Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) has been an established treatment for indicated early gastric cancer (EGC) without deterioration of quality of life (QOL) compared with surgical resection. The aim of this study was to evaluate long-term QOL in patients undergoing ESD for EGC.
Methods
Patients scheduled to undergo curative ESD for EGC were prospectively enrolled from 12 institutions between May 2010 and December 2011. Assessments of QOL with Korean versions of the European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaire-core (QLQ-C30) and a gastric cancer-specific questionnaire (STO22) were performed at baseline and at 7 days, 3 months, and 6 months after ESD.
Results
A total of 666 subjects were assessed for QLQ-C30 and QLQ-STO22. The mean QLQ-C30 score was 69.5 at baseline, 68.8 at 7 days, 73.1 at 3 months, and 73.2 at 6 months. The global health status on the EORTC QLQ-C30 was significantly improved after 3 and 6 months (p=0.0003 and p<0.0001, respectively). The QLQ-C30 and STO22 scores were not significantly different, or they only slightly deteriorated between before and immediately after ESD, but they were significantly improved after 3 and 6 months (p<0.05).
Conclusions
QOL did not deteriorate immediately after ESD, and it improved more significantly at up to 6 months in patients who underwent curative ESD for EGC without significant complications.
Keywords: Quality of life, Endoscopic submucosal dissection, Early gastric cancer
INTRODUCTION
Gastric cancer is the fourth most common malignancy worldwide and the second leading cause of cancer mortality.1 In South Korea, gastric cancer is secondary cause among all malignancies and diagnosed as early stage in 70% of patients through national cancer screening program.2,3 With early detection of gastric cancer and introduction of new techniques, endoscopic resection has largely replaced conventional surgical resection in the treatment of gastric cancers. Nowadays, endoscopic submucosal dissection (ESD) has been accepted as a standard treatment option for early gastric cancer (EGC) in the indicated case, and shown many advantages over surgical resection in terms of high quality of life (QOL), less invasiveness, preservation of organ function, low cost, and short hospital stay.4
As ESD has showed many clinical advantages without sacrifice of survival compared with surgical resection, QOL has been also considered as an important outcome. Since the European Organization for Research and Treatment of Cancer (EORTC) has developed the disease-specific QOL module for use in the patients with gastric cancer, QOL after surgical resection has been assessed in many studies.5–7 In a previous study, the fully validated Korean version of the EORTC Quality of Life questionnaire-core (QLQ)-C30 and the the gastric cancer-specific questionnaire (QLQ-STO22) were used to assess the QOL outcomes after laparoscopy-assisted distal gastrectomy compared with open distal gastrectomy in patients with EGC.8
Although many studies has reported the QOL outcomes after surgical resection for EGC, there has been few reports about the QOL outcomes after ESD for EGC, Moreover, most clinical studies has reported retrospectively the QOL outcomes as a minor result after ESD. The aim of study was to prospectively evaluate long-term QOL in the patients after ESD for EGC.
MATERIALS AND METHODS
1. Patients and study design
From May 2010 to December 2011, the patients who underwent ESD for EGC at 12 centers were prospectively enrolled in the study. The inclusion criteria were as follows: (1) adenocarcinoma with differentiated histology; (2) tumor confined to mucosa without evidence of distant metastasis; and (3) tumor size not more than 3 cm. The exclusion criteria were as follows: (1) history of malignancy within 5 years of enrollment; (2) history of partial gastrectomy; (3) severe comorbid conditions; (4) bleeding tendency; and (5) pregnancy.
All participants were provided with written informed consents for the study, and the institutional review boards of all centers approved the study. This study had been conducted in accordance with the Declaration of Helsinki.
2. ESD and pathologic evaluation
Under conscious sedation with midazolam and/or propofol, ESD was performed with cardiorespiratory monitoring. All the procedures were performed as previously described; marking, injection making submucosal cushion, submucosal dissection, and hemostasis.9 After ESD, proton pump inhibitor was given for 4 to 8 weeks for the healing of artificial ulcer. Helicobacter pylori eradication after ESD was performed by case by case.
