Abstract
Cholangiocarcinoma (CCA) is the second most common primary malignancy. Although it is more common in Asia, its incidence in Europe and North America has significantly increased in recent decades. The prognosis of CCA is dismal. Surgery is the only potentially curative treatment, but the majority of patients present with advanced stage disease, and recurrence after resection is common. Over the last two decades, our understanding of the molecular biology of this malignancy has increased tremendously, diagnostic techniques have evolved, and novel therapeutic approaches have been established. This review discusses the changing epidemiologic trends and provides an overview of newly identified etiologic risk factors for CCA. Furthermore, the molecular pathogenesis is discussed as well as the influence of etiology and biliary location on the mutational landscape of CCA. This review provides an overview of the diagnostic evaluation of CCA and its staging systems. Finally, new therapeutic options are critically reviewed, and future therapeutic strategies discussed.
Keywords: Cholangiocarcinoma, Bile duct, Cancer, Hepatobiliary, Neoplasia
INTRODUCTION
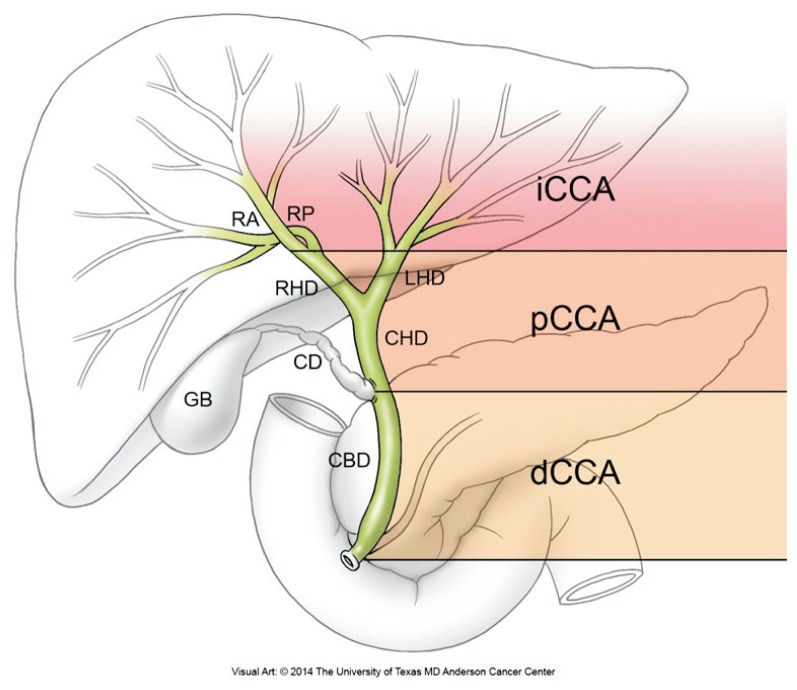
Cholangiocarcinoma (CCA) is the most common biliary tract malignancy and the second most common primary hepatic malignancy.1 It is classified into intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) subtypes (Fig. 1). The latter two subtypes were previously grouped as extrahepatic CCA but are now considered distinct entities based upon differences in their tumor biology and management. pCCA is the most common subtype. The prognosis of CCA is considered dismal. However, our understanding of its molecular tumor biology has increased, and advances in its surgical and nonsurgical management have resulted in improved outcomes and potentially curative treatments for selected patients.
Fig. 1.
Cholangiocarcinoma classification. Classification of cholangiocarcinoma based on its anatomic location within the biliary tree into intrahepatic, perihilar, and distal cholangiocarcinoma. Intrahepatic cholangiocarcinomas (iCCA) are located proximal to the secondary branches of the left and right hepatic ducts. Perihilar cholangiocarcinoma (pCCA) describes tumors located between the secondary branches of the right and left hepatic ducts and the common hepatic duct proximal to the cystic duct origin. Distal cholangiocarcinoma (dCCA) describes tumors of the common bile duct (CBD), up to but not including the ampulla Vateri. The dCCA within the intrapancreatic portion of the CBD can be difficult to distinguish from pancreatic head carcinomas.
RA, right anterior segmental duct; RP, right posterior segmental duct; RHD, right hepatic duct; LHD, left hepatic duct; CHD, common hepatic duct; CD, cystic duct; GB, gallbladder.
EPIDEMIOLOGY
Hepatobiliary malignancies account globally for 13%, and in the United States for 3% of overall cancer-related mortality.2 CCA accounts for 15% to 20% of primary hepatobiliary malignancies. CCA incidence rates are inter- and intra-continentally heterogenous. The highest CCA incidence rates have been reported in Southeast Asia and the lowest in Australia. Within Southeast Asia, its annual incidence ranges from 0.1/100,000 to 71.3/100,000. Throughout Europe, incidence rates range between 0.4/100,000 and 1.8/100,000, and in the United States from 0.6/100,000 to 1.0/100,000.3–5 During the last three decades, age-adjusted incidence rates (AAIR) of iCCA increased in Western Europe, while the incidence of extrahepatic CCA followed a stable to decreasing trend.3,5,6 Interestingly, AAIR of extrahepatic CCA in the United States had significantly increased throughout the last four decades while iCCA incidence remained overall stable.4 Causes for the changing trends in incidence have not been identified. Throughout the last decade, annual mortality rates of iCCA in the United States decreased by 2.5%, while they increased by 9% in Europe.6,7 The male-to-female ratio of CCA is 1:1.2–1.5.2 Globally, the average age at diagnosis is >50 years. In Western industrialized nations, the median age at presentation is 65 years. It is uncommon before age 40 except in patients with primary sclerosing cholangitis (PSC).
ETIOLOGY
The majority of patients develop CCA in the absence of identifiable risk factors (Table 1).8 PSC patients have a 5% to 20% lifetime risk to develop CCA. However, only 10% of CCA are attributed to PSC. Usually, CCA is diagnosed after a median of 4 years following the PSC diagnosis.9 Inflammatory bowel disease is not an independent risk factor for CCA in PSC.10 Caroli’s disease, and types I and IV biliary cysts increase the risk for cholangiocarcinogensis by 30-fold.2 Importantly, excision of cysts reduces but does not eliminate the risk.11 Hepatolithiasis has high incidence rates in Southeast Asia and is associated with a 6- to 50-fold increased risk for iCCA.2 Cirrhosis has been identified as a possible independent risk factor for iCCA.12 Data on hepatitis B virus and hepatitis C virus as risk factors for CCA show prevalence-based variability and require further validation.2 Importantly, obesity, diabetes and the metabolic syndrome have recently been suggested as risk factors for CCA but data are inconsistent.2,13 In the context of globally increasing incidence rates of obesity, metabolic syndrome and iCCA, clarification of their associations will be important.
