Abstract
The par genes of Pseudomonas aeruginosa have been studied to increase the understanding of their mechanism of action and role in the bacterial cell. Key properties of the ParB protein have been identified and are associated with different parts of the protein. The ParB- ParB interaction domain was mapped in vivo and in vitro to the C-terminal 56 amino acids (aa); 7 aa at the C terminus play an important role. The dimerization domain of P. aeruginosa ParB is interchangeable with the dimerization domain of KorB from plasmid RK2 (IncP1 group). The C-terminal part of ParB is also involved in ParB-ParA interactions. Purified ParB binds specifically to DNA containing a putative parS sequence based on the consensus sequence found in the chromosomes of Bacillus subtilis, Pseudomonas putida, and Streptomyces coelicolor. The overproduction of ParB was shown to inhibit the function of genes placed near parS. This “silencing” was dependent on the parS sequence and its orientation. The overproduction of P. aeruginosa ParB or its N-terminal part also causes inhibition of the growth of P. aeruginosa and P. putida but not Escherichia coli cells. Since this inhibitory determinant is located well away from ParB segments required for dimerization or interaction with the ParA counterpart, this result may suggest a role for the N terminus of P. aeruginosa ParB in interactions with host cell components.
Bacterial genome sequencing has revealed many conserved chromosomal features. Among them is a pair of genes located near chromosomal replication origin oriC and coding for homologues of the plasmid partitioning proteins ParA and ParB (4, 11, 41). In low-copy-number plasmids, these proteins ensure better-than-random segregation of the plasmids prior to cell division (4, 12). The majority of bacterial genomes contain a single copy of the putative partitioning operon (54), although the genomes of some species contain multiple copies of one or both genes (e.g., Vibrio cholerae, Streptomyces coelicolor, Deinococcus radiodurans, and Bacillus subtilis). Only Escherichia coli and the closely related species Salmonella enterica serovar Typhimurium and Haemophilus influenzae do not possess a conserved Par region. The chromosomal ParA and ParB counterparts are proposed to play an accessory role during regular cell division and chromosome segregation but to become crucial under specialized conditions, such as sporulation or entry into stationary phase during growth on minimal medium (14, 18, 26, 31, 48, 52). There are also data suggesting that one or both of these proteins may play important regulatory roles as molecular checkpoints in the cell cycle (6, 38, 42, 43). parB mutants of B. subtilis, S. coelicolor, or Pseudomonas putida show increased levels of anucleate cells or sporulation defects (14, 18, 26, 31). Caulobacter crescentus mutants with parA or parB disruptions could not be obtained (38) unless a wild-type copy of the gene in question was also supplied (37). The ParB homologue in B. subtilis (Spo0J) binds to at least eight sites concentrated in 20% of the genome around oriC (ori domain) (32). Although the functions of ParA and ParB in chromosome separation are still unknown, the colocalization of ParB foci with oriC (30, 33), duplication of those foci in parallel with oriC replication (13), rapid separation of both structures, and their tethering in defined sectors of the bacterial cell (49) suggest the involvement of ParB-DNA complexes in the active segregation of chromosomes. In the well-characterized plasmid partitioning systems, two components, ParA and ParB, are predicted to interact directly either at the centromere-like site (5, 24) or at the autoregulated promoter of the parAB operon (ParB potentiates repression exerted by ParA) (10, 39). Direct protein- protein interactions have been demonstrated so far for ParA and ParB of P1 (5, 51), SopA and SopB of F (16, 27), ParM and ParR of R1 (24), and IncC and KorB of RK2 (34, 46). Recent studies of spo0J mutants of B. subtilis (2) and parAB mutants of C. crescentus (9) also demonstrated the possibility of such interactions between chromosomal homologues.
