Abstract
Putative adhesins were predicted by computer analysis of the Treponema pallidum genome. Two treponemal proteins, Tp0155 and Tp0483, demonstrated specific attachment to fibronectin, blocked bacterial adherence to fibronectin-coated slides, and supported attachment of fibronectin-producing mammalian cells. These results suggest Tp0155 and Tp0483 are fibronectin-binding proteins mediating T. pallidum-host interactions.
Syphilis is a chronic infection caused by the spirochete bacterium Treponema pallidum subsp. pallidum. Numerous studies have demonstrated that T. pallidum attaches to host cells (2, 3, 11-14, 16, 21, 37, 45, 48, 50). Experimentally induced infections (7, 36) and in vitro studies (48) have shown that T. pallidum adheres to epithelial surfaces, traverses the tissue barrier, and enters the circulation by invading the tight junctions between endothelial cells. Treponemal invasion results in widespread bacterial dissemination, which sets the stage for establishment of chronic infection.
Specific attachment to the extracellular matrix (ECM) component fibronectin has been documented for many pathogenic bacteria, including the related spirochetes Borrelia burgdorferi (19, 35), Leptospira interrogans (26), and Treponema denticola (8-10, 49). Fibronectin is also likely to be involved in T. pallidum cytoadherence. The organism specifically attaches to fibronectin-coated surfaces (21, 34), and fibronectin synthesis by fibroblasts is upregulated in areas of ulceration, including syphilis chancres formed at the primary site of infection. In addition, pretreatment of host cells with antiserum to fibronectin, but not control irrelevant antiserum, inhibits attachment of T. pallidum (21, 34, 45). Finally, three T. pallidum fibronectin-binding proteins, designated P1, P2, and P3, were previously identified by fibronectin affinity chromatography and radioimmunoprecipitation techniques (1, 33, 34, 46). The molecular masses of these proteins were determined to be 89.5, 37, and 32 kDa for P1, P2, and P3, respectively (46); however, their molecular identities remain unknown.
T. pallidum fibronectin-binding proteins.
To identify T. pallidum fibronectin-binding proteins, 10 potential adhesins were tested for their capacity to mediate attachment of fibronectin. These putative adhesins were identified via bioinformatic analysis of the T. pallidum genome (18) and expressed as recombinant proteins, as described in detail elsewhere (5). Enzyme-linked immunosorbent assay (ELISA) plates (Nalge Nunc International, Rochester, N.Y.) were coated for 16 h at room temperature with 100 μl of the recombinant T. pallidum proteins, a positive control S. aureus fibronectin-binding protein (FnbpA) (41), and a negative control recombinant protein (SA85-1.1) (23), all at a concentration of 5 μg/ml in phosphate-buffered saline (PBS). Wells were subsequently washed three times with PBS. For the adherence assays, 100 μl of either soluble or matrix fibronectin (Sigma, St. Louis, Mo.) was added to the wells at a concentration of 5 μg/ml. To test for the dose-dependent attachment of fibronectin, 100 μl of various matrix or soluble fibronectin concentrations ranging from 0 to 5 μg/ml in PBS was added to the wells. After incubation for 1 h at room temperature, wells were washed six times with PBS with 0.05% Tween-20 (PBST) and bound fibronectin was detected with rabbit anti-human fibronectin (1:500 dilution; Sigma) and goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (1:2,000; Sigma) followed by the TMB Microwell peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). Optical densities were read at 600 nm with an ELISA plate reader (Bio-Tek Instruments, Winooski, Vt.).