Table 1.
Risk Factors for Cholangiocarcinogenesis
| Established risk factors |
| Primary sclerosing cholangitis |
| Hepatobiliary parasites (Opisthorchis viverrini, Clonorchis sinensis) |
| Hepatolithiasis |
| Caroli’s disease |
| Choledochal cysts (types I and IV) |
| Thorotrast |
| Possible risk factors |
| Cirrhosis |
| HBV |
| HCV |
| Diabetes mellitus |
| Obesity |
| Chronic alcohol use (>80 g/day) |
| Tobacco |
| Biliary enteric drainage procedures |
| Toxins (dioxins, polyvinyl chloride) |
HBV, hepatitis B virus; HCV, hepatitis C virus.
PATHOLOGY
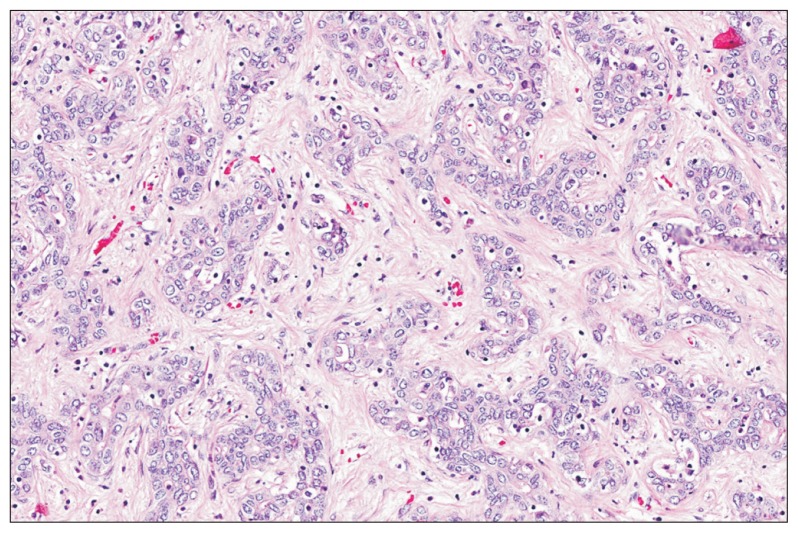
Based upon their macroscopic growth pattern, CCA are classified as mass-forming, periductal-infiltrating or intraductal-papillary. iCCA are predominantly mass-forming, while pCCA are typically periductal-infiltrating. Histopathologically, 90% to 95% of CCA are adenocarcinomas of moderate to poor differentiation, with characteristic mucin expression and highly desmoplastic stroma (Fig. 2).8,14 CK7 and CK19 expression are characteristic of CCA, but both proteins can also be expressed in hepatocellular carcinoma (HCC) and metastatic adenocarcinomas.
Fig. 2.
Cholangiocarcinoma (CCA) histopathology. Characteristic histopathology of CCA (HE, ×20). Between 90% and 95% of CCA are adenocarcinomas of poor to moderate differentiation. Tumor cells are cuboidal to columnar and form glandular and tubular structures. Highly desmoplastic stroma and mucin are characteristic of CCA. (Courtesy of Dr. Wai Chin Foo, MD Anderson Cancer Center, Houston, TX, USA).
PATHOGENESIS
CCA is an epithelial malignancy originating from transformed cholangiocytes, with preclinical studies suggesting hepatic progenitor cells as cells of origin.15 Inflammation and cholestasis are key factors in cholangiocarcinogenesis. Proinflammatory cytokines (i.e., interleukin-6 [IL-6]) activate inducible nitric oxide synthase resulting in excess nitric oxide that mediates oxidative DNA-damage, inhibition of DNA repair enzymes and expression of cyclooxygenase 2 (COX-2). Proinflammatory pathways downregulate hepatobiliary transporters, thereby, contributing to cholestasis.16 Bile acids and oxysterols activate epidermal growth factor receptor (EGFR) and enhance COX-2 expression.17 COX-2 dysregulates CCA growth and apoptosis-resistance, and positively regulates pro-oncogenic signaling pathways such as hepatocyte growth factor (HGF), IL-6, and EGFR.
Next generation sequencing identified somatic mutations in oncogenes (i.e., KRAS), tumor suppressor (i.e., TP53 and SMAD4) and chromatin modifying genes (i.e., ARID1A, BAP1, and PBMR1) in CCA.18–20 These studies have also shown the distinct mutational landscape of different etiologies and anatomic locations.18–21 KRAS mutations are more common in pCCA (22% to 53%) than iCCA (9% to 17%), while IDH1/2 mutations are more characteristic of iCCA.18,21 Mutant IDH1/2 (isocitrate dehydrogenase) inhibits hepatocyte but not biliary differentiation and causes expansion of hepatic progenitor cells, resulting in iCCA-formation in genetic mouse models.22 Liver fluke-associated CCA is more commonly associated with mutations of TP53 and SMAD4, while BAP1 and IDH1/2 mutations are more frequent in non-liver fluke associated CCA.18,20 Mutations of genes coding for components of oncogenic pathways such as PI3KCA and MET have been described in iCCA and pCCA.23 Recently, gene rearrangements resulting in oncogenic fibroblast growth factor 2 (FGFR2) fusion proteins were identified in up to 45% of iCCA patients.24,25 Also nongenomic upregulation of EGFR, human epidermal growth factor receptor 2 (HER2) and MET was found, especially in patients with poor outcomes.21 Whole-genome expression profiling confirmed activation of pathways driving proliferation (i.e., EGF, RAS, AKT, and MET), angiogenesis (i.e., vascular endothelial growth factor receptor [VEGFR], platelet-derived growth factor receptor) and inflammation (i.e., IL-6).26
Receptor tyrosine kinases such as IL-6 receptor, c-MET and the EGFR family members ERBB2 and ERBB1 are key signaling pathways in cholangiocarcinogenesis. CCA cells and cancer-associated fibroblasts express and secrete cytokines and other mitogenic growth factors (i.e., IL-6 and HGF) with subsequent auto- and paracrine stimulation of their cognate receptors. Receptor-overexpression (i.e., IL-6R, c-MET, and EGFR), inactivation of negative feedback mechanisms, and transactivation between receptors (i.e., c-MET/EGFR and COX-2/IL-6) further contribute to constitutive pathway activation. Aberrant activation of these receptor tyrosine kinases causes constitutive activation of downstream signaling cascades (i.e., Janus kinase [JAK]/signal transducer and activator of transcription 3 [STAT3], PI3K/Akt, ERK1/2, and p38MAPK) resulting in dysregulation of cell senescence, cell cycle regulation and proliferation, and apoptosis.
