Abstract
Aims
The tonographic effect is a phenomenon of intraocular pressure (IOP) reduction following repeated tonometry. This study examines whether the tonographic effect occurs following IOP measurement performed with Ocular Response Analyzer (ORA).
Methods
Both eyes of 31 glaucoma patients and 35 healthy controls underwent nine IOP-measurements performed with GAT and ORA. The number of GAT and ORA measurements performed on each eye differed depending on the randomly allocated investigation scheme. Central corneal thickness (CCT), anterior chamber volume (ACV) and anterior chamber depth (ACD) were assessed with Pentacam before and after the repeated GAT/ORA measurements.
Results
There was no statistically significant tonographic effect for IOP readings obtained by the ORA: corneal compensated intraocular pressure (IOPcc) (-0.11 ± 3.06 mmHg, p = 0.843 in patients and -0.71 ± 3.28 mmHg, p = 0.208 for controls) and Goldmann-correlated intraocular pressure (IOPg) (-0.31 ± 2.38 mmHg, p = 0.469 in patients and -0.31 ± 2.37 mmHg, p = 0.441 in controls) measured with ORA. There was a significant IOP reduction from the first to the second GAT measurement, i.e. tonographic effect (-0.55 ± 2.00 mmHg, p = 0.138 in patients and -1.15 ± 1.52 mmHg, p < 0.001 in controls). CCT, corneal hysteresis (CH) and corneal resistance factor (CRF) were lower in glaucoma patients. The repeated IOP measurements resulted in an increase of CCT in all subjects (but no change of ACV and ACD). The tonographic effect of GAT correlated with CCT in glaucoma patients (r = 0.37).
Conclusion
In contrast to GAT, repeated ORA measurements do not result in the tonographic effect. Repeated IOP measurements resulted in an increase of central corneal thickness, but did not influence the volume and depth of anterior chamber.
Introduction
The measurement of intraocular pressure (IOP) is an important means to detect and manage glaucoma [1, 2]. The gold standard for IOP measurement still is Goldmann applanation tonometry (GAT). There are multiple sources of error that may influence the accuracy of GAT measurements [3, 4]. One of the known phenomena is the reduction of the IOP through repeated applanation, also called “tonographic effect” [5–11]. Stocker [5] mentions the amount of aqueous humor pressed out of the anterior chamber as one of the reasons for the IOP drop. However, he hypothesized that the mechanic process of forcing out the aqueous humor alone cannot explain the tonographic phenomenon, but suspected a reflectory change of aqueous formation as a further variable. Moses [6] suspected topical anesthetics as being relevant for the tonographic effect.
In order to increase the measurement reliability in clinical trials, a mean value of several repeated IOP-measurements is used [12–15]. Likewise, young ophthalmologists or optometrists at the beginning of their learning curve often need more tries to measure IOP properly. However, repeated GAT measurements bear the risk of false results through the tonographic effect.
Ocular Response Analyzer (ORA) is an IOP measurement technique, which utilizes a visco-elastic structure of the human corneal tissue in a non-contact way. ORA provides both: IOP and biomechanical properties of the cornea such as corneal hysteresis (CH) and corneal resistance factor (CRF). A corneal-compensated intraocular pressure (IOPcc) has been reported to be less affected by the corneal properties than GAT [16–18]. Furthermore, CH and CRF seem to be predictors for the development of glaucoma [19]. The lack of contact with the cornea eliminates the need for topical anesthetics and reduces the risk of cross-infection and corneal damage [20–22].
The ORA-measurements have shown to be reproducible in several studies [17, 18, 23]. There are only few studies evaluating an influence of repeated ORA measurements or ocular massage on the IOP in healthy subjects [17, 24, 25].
The purpose of our prospective, randomized clinical trial was to compare ORA and GAT regarding the tonographic effect in glaucoma patients and in healthy controls. Furthermore, we wanted to evaluate the possible correlation between the tonographic effect and parameters as central corneal thickness (CCT), anterior chamber volume (ACV), anterior chamber depth (ACD), CH, and CRF.
Materials and Methods
This prospective, randomized, single-center, open, controlled study with two parallel study groups was carried out in accordance with the Declaration of Helsinki. Ethics approval was obtained from the Ethics committee of Rhineland-Palatinate, Germany.
Glaucoma patients were recruited from the glaucoma section of the Department of Ophthalmology of the University Medical Center of the Johannes Gutenberg-University Mainz. In order to obtain similar study groups, sex- and age-matched healthy controls were recruited from the staff of the University Medical Center of Mainz and from the patients of a family practice in Mainz, Germany. After a detailed personal survey conversation, a written informed consent was obtained from all study participants. All subjects were then evaluated at the Department of Ophthalmology of the University Medical Center of the Johannes Gutenberg-University Mainz between December 2013 and March 2015. Inclusion criteria for both groups were: female or male of any race aged 18 years or older, ability to understand the character and individual consequences of the clinical trial, signed and dated informed consent must have been available before the start of any specific trial procedure; in the control group: normal ophthalmological status, no glaucoma or other relevant eye diseases, IOP ≤ 21 mmHg; in the patients´ group: diagnosis of primary open-angle glaucoma (POAG), pseudoexfoliation glaucoma (PEXG) or pigment dispersion glaucoma (PG) with characteristic alterations of the optic nerve head and correlating visual field deficiencies.
The exclusion criteria in both groups were: astigmatism > 2.0 diopters; spherical refraction > 3.0 diopters; CCT < 500 μm; CCT > 600 μm; pseudophakia; corneal, conjunctival, or intraocular inflammatory eye disease; use of contact lenses within three months before study examination; any corneal pathologic condition; history of previous ocular surgery.
Study procedure
All subjects underwent an assessment of an objective refraction, the best corrected visual acuity (BCVA) with Snellen charts, a slit lamp examination and indirect ophthalmoscopy in miosis.
Each eye underwent nine measurements of the IOP, with both GAT and ORA. The number of IOP measurements differed according to the examination scheme that was assigned in a randomized order to each patient or healthy control (Table 1). For instance, in scheme 1, the left eye (OS) was measured first with triple ORA, triple GAT, and then triple ORA again. Afterwards, the right eye (OD) was measured with triple GAT, triple ORA, and then triple GAT. The time lapse between measurements with different devices was kept as short as possible, i.e. between 25–130 seconds, depending on the mobility of the patients or individual positioning difficulties.