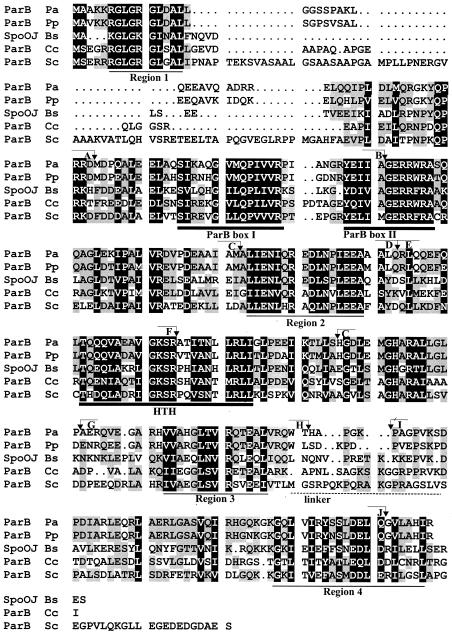
Previous studies suggested a number of basic generalizations about the structure and function of the chromosomal ParA and ParB homologues. These include a strongly conserved ATPase motif in ParA, thought to provide the energy for partitioning, and putative α-helix-turn-α-helix (H-T-H) motifs in ParB, allowing it to selectively bind the centromere-like sequence. N-terminal parts of chromosomal ParB family members contain two highly conserved sequences, previously identified as box I and box II (54) (Fig. 1). A Comparison of ParB of Pseudomonas aeruginosa with the four best-studied representatives of the chromosomal ParB subfamily revealed additional regions of high conservation separated by stretches with fewer conserved amino acids (regions 1 to 4 in Fig. 1). One of the variable regions, between W228 and P238 (coordinates from the P. aeruginosa sequence), corresponds exactly to the nonconserved region between KorB from plasmid RK2 (KorBRK2) (IncP1α) and KorB from plasmid R751 (IncP1β) (34), the closest of the plasmid homologues of chromosomal ParB proteins of Pseudomonas. This variable region was designated by us the putative linker region between the N-terminal and the C-terminal domains of KorB proteins (34), and tentatively we gave it the same designation for chromosomal ParB proteins (Fig. 1). Our studies with ParB focused first on determining whether the P. aeruginosa par system obeys the rules emerging for other chromosome- and plasmid-encoded homologues. Whereas the DNA-binding and dimerization domains conform to these rules, the major difference that we found was the location of the region of ParB that interacts with ParA. These results suggest that ParA-ParB interactions occur in an area where specificity may be exerted by different systems. We also localized a region in ParB that was not involved in dimerization or interactions with ParA and, whose overproduction caused growth inhibition. We confirmed that the centromere-like sequence similar to that identified for the B. subtilis and S. coelicolor genomes was recognized specifically by ParB of P. aeruginosa in in vivo and in vitro experiments. We also demonstrated that the chromosomal ParB protein might “silence” the neighboring genes in a manner similar to that of its plasmid counterparts—SopB of F (35) and ParB of P1 (45)—and that the silencing was DNA sequence dependent and therefore might contribute to the process that regulates the activity of the oriC region after replication.
FIG. 1.
Comparison of P. aeruginosa ParB with the other well-studied chromosomal members of the ParB family (P. aeruginosa, Pa; P. putida, Pp; B. subtilis, Bs; C. crescentus, Cc; S. coelicolor, Sc). A black background indicates homology in all five proteins, and a gray background indicates homology in three or four proteins. Regions of conservation are underlined; a thick line corresponds to previously identified blocks (54), and a thin line corresponds to blocks identified here (for the first time). A broken line indicates the proposed linker region referred to in the text. Positions defining the ends of the deletions (see Fig. 3) are marked with coordinate numbers and letters for cross-referencing.
MATERIALS AND METHODS
Bacterial strains and growth.