STAGING
Staging systems optimally provide a systemic approach to prognostication, therapeutic stratification and outcome comparison. However, none of the currently existing CCA staging systems fulfills these criteria.
1. Intrahepatic CCA
Currently, there are three major staging systems for iCCA: (1) the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC); (2) the Liver Cancer Study Group of Japan (LCSGJ); and (3) the National Cancer Center of Japan (NCCN) staging systems. The AJCC/UICC staging system (Table 2) is the only system that has shown stage-survival correlation, but it is limited by its requirement for histology to determine Tis and T4.27 Recently developed prognostic nomograms and modifications of the existing LCSGJ staging system outperformed the seventh edition AJCC/UICC staging system but further validation is required.28,29
Table 2.
TNM and American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) Staging Systems for Intrahepatic Cholangiocarcinoma
| TNM stage | Criteria | ||
|---|---|---|---|
| Tx | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ (intraductal tumor) | ||
| T1 | Solitary tumor without vascular invasion | ||
| T2a | Solitary tumor with vascular invasion | ||
| T2b | Multiple tumors, with or without vascular invasion | ||
| T3 | Tumor perforating the visceral peritoneum or involving the local extrahepatic structures by direct invasion | ||
| T4 | Tumor with periductal invasion | ||
| Nx | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastases | ||
| N1 | Regional lymph node metastases present | ||
| M0 | No distant metastases | ||
| M1 | Distant metastases | ||
|
| |||
| AJCC/UICC stage | Tumor | Node | Metastasis |
|
| |||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| IV A | T4 | N0 | M0 |
| Any T | N1 | M0 | |
| IV B | Any T | Any N | M1 |
TNM, tumor, node, metastasis.
2. Perihilar CCA
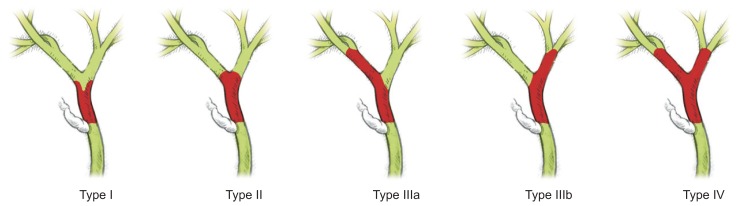
The Bismuth-Corlette classification (Fig. 3) was developed to guide surgical therapy but it is not a staging system per se. The two most commonly used staging systems for pCCA include the AJCC/UICC and the Memorial Sloan-Kettering Cancer Center (MSKCC) staging systems. In the most current seventh edition of the AJCC/UICC staging system, pCCA (Table 3) and dCCA are for the first time staged separately; thus, it requires further validation of its prognostic value. The MSKCC staging system failed to stratify resectable from unresectable patients with sufficient accuracy. New staging systems have been proposed awaiting further validation.30 Very recently, a staging system was developed solely based on clinical information, which had excellent treatment-dependent and -independent prognostic performance, and outperformed the current TNM-staging system.31
Fig. 3.
Bismuth-Corlette classification of perihilar cholangiocarcinoma (pCCA). Bismuth-Corlette classification of pCCA as types I to IV. The tumor is depicted in red, normal bile ducts are in green, and the cystic duct is in white.
Table 3.
TNM and American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) Staging Systems for Perihilar Cholangiocarcinoma
| TNM stage | Criteria | ||
|---|---|---|---|
| Tx | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumor confined to the bile duct, with extension up to the muscle layer of fibrous tissue | ||
| T2a | Tumor invades beyond the wall of the bile duct to surrounding adipose tissue | ||
| T2b | Tumors invades adjacent hepatic parenchyma | ||
| T3 | Tumor invades unilateral branches of the portal vein or hepatic artery | ||
| T4 | Tumor invades main portal vein or its branches bilaterally OR tumor invades the common hepatic artery OR tumor invades second-order biliary radicals bilaterally or tumor invades unilateral second-order biliary radicals with contralateral portal vein ORhepatic artery involvement | ||
| Nx | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastases | ||
| N1 | Regional lymph node metastases (incl. nodes along the cystic duct, common bile duct, hepatic artery and portal vein) | ||
| N2 | Metastases to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes | ||
| M0 | No distant metastases | ||
| M1 | Distant metastases | ||
|
| |||
| AJCC/UICC stage | Tumor | Node | Metastasis |
|
| |||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2a–b | N0 | M0 |
| III A | T3 | N0 | M0 |
| III B | T1–3 | N1 | M0 |
| IV A | T4 | N0–1 | M0 |
| IV B | Any T | N2 | M0 |
| Any T | Any N | M1 | |
TNM, tumor, node, metastasis.
3. Distal extrahepatic
The seventh edition of the AJCC/UICC is currently the only staging system for dCCA (Table 4). Its use is limited by a lack of correlation of its T-stages to outcomes after resection, and the need for microscopic evaluation of tumor invasion depth.32
Table 4.
TNM and American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) Staging Systems for Distal Extrahepatic Cholangiocarcinoma
| TNM stage | Criteria | ||
|---|---|---|---|
| Tx | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ (intraductal tumor) | ||
| T1 | Tumor confined to the bile duct histologically | ||
| T2a | Tumor invades beyond the wall of the bile duct | ||
| T3 | Tumor invades the gallbladder, pancreas, duodenum or other adjacentorgans without involvement of the celiac axis or the superior mesenteric artery | ||
| T4 | Tumor involves the celiac axis or the superior mesenteric artery | ||
| Nx | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastases | ||
| N1 | Regional lymph node metastases present | ||
| M0 | No distant metastases | ||
| M1 | Distant metastases | ||
|
| |||
| AJCC/UICC stage | Tumor | Node | Metastasis |
|
| |||
| 0 | Tis | N0 | M0 |
| I A | T1 | N0 | M0 |
| I B | T2 | N0 | M0 |
| II A | T3 | N0 | M0 |
| II B | T1–3 | N1 | M0 |
| III | T4 | Any N | M0 |
| IV | Any T | Any N | M1 |
TNM, tumor, node, metastasis.