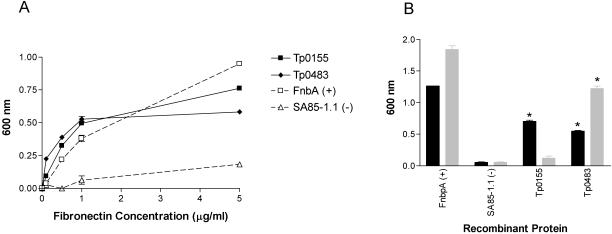
Of the panel of expressed T. pallidum recombinant proteins, fibronectin exhibited a significant level of attachment to Tp0155 and Tp0483 (P < 0.05) (Fig. 1). Each of these proteins, as well as the positive control protein, bound increasing concentrations of fibronectin in a dose-dependent manner, compared to a minimal level of fibronectin attachment observed to the negative control recombinant protein (Fig. 2A). The capacity of Tp0155 and Tp0483 to interact with two different forms of fibronectin was also investigated: soluble dimeric fibronectin, which resembles the form found in plasma, and superfibronectin, a multimeric form of fibronectin that most closely resembles the form found in the extracellular matrix (27, 28). Tp0155 preferentially bound the matrix form of fibronectin, whereas Tp0483 bound both the soluble and matrix forms of fibronectin (Fig. 2B).
FIG. 1.
Binding of fibronectin to the recombinant T. pallidum proteins Tp0155 and Tp0483 and positive (FnbpA) and negative (SA85-1.1) control proteins. Statistical analyses compared the fibronectin attachment level of each recombinant protein to that of the negative control by Student's two-tailed t test (*, P < 0.05). Bars represent the mean absorbance values at 600 nm ± standard error for triplicate wells, and the results are representative of three independent experiments.
FIG. 2.
(A) Dose-dependent attachment of fibronectin to recombinant Tp0155 and Tp0483. (B) Attachment of matrix fibronectin (black bars) and soluble fibronectin (gray bars) to recombinant Tp0155 and Tp0483. For each condition, attachment to Tp0155 and Tp0483 was compared to attachment to the negative control for the respective fibronectin preparation by Student's two-tailed t test (*, P < 0.05). Shown are the mean absorbance values at 600 nm ± standard error for triplicate wells, and the results are representative of three independent experiments.
Inhibition experiments.
The involvement of Tp0155 and Tp0483 in mediating attachment of T. pallidum to fibronectin was directly assayed via in vitro inhibition experiments. Slides were coated with 4 μg of matrix fibronectin in PBS on Lab-Tek II chamber slides (Nalge Nunc International) by incubation for 16 h at room temperature. After washing with PBS, slides were blocked for 2 h with 3% bovine serum albumin. Inhibition experiments were performed by preincubating fibronectin-coated slides with 200-μg/ml samples of either Tp0155 or Tp0483, both Tp0155 and Tp0483, or the negative control recombinant proteins Tp0751 and Tp0952. After washing with PBS, slides were incubated for 2 h at 34°C with 3 × 107 Percoll-purified T. pallidum (20). After gentle washing with saline (10 times for 5 min each), the attached spirochetes were visualized by dark-field microscopy and quantitative attachment was determined by calculating the number of attached treponemes per field. As shown in Fig. 3, preincubation of fibronectin-coated slides with Tp0155 or Tp0483, either alone or in combination, significantly decreased the number of adherent treponemes (P < 0.05). In contrast, the addition of the two negative control proteins did not significantly decrease the number of adherent treponemes. These results demonstrate that Tp0155 and Tp0483 can directly compete with the binding of T. pallidum to fibronectin.
FIG. 3.
Inhibition of T. pallidum attachment to fibronectin-coated slides by preincubation with Tp0155 and/or Tp0483 or two negative control recombinant proteins (Tp0751 and Tp0952). Bars represent the average number of treponemes bound ± standard error for six fields, and the results are representative of three independent experiments. Significance was assessed by comparison with the “no addition” wells by Student's two-tailed t test (*, P < 0.05).
Attachment of mammalian cells to Tp0155 and Tp0483.
