4 |
| fepC | Ferric enterobactin transport protein FepC | 3.2 ± 0.08 |
| fhuA | Ferrichrome-iron receptor precursor | 3.9 ± 0.4 |
| fhuF | Ferric hydroxamate transport protein | 7 ± 0.2 |
| livK | Leucine transport protein LivK precursor | 4.8 ± 0.4 |
| Genes involved in cell metabolism | ||
| acpS | Holo-[acyl-carrier protein] synthase | 3 ± 0.1 |
| aroE | Shikimate 5-dehydrogenase | 4.3 ± 0.25 |
| aroG | Phospho-2-dehydro-3-deoxyheptonate aldolase | 4.2 ± 0.3 |
| cobU | Cobinamide kinase | 3.9 ± 0.2 |
| ddg | Ddg protein | 5.4 ± 0.4 |
| gltD | Glutamate synthase | 5.5 ± 0.18 |
| gltF | GltF protein | 4.8 ± 0.4 |
| Genes encoding proteins with diverse functions | ||
| creA | CreA protein | 5.1 ± 0.05 |
| deaD | ATP-dependent RNA helicase | 5 ± 1 |
| fimE | Type 1 fimbriae regulatory protein FimE | 5.5 ± 1 |
| fimF | FimF protein | 7 ± 1.1 |
| hha | Hha protein | 3.6 ± 0.5 |
| intA | Prophage cp4-57 integrase | 3.5 ± 0.19 |
| rhlE | Putative ATP-dependent RNA helicase RhIE | 4.1 ± 0.4 |
| rpoE | RNA polymerase sigma-E factor (sigma-24) | 4.6 ± 0.44 |
| rseB | RseB protein | 3.2 ± 0.5 |
| rseC | Sigma-E factor regulatory protein | 5.5 ± 0.4 |
| sdiA | SdiA regulatory protein | 3.5 ± 0.12 |
| tnpR | Resolvase | 5.1 ± 0.6 |
In addition to the genes listed in Table 2, most of the genes encoding ribosomal L proteins showed transient reduced levels during acclimation phase in the wild-type strain, although in the latter the effect was not severe (approximately two- to threefold) and their synthesis recovered after continued growth at 15°C for 5 h. This result is consistent with cold shock two-dimensional gel electrophoresis data published for E. coli (26).
In addition, the products of a number of genes, such as ybdQ, ycfP, ydaA, ydhO, yeaA, yedU, yeeX, yefI, yfbU, yfiA, yfjL, yggG, ygjR, yhbT, yhjH, yieP, yqeB, yqhD, and yzzQ, increased significantly, although the products have not been assigned any functions. On the other hand, priB, yaeG, yafK, ybiR, ybiT, ycaJ, ycaO, yccA, yceD, yceP, ycfC, ycfV, ycfX, ycgE, ydgR, ydiU, yedA, yedl, yfcA, yfK, yfgL, yfgM, yfiH, yfiR, ygdE, yggN, yhaD, yhaE, yhaF, yhaU, yhbM, yheQ, yhiN, yjgP, yqgE, yqgF, and yrbE were repressed.
Genes showing prolonged induction and repression upon cold shock.
Genes encoding flagellar proteins were induced and maintained at high levels even after 5 h at 15°C in the wild-type strain and were down-regulated in the quadruple-deletion strain. Spermidine acetyltransferase encoded by speG is required to prevent spermidine toxicity at low temperatures in E. coli (18). Our DNA microarray analysis showed a steady increase in speG levels from three- to fivefold at 1 to 5 h after temperature downshift (Table 3). On the other hand, genes such as tas (Tas protein), artP (ArtP protein), those mainly involved in amino acid and nucleotide biosynthesis, such as trpB, and leu, pur, and pyr operon genes were repressed even 5 h after cold shock. All of these showed further down-regulation in the quadruple-deletion strain.
Cold shock induction of genes encoding heat shock proteins.