CLINICAL PRESENTATION AND DIAGNOSIS
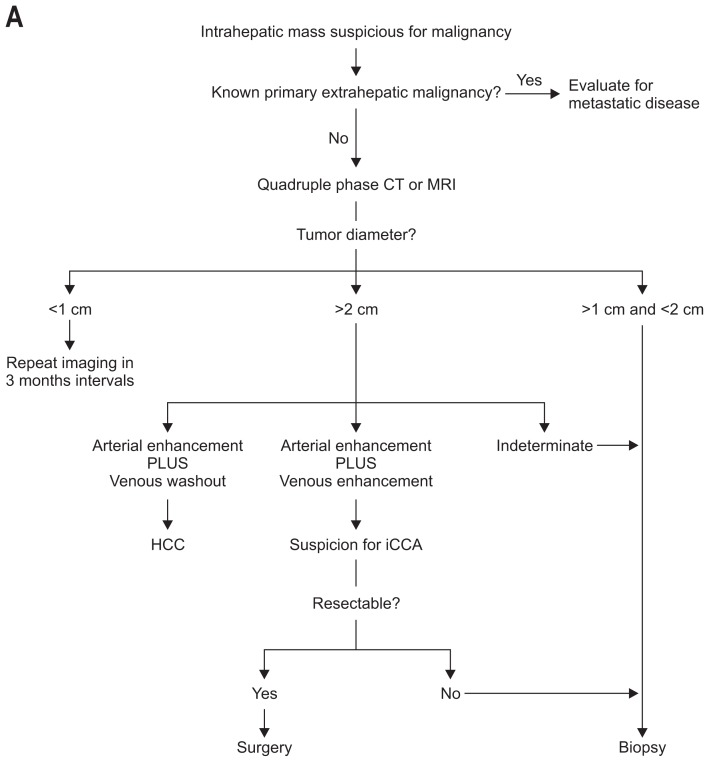
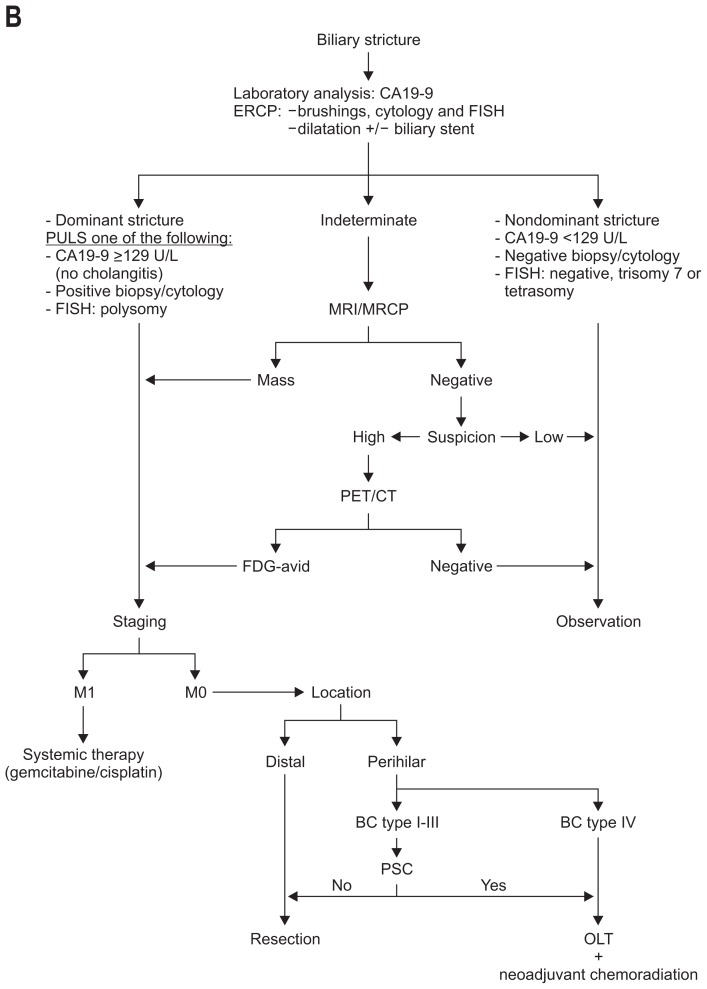
The clinical presentation of CCA is unspecific. Its diagnosis requires the combined interpretation of different diagnostic modalities (Fig. 4A and B).
Fig. 4.
Algorithm for the management and diagnosis of cholangiocarcinoma. (A) Algorithm for intrahepatic cholangiocarcinoma. (B) Algorithm for perihilar cholangiocarcinoma.
CT, computed tomography; MRI, magnetic resonance imaging; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; CA 19-9, carbohydrate antigen 19-9; ERCP, endoscopic retrograde cholangiopancreaticography; FISH, fluorescent in situ hybridization; MRCP, magnetic resonance cholangiopancreatography; PET/CT, positron emission tomography/computer tomography; BC, Bismuth-Corlette; PSC, primary sclerosing cholangitis; OLT, orthotopic liver transplantation.
1. Intrahepatic CCA
Nineteen percent to 43% of iCCA are diagnosed incidentally.14,33 Patients usually become symptomatic at advanced stage disease with unspecific symptoms such as abdominal pain, malaise, night sweats, and cachexia. Twenty-five percent of symptomatic patients have resectable disease versus 58% of nonsymptomatic patients.8 The most commonly used tumor marker for CCA is carbohydrate antigen 19-9 (CA 19-9). Its accuracy in distinguishing iCCA from HCC is 63% to 67%.34 However, CA 19-9 can also be elevated in other gastrointestinal, pancreatic and gynecologic malignances, and benign cholangiopathies. Eight percent of the population are Lewis antigen-negative and, therefore, do not express CA 19-9 regardless of tumor burden. The use of other tumor markers is limited by their low specificity (i.e., CEA and CA-125) or need for further validation (i.e., CA242 and CYFRA 21-1).