Table 1. Four examination schemes for IOP measurements.
| Scheme | 1st Eye | Period 1 | Period 2 | Period 3 | 2nd Eye | Period 1 | Period 2 | Period 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | OS | ORA | GAT | ORA | OD | GAT | ORA | GAT |
| 2 | OS | GAT | ORA | GAT | OD | ORA | GAT | ORA |
| 3 | OD | ORA | GAT | ORA | OS | GAT | ORA | GAT |
| 4 | OD | GAT | ORA | GAT | OS | ORA | GAT | ORA |
ORA, Ocular Response Analyzer; GAT, Goldmann applanation tonometry; OD, right eyes; OS, left eyes.
GAT was performed by one experienced ophthalmologist and the same calibrated tonometer was used through the whole study. ORA- and Pentacam measurements were performed by a medical student, who was experienced and trained in handling of these devices. The measurement of IOP with GAT required instilling oxybuprocaine-HCl/fluorescein-Na (Thilorbin®, OmniVision) eye drops in the lower conjunctival cul-de-sac in both eyes and was performed without pupil dilatation in all cases. The application of one anesthetizing eye drop was performed once per eye, regardless of whether the eye was measured with GAT once or twice. Each GAT measurement was performed three times and the mean value was taken for the statistical analysis.
The ORA (Reichert Inc., Depew, USA, software version 2.0) utilizes a visco-elastic structure of the human corneal tissue in a dynamic bi-directional applanation process. The difference in inward and outward pressure values is called CH and the average of both values provides Goldmann-correlated intraocular pressure (IOPg). Calculated on the basis of the measured CH, the ORA provides two other parameters: CRF and IOPcc. According to our study protocol, three measurements were performed and the value with the best wave-score was taken for the statistical analysis.
The Pentacam (Oculus Pentacam HR Typ 70900, Oculus, Weimar, Germany) is a device combining a slit illumination and Scheimpflug camera, which rotate together around the eye. The CCT, ACD and ACV were assessed before and after the IOP session. The average of three values was taken for further analysis.
Statistical analysis
The sample size was chosen such that six pairwise t-tests could be performed on a 5% significance level, controlling for multiple testing by using a Bonferroni correction, i.e. a significance level of 0.83% for six pairwise t-tests. A power of 81% was calculated for 70 study participants in order to be able to detect an IOP difference of 1 mmHg with a standard deviation of 3 mmHg. The statistical analysis was performed using Microsoft Excel 2007, IBM SPSS Statistics Version 20, and SAS 9.4. First, descriptive were obtained, i.e. absolute and relative frequencies for categorical variables, mean, standard deviation, minimum, maximum, median and quartiles for quantitative variables. Second, six confirmatory t-tests were performed to identify differences between measurements taken before and after ORA and GAT and between glaucoma patients and probands. The results of these confirmatory t-tests were complemented with a three-period crossover analysis employing a linear model. Moreover, several exploratory t-tests concerning IOP and corneal differences between glaucoma patients and probands were performed.
The Pearson correlation coefficient was used to assess correlations between IOP values (GAT-IOP, IOPg, IOPcc), corneal parameters (CH, CRF), and Pentacam measurements (CCT, ACV and ACD). A correlation coefficient ≥ 0.7 was considered a strong correlation; ≥ 0.5 was considered a moderate correlation; and < 0.5 a weak correlation.
Results
Both eyes of 31 glaucoma patients (21 female, aged 63.3 ± 8.2 years, range = 40–75 years) and sex- and age-matched 35 healthy probands (21 female, aged 65.2 ± 9.2 years, range = 44–84 years) were enrolled in this study. Originally, 35 patients were included. However, four patients were excluded after measurement values did not meet the inclusion criteria. Thirty patients were diagnosed with primary open angle glaucoma (POAG), while one patient was diagnosed with pseudoexfoliation glaucoma (PEXG). All patients and probands were phakic. All glaucoma patients were treated with topical antiglaucoma agents.
IOP values
Concerning mean GAT-IOP, IOPg, IOPcc, no differences between patients and healthy controls were identified (Table 2). In the case of multiple measurement sessions for the same eye, the mean of these sessions was employed.
Table 2. Mean IOP values ± standard deviation.
| GAT-IOP OD | GAT-IOP OS | IOPcc OD | IOPcc OS | IOPg OD | IOPg OS | |
|---|---|---|---|---|---|---|
| Patients | 13.86 ± 3.39 | 13.47 ± 3.19 | 16.23 ± 4.35 | 16.43 ± 4.18 | 14.32 ± 4.44 | 15.16 ± 5.01 |
| Probands | 13.90 ± 3.44 | 14.71 ± 2.66 | 16.40 ± 4.96 | 16.14 ± 4.53 | 15.95 ± 4.29 | 16.58 ± 4.56 |
| p-value | 0.960 | 0.091 | 0.881 | 0.788 | 0.134 | 0.231 |
GAT-IOP, Goldmann applanation tonometry; IOPcc, corneal-compensated IOP; IOPg, Goldmann-correlated IOP; OD, right eyes; OS, left eyes; in mmHg.
GAT-IOP was systematically lower than IOPcc: mean differences between IOPcc and GAT-IOP of 2.44 ± 3.07 mmHg for OD (p < 0.001) and 2.15 ± 3.37 mmHg for OS (p < 0.001) were found.
IOPcc, IOPg and GAT showed no differences between the right and the left eyes.
Tonographic effect
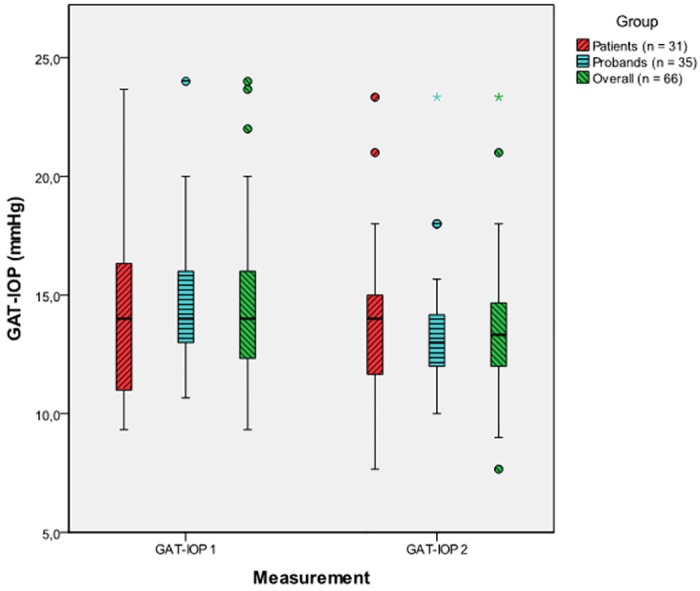
The significant mean IOP decrease from the first to the second GAT measurement was -0.87 ± 1.77 mmHg, p < 0.001 in the same eye for patients and probands. This was also confirmed by the results of the linear model. The average difference between the first and the second mean GAT-IOP was -0.55 ± 2.00 mmHg (p = 0.138) for the glaucoma patients and -1.15 ± 1.52 mmHg (p < 0.001) for controls (Fig 1). No significant difference between glaucoma patients and healthy probands concerning the magnitude of the tonographic effect could be found (p = 0.196).