Protein misfolding was previously not considered a major problem upon cold shock. But increasing numbers of recent reports of a heat shock protein being induced by cold shock even in higher systems suggest that proper folding of proteins as well as refolding of cold-damaged proteins is important after cold shock. However, in most of these cases the heat shock induction of proteins is after prolonged incubation at low temperature (10, 28). On the other hand, in the present study, a number of genes encoding heat shock-inducible proteins and molecular chaperones such as htpG, mopA, mopB, and ppiA (encoding HtpG, GroEL, GroES, and peptidyl-prolyl-cis-trans-isomerase, respectively) showed transient induction immediately following cold shock. ClpB, which is both heat and cold shock-inducible in Synechococcus sp. strain PCC 7942 (28) was induced 10-fold and maintained at this level even at 5 h after temperature downshift (Table 3). ppiA, encoding peptidyl-prolyl-cis-trans-isomerase, is also reported from Bacillus, is involved in accelerating proline-limited steps in protein folding, and is important in helping protein folding at low temperatures (7, 8). Trigger factor encoded by tig is another interesting chaperone, which is moderately induced 2 to 3 h after cold shock (15). It is not included in Table 1, as it does not fulfill the criteria of the required n-fold increase; however, we did find moderate (1.7-fold) induction of this gene. Previously, it was also shown that when E. coli is grown at 16°C, GroEL expression is reduced (15); however, in that study the cells were grown to an OD600 of 0.5, and then the protein expression was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In the present study, the immediate effect of cold shock is being analyzed (1 h). Both mopA and mopB were induced 9.3- and 8-fold after 1 h and then reduced to a basal level of 1.3- and 0.9-fold, respectively, after 5 h. Thus, our data are consistent with the low level of GroEL observed by these authors after cells reached the OD600 of 0.5 at 16°C. Note that the OD600 increased by 0.4 at 5 h after cold shock in the present study. This suggests that the GroELS system is transiently induced immediately after the temperature downshift, along with the induction of CspA homologues, and is then reduced to a basal level. It should be mentioned that GroEL is induced in E. coli at 37°C by the overexpression of CspC and CspE, although this induction is lesser than its heat shock induction (22). On the same note, the present study showed that deletion of four CspA homologues leads to repression of cold shock induction of mopA and mopB (Table 1). This suggests a possibility that cold shock induction of GroEL may be linked to the higher levels of CspA homologues.
Comparison of cold shock response of E. coli and B. subtilis.
DNA microarray analysis of the cold shock response of B. subtilis has been carried out by two groups (2, 13). Our study showed that there are a number of common genes such as leuBCD (amino acid biosynthesis) and purBCDEFHKLMN (purine biosynthesis) that are affected by cold shock in E. coli and Bacillus spp. Other such examples include topA (DNA topoisomerase I), gltB, arg, and aro genes (amino acid biosynthesis) (Table 2). There are certain genes that are not included in the tables, as their ratios do not fulfill the criteria of the required n-fold difference; however, these are worth mentioning as they are affected by cold shock in Bacillus spp. The genes and the respective n-fold differences are as follows: (i) amino acid biosynthesis, aroF and aroH (0.5 and 0.6, respectively), metC (0.7), and serC (0.6); (ii) tRNA synthetases, aspS (0.6), hisS (0.5), and thrS (0.75); (iii) NAD biosynthesis, nifS (0.7) and nadC (0.7); (iv) ATP synthase, atpA, atpB, atpE, atpF, atpH, and atpI (approximately 0.5); (vi) pyrimidine biosynthesis, pyrC (0.75); (vii) citric acid cycle, sdhC (0.8); and (viii) metabolism, bioA and bioD (0.5 to 0.6) and ptb (2). However, there were also differences between E. coli and Bacillus cold shock response. For example, in the case of Bacillus spp., the ribosomal proteins were induced by cold shock, while in E. coli, these were either repressed or showed no significant change. One of these two analyses in Bacillus showed repression of GroEL 70 min after cold shock (2), while the present study shows induction of the GroELS system in E. coli 1 h after cold shock. This suggests that in spite of common basic principles in the cold shock response of E. coli and Bacillus spp., there are certain distinct differences.
CspA homologues are needed at acclimation phase.
At low temperature, the secondary structures of RNA stabilize, which should slow down (i) transcription elongation and (ii) ribosomal movement on RNA and thus translation. The Csps are transiently and dramatically induced in the acclimation phase upon cold shock. These presumably act as RNA chaperones (1, 9, 11, 23, 25) by destabilizing the secondary structures in RNA and thus facilitating transcription and translation. Increased levels of CspA homologues after cold shock may be important for compensating for higher stability of secondary structures in RNA at low temperatures (11). The RNA chaperone effect of CspA homologues is apparent in the present microarray analysis, as cold shock induction of a number of diverse genes was repressed by deletion of four csp genes. These may be the genes that need help to transcribe and translate efficiently at low temperature, possibly due to stabilization of secondary structures in their mRNAs, and the high level of Csps ensures their effective production. It is noteworthy that, except for the flagellar operon, deletion of four csp genes mainly affected genes that are transiently induced during acclimation phase. This emphasizes the need for the RNA chaperones immediately upon cold shock, and once the cells are acclimated to cold, their presence is no longer required. This is supported by the observations that cold shock induction of Csps is transient and the quadruple-deletion strain shows a prolonged lag period after cold shock. Further studies on the effect of Csps on the transcription and translation of genes, especially those encoding GroELS, maltose, the ribose operon, and flagellar proteins, should prove to be useful in this aspect.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM 19043) to M.I.
We thank Takara Bio Inc. Japan for DNA array chips and help in analysis of the array scans. We also thank Anirvan Sengupta and Ruadhan O'Flanagan for their help in statistical analysis of the data and K. V. Chin for his useful suggestions in the scanning of the DNA arrays.