The E. coli strains used were K-12 strains DH5α [F− (φ80dlacZΔM15) recA1 endA1 gyrA96 thi-1 hsdR17 (rk− mk+) supE44 relA1 deoR Δ(lacZYA-argF)U196] and S17-1 (pro hsdR recA Tpr Smr ΩRP4-Tc::Mu-Kn::Tn7) (C. M. Thomas), E. coli B strain BL21 [F− ompT hsdSB (rB− mB−) gal dcm (λDE3)] (Novagen Inc.), and E. coli C strain C2110 (polA1 his rha) (D. R. Helinski, University of California at San Diego). P. aeruginosa PAO1161 (leu r−) was kindly provided by B. M. Holloway (Monash University, Clayton, Victoria, Australia), and PAO1161 parA::smh was constructed by K. Lasocki by allele exchange with strain S17-1 and suicide vector derivative pAKE600 (3). P. putida prototrophic strain KT2442 gfp Rifr was kindly provided by K. Smalla (Braunschweig, Germany). Bacteria were generally grown in L broth (25) at 37 or 30°C or on L agar (L broth with 1.5% [wt/vol] agar) supplemented with antibiotics as appropriate: benzylpenicillin sodium salt at 150 μg ml−1 in liquid medium and 300 μg ml−1 on agar plates for penicillin resistance in E. coli, kanamycin sulfate at 50 μg ml−1 for kanamycin resistance in E. coli, and streptomycin sulfate at 30 μg ml−1 for streptomycin resistance in E. coli, 60 μg ml−1 for streptomycin resistance in P. putida, and 200 μg ml−1 for streptomycin resistance in P. aeruginosa.
Yeast strains and growth conditions.
Saccharomyces cerevisiae strain L40 (MATa trp1 leu2 his3 ade2 LYS2::lexA-HIS3 URA3::lexA- lacZ) was used for transformation. Yeast cells were grown in 1% yeast extract- 2% Bacto Peptone-2% glucose medium. Plasmid-containing yeast strains were grown in 0.67% yeast nitrogen base-3% glucose (YNB) medium supplemented with a mixture of appropriate nutrients lacking tryptophan (for selection of pBTM116 derivatives), leucine (for selection of pGAD424 derivatives), or both (for selection of double transformants) as required. Agar was added to a concentration of 2% (wt/vol) for agar plates. To grow transformants for the β-galactosidase filter lift assay, YNB agar contained 0.5% glucose instead of 3% glucose. For the β-galactosidase assay with liquid cultures, the yeast cells were grown to logarithmic phase in YNB medium with 0.5% glucose and then transferred to YNB-ethanol (3%) medium for several hours. For testing of the expression of the His reporter gene in strain L40, YNB agar plates were supplemented with a mixture of amino acids lacking tryptophan, leucine, and histidine. 3-Amino-1,2,4-triazole (3-AT) (25 mM) was added to the plates to repress the basal activity of the His3 reporter gene. The plates were incubated at 30°C for 3 to 4 days.
Plasmids.
The plasmids used in this study are listed in Table 1. Plasmids used to create transcriptional fusions with regulated levels of expression were constructed as follows. For the expression of cloned open reading frames (ORFs), the high-copy-number Penr vector pGBT30 (19), based on the pMB1 replicon with lac1q and tacp separated from λtR1 by the multiple cloning site from pUC18, was used. To make use of the associated tacp Shine-Dalgarno sequence, the ATG codon of the inserted ORF must directly follow the EcoRI site. A BamHI-SalI fragment of pGBT30 derivatives with the ORF of interest and all upstream control elements was recloned into the broad-host-range Smr IncQ-based replicon pGBT400 (20). N-terminal parB deletions were obtained by using unique restriction sites. The linearized plasmid with parB was treated (if necessary) with the DNA PolI Klenow fragment and ligated with an oligonucleotide carrying an EcoRI recognition site followed by an ATG codon in frame with the rest of parB. C-terminal deletions of parB were also obtained by use of suitable restriction sites, which were blunt ended and then ligated with an oligonucleotide carrying a SalI site (italics) and stop codons (underlining) in all three frames (TGACCTAGTCGACTAGGTCA). All constructions were checked by sequencing of the new junction point. EcoRI-SalI fragments carrying the chosen parB alleles were recloned into pGBT30 and pGBT400 under tacp control.
TABLE 1.