After ESD, the specimen was fixed in 10% formalin and sectioned serially at 2-mm intervals, parallel to a line that includes the closest resection margin of the specimen. Final pathological diagnosis was made by the central committee of pathologists with the agreement over 80% on the basis of the third edition of Japanese Classification of Gastric Carcinoma.10 In final pathologic report, the pathologic type, depth of tumor invasion, tumor size, lymphatic and venous involvement, and presence of tumor at the resection margin (horizontal and vertical margin) were evaluated.
Complete resection was defined as a differentiated-type adenocarcinoma limited to the mucosal layer without lymphovascular invasion, and with tumor-free horizontal and vertical margins. Post hoc analyses were also performed for curative resection, which was defined based on the Japanese Gastric Cancer Association recommendation for curability criteria. Lesions meeting the absolute or expanded indication were considered as curative resection.
3. Assessment of QOL
QOL outcomes were evaluated with Korean version of EORTC QLQ-C30 (version 3.0) and QLQ-STO22 at baseline (before ESD), 7 days, 3 months, and 6 months after ESD. EORTC QLQ-C30 scores were calculated according to standard guidelines yielding a range of 0 to 100, which is composed of multi-item scales and single-item measures, a global health status, five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea and vomiting), and six single items (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea). All items were scored on 4-point Likert scales without two items in the global health status using 7-point scales. Each of the multi-item scales includes a different set of items. All of the scales and single-item measures range in score from 1 to 100, a higher score represents a higher level of functioning, or a higher level of symptoms.
EORTC QLQ-STO22 is gastric cancer module for use among a wide range of patients with adenocarcinoma of the stomach. The module is composed of 22 items concerning disease, treatment-related symptoms, complications, dysphagia, nutritional aspects, and the emotional problems of gastric cancer.
This questionnaire was self-reported by patients at the points. If a patient did not created directly, researcher in hospital explained the questions to patients and recorded the answers.
4. Statistical analyses
Continuous variables were presented as mean±standard deviation (SD) and compared by using Student t-test. Categorical variables were presented as proportion and compared by using chi-square test. Statistical analyses of the QOL outcomes evaluated the differences between the baseline and the 7 days, 3 months, and 6 months after ESD with respect to the overall changes. For assessing change of QOL scores and to adjust for possible time effects, we performed generalized estimating equation. We included main effects of time, main effect of covariance in fixed effect model, used unstructured covariance matrix.
Data were analyzed with SAS statistical software version 9.2 for Windows (SAS, Cary, NC, USA); two-sided probability value less than 0.05 were considered of statistical significance.
RESULTS
1. Baseline demographics
A total of 712 patients who underwent ESD for EGC from 12 centers were enrolled in the study. Among those, 37 patients were excluded for additional treatment such as surgical resection or ablation therapy with argon plasma coagulation for incomplete resection. Also, nine patients who did not fulfill the questionnaires at baseline were excluded, and 666 patients were finally analyzed.
The mean age of the patients was 62.8±9.2 years and 77% were men (Table 1). Complete resection was achieved in 82.6% (588/712), and curative resection in 87.6% (624/712). Immediate complications such as bleeding or perforation occurred in 8.6% (61/712), which were immediately controlled by endoscopic treatment without significant sequelae.
Table 1.
Baseline Characteristics
| Characteristic | Value |
|---|---|
| Gender | |
| Male | 548 (77.0) |
| Female | 164 (23.0) |
| Age, yr | 62.8±9.2 |
| Body mass index, kg/m2 | 24.2±2.8 |
| Complete resection | 588/712 (82.6) |
| Curative resection | 624/712 (87.6) |
| Complication | |
| Bleeding | 49 (6.9) |
| Perforation | 12 (1.7) |
Data are presented as number (%) or mean±SD.
2. Assessment of QOL
Among the patients who were assessed for QLQ-C30 and QLQ-STO22 at baseline, 82.1% of the patients were followed-up at 7 days, 74.3% at 3 months, and 65.2% at 6 months after ESD, respectively. In cases of missing value, most frequent answers were used, and the missing was considered as “missing at random” because there were no no-nignorable patterns of missing.