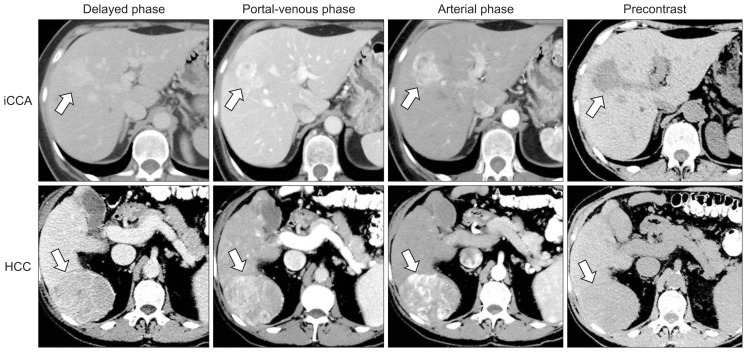
Dynamic cross-sectional imaging is essential in the characterization of intrahepatic masses and the preoperative planning for CCA. The major limitation of ultrasound, including contrast-enhanced ultrasound, is its inability to reliably distinguish HCC from iCCA with misdiagnosis rates of up to 52%.35 Computed tomography (CT) and magnetic resonance imaging (MRI) can accurately distinguish iCCA from HCC in tumors >2 cm (Fig. 5).36 18F-FDG-positron emission tomography (PET) with CT (PET/CT) has a sensitivity and specificity of up to 95% and 83% in the evaluation of the primary tumor, but it does not provide a significant advantage over CT or MRI.37 Also, positive FDG-uptake can be observed in other malignancies (i.e., lymphomas), inflammatory and infectious processes. In the evaluation for lymph node metastases, 18F-FDG-PET has a sensitivity and specificity of 80% and 92%, compared to 20% and 86% with CT.38 The diagnostic accuracy of 18F-FDG-PET for distant metastases is 88% versus 79% with CT.39
Fig. 5.
Hepatocellular carcinoma (HCC) versus intrahepatic CCA (iCCA) on dynamic cross-sectional imaging. On quadruple phase imaging, iCCA (upper panel, white arrow) presents with progressive, heterogenous contrast-enhancement, whereas HCC (lower panel, white arrow) is characterized by homogenous contrast-enhancement followed by washout in the portal venous and delayed phases.36 Other radiologic iCCA characteristics include homogeneous low-attenuation mass with irregular peripheral enhancement, a lobulated morphology, hepatic capsular retraction, local vascular invasion, and proximal biliary dilatation. (Courtesy of Dr. Janio Szklaruk, MD Anderson Cancer Center, Houston, TX, USA).
While there are no data on tumor spread following tumor biopsy in iCCA, it is recommended in cases with inconclusive or contradictory test results given the differences in management and prognosis of iCCA versus other primary hepatic tumors.
2. Perihilar and distal CCA
Painless jaundice is the presenting symptom in 90% of pCCA patients, and acute cholangitis in 10%. Fifty-six percent of pCCA patients have systemic signs of malignancy (i.e., anorexia, weight loss, and fatigue) at their initial presentation. A palpable prominence of one hepatic lobe can occasionally be noted as manifestation of a hypertrophyatrophy complex (unilobar biliary obstruction with ipsilateral vascular encasement resulting in ipsilateral hepatic lobe atrophy with contralateral hepatic lobe hypertrophy).
A frequent diagnostic dilemma is the distinction of benign from malignant biliary duct strictures. Up to 15% of suspicious biliary strictures are postoperatively found to be benign. IgG4 serum concentrations need to be evaluated to rule out IgG4 cholangiopathy. In non-PSC patients with benign biliary strictures, CA 19-9 serum concentrations of <100 U/L have a negative predictive value (NPV) of 92%.40 In PSC patients, a CA 19-9 cutoff of 129 U/L has a positive predictive value of 57% and a NPV of 99% for CCA.41 However, CA 19-9 elevations >129 U/L have been reported in 30% to 37% of PSC patients without cholangiocarcinogenesis on long-term follow-up, and its specificity significantly decreases in cholestasis and cholangitis.42
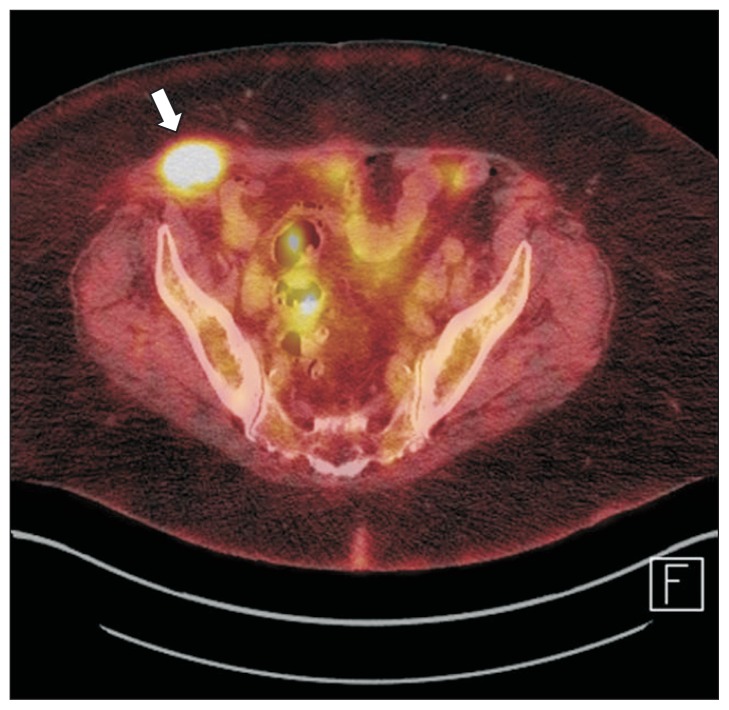
Cross-sectional imaging should be obtained prior to biliary interventions and surgery. Multidetector CT can identify anatomic variations and tumor extent, thereby, guiding the surgical approach. MRI with magnetic resonance cholangiopancreatography allows evaluation of the biliary tree and the hepatic parenchyma. Its accuracy in the evaluation of the local extent and resectability is up to 95%, but only 67% to 73% and 75% to 80% in detection of vascular and parenchymal invasion.43,44 Portal venous and hepatic arterial invasion can be assessed with CT with accuracies of up to 96% and 93%.45 The sensitivity and specificity of 18F-FDG-PET for the primary tumor in pCCA is 60% to 92% and 93% to 100%, while its sensitivity for regional lymph node metastases is only 13% to 50%.46,47 The predominant role of 18F-FDG-PET in the evaluation of pCCA is the identification of distant metastases (Fig. 6).
Fig. 6.
Positron emission tomography/computer tomography (PET/CT) in the evaluation of metastatic disease in cholangiocarcinoma. A peritoneal metastasis in a patient with perihilar cholangiocarcinoma. The mass (arrow) was identified as a FDG-avid mass within the abdominal wall on PET/CT imaging (Courtesy of Dr. Janio Szklaruk, MD Anderson Cancer Center, Houston, TX, USA).