Plasmids used in this study
| Category of derivatives | Plasmid | Relevant featuresa | Reference or source |
|---|---|---|---|
| pBBR1MCS1 | Cmrmob; BHR | 28 | |
| pJMB500 | pBBR1MCS1 derivative; lacIqtacp-parBPa | This work | |
| pGBT400 derivatives | pGBT400 | SmrlacIqtacp expression vector; IncQ rep | 20 |
| pABB100 | tacp-parB | This work | |
| pABB111 | tacp-parBΔ230-290 | This work | |
| pABB114 | tacp-parBΔ91-290 | This work | |
| pABB128 | tacp-parBΔ1-234 | This work | |
| pABB130 | tacp-parBΔ121-183 | This work | |
| pET28mod derivatives | pET28a(+) | Knr T7p expression vector; His6 tag T7tag pMB1 ori | Novagen |
| pET28mod | Knr T7p expression vector; His6 tag pMB1 ori | 21 | |
| pABB211 | T7p-parBΔ230-290 | This work | |
| pABB216 | T7p-parBΔ230-290-korBRK2Δ1-257 | This work | |
| pABB228 | T7p-parBΔ1-234 | This work | |
| pKLB28 | T7p-parB | This work | |
| pMLB6.0 | T7p-korBRK2Δ1-257 | 34 | |
| pGBT30 derivatives | pGBT30 | PnrlacIqtacp expression vector; pMB1 ori | 19 |
| pKLB1 | tacp-parA | This work | |
| pKLB2 | tacp-parB | This work | |
| pKLB3 | tacp-parAB | This work | |
| pABB311 | tacp-parBΔ230-290 | This work | |
| pABB316 | tacp-parBΔ230-290-korBRK2Δ1-257 | This work | |
| pABB323 | tacp-parBΔ1-18 | This work | |
| pABB324 | tacp-parBΔ1-53 | This work | |
| pABB326 | tacp-parBΔ1-142 | This work | |
| pABB328 | tacp-parBΔ1-234 | This work | |
| pABB330 | tacp-parBΔ121-183 | This work | |
| pGAD424 derivatives | pGAD424 | Pnrleu2 shuttle vector; pMB1 2μm ori gal4AD fusions | Clontech |
| pKLB1.4 | gal4AD-parA translational fusion | This work | |
| pKLB2.4 | gal4AD-parB translational fusion | This work | |
| pBTM116 derivatives | pBTM116 | Pnrtrp1 shuttle vector; pMB1 2μm ori lexADB fusions | Clontech |
| pKLB1.6 | lexADB-parA translational fusion | This work | |
| pKLB2.6 | lexADB-parB translational fusion | This work | |
| pABB511 | lexADB-parBΔ230-290 translational fusion | This work | |
| pABB512 | lexADB-parBΔ163-290 translational fusion | This work | |
| pABB513 | lexADB-parBΔ143-290 translational fusion | This work | |
| pABB514 | lexADB-parBΔ91-290 translational fusion | This work | |
| pABB515 | lexADB-parBΔ55-290 translational fusion | This work | |
| pABB526 | lexADB-parBΔ1-142 translational fusion | This work | |
| pABB527 | lexADB-parBΔ1-200 translational fusion | This work | |
| pABB528 | lexADB-parBΔ1-234 translational fusion | This work | |
| pABB529 | lexADB-parBΔ1-234-Δ284-290 translational fusion | This work | |
| pABB530 | lexADB-parBΔ122-184 translational fusion | This work | |
| pOG04 derivatives | pOG04 | Pnr pMB1/P7 rep; no par | 53 |
| pABB70 | tacp-parAB lacIq | This work | |
| pABB71 | parS2/3 | This work | |
| pABB72 | parS2/3tacp-parAB lacIq | This work | |
| pUC18 derivatives | pUC18 | Pnr pMB1 ori | 55 |
| pKLB181 | parS2/3 | This work | |
| pABB182 | parS1/4 | This work | |
| pABB183 | parS7II | This work | |
| pABB184 | parSSc | This work | |
| pGB2 derivatives | pGB2 | Smr Spr pSC101 rep par | 7 |
| pABB811 | parS2/3I | This work | |
| pABB812 | parS2/3II | This work | |
| pABB821 | parS1/4I | This work | |
| pABB822 | parS1/4II | This work | |
| pABB831 | parS7I | This work | |
| pABB832 | parS7II | This work | |
| pABB841 | parSScI | This work |
DB, DNA-binding domain; I, orientation I; II, orientation II.