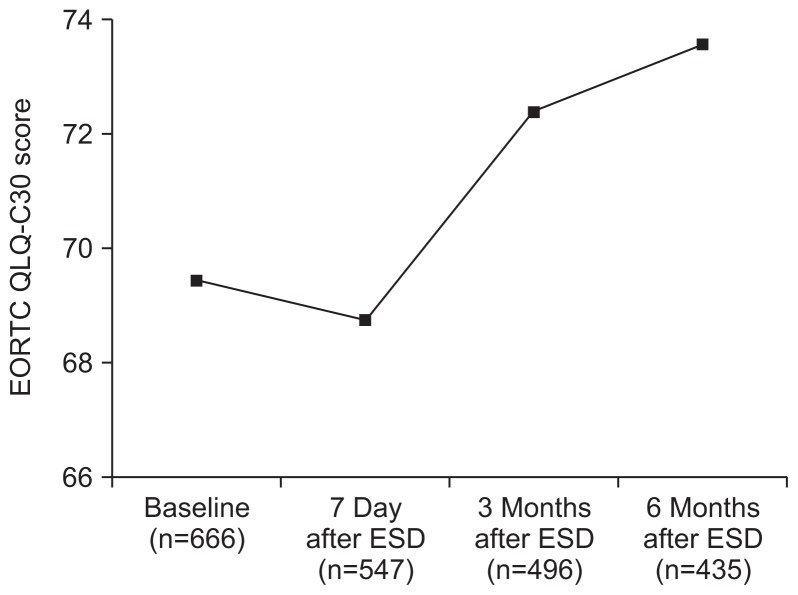
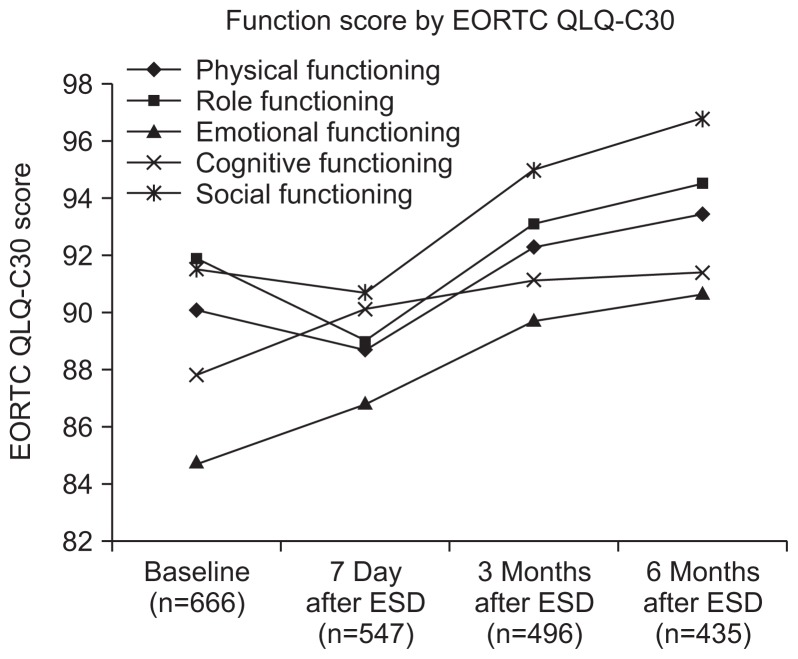
Although the global health status in the EORTC QLQ-C30 was not significantly different between before and immediately after ESD (p=0.39), it was significantly improved after 3 and 6 months (p=0.0003 and p<0.0001) (Table 2, Fig. 1). Most of functional scales were steadily improved after ESD. In symptom scales, the scores of pain, fatigue, and appetite loss had increased significantly at 7 days after ESD, but had decreased at 3 and 6 months, and the scores of nausea and vomiting, dyspnea, and insomnia had decreased progressively during follow-up (Fig. 2).
Table 2.