Fig 1. Tonographic effect from the first (GAT-IOP 1) to the second (GAT-IOP 2) GAT measurement for patients, probands and the overall sample (patients and probands combined), [mmHg].
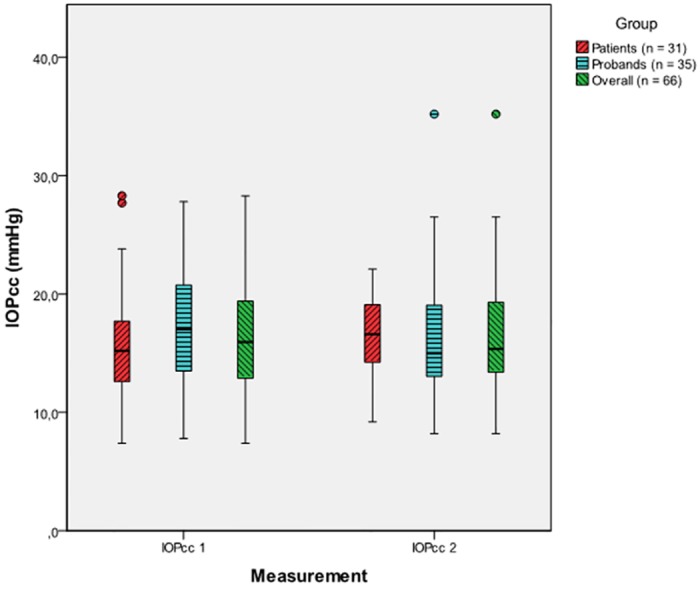
The mean difference between the first and the second IOPcc for both study groups combined was -0.43 ± 3.17 mmHg (p = 0.276), thus being non-significant. This was also confirmed by the results of the linear model. The average difference between the first and the second IOPcc was -0.11 ± 3.06 mmHg in patients (p = 0.843) and -0.71 ± 3.28 mmHg for controls (p = 0.208), no significant difference between patients and probands could be found (p = 0.446) (Fig 2).
Fig 2. Tonographic effect from the first (IOPcc 1) to the second (IOPcc 2) IOPcc measurement for patients, probands and the overall sample (patients and probands combined), [mmHg].
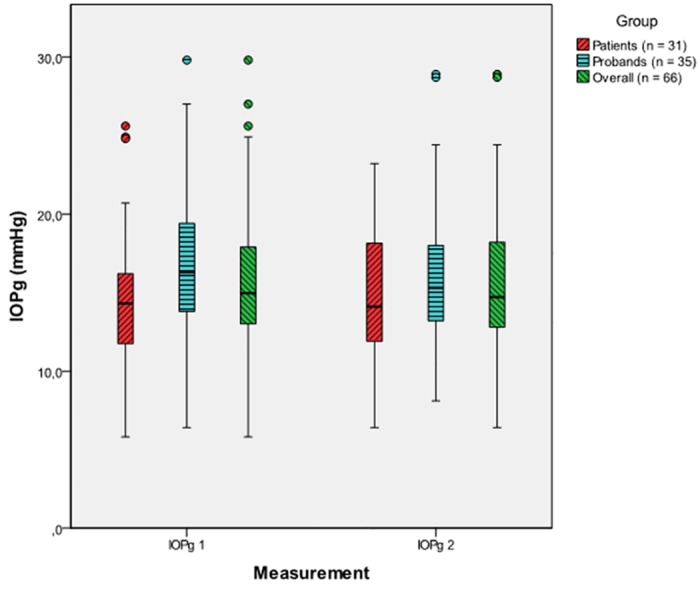
The mean difference between the first and the second IOPg was -0.31.± 2.35 mmHg, (p = 0.285) for both groups combined (non-significant). This was also confirmed by the results of the linear model. The average difference between the first and the second IOPg was -0.31± 2.38 mmHg (p = 0.469) in glaucoma patients and -0.31 ± 2.37 mmHg (p = 0.441) in controls. There was no significant difference between patients and probands (p = 0.998) (Fig 3).
Fig 3. Tonographic effect from the first (IOPg 1) to the second (IOPg 2) IOPg measurement for patients, probands and the overall sample (patients and probands combined), [mmHg].
Pentacam parameters
Mean CCT was noticeably thinner in glaucoma patients in comparison to healthy controls in both measurements in both eyes (Table 3).
Table 3. Mean values and standard deviations of central corneal thickness before and after IOP measurements for patients and probands.
| CCT 1_OD | CCT 1_OS | CCT 2_OD | CCT 2_OS | |
|---|---|---|---|---|
| Patients | 538.46 ± 19.65 | 535.74 ± 20.03 | 541.05 ± 20.31 | 541.63 ± 18.99 |
| Probands | 556.20 ± 24.34 | 557.56 ± 24.49 | 564.48 ± 25.44 | 562.02 ± 26.48 |
| p-value | 0.002 | < 0.001 | < 0.001 | 0.001 |
CCT, central corneal thickness; CCT 1, CCT before IOP measurements; CCT 2, CCT after IOP measurements; OD, right eyes; OS, left eyes; μm.
The repeated GAT/ORA IOP measurements led to a relevant increase of CCT in all subjects (Table 4).
Table 4. Mean values and standard deviations for differences between first and second CCT measurements.
| CCT1_2_OD | CCT1_2_OS | |
|---|---|---|
| Value ± SD | 5.61 ± 9.41 | 5.13 ± 7.39 |
| p-value | < 0.001 | < 0.001 |
SD, standard deviation; CCT1_2, mean values and SDs for differences between first and second CCT measurements; OD, right eyes; OS, left eyes; μm.