Plasmids used for protein purification were constructed by inserting parB alleles as described above into a derivative of pET28a Kmr (Novagen) modified with the help of a synthetic oligomer so that a His6 tag and a thrombin cleavage site precede almost directly the EcoRI site (34). Purified His-tagged ParB derivatives were N-terminally extended with the same amino acid sequence: MGSSH6SSGLVPRGSHSEF.
E. coli-S. cerevisiae shuttle plasmids used in the two-hybrid system were constructed by using vectors pBTM116 and pGAD424 (Clontech Matchmaker) to fuse the polypeptides encoded by the EcoRI-SalI inserts to the C terminus of LexA (DNA-binding domain) and the Gal4 activation domain (Gal4AD), respectively. The system provides Penr selection in E. coli cells and LEU+ (pGAD424) or TRP+ (pBTM116) selection in yeast cells. All parB mutant alleles and the parA gene were transferred to the shuttle vectors.
Plasmid DNA isolation, analysis, cloning, and manipulation of DNA.
Plasmid DNA was isolated by standard procedures (47), digested with restriction enzymes under conditions recommended by the suppliers, and run on 0.8 to 2.0% (wt/vol) agarose gels. DNA sequencing was performed at an internal sequencing facility (Institute of Biochemistry and Biophysics, Warsaw, Poland) by using the dye terminator method in conjunction with an ABI 373 automated DNA sequencer. Standard PCRs (40) were performed as described previously (22) with the following pairs of primers for parA and parB amplification: ParA1 (CCGAATTCATGGCTAAGGTATTCGC) and ParA2 (CCGTCGACCCAGTCCACGTTTCTTG), ParB1 (CCGAATTCATGGCAGCCAAGAAACGTGG) and ParB2 (CCGTCGACCCGACTACCCGCTACAACCC), ParB1 and ParB3 (GGGGATCCGTCCACTGCCGAACCAGGGC), and ParB4 (GGGGATCCCCTGCGGGACCGGTCAAGAG) and ParB2.
To amplify the parAparB fragment, primers ParA3 (CCGAATTCCTGCTGATACTGCGGCGC) and ParB2 were used. Chromosomal DNA of P. aeruginosa PAO1161 was used as the template. Cells from 1 ml of an overnight culture were spun down, washed with H2O, and resuspended in 500 μl of H2O. After the suspension was boiled for 5 min, the cell debris was separated by centrifugation, and 10 μl of the supernatant was used in the PCR. PCR products were cloned into vector pGEM-T Easy (Promega) and later recloned as EcoRI-SalI fragments into appropriate vectors. All PCR-derived clones were analyzed by DNA sequencing to check their fidelity.
E. coli and Pseudomonas transformation.
Competent cells of E. coli and P. putida were prepared by the standard CaCl2 method (47). Competent cells of P. aeruginosa were prepared according to the modifications described by Irani and Rowe (17). Transformation was accomplished either by 50°C heat-pulse treatment or by electroporation with a BioPulser (Bio-Rad).
Yeast transformation.
Yeast transformation was performed by the standard polyethylene glycol-lithium acetate method recommended by Clontech. Either single or double transformations were conducted, and transformants were selected and stored on minimal YNB agar.
Determination of LacZ activity in yeast cells.
β-Galactosidase activity was monitored by the filter lift assay with 5-bromo-4- chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as a substrate and by the quantitative liquid culture assay with 2-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate (Clontech).
Purification of His6-tagged polypeptides.