Changes in the EORTC QLQ-C30 Scores
| EORTC-C30 | Baseline | 7 day | 3 mo | 6 mo | p-value* | p-value† | p-value‡ | p-value§ |
|---|---|---|---|---|---|---|---|---|
| Global health status | 69.47±18.17 | 68.80±17.63 | 72,42±15.16 | 73.59±15.55 | <0.0001 | 0.39 | 0.0003 | <0.0001 |
| Functional scales | ||||||||
| Physical functioning | 90.02±13.81 | 88.82±15.43 | 92.29±11.32 | 93.40±9.60 | <0.0001 | 0.0016 | 0.0009 | <0.0001 |
| Role functioning | 91.74±16.58 | 88.96±19.12 | 93.08±14.48 | 94.53±11.64 | <0.0001 | <0.0001 | 0.2759 | <0.0006 |
| Emotional functioning | 84.75±17.62 | 86.84±18.05 | 89.75±14.07 | 90.71±14.39 | <0.0001 | 0.0377 | <0.0001 | <0.0001 |
| Cognitive functioning | 87.77±14.63 | 90.08±14.62 | 91.14±12.58 | 91.40±12.35 | <0.0001 | 0.0041 | <0.0001 | <0.0001 |
| Social functioning | 91.43±17.42 | 90.68±18.82 | 95.01±12.91 | 96.74±10.21 | <0.0001 | 0.0416 | 0.0002 | <0.0001 |
| Symptom scales/items | ||||||||
| Fatigue | 19.22±18.25 | 22.00±20.02 | 17.96±16.36 | 15.27±16.85 | <0.0001 | 0.0002 | 0.2822 | <0.0001 |
| Nausea and vomiting | 5.90±12.83 | 5.69±11.84 | 2.56±8.63 | 2.22±6.71 | <0.0001 | 0.7600 | <0.0001 | <0.0001 |
| Pain | 8.71±17.16 | 15.54±19.72 | 5.72±11.89 | 4.20±10.49 | <0.0001 | <0.0001 | 0.0001 | <0.0001 |
| Dyspnea | 9.38±17.84 | 7.19±15.92 | 6.48±14.96 | 4.67±12.64 | <0.0001 | 0.0108 | 0.0008 | <0.0001 |
| Insomnia | 12.26±21.35 | 11.01±19.10 | 9.90±18.57 | 9.66±19.82 | <0.0001 | 0.2027 | 0.0280 | 0.0116 |
| Appetite | 8.61±18.08 | 11.44±21.43 | 5.32±14.72 | 3.92±12.72 | <0.0001 | 0.0022 | <0.0001 | <0.0001 |
| Constipation | 11.81±20.72 | 11.56±21.46 | 9.36±17.74 | 8.03±17.04 | <0.0001 | 0.6068 | 0.0604 | 0.0004 |
| Diarrhea | 10.46±18.25 | 7.57±17.82 | 5.79±13.67 | 5.66±13.88 | <0.0001 | 0.0083 | <0.0001 | <0.0001 |
| Financial | 12.75±23.38 | 13.22±23.54 | 7.07±17.75 | 7.14±17.48 | <0.0001 | 0.1504 | <0.0001 | <0.0001 |
Data are presented as mean±SD.
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire-core.
p-value for the time effect using the generalized estimating equation (GEE) method;
p-value for comparison between screening and 7 days using the GEE method;
p-value for comparison between screening and 3 months using the GEE method;
p-value for comparison between screening and 6 months using the GEE method.
Fig. 1.
Changes in EORTC QLQ-C30 global health status. The score was not different between baseline and 7 days, and it improved after 3 and 6 months.
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire-core; ESD, endoscopic submucosal dissection.
Fig. 2.
Changes in EORTC QLQ-C30 functional scales. Most scores decreased progressively during follow-up.
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire-core; ESD, endoscopic submucosal dissection.