ACV and ACD did not differ between patients and probands (Table 5). The repeated IOP-measurements did not influence the ACD in both eyes and ACV in the right eyes. Only the ACV in the left eyes showed a decrease between the first and the second Pentacam measurement (p = 0.02).
Table 5. Anterior chamber volume and anterior chamber depth before and after repeated GAT/ORA IOP measurements for patients and probands for the right and the left eyes.
| ACV 1_OD | ACV 1_OS | ACV 2_OD | ACV 2_OS | ACD 1_OD | ACD 1_OS | ACD 2_OD | ACD 2_OS | |
|---|---|---|---|---|---|---|---|---|
| Patients | 142.65 ± 32.67 | 140.18 ± 33.00 | 141.59 ± 32.73 | 140.02 ± 33.43 | 2.66 ± 0.33 | 2.67 ± 0.31 | 2.65 ± 0.33 | 2.66 ± 0.33 |
| Probands | 129.60 ± 34.54 | 132.74 ± 33.42 | 128.74 ± 32.37 | 130.55 ± 31.08 | 2.55 ± 0.39 | 2.57 ± 0.38 | 2.55 ± 0.38 | 2.57 ± 0.38 |
| p-value | 0.121 | 0.367 | 0.114 | 0.238 | 0.241 | 0.255 | 0.265 | 0.325 |
ACV, anterior chamber volume [mm3]; ACD, anterior chamber depth [mm]; ACV 1/ACD 1, values before repeated GAT/ORA measurements; ACV 2/ACD 2, values after repeated GAT/ORA IOP measurements; OD, right eyes; OS, left eyes.
Crossover model
The confirmatory t-tests verify the results of the three period crossover analyses, with GAT values on average at 1.98 mmHg (p = 0.0028) lower than IOPcc values (95% CI: [-3.26, -0.69]). When comparing IOPcc (ORA) with GAT we found no carry-over effects (p = 0.29), we also found no period effect (p = 0.29). However, we found a significant treatment effect. Therefore, still no tonographic effect could be observed for the ORA measurements. No difference was found for a right versus left eye comparison.
Discussion
In order to find out whether the tonographic effect concerns ORA measurements, we employed a cross-over analysis with multiple GAT and ORA IOP-measurements randomly alternating between the right and the left eyes of healthy controls and glaucoma patients. In contrast to GAT, we did not find any significant decrease of IOPcc and IOPg between the first and the second ORA measurement and therefore no statistically or clinically significant tonographic effect in either glaucoma patients or healthy controls. Only two studies report about the effect of repeated ORA measurements on IOP: Goebels et al. [24] found IOPcc and IOPg values decreasing by -1.05 and -1.19 mmHg within a sequence of five sequential ORA readings in healthy subjects. This effect was more pronounced than in the healthy population of our study (-0.71 ± 3.28 mmHg, p = 0.208 for IOPcc and -0.31 ± 2.37 mmHg, p = 0.441, for IOPg). This may be explained by a higher number of single ORA measurements (5 sets with 4 readings each) or by the younger age of their population (mean 35 years, range 17–59 years) compared to the age of our healthy population (65.2 years, range 44–84 years). Corneal stiffness was shown to increase with age [26–32]. However, the authors do not mention any significances or the time intervals between the ORA measurements, thus making comparisons difficult. David et al. [17] compared 12 readings (3 sets with 4 readings each) of ORA parameters (IOPcc, IOPg, CH, and CRF) obtained within a 30-minute period in healthy subjects. They found significantly higher IOPcc and IOPg values in the first set of measurements compared to later readings with a maximum difference of -0.52 mmHg (p = 0.01) for IOPcc and -0.49 mmHg (p = 0.004) for IOPg (difference between sessions one and two for right eyes). Lam and Chen [25] evaluated the effect of ocular massage on ORA parameters in young healthy subjects, finding a significant decrease of IOPcc of about 2.8 mmHg. As important ameliorations, our study compared ORA results with GAT and took wave score as a quality criterion into consideration. A purpose-built additional three period cross-over analysis helped to detect any tonographic effects. Moreover, to the best of our knowledge our study examined the tonographic effect of ORA for the first time in glaucoma patients and in age-matched controls.
There have been only few attempts to determine the tonographic effect for other non-contact tonometers [11, 33]. Al-Mubrad and Ogbuehi [34] examined the tonographic effect of Keeler Pulsair EasyEye and Topcon CT80 in 120 healthy subjects by using a randomized examination scheme with different combinations of CT80, Keeler and GAT, measuring three times (Keeler, GAT) or four times (CT80). They found an IOP-decrease of -1.0 ± 2.3 mmHg (p < 0.05) for Keeler Pulsair EasyEye and -0.6 ± 1.7 mmHg (p < 0.05) for Topcon CT80 in healthy subjects and therefore a statistically significant tonographic effect.
GAT-IOP was systematically lower in comparison to IOPcc in our study. Similar results were found by several researchers [12, 14, 35–41]. With regard to mean GAT-IOP, IOPcc and IOPg values, no differences could be found between patients and healthy probands. The fact that all glaucoma patients were using IOP-lowering medications at the time of the study might explain the lack of IOP differences between patients and probands. The phenomenon of similar IOP values between glaucoma patients under therapy and probands can be observed in other studies such as these of Costin et al. [42] or Sullivan-Mee et al. [19].
In our study we found a statistically significant tonographic effect of GAT. We recorded the GAT-IOP reduction of mean -0.55 ± 2.00 mmHg for glaucoma patients and -1.15 ± 1.52 mmHg for healthy controls. The more pronounced tonographic effect in healthy controls in comparison to glaucoma patients may be explained by a pathologically changed trabecular meshwork of the glaucomatous eyes [43, 44] The time gap between the two GAT measurements was approximately three minutes. Since Stocker [5] there have been numerous attempts to analyze the tonographic effect of GAT (Table 6). The study applying a quite similar study design concerning GAT is that of Recep et al. [10], who obtained similar GAT-IOP differences after three minutes in healthy subjects after employing oxybuprocaine and fluorescein for GAT-IOP measurement. Our results are also comparable with other mentioned studies. However, they either measured only normal subjects or only glaucomatous patients [5–8, 45–47] or only glaucomatous patients [9], or do not give any information about the significance of their results. [8, 45, 47]. The other works employed a slightly different setting with very short time gaps between GAT-IOP measurements [33, 48, 49]. Overall, despite the statistical significance of the tonographic effect for GAT-IOP, an IOP change in the range of -0.5 to -1.15 mmHg does not seem to have decisive relevance in a clinical setting.