Adhesion assays were performed to investigate the ability of Tp0155 and Tp0483 to mediate attachment of mammalian cells. Cell lines were obtained from the American Type Culture Collection (Manassas, Va.) and included the colon carcinoma cell line SW480, which produces fibronectin, and the pituitary cell line AtT20, which does not synthesize fibronectin or express fibronectin-binding receptors. Assays were performed as previously described (38). Non-tissue culture-treated ELISA plates (Fisher Scientific, Pittsburgh, Pa.) were coated for 24 h at 4°C with 100 μl of either of the recombinant T. pallidum proteins Tp0155 and Tp0483 or the negative control protein bovine serum albumin at a concentration of 20 μg/ml in PBS. Wells coated with Tp0155 and Tp0483 demonstrated a fourfold-higher level of attachment of SW480 than AtT20 cells (data not shown). These results are consistent with the capacity of Tp0155 and Tp0483 to attach to fibronectin.
Summary.
This study extends previous investigations on the specific interaction of T. pallidum with fibronectin (1, 4, 15, 16, 21, 33, 34, 45-47). Adherence assays identified the T. pallidum open reading frames Tp0155 and Tp0483 as encoding proteins that bind fibronectin. The predicted sizes of Tp0155 (35.8 kDa) and Tp0483 (40 kDa) are similar to the sizes estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis for the previously identified T. pallidum fibronectin-binding proteins P3 (32 kDa) and P2 (37 kDa) (46). The binding of fibronectin to Tp0155 and Tp0483 is characteristic of specific receptor-ligand interactions, in that each molecule bound increasing fibronectin concentrations in a dose-dependent manner. Recombinant Tp0155 and Tp0483 inhibited attachment of T. pallidum to fibronectin, and the two proteins mediated attachment of the fibronectin-producing SW480 cell line. These results identify Tp0155 and Tp0483 as T. pallidum fibronectin-binding proteins.
As discussed in this report, Tp0155 preferentially bound the matrix form of fibronectin, whereas Tp0483 bound both the soluble and matrix forms of fibronectin. The two forms of fibronectin exist in different conformational states, with cryptic epitopes becoming exposed during fibronectin matrix assembly (44, 51). Similar differential fibronectin binding abilities have been observed in group B streptococci (43), Streptococcus sanguis (25), Yersinia sp. (40), and human immunodeficiency virus (44), which bind preferentially to the matrix form of fibronectin, and Streptococcus pyogenes (30) and Staphylococcus aureus (24), which bind to both soluble and matrix forms. The differential fibronectin binding capabilities of Tp0155 and Tp0483 would each result in T. pallidum attachment to cells and tissues. Tp0155 could mediate attachment of T. pallidum through direct binding of matrix-associated fibronectin. Further, Tp0483 could mediate both direct binding to cells via matrix-associated fibronectin, as well as indirect binding via soluble fibronectin serving as a bridging molecule between the T. pallidum Tp0483 receptor and cells. Such an attachment mechanism has been observed with other bacterial pathogens, including S. aureus (17, 32, 42), S. pyogenes (6, 29, 31), and Mycobacterium leprae (39). These alternative mechanisms for promoting attachment of T. pallidum to fibronectin may allow the pathogen to colonize different niches in the host.
The presence of multiple fibronectin-binding proteins within one organism has been observed for other pathogenic bacteria, including Mycobacterium, Streptococcus, and Staphylococcus spp. (22). We now show that T. pallidum similarly expresses multiple fibronectin-binding adhesins. In addition, T. pallidum also possesses a laminin-binding adhesin (5). The exact contribution of each of these adhesins, as well as other currently unidentified T. pallidum adhesins, to the infection process remains to be determined. However, it is likely that the role in treponemal pathogenesis played by an individual adhesin is particularly suited to the stage of infection and/or tissue niche, with appropriate redundancy existing between these adhesins to ensure successful establishment of infection. Additional characterization of these treponemal fibronectin-binding adhesins, as well as other T. pallidum molecules that interact with host components, will further our understanding of the pathogenesis of T. pallidum.
Acknowledgments
We are grateful to Lynn Barrett for assistance with recombinant expression, Julie Yabu and Melissa Steadele for their assistance with the cell adhesion assays, and Barbara Molini and Sheila Lukehart for their gift of T. pallidum.