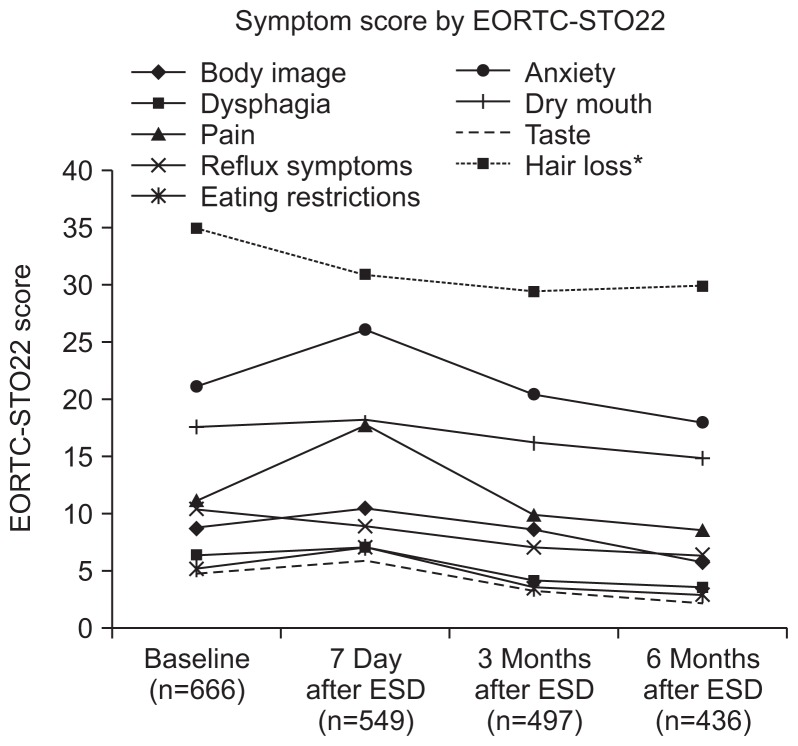
The scores of the EORTC QLQ-STO22 showed similar patterns to those of the EORTC QLQ-30 (Table 3). Although the scores of body image, pain, emotional change, and dietary restriction had significantly increased at 7 days after ESD, those had significantly decreased during follow-up. Reflux score had decreased from baseline to 6 months of follow-up, and other scores had decreased from 7 days during follow-up (Fig. 3).
Table 3.
Changes in EORTC STO-22 Scores
| EORTC-STO 22 | Baseline | 7 day | 3 mo | 6 mo | p-value* | p-value† | p-value‡ | p-value§ |
|---|---|---|---|---|---|---|---|---|
| Body image | 8.61±18.15 | 10.28±19.86 | 8.48±17.63 | 5.67±14.99 | <0.001 | 0.0272 | 0.83 | 0.0052 |
| Dysphagia | 6.38±11.68 | 6.85±12.51 | 4.03±7.61 | 3.45±6.56 | <0.001 | 0.2608 | <0.001 | <0.001 |
| Pain | 11.05±13.68 | 17.63±16.70 | 9.74±11.13 | 8.43±11.26 | <0.001 | <0.001 | 0.0513 | 0.0001 |
| Reflux | 10.32±13.94 | 8.78±14.19 | 7.01±11.05 | 6.21±11.59 | <0.001 | 0.0182 | <0.001 | <0.001 |
| Eating | 5.04±9.51 | 7.05±12.72 | 3.42±8.53 | 2.77±7.44 | <0.001 | <0.001 | 0.0007 | <0.001 |
| Anxiety | 21.14±20.34 | 25.96±18.36 | 20.38±15.77 | 17.92±15.14 | <0.001 | <0.001 | 0.725 | 0.0007 |
| Dry mouth | 17.58±23.27 | 17.97±22.73 | 16.09±21.27 | 14.70±20.69 | <0.001 | 0.7601 | 0.2532 | 0.0158 |
| Taste | 4.73±13.26 | 5.78±15.77 | 3.16±11.06 | 1.99±9.11 | <0.001 | 0.1566 | 0.0137 | <0.001 |
| Hair loss | 34.77±29.33 | 30.87±24.31 | 29.30±21.72 | 29.73±23.30 | <0.001 | 0.0873 | 0.029 | 0.1201 |
Data are presented as mean±SD.
EORTC STO-22, European Organization for Research and Treatment of Cancer gastric cancer-specific questionnaire.
p-value for the time effect using the generalized estimating equation (GEE) method;
p-value for comparison between screening and 7 days using the GEE method;
p-value for comparison between screening and 3 months using the GEE method;
p-value for comparison between screening and 6 months using the GEE method.
Fig. 3.
Changes in EORTC STO-22. Most scores decreased progressively during follow-up.
EORTC STO-22, European Organization for Research and Treatment of Cancer gastric cancer-specific questionnaire; ESD, endoscopic submucosal dissection.
DISCUSSION
As the proportion of EGC in the newly-diagnosed gastric cancer has been increased by national cancer screening program in Korea, ESD has become a mainstay of curative modality for EGC. Also, laparoscopic or robot-assisted surgery has replaced the open surgery for resection of EGC. With advances of less-invasive modalities, QOL as well as long-term survival has been considered as an important clinical outcome.
In EORTC-C30, global health status showed a tendency of decrement immediately after ESD, which was not significant. Although physical, role and social functioning were decreased in 7 days with the adverse symptoms and hospitalization by ESD itself, those were significantly increased in 3 and 6 months after ESD compared with baseline. Emotional and cognitive functioning had showed a steady increment after ESD during follow-up.