Table 6. Overview of the studies analyzing the tonographic effect of contact and non-contact tonometers.
| Author(s) | Year | IOP/IOP change | Sig. (p) | Setup | Sample (n) | Eye status | Device |
|---|---|---|---|---|---|---|---|
| Stocker [5] | 1958 | -0.97 mmHg | n.a. | 30s after first reading on same eye | 20 | normal | Schiötz tonometer |
| -1.90 mmHg | n.a. | 4 mins after first reading on same eye | 20 | normal | |||
| -2.72 mmHg | n.a. | 4 mins after the tonometer resting on other eye | 20 | normal | |||
| -2.37 mmHg | n.a. | on healthy eyes | 12 | normal | |||
| -3.25 mmHg | n.a. | on glaucomatous eyes | 8 | glaucomatous | |||
| Armaly & Rubin [37] | 1961 | -0.26 ± 0.75 to -0.39 ± 0.81 mmHg | sig. | GAT values after GAT once a minute for six minutes | 20 | normal | GAT |
| Moses [6] | 1961 | -0.9 mmHg* | n.a. | 1 min after first reading on right eye | 25 | normal | GAT |
| +0.1 mmHg* | n.a. | 2 mins after first reading on right eye | 25 | ||||
| -1 mmHg* | n.a. | 3 mins after first reading on right eye | 25 | ||||
| -0.05 mmHg* | n.a. | 4 mins after first reading on right eye | 25 | ||||
| -0.9 mmHg* | n.a. | 5 mins after first reading on right eye | 25 | ||||
| -0.1 mmHg* | n.a. | 1 min after first reading on left eye, 7 mins after first reading on right eye | 25 | ||||
| +0.1 mmHg* | n.a. | 2 mins after first reading on left eye, 8 mins after first reading on right eye | 25 | ||||
| -0.5 mmHg* | n.a. | 3 mins after first reading on left eye, 9 mins after first reading on right eye | 25 | ||||
| -0.4 mmHg* | n.a. | 4 mins after first reading on left eye, 10 mins after first reading on right eye | 25 | ||||
| -0.1 mmHg* | n.a. | 5 mins after first reading on left eye, 11 mins after first reading on right eye | 25 | ||||
| Bechrakis [9] | 1966 | -3.70 mmHg | n.a. | 4 mins after first reading on same eye | 17 | glaucomatous | GAT |
| -5.7 ± 1.3 mmHg | n.a. | 12 mins after first reading on same eye | 17 | ||||
| -5.1 mmHg | n.a. | 12 mins after first reading on same eye | 17 | ||||
| -3.58 ± 1.2 mmHg | n.a. | 4 mins after first reading on same eye | 44 | ||||
| Moses & Liu [39] | 1968 | -0.40 ± 1.40 mmHg | < 0.05 | right eye, rising and approx. 30 feet of walking between measurements | 74 | glaucomatous | GAT |
| -0.16 ± 1.43 mmHg | n.a. | left eye, rising and approx. 30 feet of walking between measurements | 74 | ||||
| Krakau & Wilke [7] | 1971 | -2.9 mmHg | n.a. | 5 mins after the first measurement and one measurement each minute on the same eye | 16 | normal | GAT |
| -4.6 mmHg | n.a. | 5 mins after the first measurement and one measurement each minute on the same eye | 5 | ||||
| Wilke [8] | 1972 | -0.4 mmHg | > 0.10 | A) 5 mins on the same eye after measurement on both eyes | 6 | normal | GAT |
| -2.6 mmHg | < 0.001 | B) 5 minutes on the same eye after measurement once a minute for 5 minutes | 6 | ||||
| -3.2 mmHg | < 0.001 | C) Patients seated for 5 mins after having local anesthesia, then measurements as in B) | 6 | ||||
| -2.9 mmHg | < 0.001 | Sham measurements without contact to cornea, then as in B) | 6 | ||||
| Phelps & Phelps [36] | 1976 | -0.4 ± 2.6 mmHg | < 0.05 | Mean IOP change in the right eye after 15 (5–30 minutes range) minutes of walking | 210 | normal | GAT |
| -0.2 ± 2.4 mmHg | > 0.05 | Mean IOP change in the left eye after 15 (5–30 minutes range) minutes of walking | 210 | ||||
| Thorburn [38] | 1979 | -0.5 ± 0.9 mmHg | n.a. | IOP change with same investigator, time interval unclear | 20 | in-patients | GAT |
| -0.5 ± 0.9 mmHg | n.a. | IOP change with two different investigators for 1st and 2nd measurement, time interval unclear | 72 | ||||
| Motolko et al. | 1982 | 18.81 mmHg | Mean of first three GAT measurements in 30s intervals | 9 | normal | GAT | |
| [35] | 17.48 mmHg | < 0.05 | Mean of fourth through sixth GAT mesaurement in 30s intervals | 9 | |||
| 16.78 mmHg | < 0.05 | Mean of seventh through ninth GAT measurement in 30s intervals | 9 | ||||
| Recep et al. [10] | 1998 | -1.33 ± 1.86 mmHg | < 0.05 | 1 min on the same eye after first measurement | 34 | normal | GAT |
| -0.53 ± 1.78 mmHg | > 0.05 | 2 mins on the same eye after first measurement | 29 | ||||
| -1.89 ± 1.57 mmHg | < 0.05 | 3 mins on the same eye after first measurement | 27 | ||||
| -2.29 ± 1.07 mmHg | < 0.05 | 4 mins on the same eye after first measurement | 41 | ||||
| -1.92 ± 2.08 mmHg | < 0.05 | 5 mins on the same eye after first measurement | 33 | ||||
| -0.00 ± 1.17 mmHg | > 0.05 | 10 mins on the same eye after first measurement | 28 | ||||
| Stewart et al. [40] | 2004 | IOP 1) 24.9 ± 2.7, IOP 2) 25.0 ± 2.8, IOP 3) 24.9 ± 2.8 mmHg | > 0.05 | 3 IOP readings of untreated baseline IOPs within a few seconds at 8 am | 33 | glaucomatous | GAT |
| IOP 1) 16.3 ± 2.9, IOP 2)16.1 ± 3.0, IOP 3) 16.3 ± 2.9 mmHg | > 0.05 | 3 IOP readings of treated final IOPs within a few seconds at 8 am | 33 | ||||
| Lam et al. [25] | 2007 | 1) 15.32 ± 2.30 2) 12.56 ± 3.08 | sig. | IOPcc before (1) and after (2) ocular massage for 5 mins | 53 | normal | ORA |
| 1) 11.03 ± 1.31 2) 11.63± 1.46 | sig. | CH before (1) and after (2) ocular massage for 5 mins | 53 | ||||