Exponentially growing BL21(DE3) strains with pET28 derivatives (1, 50) were induced with 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) at a cell density of approximately 2 × 108 CFU/ml and grown for an additional 2 h with shaking at 37°C. Cells were harvested by centrifugation and sonicated. Overproduced His-tagged proteins were purified on Ni-agarose columns as recommended by Qiagen for soluble native proteins with an imidazole gradient in phosphate buffer at pH 6.0. The purification procedure was monitored by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with a Pharmacia PHAST gel system.
Cross-linking with glutaraldehyde.
His-tagged polypeptides purified on Ni-agarose columns were cross-linked by use of glutaraldehyde (23) and separated by SDS-PAGE with a PHAST gel system.
Analysis of protein-DNA interactions by gel retardation.
The pairs of complementary oligonucleotides were annealed and then cloned between SalI and HindIII in pUC18 (37). AGCTTGTTGCTTGTTCCACGTGGAACAAGGCCG and TCGACGGCCTTGTTCCACGTGGAACAAGCAACA were used to form parS2/3, AGCTTGTTGCTTGTTCCACGTGGAACCAGGCCG and TCGACGGCCTGGTTCCACGTGGAACAAGCAACA were used to form parS1/4, AGCTTGTTGCTTGTTCCACGAGGAACGAGGCCG and TCGACGGCCTCGTTCCTCGTGGAACAAGCAACA were used to form parS7, and AGCTTGTTGCTTGTTTCACGTGAAACAAGGCCG and TCGACGGCCTTGTTTCACGTGAAACAAGCAACA were used to form S. coelicolor parS (parSSc).
An NdeI-EcoRI fragment of 284 nucleotides (nt) containing the palindrome was purified, dephosphorylated, and 5′-end labeled with polynucleotide kinase (Bethesda Research Laboratories) and [γ-32P]ATP. The labeled fragment was then cleaved by PvuI into a 191-nt fragment with the palindrome and a control fragment of 93 nt. For gel retardation experiments, radioactive fragments were incubated for 15 min at 37°C with purified His6-ParB (wild type or truncated derivatives) in binding buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol) and then separated by PAGE under previously described conditions (23). The gels were dried and autoradiographed by using a PhosphorImager system.
Preparation of anti-ParB antiserum.
Purified His-tagged ParB protein (0.6 mg/ml) was used to inject a rabbit. The blood collected from the rabbit was allowed to clot at room temperature before antiserum was collected. To decrease cross-reactivity with E. coli proteins, 1 ml of antiserum was pretreated with E. coli extracts as previously described (23) and diluted in 20 ml of phosphate-buffered saline (PBS; phosphate buffer [pH 6.5]-150 mM NaCl).
Western blotting.
The growth of bacteria was monitored by measuring the optical density at 600 nm (OD600); the culture was diluted and plated on L agar to establish CFU per milliliter. Bacteria were harvested, washed in water, and resuspended in 200 μl of sonication buffer (50 mM phosphate buffer [pH 8.0]-300 mM NaCl). Bacteria were kept on ice and disrupted by sonication in short bursts for 2 min. Crude extracts were cleared by centrifugation in a microcentrifuge at 4°C for 15 min at 12,000 × g, and proteins from the cleared extracts were separated by SDS-12.5% (wt/vol) PAGE and electroblotted onto nitrocellulose membranes (Protron; Schleicher & Schuell). Probing of the blots was carried out by using the amplified alkaline phosphatase-goat anti-rabbit antibody immunoblot assay (Promega) with a 1:100,000 dilution of anti-ParB antiserum as the primary antibody. The cell extracts were compared to a range of concentrations of purified His-tagged ParB on the same gel. Band intensity on Western blots was determined by using ImageQuant (Molecular Dynamics).
Fluorescence microscopy.
P. aeruginosa PAO1161 with appropriate plasmids was grown in L broth with selection, and the production of ParB derivatives was induced with various amounts of IPTG. At an OD600 of 0.4, the cells were collected and used to prepare microscope slides. Fixation and permeabilization of cells and subsequent 4,6-diamidino-2-phenylindole (DAPI) staining were carried out as described previously (3). A coverslip was placed on a slide with a 1:4 (vol/vol) solution of DAPI (1 μg/ml) and Vectorshield (Vector Laboratories mounting medium). Cells were studied with an Olympus IX70 inverted reflected-light fluorescence microscope fitted with a Sensys charge-coupled device camera (Photometrics). Images were captured and manipulated on a Macintosh G3 computer with the Smartcapture I program (Digital Scientific).