Also, fatigue, pain and appetite loss were increased immediately after ESD. Although the stomach is saved even after ESD unlikely surgical resection, various discomforts can be sustained for several days after ESD. However, those were significantly decreased in 3 and 6 months of follow-up compared with baseline, which means the improvement of patients’ subjective comfort after ESD for EGC. The symptom scores of nausea and vomiting, dyspnea, insomnia, constipation, diarrhea, and finance were also decreased after ESD during follow-up.
In EORTC STO-22, the scores of body image, pain, eating and anxiety were significantly increased immediately after ESD compared with baseline, which were significantly decreased in 3 and 6 months of follow-up. The scores of dysphagia, reflux, dry mouth, taste, and hair loss had showed a tendency of steady decrement, which shows the excellent clinical outcome in terms of QOL after ESD for EGC.
The most distinguished benefit of ESD is patients’ comfort by less-invasiveness and organ preservation compared with surgical resection. Whereas more than 1 week is required for recovery from discomforts such as pain after surgical resection, the patient can recover from pain and return to ordinary life and work in several days after ESD. In this study, functional scales showed a tendency of slight decrement immediately after ESD, but were improved in 3 and 6 months of follow-up. The symptom scores of pain and appetite loss were decreased during follow-up rather than before ESD except immediate after ESD. The immediate symptom scores may show significant differences if compared with surgical resection. In a previous study, the scores of pain showed around 30 points, which is more than double compared with ESD.8
In comparison with surgical resection, stomach is preserved after ESD, which means that all functions of stomach are maintained even after resection of EGC. In this study, stomach cancer-specific EORTC STO-22 scores were preserved or improved after ESD, whereas most scores of treatment-related symptoms were not recovered compared with baseline after surgical resection.8 In terms of nutrition, it may be also influenced by surgical resection, especially in absorption of iron and vitamin B12. With preservation of stomach, ESD has a benefit of long-term maintenance of nutrition compared with surgical resection.
As this study did not evaluate QOL after ESD compared with after surgical resection, it has a limitation of single-arm study. The results may show the difference of QOL between ESD and surgical resection if the QOL was compared between two groups. Besides, one third of the patients did not participate in the response to questionnaire at 6 months, which may influence the results of this study as a selection bias. Despite the limitation, this study has showed the excellent maintenance of QOL after ESD for EGC, and the strength of this study is the first prospective report about the comparison of QOL before and after ESD for EGC. Further studies are needed to compare the differences of QOL between ESD and surgical resection.
ACKNOWLEDGEMENTS
This work was funded by the National Evidence-based Healthcare Collaborating Agency (NECA), project number, NA 2010-001.
Author contributions: Na Rae Lee, Seung-Hee Park and Ji Hye You had contributed to analysis and interpretation of data of study. Il Ju Choi, Wan Sik Lee, Seun Ja Park, Jun Haeng Lee, Sang-Yong Seol, Ji Hyun Kim, Chul-Hyun Lim, Joo Young Cho, Gwang Ha Kim, Hoon Jai Chun, Yong Chan Lee, and Hwoon-Yong Jung had contributed to acquisition of data, drafting of the manuscript of study. Jae J. Kim had contributed to critical revision of the manuscript for important intellectual content and obtained funding.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi IJ. Gastric cancer screening and diagnosis. Korean J Gastroenterol. 2009;54:67–76. doi: 10.4166/kjg.2009.54.2.67. [DOI] [PubMed] [Google Scholar]
- 4.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 5.Vickery CW, Blazeby JM, Conroy T, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/S0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 6.Blazeby JM, Vickery CW. Quality of life in patients with cancers of the upper gastrointestinal tract. Expert Rev Anticancer Ther. 2001;1:269–276. doi: 10.1586/14737140.1.2.269. [DOI] [PubMed] [Google Scholar]
- 7.Blazeby JM, Conroy T, Bottomley A, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 9.Yoon H, Kim SG, Choi J, et al. Risk factors of residual or recurrent tumor in patients with a tumor-positive resection margin after endoscopic resection of early gastric cancer. Surg Endosc. 2013;27:1561–1568. doi: 10.1007/s00464-012-2627-3. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]