| 1) 10.96 ± 1.60 2) 10.72 ± 1.65 | sig. | CRF before (1) and after (2) ocular massage for 5 mins | 53 | ||||
| > +1.00 mmHg | n.a. | Distribution of change in IOPcc from ocular massage | 2 | ||||
| +1.00 to -1.00 mmHg | n.a. | Distribution of change in IOPcc from ocular massage | 11 | ||||
| -1.01 to -3.00 mmHg | n.a. | Distribution of change in IOPcc from ocular massage | 14 | ||||
| -3.01 to -5.00 mmHg | n.a. | Distribution of change in IOPcc from ocular massage | 18 | ||||
| > -5.00 mmHg | n.a. | Distribution of change in IOPcc from ocular massage | 8 | ||||
| AlMubrad & Ogbuehi [23] | 2010 | 1.0 ± 2.3 mmHg | < 0.05 | IOP difference before and after GAT, min. 48 hrs between sessions, interval between GAT and non-contact tonometry 2–5 mins | 60 | normal | Keeler Pulsair EasyEye |
| 0.6 ± 1.7 mmHg | < 0.05 | IOP difference before and after GAT, min. 48 hrs between sessions, interval between GAT and non-contact tonometry 2–5 mins | 60 | Topcon CT80 | |||
| Gaton et al. [26] | 2010 | IOP 1) 15.94 ± 4.3 mmHg | < 0.0001 | GAT readings in interval of few seconds | 67 | glaucomatous | GAT |
| IOP 2) 14.90 ± 4.5 mmHg | 67 | ||||||
| IOP 1) 13.64 ± 3.8 mmHg | 0.83 | GAT readings in interval of few seconds | 70 | normal | |||
| IOP 2) 13.73 ± 3.2 mmHg | 70 | ||||||
| Goebels et al. [24] | 2012 | 14.92 mmHg | - | Mean IOPcc measurement 1 | 45 | normal | ORA |
| 14.14 mmHg | - | Mean IOPcc measurement 2 | 45 | ||||
| 13.78 mmHg | - | Mean IOPcc measurement 3 | 45 | ||||
| 13.91 mmHg | - | Mean IOPcc measurement 4 | 45 | ||||
| 13.87 mmHg | - | Mean IOPcc measurement 5 | 45 | ||||
| 15.72 mmHg | - | Mean IOPg measurement 1 | 45 | ||||
| 14.92 mmHg | - | Mean IOPg measurement 2 | 45 | ||||
| 14.49 mmHg | - | Mean IOPg measurement 3 | 45 | ||||
| 14.58 mmHg | - | Mean IOPg measurement 4 | 45 | ||||
| 14.53 mmHg | - | Mean IOPg measurement 5 | 45 | ||||
| David et al. [17] | 2013 | -0.493 mmHg | 0.004 | IOPg difference between sessions 1 and 2, right eyes | 100 | normal | ORA |
| -0.241 mmHg | 0.08 | IOPg difference between sessions 1 and 2, left eyes | 100 | ||||
| -0.521 mmHg | 0.01 | IOPcc difference between sessions 1 and 2, right eyes | 100 | ||||
| -0.269 mmHg | 0.11 | IOPcc difference between sessions 1 and 2, left eyes | 100 | ||||
| 0.011 mmHg | 0.93 | IOPg difference between sessions 2 and 3, right eyes | 100 | ||||
| -0.168 mmHg | 0.10 | IOPg difference between sessions 2 and 3, left eyes | 100 | ||||
| 0.141 mmHg | 0.43 | IOPcc difference between sessions 2 and 3, right eyes | 100 | ||||
| -0.148 mmHg | 0.35 | IOPcc difference between sessions 2 and 3, left eyes | 100 | ||||
| -0.482 mmHg | 0.002 | IOPg difference between sessions 1 and 3, right eyes | 100 | ||||
| -0.409 mmHg | 0.008 | IOPg difference between sessions 1 and 3, left eyes | 100 | ||||
| -0.380 mmHg | 0.03 | IOPcc difference between sessions 1 and 3, right eyes | 100 | ||||
| -0.417 mmHg | 0.02 | IOPcc difference between sessions 1 and 3, left eyes | 100 |
* IOP estimations as observed in graphic.
Another aspect of this study was to analyze whether a correlation between corneal parameters and the tonographic effect exists. We identified a weak (r = 0.37), but mentionable (p = 0.042) correlation between the tonographic effect of GAT and CCT. This was true only for patients, but not for the healthy controls (r = -0.107). To the best of our knowledge, we executed the only study that evaluated the relationship of CCT with the GAT-IOP change, i.e. the tonographic effect for glaucomatous eyes. We can speculate, the higher the initial CCT, the higher the tonographic effect in glaucoma patients or vice versa. Baseline CCT was thinner in glaucoma patients in comparison to healthy controls. Similar effects were found by other authors either [34, 41, 42, 46, 47–50].
Repeated IOP-measurements resulted in a noticeable increase of CCT, but no change of the anterior chamber depth and volume. Ocular massage through applanation tonometry might cause damage to the corneal epithelium and thus soften structures and/or increase penetration of anesthetics [3, 22]. This might be crucial for a CCT change as well: Al-Mubrad and Ogbuehi [20], p.307, mention a study by Weekers [50], stating “that topical anesthetics caused an alteration of the endothelial Na+/K+ pump resulting increased stromal hydration and as a consequence, increased corneal thickness.” Herse and Siu [51] also suspect the appearance of transitory edema of the corneal stroma as a cause for the CCT increase. Rosa et al. [52] mention the preservatives present in the ophthalmic solutions as a trigger for structural/functional damage to both the corneal epithelium and endothelium, thus increasing CCT in other studies. The increased corneal penetration caused by applanation tonometry could explain why a CCT increase with potential edema etc. was observed without any change in ACV or ACD. Moreover, since the restoration of ACV presumably takes between 2–5 minutes, it is very likely that any ACV reduction was compensated for before the final Pentacam measurements [11, 53].