Dependent on accessibility of the biliary tree, biliary strictures can be evaluated by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography. pCCA typically presents as a dominant stricture or filling defect. Interventional cholangiography allows sampling of the strictures and therapeutic biliary stent placement. The sensitivity of cytology of biliary brushings is only 20% to 43%, but can be increased up to 46% to 68% through additional analysis for chromosomal aneuploidy using fluorescent in situ hybridization (FISH).48,49
Single-operator peroral cholangioscopy (SOP) allows direct visualization of biliary defects, and guided brushings and biopsies. The accuracy of SOP with tissue sampling in patients in whom ERCP-guided tissue sampling was inconclusive is 58% to 95%. However, bile duct canniculation is unsuccessful in 18% to 41%, and interobserver agreement is suboptimal.50,51 Endoscopic ultrasound can detect lymph node metastases in up to 17% of patients negative for N1 disease on CT.52 Although its sensitivity in diagnosing pCCA has been reported as up to 86%, biopsies of the primary tumor must be avoided as it can result in exclusion of these patients from potentially curative liver transplantation due to potential tumor cell spread.
MANAGEMENT
Surgical treatments are the only potentially curative therapeutic options for CCA. However, the majority of CCA patients are diagnosed at late stage disease, and 10% to 45% of patients considered resectable are found to be unresectable during explorative laparotomy.8,33,53
More aggressive surgical approaches and improved radiologic techniques have resulted in improved R0 (tumor-free margins) resection rates (Table 5) but recurrence rates remain high with 49% to 64%. Recurrences are predominantly intrahepatic and usually occur within 2 to 3 years postresection.8,14,33,54,55
Table 5.
Outcomes after Resection of Cholangiocarcinoma
| Author | Year | Case, n | R0, % | Morbidity, % | Mortality, % | 5-Year survival, % | R0 5-year survival, % | N+ 5-year survival, % |
|---|---|---|---|---|---|---|---|---|
| iCCA | ||||||||
| Weber et al.14 | 2001 | 33 | 88 | 19 | 3.9 | 31 | - | 25 (at 3-year) |
| Morimoto et al.33 | 2003 | 49 | 69 | 35 | 3.8 | 32 | 41 | 9 |
| DeOliveira et al.76 | 2007 | 44 | 45 | 35 | 4.5 | 40 | 63 | - |
| Konstadoulakis et al.53 | 2008 | 54 | 78 | 11 | 7.0 | 25 | - | |
| Choi et al.54 | 2009 | 64 | 86 | 22 | 1.6 | 40 | 54 | 11 |
| Lang et al.55 | 2009 | 83 | 64 | 44 | 7.1 | 21 | 30 | 17 |
| Murakami et al.77 | 2011 | 21 | 62 | - | - | 37 | - | - |
| de Jong et al.78 | 2011 | 449 | 81 | - | - | 31 | - | - |
| Ribero et al.79 | 2012 | 434 | 85 | 35 | 5.3 | 33 | 40 | 16 |
| pCCA | ||||||||
| DeOliveira et al.76 | 2007 | 281 | 19 | 62 | 5.4 | 10 | 30 | - |
| Hirano et al.80 | 2010 | 146 | 87 | 45 | 3.4 | 36 | - | - |
| Lee et al.58 | 2010 | 302 | 71 | 43 | 1.7 | 33 | 47 | - |
| Ercolani et al.81 | 2010 | 51 | 73 | 51 | 9.8 | 34 | 44 | 0 |
| Unno et al.82 | 2010 | 125 | 63 | 49 | 8.0 | 35 | 46 | - |
| Shimizu et al.83 | 2010 | 172 | 66 | 44 | 6.4 | 29 | 39 | 29 |
| Saxena et al.84 | 2011 | 42 | 64 | 45 | 2.4 | 24 | - | - |
| Young et al.85 | 2011 | 83 | 46 | 64 | 7.2 | 20 | 33 | 15 |
| Murakami et al.77 | 2011 | 50 | 74 | - | - | 37 | - | - |
| Matsuo et al.86 | 2012 | 157 | 76 | 59 | 7.6 | 32 | - | - |
| de Jong et al.87 | 2012 | 305 | 65 | - | 5.2 | 20 | - | - |
| Nagino et al.56 | 2013 | 574 | 67 | 57 | 4.7 | 33 | 67 | 22 |
| dCCA | ||||||||
| Yoshida et al.62 | 2002 | 27 | 85 | 22 | 3.7 | 37 | - | 20 |
| Sakamoto et al.63 | 2005 | 55 | 84 | - | 3.6 | 24 | 34 | 16 |
| DeOliveira et al.76 | 2007 | 239 | 78 | 56 | 3.0 | 23 | 27 | - |
| Shimizu et al.88 | 2008 | 34 | - | 21 | 2.9 | 45 | - | - |
| Murakami et al.77 | 2011 | 56 | 80 | - | - | 43 | - | 21 |
iCCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; dCCA, distal cholangiocarcinoma.
1. Surgical resection
Surgical resection is the preferred treatment for CCA. Contraindications to surgical resection include bilateral, multifocal disease, distant metastases and comorbidities associated with operative risks outweighing the expected benefits of a surgery. PSC patients with pCCA should preferentially be treated with liver transplantation due to the field defect in PSC and frequent underlying advanced fibrosis. Regional lymph node metastases are not considered an absolute contraindication to resection, although N1 disease is an independent prognostic factor for worse outcomes (Table 5). Dependent on the tumor extent, iCCA are resected by segmentectomy with 78% to 82% of patients requiring major segmentectomy (>3 segments).8,14 In cases in which low volume of the remaining hepatic lobe is prohibitive to resection, preoperative portal vein embolization of the ipsilateral hepatic lobe can result in compensatory hypertrophy of the contralateral lobe, thereby, permitting subsequent hemihepatectomy. While resection of Bismuth-Corlette type IV pCCA is not considered an absolute contraindication to resection, neoadjuvant chemoradiation followed by orthotopic liver transplantation (OLT) is the preferable treatment based upon its excellent outcomes (vide infra). dCCA are most commonly resected by conventional or pylorus-preserving pancreaticoduodenectomy with lymphadenectomy. pCCA tend to invade the right hepatic artery (RHA) due to its proximity to the biliary confluence. Recently, successful RHA-resection and -reconstruction in pCCA patients has been reported with perioperative mortality rates of less than 5% and 5-year survival rates of up 30%.56 In selected patients with portal vein- or local invasion, pancreaticoduodenectomy with portal vein resection or hepatopancreaticoduodenectomy has been performed at specialized centers with R0 resection rates of 74% to 85% and 5-year survival rates of 11% to 37%; however, it is limited by high morbidity and mortality rates.57
Postoperative morbidity and mortality rates have decreased in recent years (Table 5) Major postoperative complications include intraabdominal abscesses and bile duct leaks.8,33,53,58 Preoperative biliary drainage in patients with malignant jaundice is an area of controversy. Uncorrected preoperative jaundice has been associated with higher rates of postsurgical abscesses, bile leaks, biliary fistulae and sepsis. However, several studies failed to show significant improvements in morbidity and mortality with presurgical biliary drainage, and some reported increased overall and postsurgical complication rates.59,60 Presurgical biliary drainage should be pursued in patients with a bilirubin of >10 mg/dL, cholangitis, neoadjuvant treatment, and delayed surgery.