For immunofluorescence microscopy, anti-ParB antibodies were affinity purified (44) as previously described (3). Affi-Gel 10 (Bio-Rad) was used as the support for purified ParB in 20-μl columns made with protein gel loading tips. Affinity-purified anti-ParB antibodies (10 μl) were used as the primary antibodies (1:100 dilution in 2% [wt/vol] bovine serum albumin-PBS), followed by 10 μl of anti-rabbit immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (FITC) (6.9 μg/ml in 2% [wt/vol] bovine serum albumin-PBS) (Sigma). The images were analyzed as described above.
Determination of plasmid stability.
For pOG04 (53) derivatives, E. coli C2110 transformants were grown under selection and then diluted 105-fold in nonselective L broth. Samples of the culture were collected at hourly intervals for the first 12 h and then every 12 h (at which point the cultures were diluted 105-fold and transferred to fresh medium), diluted, and plated on L agar and L agar with penicillin. The CFU were counted to determine the proportions of bacteria retaining plasmids. DNA was extracted from each overnight culture to check the plasmid identity and to determine whether the copy number had changed significantly.
For pGB2 (7) derivatives, the recipient strains were transformed with an estimated 1 μg of DNA from the pGBT30 expression vector and its derivatives. Undiluted and serially diluted transformation mixture (100 μl) were plated in repetition on different selection plates. The selection was either for an incoming plasmid only (L agar with penicillin) or for both resident and incoming plasmids (L agar with penicillin and streptomycin). We also used selective media supplemented with 0.5 mM IPTG.
RESULTS
Cloning and manipulation of P. aeruginosa par genes.
PCR was used to amplify the parA and parB ORFs of P. aeruginosa as well as the segment containing parA, parB, and the putative parA ribosome-binding site to ensure the natural balance in the production of both proteins (Fig. 2A). The products were cloned into the T-tailed vector pGEM-T Easy and checked by DNA sequencing. The segments were transferred to specialized vectors for the various functional analyses described below. Derivatives were created by restriction and ligation or site-directed mutagenesis in pGEM-T Easy or other vectors and recloned as appropriate.
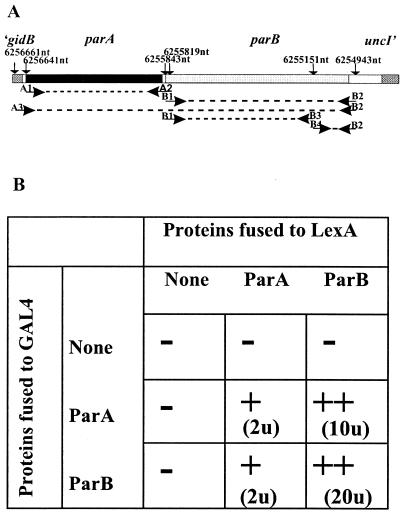
FIG. 2.
Detection of ParA-ParA, ParA-ParB, and ParB-ParB interactions with the yeast two-hybrid system (YTH). (A) Region of the genome used as the template in PCRs; genome coordinates define the primer sites. Coordinates shown correspond to P. aeruginosa genome coordinates. Primer A3 includes the ribosome-binding site for parA. Primers B3 and B4 introduce a BamHI site into the DNA sequence without changing the protein sequence. (B) Summary of results of the YTH assay. S. cerevisiae strain L40 was transformed with two compatible plasmids expressing only Gal4AD, LexA, or products of translational fusions between Gal4AD or LexA and ParA or ParB (parA was amplified with primers A1 and A2; parB was amplified with primers B1 and B2). −, no interactions (β-galactosidase activity of <0.2 U); +, weak interactions (β-galactosidase activity of 1 to 3 U); ++, strong interactions (β-galactosidase activity of >10 U).