The study has certain limitations: concerning participating glaucoma patients, an inhomogeneous group with supposedly different kinds and numbers of topical medication were included. The differences in medication might have differing effects on corneal properties etc. As a further consequence, only glaucoma patients with normal IOP values were included in the study. Thus, conclusions cannot be drawn for (untreated) glaucoma patients with elevated IOP values.
Acknowledgments
The results are part of the doctoral thesis of Martin Zimmermann.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Survey of ophthalmology. 2008;53(6):S3–S10. [DOI] [PubMed] [Google Scholar]
- 2.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of ophthalmology. 2002;120(6):714–20. [DOI] [PubMed] [Google Scholar]
- 3.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Survey of ophthalmology. 1993;38(1):1–30. [DOI] [PubMed] [Google Scholar]
- 4.Rüfer PDF. Fehlerquellen bei der Goldmann-Applanationstonometrie. Der Ophthalmologe. 2011;108(6):546–52. [DOI] [PubMed] [Google Scholar]
- 5.Stocker FW. On changes in intraocular pressure after application of the tonometer; in the same eye and in the other eye. American journal of ophthalmology. 1958;45(2):192 [DOI] [PubMed] [Google Scholar]
- 6.Moses R. Repeated applanation tonometry. Ophthalmologica. 1961;142(6):663–8. [DOI] [PubMed] [Google Scholar]
- 7.Krakau CET, Wilke K. On repeated tonometry. Acta ophthalmologica. 1971;49(4):611–4. [DOI] [PubMed] [Google Scholar]
- 8.Wilke K. Effects of repeated tonometry: genuine and sham measurements. Acta ophthalmologica. 1972;50(4):574–82. [DOI] [PubMed] [Google Scholar]
- 9.Bechrakis E. On spontaneous decrease of pressure in applanation tonometry. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift für Augenheilkunde. 1966;151(5):604–14. [DOI] [PubMed] [Google Scholar]
- 10.Recep ÖF, Hasiripi H, Vayisoğlu E, Kalayci D, Sarikatipoğlu H. Accurate time interval in repeated tonometry. Acta Ophthalmologica Scandinavica. 1998;76(5):603–5. [DOI] [PubMed] [Google Scholar]
- 11.Jóhannesson G, Hallberg P, Eklund A, Behndig A, Lindén C. Effects of topical anaesthetics and repeated tonometry on intraocular pressure. Acta ophthalmologica. 2014;92(2):111–5. 10.1111/aos.12058 [DOI] [PubMed] [Google Scholar]
- 12.Carbonaro F, Andrew T, Mackey D, Spector T, Hammond C. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye. 2010;24(7):1165–70. 10.1038/eye.2010.11 [DOI] [PubMed] [Google Scholar]
- 13.Ayala M, Chen E. Measuring corneal hysteresis: threshold estimation of the waveform score from the Ocular Response Analyzer. Graefe's Archive for Clinical and Experimental Ophthalmology. 2012;250(12):1803–6. 10.1007/s00417-012-2053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotecha A, White E, Schlottmann PG, Garway-Heath DF. Intraocular pressure measurement precision with the Goldmann applanation, dynamic contour, and ocular response analyzer tonometers. Ophthalmology. 2010;117(4):730–7. 10.1016/j.ophtha.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 15.Xu G, Lam DS, Leung CK-S. Influence of ocular pulse amplitude on ocular response analyzer measurements. Journal of glaucoma. 2011;20(6):344–9. 10.1097/IJG.0b013e3181efb388 [DOI] [PubMed] [Google Scholar]
- 16.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. Journal of Cataract & Refractive Surgery. 2005;31(1):156–62. [DOI] [PubMed] [Google Scholar]
- 17.David VP, Stead RE, Vernon SA. Repeatability of Ocular Response Analyzer Metrics: A Gender-Based Study. Optometry & Vision Science. 2013;90(7):691–9. [DOI] [PubMed] [Google Scholar]
- 18.Wasielica-Poslednik J, Berisha F, Aliyeva S, Pfeiffer N, Hoffmann EM. Reproducibility of ocular response analyzer measurements and their correlation with central corneal thickness. Graefe's Archive for Clinical and Experimental Ophthalmology. 2010;248(11):1617–22. 10.1007/s00417-010-1471-1 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. Ocular Response Analyzer in subjects with and without glaucoma. Optometry & Vision Science. 2008;85(6):463–70. [DOI] [PubMed] [Google Scholar]
- 20.Al-Mubrad TM, Ogbuehi KC. Clinical investigation of the effect of topical anesthesia on intraocular pressure. Clinical ophthalmology. 2007;1(3):305–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38. [DOI] [PubMed] [Google Scholar]
- 22.Worthen DM, Zimmerman TJ, Wind CA. An evaluation of the pilocarpine Ocusert. Investigative Ophthalmology & Visual Science. 1974;13(4):296–9. [PubMed] [Google Scholar]
- 23.Kopito R, Gaujoux T, Montard R, Touzeau O, Allouch C, Borderie V, et al. Reproducibility of viscoelastic property and intraocular pressure measurements obtained with the Ocular Response Analyzer. Acta ophthalmologica. 2011;89(3):e225–e30. 10.1111/j.1755-3768.2010.01957.x [DOI] [PubMed] [Google Scholar]
- 24.Goebels SC, Seitz B, Langenbucher A. Precision of ocular response analyzer. Current eye research. 2012;37(8):689–93. 10.3109/02713683.2012.660592 [DOI] [PubMed] [Google Scholar]
- 25.Lam A, Chen D. Effect of ocular massage on intraocular pressure and corneal biomechanics. Eye. 2007;21(9):1245–6. 10.1038/sj.eye.6702928 [DOI] [PubMed] [Google Scholar]
- 26.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Investigative ophthalmology & visual science. 1998;39(3):644–8. [PubMed] [Google Scholar]
- 27.Friedenwald JS. Contribution to the theory and practice of tonometry. American Journal of Ophthalmology. 1937;20(10):985–1024. [Google Scholar]
- 28.Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF. Corneal thickness-and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Investigative ophthalmology & visual science. 2006;47(12):5337–47. [DOI] [PubMed] [Google Scholar]