Lymph node metastases and R0 status are the two major prognostic factors influencing outcomes after resection.8,33,54 Approximately 45% of patients undergoing resection are found to be N+.61 Five-year survival of N+ versus N0 disease is 0% to 9% versus 36% to 43% in iCCA, 0% to 29% versus 32% to 67% in pCCA, and 16% to 21% versus 42% to 61% in dCCA.33,62,63 Based on a recent meta-analysis, the National Comprehensive Cancer Network (NCCN) recommends adjuvant chemotherapy with fluoropyrimidine- or gemcitabine-based regimens for patients with R1-resection and/or N1 disease.64 However, there are no prospective, large randomized controlled trials that have yet shown a survival benefit from either neoadjuvant or adjuvant treatments.
2. Orthotopic liver transplantation
OLT is not recommended as monotherapy for CCA due to high recurrence rates and long-term survival of less than 20%. Recently, neoadjuvant chemoradiation followed by OLT has been established as an effective treatment for pCCA. Recurrence rates following this transplant protocol are 20%, and recurrence-free 5-year survival 68%.65 However, patient-selection criteria are stringent (Tables 6 and 7), and 25% to 31% of patients develop progression of their disease while awaiting OLT, resulting in exclusion from the protocol.65
Table 6.
Diagnostic Criteria for Perihilar Cholangiocarcinoma89
| Biopsy or cytology positive for adenocarcinoma |
| Malignant appearing stricture with cytology suspicious for adenocarcinoma |
| PLUS |
| fluorescent in situ hybridization positive for polysomy |
| Mass lesion on cross sectional imaging studies |
| Malignant biliary stricture with CA 19-9 >100 U/mL in the absence of bacterial cholangitis |
CA 19-9, carbohydrate antigen 19-9.
Table 7.
Liver Transplant Exclusion Criteria for Perihilar Cholangiocarcinoma89
| Radial diameter on cross-sectional imaging >3 cm |
| Perihilar lymph node metastases |
| Intra- or extrahepatic metastases |
| Transperitoneal (percutaneous or endoscopic ultrasound) biopsy of primary tumor |
| Prior attempt at resection with violation of bile ducts |
| Prior radiation treatment |
| Uncontrolled infection |
| Significant comorbidities prohibitive to surgery |
Few studies have evaluated the benefit of OLT with or without adjuvant treatment for iCCA; these studies were limited by their retrospective nature, small sample size, and differences in tumor characteristics and adjuvant treatment regimens. Recurrence rates were as high as 35% to 75% and 5-year survival only 34% to 51%; therefore, OLT is currently not recommended for iCCA.66–68
3. Nonsurgical therapies
Traditionally considered chemotherapy-resistant, the phase III randomized controlled ABC-02 trial reported a 6-month survival benefit in CCA patients treated with gemcitabine/cisplatin-combination therapy versus gemcitabine monotherapy.69 A recent phase III randomized controlled trial showed higher objective response rates (31% vs 14%, p=0.004) and longer progression-free survival (5.9 months vs 3.0 months, p=0.049) through the addition of the EGFR-inhibitor erlotinib to gemcitabine/cisplatin.70
In the palliative setting, specialized centers offer locoregional therapies such as radiofrequency ablation, transarterial chemoembolization (TACE), drug-eluting bead-TACE, selective intra-arterial radiotherapy with 90Y microspheres or external beam radiation therapy. Few studies suggested a benefit of such therapies in regard to tumor progression and survival. These studies are limited by their retrospective nature, small sample size, different chemotherapeutic agents and inclusion of other biliary tract cancers. Currently, there are no prospective, randomized controlled trials that have shown a survival benefit of locoregional therapies. Grade III/IV toxicity rates of up to 36% have been reported with the above described local ablative therapies.71
Stenting with photodynamic therapy (PDT) has been compared to stenting without PDT. The results indicated a benefit in regard to survival, biliary drainage and quality of life; however this will need to be confirmed by larger studies.72
In patients with unresectable CCA and cholestasis, drainage of >50% of the liver parenchyma can improve patient survival. Outcomes of percutaneously versus endoscopically placed biliary metal stents are comparable and the route of placement should be chosen based upon accessibility and comorbidities.73
FUTURE DIRECTIONS
Limitations of current clinical trials include small sample size, combined analysis of CCA and gallbladder carcinoma, and lack of randomization. Very few studies evaluated the therapeutic efficacy of targeted agents combined with retrospective analysis of transcriptomic data. However, preclinical and early clinical trials support targeting EGFR in combination with other molecular targets (i.e., HER2 and VEGFR) and/or chemotherapeutics.70 Interestingly, mutations in KRAS are associated with resistance to receptor tyrosine kinase inhibition and poor survival.20,21 Targeting JAK/STAT3 is another highly promising approach based on preclinical studies.26 Inhibitors of IDH1/2-mutations are currently evaluated in clinical phase I trials in solid tumors including iCCA. Interestingly, IDH1/2-mutations sensitize AML-cells to BH3-mimetics such as Navitoclax, a drug shown to effectively eradicate cancer associated fibroblasts in preclinical CCA models, thereby, offering an alternative approach for targeted treatment in selected patients with IDH-mutations.74,75 The identification of fusion FGFR2 in iCCA provides another druggable target currently evaluated in clinical phase I and II trials in CCA patients.25 However, CCA are genetically highly heterogeneous and, therefore, precision medicine will be required to improve outcomes.19 Transcriptomic analysis revealed prognostic classifiers that were independent of anatomical location,19,21 and next generation gene profiling demonstrated distinct subclasses prediciting survival and recurrence, thereby, allowing patient stratification based upon their prognostic and oncogenic pathway analysis.26 Expansion of OLT or resection criteria could be based upon such classifiers in the future. Randomized controlled trials are needed that prospectively assign patients based upon their transcriptomic and genetic profile, but for outcome comparison, better staging systems will be needed.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110. doi: 10.1007/s10620-014-3276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witjes CD, Karim-Kos HE, Visser O, et al. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB (Oxford) 2012;14:777–781. doi: 10.1111/j.1477-2574.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Hahn T, Ciesek S, Wegener G, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46:1092–1098. doi: 10.3109/00365521.2011.589472. [DOI] [PubMed] [Google Scholar]
- 7.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667–1674. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 8.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 9.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7–11. doi: 10.1002/hep.510310103. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T, Ochiai T. Risk of bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery. 1999;126:939–944. doi: 10.1016/S0039-6060(99)70036-X. [DOI] [PubMed] [Google Scholar]
- 12.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, Mc-Glynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/S1072-7515(01)01016-X. [DOI] [PubMed] [Google Scholar]