This work was supported by Public Health Service grant AI-51334 from the National Institutes of Health, faculty awards from the University of Washington (Royalty Research Fund and STD New Investigator Award, AI-31448), and the Canadian Institutes of Health Research.
REFERENCES
- 1.Alderete, J. F., and J. B. Baseman. 1980. Surface characterization of virulent Treponema pallidum. Infect. Immun. 30:814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baseman, J. B. and J. F. Alderete. 1983. The parasitic strategies of Treponema pallidum, p. 229-239. In R. Schell and D. Musher (ed.), Pathogenesis and immunology of Treponema infections. Marcel Dekker, New York, N.Y.
- 3.Baseman, J. B., and E. C. Hayes. 1980. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J. Exp. Med. 151:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baughn, R. E. 1986. Antibody-independent interactions of fibronectin, C1q, and human neutrophils with Treponema pallidum. Infect. Immun. 54:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron, C. E. 2003. Identification of a Treponema pallidum laminin-binding protein. Infect. Immun. 71:2525-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cumberland, M. C., and T. B. Turner. 1949. Rate of multiplication of Treponema pallidum in normal and immune rabbits. Am. J. Syph. 33:201-212. [PubMed] [Google Scholar]
- 8.Dawson, J. R., and R. P. Ellen. 1990. Tip-oriented adherence of Treponema denticola to fibronectin. Infect. Immun. 58:3924-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson, J. R., and R. P. Ellen. 1994. Clustering of fibronectin adhesins toward Treponema denticola tips upon contact with immobilized fibronectin. Infect. Immun. 62:2214-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenno, J. C., K.-H. Müller, and B. C. McBride. 1996. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J. Bacteriol. 178:2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald, T. J., P. Cleveland, R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J. Bacteriol. 130:1333-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald, T. J., R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect. Immun. 18:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald, T. J., R. C. Johnson, J. A. Sykes, and J. N. Miller. 1977. Interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells: effects of oxygen, reducing agents, serum supplements, and different cell types. Infect. Immun. 15:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald, T. J., J. N. Miller, and J. A. Sykes. 1975. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect. Immun. 11:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald, T. J., and L. A. Repesh. 1985. Interactions of fibronectin with Treponema pallidum. Genitourin. Med. 61:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald, T. J., L. A. Repesh, D. R. Blanco, and J. N. Miller. 1984. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br. J. Vener. Dis. 60:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler, T., E. R. Wann, D. Joh, S. Johansson, T. J. Foster, and M. Höök. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 79:672-679. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, L. Watthey, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 19.Grab, D. J., C. Givens, and R. Kennedy. 1998. Fibronectin-binding activity in Borrelia burgdorferi. Biochim. Biophys. Acta 1407:135-145. [DOI] [PubMed] [Google Scholar]
- 20.Hanff, P. A., S. J. Norris, M. A. Lovett, and J. N. Miller. 1984. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex. Transm. Dis. 11:275-286. [DOI] [PubMed] [Google Scholar]
- 21.Hayes, N. S., K. E. Muse, A. M. Collier, and J. B. Baseman. 1977. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect. Immun. 17:174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Höök. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 23.Kahn, S. J., and M. Wleklinski. 1997. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J. Immunol. 159:4444-4451. [PubMed] [Google Scholar]
- 24.Kuusela, P., T. Vartio, M. Vuento, and E. B. Myhre. 1985. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect. Immun. 50:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowrance, J. H., D. L. Hasty, and W. A. Simpson. 1988. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect. Immun. 56:2279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merien, F., J. Truccolo, G. Baranton, and P. Perolat. 2000. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol. Lett. 185:17-22. [DOI] [PubMed] [Google Scholar]