- 29.Kotecha A, White E, Shewry J, Garway-Heath D. The relative effects of corneal thickness and age on Goldmann applanation tonometry and dynamic contour tonometry. British Journal of Ophthalmology. 2005;89(12):1572–5. 10.1136/bjo.2005.075580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherrard E, Novakovic P, Speedwell L. Age-related changes of the corneal endothelium and stroma as seen in vivo by specular microscopy. Eye. 1987;1(2):197–203. [DOI] [PubMed] [Google Scholar]
- 31.Tonnu P, Ho T, Newson T, El Sheikh A, Sharma K, White E, et al. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. British Journal of Ophthalmology. 2005;89(7):851–4. 10.1136/bjo.2004.056622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: structural and biochemical changes. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1992;1138(3):222–8. [DOI] [PubMed] [Google Scholar]
- 33.Gaton DD, Ehrenberg M, Lusky M, Wussuki-Lior O, Dotan G, Weinberger D, et al. Effect of repeated applanation tonometry on the accuracy of intraocular pressure measurements. Current eye research. 2010;35(6):475–9. 10.3109/02713681003678824 [DOI] [PubMed] [Google Scholar]
- 34.Al-Mubrad TM, Ogbuehi KC. On repeated corneal applanation with the Goldmann and two non‐contact tonometers. Clinical and Experimental Optometry. 2010;93(2):77–82. 10.1111/j.1444-0938.2010.00453.x [DOI] [PubMed] [Google Scholar]
- 35.Hager A, Loge K, Schroeder B, Füllhas M-O, Wiegand W. Effect of central corneal thickness and corneal hysteresis on tonometry as measured by dynamic contour tonometry, ocular response analyzer, and Goldmann tonometry in glaucomatous eyes. Journal of glaucoma. 2008;17(5):361–5. 10.1097/IJG.0b013e31815c3ad3 [DOI] [PubMed] [Google Scholar]
- 36.Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, Mendez-Hernandez C, Garcia-Sanchez J. Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Investigative ophthalmology & visual science. 2006;47(10):4410–4. [DOI] [PubMed] [Google Scholar]
- 37.Morita T, Shoji N, Kamiya K, Hagishima M, Fujimura F, Shimizu K. Intraocular pressure measured by dynamic contour tonometer and ocular response analyzer in normal tension glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology. 2010;248(1):73–7. 10.1007/s00417-009-1169-4 [DOI] [PubMed] [Google Scholar]
- 38.Renier C, Zeyen T, Fieuws S, Vandenbroeck S, Stalmans I. Comparison of ocular response analyzer, dynamic contour tonometer and Goldmann applanation tonometer. International ophthalmology. 2010;30(6):651–9. 10.1007/s10792-010-9377-9 [DOI] [PubMed] [Google Scholar]
- 39.Kynigopoulos M, Schlote T, Kotecha A, Tzamalis A, Pajic B, Haefliger I. Repeatability of intraocular pressure and corneal biomechanical properties measurements by the ocular response analyser. Klinische Monatsblätter fur Augenheilkunde. 2008;225(5):357–60. 10.1055/s-2008-1027256 [DOI] [PubMed] [Google Scholar]
- 40.Lam AK, Chen D, Chiu R, Chui W-S. Comparison of IOP measurements between ORA and GAT in normal Chinese. Optometry & Vision Science. 2007;84(9):909–14. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang P-B, Li C-Y, Zhu X-H, Duan X-C. Assessment of intraocular pressure measured by Reichert Ocular Response Analyzer, Goldmann Applanation Tonometry, and Dynamic Contour Tonometry in healthy individuals. International journal of ophthalmology. 2012;5(1):102–7. 10.3980/j.issn.2222-3959.2012.01.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costin BR, Fleming GP, Weber PA, Mahmoud AM, Roberts CJ. Corneal biomechanical properties affect Goldmann applanation tonometry in primary open-angle glaucoma. Journal of glaucoma. 2014;23(2):69–74. 10.1097/IJG.0b013e318269804b [DOI] [PubMed] [Google Scholar]
- 43.Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Survey of ophthalmology. 2007;52(6):S101–S4. [DOI] [PubMed] [Google Scholar]
- 44.Tektas O-Y, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Experimental eye research. 2009;88(4):769–75. 10.1016/j.exer.2008.11.025 [DOI] [PubMed] [Google Scholar]
- 45.Motolko M, Feldman F, Hyde M, Hudy D. Sources of variability in the results of applanation tonometry. Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 1982;17(3):93–5. [PubMed] [Google Scholar]
- 46.Phelps CD, Phelps GK. Measurement of intraocular pressure: a study of its reproducibility. Graefe's Archive for Clinical and Experimental Ophthalmology. 1976;198(1):39–43. [DOI] [PubMed] [Google Scholar]
- 47.Armaly MF, Rubin ML. Accommodation and applanation tonometry. Archives of Ophthalmology. 1961;65(3):415–23. [DOI] [PubMed] [Google Scholar]
- 48.Moses R, Liu C-H. Repeated applanation tonometry. American journal of ophthalmology. 1968;66(1):89–91. [DOI] [PubMed] [Google Scholar]
- 49.Stewart WC, Geiger AC, Jenkins JN. The Benefit of Repeated Intraocular Pressure Measurements in ClinicalTrials. Archives of ophthalmology. 2004;122(6):936–7. [DOI] [PubMed] [Google Scholar]
- 50.Weekers J. Recherches experimentales sur la genese des lesions corneennes dues aux anesthesiques. Arch Ophthalmol (Paris). 1974;34:121–32. [PubMed] [Google Scholar]
- 51.Herse P, Siu A. Short‐term effects of proparacaine on human corneal thickness. Acta ophthalmologica. 1992;70(6):740–4. [DOI] [PubMed] [Google Scholar]
- 52.Rosa N, De Bernardo M, Borrelli M, Filosa ML, Lanza M. Effect of oxybuprocaine eye drops on corneal volume and thickness measurements. Optometry & Vision Science. 2011;88(5):640–4. [DOI] [PubMed] [Google Scholar]
- 53.Brubaker R. Flow of aqueous humor in humans [The Friedenwald Lecture]. Investigative ophthalmology & visual science. 1991;32(13):3145–66. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