- 15.Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol. 2015;62:198–207. doi: 10.1016/j.jhep.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis. 2010;30:186–194. doi: 10.1055/s-0030-1253227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 18.Chan-On W, Nairismägi ML, Ong CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 19.Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 21.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031.e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 25.Borad MJ, Gores GJ, Roberts LR. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31:264–268. doi: 10.1097/MOG.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: Bby the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 29.Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: a multicenter analysis by the study group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:499–508. doi: 10.1002/jhbp.92. [DOI] [PubMed] [Google Scholar]
- 30.Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 31.Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881–1890. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146:250–257. doi: 10.1016/j.surg.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto Y, Tanaka Y, Ito T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:432–440. doi: 10.1007/s00534-002-0842-3. [DOI] [PubMed] [Google Scholar]
- 34.Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg. 2010;76:1210–1213. [PubMed] [Google Scholar]
- 35.Galassi M, Iavarone M, Rossi S, et al. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771–779. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 36.Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791–798. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 37.Annunziata S, Caldarella C, Pizzuto DA, et al. Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in the evaluation of the primary tumor in patients with cholangiocarcinoma: a meta-analysis. Biomed Res Int. 2014;2014:247693. doi: 10.1155/2014/247693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park TG, Yu YD, Park BJ, et al. Implication of lymph node metastasis detected on 18F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinoma. Clin Nucl Med. 2014;39:1–7. doi: 10.1097/RLU.0b013e3182867b99. [DOI] [PubMed] [Google Scholar]
- 39.Kim JY, Kim MH, Lee TY, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 40.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 41.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 42.Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:434–439.e1. doi: 10.1016/j.cgh.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 44.Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213–2221. doi: 10.1007/s00330-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 45.Chen HW, Lai EC, Pan AZ, Chen T, Liao S, Lau WY. Preoperative assessment and staging of hilar cholangiocarcinoma with 16-multidetector computed tomography cholangiography and angiography. Hepatogastroenterology. 2009;56:578–583. [PubMed] [Google Scholar]
- 46.Kluge R, Schmidt F, Caca K, et al. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029–1035. doi: 10.1053/jhep.2001.23912. [DOI] [PubMed] [Google Scholar]
- 47.Moon CM, Bang S, Chung JB, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23:759–765. doi: 10.1111/j.1440-1746.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 48.Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:943–950.e3. doi: 10.1016/j.gie.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783–789. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Manta R, Frazzoni M, Conigliaro R, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013;27:1569–1572. doi: 10.1007/s00464-012-2628-2. [DOI] [PubMed] [Google Scholar]
- 51.Kalaitzakis E, Webster GJ, Oppong KW, et al. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol. 2012;24:656–664. doi: 10.1097/MEG.0b013e3283526fa1. [DOI] [PubMed] [Google Scholar]
- 52.Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 53.Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 55.Lang H, Sotiropoulos GC, Sgourakis G, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 57.Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 2012;256:297–305. doi: 10.1097/SLA.0b013e31826029ca. [DOI] [PubMed] [Google Scholar]
- 58.Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 59.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 60.Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17–27. doi: 10.1097/00000658-200207000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 2013;257:718–725. doi: 10.1097/SLA.0b013e3182822277. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. doi: 10.1001/archsurg.137.1.69. [DOI] [PubMed] [Google Scholar]
- 63.Sakamoto Y, Kosuge T, Shimada K, et al. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396–402. doi: 10.1016/j.surg.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 64.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology: hepatobiliary cancers [Internet] Fort Washington: NCCN; c2016. [cited 2015 Nov 5]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pre-transplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–981. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 2011;146:683–689. doi: 10.1001/archsurg.2011.116. [DOI] [PubMed] [Google Scholar]
- 67.Sotiropoulos GC, Kaiser GM, Lang H, et al. Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc. 2008;40:3194–3195. doi: 10.1016/j.transproceed.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 68.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 70.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 71.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213–220. doi: 10.1002/jso.23781. [DOI] [PubMed] [Google Scholar]
- 72.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 73.Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Chan SM, Thomas D, Corces-Zimmerman MR, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 78.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 79.Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 80.Hirano S, Kondo S, Tanaka E, et al. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 81.Ercolani G, Zanello M, Grazi GL, et al. Changes in the surgical approach to hilar cholangiocarcinoma during an 18–year period in a Western single center. J Hepatobiliary Pancreat Sci. 2010;17:329–337. doi: 10.1007/s00534-009-0249-5. [DOI] [PubMed] [Google Scholar]
- 82.Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2010;17:463–469. doi: 10.1007/s00534-009-0206-3. [DOI] [PubMed] [Google Scholar]
- 83.Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- 84.Saxena A, Chua TC, Chu FC, Morris DL. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg. 2011;202:310–320. doi: 10.1016/j.amjsurg.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 85.Young AL, Igami T, Senda Y, et al. Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB (Oxford) 2011;13:483–493. doi: 10.1111/j.1477-2574.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215:343–355. doi: 10.1016/j.jamcollsurg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 87.de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118:4737–4747. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu Y, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. The morbidity, mortality, and prognostic factors for ampullary carcinoma and distal cholangiocarcinoma. Hepatogastroenterology. 2008;55:699–703. [PubMed] [Google Scholar]
- 89.Gores GJ, Darwish Murad S, Heimbach JK, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma. Dig Dis. 2013;31:126–129. doi: 10.1159/000347207. [DOI] [PubMed] [Google Scholar]