- 27.Morla, A., and E. Ruoslahti. 1992. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J. Cell Biol. 118:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morla, A., Z. Zhang, and E. Ruoslahti. 1994. Superfibronectin is a functionally distinct form of fibronectin. Nature 367:193-196. [DOI] [PubMed] [Google Scholar]
- 29.Okada, N., I. Tatsuno, E. Hanski, M. Caparon, and C. Sasakawa. 1998. Streptococcus pyogenes protein F promotes invasion of HeLa cells. Microbiology 144:3079-3086. [DOI] [PubMed] [Google Scholar]
- 30.Okada, N., M. Watarai, V. Ozeri, E. Hanski, M. Caparon, and C. Sasakawa. 1997. A matrix form of fibronectin mediates enhanced binding of Streptococcus pyogenes to host tissue. J. Biol. Chem. 272:26978-26984. [DOI] [PubMed] [Google Scholar]
- 31.Ozeri, V., I. Rosenshine, D. F. Mosher, R. Fassler, and E. Hanski. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625-637. [DOI] [PubMed] [Google Scholar]
- 32.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 33.Peterson, K., J. B. Baseman, and J. F. Alderete. 1987. Molecular cloning of Treponema pallidum outer envelope fibronectin binding proteins, P1 and P2. Genitourin. Med. 63:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson, K. M., J. B. Baseman, and J. F. Alderete. 1983. Treponema pallidum receptor binding proteins interact with fibronectin. J. Exp. Med. 157:1958-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 36.Raiziss, G. W., and M. Severac. 1937. Rapidity with which Spirochaeta pallida invades the bloodstream. Arch. Dermatol. Syphilol. 35:1101-1109. [Google Scholar]
- 37.Rice, M., and T. J. Fitzgerald. 1985. Detection and functional characterization of early appearing antibodies in rabbits with experimental syphilis. Can. J. Microbiol. 31:62-67. [DOI] [PubMed] [Google Scholar]
- 38.Schnapp, L. M., N. Hatch, D. M. Ramos, I. V. Klimanskaya, D. Sheppard, and R. Pytela. 1995. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 270:23196-23202. [DOI] [PubMed] [Google Scholar]
- 39.Schorey, J. S., Q. Li, D. W. McCourt, M. Bong-Mastek, J. E. Clark-Curtiss, T. L. Ratliff, and E. J. Brown. 1995. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 63:2652-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze-Koops, H., H. Burkhardt, J. Heesemann, T. Kirsch, B. Swoboda, C. Bull, S. Goodman, and F. Emmrich. 1993. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect. Immun. 61:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signäs, C., G. Raucci, K. Jonsson, P. E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 43.Tamura, G. S., and C. E. Rubens. 1995. Group B streptococci adhere to a variant of fibronectin attached to a solid phase. Mol. Microbiol. 15:581-589. [DOI] [PubMed] [Google Scholar]
- 44.Tellier, M. C., G. Greco, M. Klotman, A. Mosoian, A. Cara, W. Arap, E. Ruoslahti, R. Pasqualini, and L. M. Schnapp. 2000. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ T lymphocytes. J. Immunol. 164:3236-3245. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J. Exp. Med. 161:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Putative Treponema pallidum cytadhesins share a common functional domain. Infect. Immun. 49:833-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1986. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect. Immun. 52:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA. 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umemoto, T., Y. Nakatani, Y. Nakamura, and I. Namikawa. 1993. Fibronectin-binding proteins of a human oral spirochete Treponema denticola. Microbiol. Immunol. 37:75-78. [DOI] [PubMed] [Google Scholar]
- 50.Wong, G. H. W., B. Steiner, and S. Graves. 1983. Effect of syphilitic rabbit sera taken at different periods after infection on treponemal motility, treponemal attachment to mammalian cells in vitro, and treponemal infection in rabbits. Br. J. Vener. Dis. 59:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong, C., M. Chrzanowska-Wodnicka, J. Brown, A. Shaub, A. M. Belkin, and K. Burridge. 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]