REFERENCES
- 1.Bae, W., B. Xia, M. Inouye, and K. Severinov. 2000. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc. Natl. Acad. Sci. USA 97:7784-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckering, C. L., L. Steil, M. H. Weber, U. Volker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, A. W., S. D. Buckel, J. M. Groarke, J. N. Hope, D. H. Kingsley, and M. A. Hermodson. 1986. The nucleotide sequences of the rbsD, rbsA, and rbsC genes of Escherichia coli K12. J. Biol. Chem. 261:7652-7658. [PubMed] [Google Scholar]
- 4.Boos, W., and H. Shuman. 1998. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62:204-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermolenko, D. N., and G. I. Makhatadze. 2002. Bacterial cold-shock proteins. Cell. Mol. Life Sci. 59:1902-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein, J., N. S. Pollitt, and M. Inouye. 1990. Major cold shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graumann, P., K. Schroder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:203-209. [PubMed] [Google Scholar]
- 9.Graumann, P. L., and M. A. Marahiel. 1998. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 23:286-290. [DOI] [PubMed] [Google Scholar]
- 10.Hossain, M. M., and H. Nakamoto. 2002. HtpG plays a role in cold acclimation in cyanobacteria. Curr. Microbiol. 44:291-296. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, W., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272:196-202. [DOI] [PubMed] [Google Scholar]
- 12.Jones, P. G., R. A. VanBogelen, and F. C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 169:2092-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaan, T., G. Homuth, U. Mader, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 14.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandror, O., and A. L. Goldberg. 1997. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc. Natl. Acad. Sci. USA 94:4978-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan, F., and C. L. Guy. 2004. Beta-amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 135:1674-1684. (First published 9 July 2004; 10.1104/pp.104.040808.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, S. J., A. Xie, W. Jiang, J. P. Etchegaray, P. G. Jones, and M. Inouye. 1994. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol. Microbiol. 11:833-839. [DOI] [PubMed] [Google Scholar]
- 18.Limsuwun, K., and P. G. Jones. 2000. Spermidine acetyltransferase is required to prevent spermidine toxicity at low temperatures in Escherichia coli. J. Bacteriol. 182:5373-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindler, L. E. 1994. Nucleotide sequence of the Escherichia coli groE promoter. Gene 146:129-130. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima, K., K. Kanamaru, T. Mizuno, and K. Horikoshi. 1996. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phadtare, S. 2004. Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 6:125-136. [PubMed] [Google Scholar]
- 22.Phadtare, S., and M. Inouye. 2001. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J. Bacteriol. 183:1205-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phadtare, S., M. Inouye, and K. Severinov. 2002. The nucleic acid melting activity of Escherichia coli CspE is critical for transcription antitermination and cold acclimation of cells. J. Biol. Chem. 277:7239-7245. [DOI] [PubMed] [Google Scholar]
- 24.Phadtare, S., I. Kato, and M. Inouye. 2002. DNA microarray analysis of the expression profile of Escherichia coli in response to treatment with 4,5-dihydroxy-2-cyclopenten-1-one. J. Bacteriol. 184:6725-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phadtare, S., S. Tyagi, M. Inouye, and K. Severinov. 2002. Three amino acids in Escherichia coli CspE surface-exposed aromatic patch are critical for nucleic acid melting activity leading to transcription antitermination and cold acclimation of cells. J. Biol. Chem. 277:46706-46711. [DOI] [PubMed] [Google Scholar]
- 26.Phadtare, S., Yamanaka, K., and M. Inouye. 2000. The cold shock response, p. 33-45. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 27.Polissi, A., W. De Laurentis, S. Zangrossi, F. Briani, V. Longhi, G. Pesole, and G. Deho. 2003. Changes in Escherichia coli transcriptome during acclimatization at low temperature. Res. Microbiol. 154:573-580. [DOI] [PubMed] [Google Scholar]
- 28.Porankiewicz, J., and A. K. Clarke. 1997. Induction of the heat shock protein ClpB affects cold acclimation in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 179:5111-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pressler, U., H. Staudenmaier, L. Zimmermann, and V. Braun. 1988. Genetics of the iron dicitrate transport system of Escherichia coli. J. Bacteriol. 170:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, vol. 2. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 31.Wang, N., K. Yamanaka, and M. Inouye. 1999. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber, M. H., and M. A. Marahiel. 2003. Bacterial cold shock responses. Sci. Prog. 86:9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia, B., H. Ke, and M. Inouye. 2001. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol. Microbiol. 40:179-188. [DOI] [PubMed] [Google Scholar]
- 34.Xia, B., H. Ke, W. Jiang, and M. Inouye. 2001. The Cold Box stem-loop proximal to the 5′-end of the Escherichia coli cspA gene stabilizes its mRNA at low temperature. J. Biol. Chem. 277:6005-6011. [DOI] [PubMed] [Google Scholar]
- 35.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]