The C-terminal region of ParB is responsible for the dimerization domain and the interaction with ParA.
The yeast two-hybrid system (36) was used to probe the interactions between the putative ParA and ParB homologues of P. aeruginosa. The ORFs for P. aeruginosa ParA and ParB (ParAPa and ParBPa, respectively) were cloned into shuttle vectors pBTM116 and pGAD424 in frame with the DNA-binding protein LexA and Gal4AD, respectively. S. cerevisiae strain L40, which was used to monitor the interactions between the hybrid proteins, contains two genes under the control of Gal4-activated promoters with LexA-binding sites: HIS3 from yeasts, needed for growth without histidine, and lacZ from E. coli, coding for β-galactosidase. Qualitative filter tests for β-galactosidase activity indicated the presence of weak interactions between ParA molecules and strong interactions between ParB molecules. The apparent strength of the ParA-ParB interactions depended on the protein (Gal4 or LexA) to which ParA and ParB were fused (Fig. 2B). The results were verified by quantitative assays of β-galactosidase activity in liquid cultures and by the histidine prototrophy of the tested derivatives of strain L40.
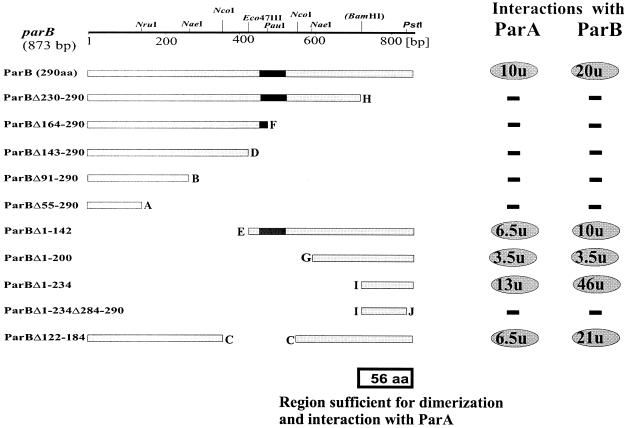
To facilitate mapping of the regions of interaction, a BamHI site was engineered by PCR into the linker region to allow separate cloning of the N-terminal and C-terminal parts of the ParB protein. Two sets of N-terminal and C-terminal deletions of parB and an internal deletion removing the putative H-T-H motif were constructed by using suitable restriction sites. The different parB deletion mutants were first transferred into the BL21(pET28) system to check the stability of the truncated products. All ParB derivatives seemed to be stable when overproduced in E. coli (data not shown), and so they were fused to the C termini of Gal4AD and LexA. The data summarized in Fig. 3 show that a region of C-terminal 56 amino acids (aa) of ParB (ParBΔ1-234) interacts strongly with itself and with ParA. Removal of the C-terminal 7 aa from ParBΔ1-234 (ParBΔ1-234Δ284-290) abolishes both of these interactions. The data also show that the removal of different parts of the ParB protein from the C terminus (from 61 to 236 aa) does not unmask a secondary dimerization domain in the more N-terminal region, as was observed previously for the homologues ParB of plasmid P1 (51) and KorB of plasmid RK2 (34).
FIG. 3.
Mapping of ParB-ParB and ParB-ParA interaction domains with the yeast two-hybrid system. The linear map at the top shows the restriction sites used for manipulations. The BamHI site was engineered by PCR (Fig. 2A). The putative H-T-H motif in ParB is indicated by black box. Lettering at the ends of deletions is the same as in Fig. 1. Interactions between deletion derivatives of ParB fused to LexA (pBTM derivatives) and intact ParA or ParB fused to Gal4AD (pGAD424 derivatives) were monitored. The two panels on the right summarize β-galactosidase activity: −, no interactions (β- galactosidase activity of <0.2 U); ovals, positive interactions (numbers in ovals correspond to mean units of β-galactosidase activity averaged from at least three assays).
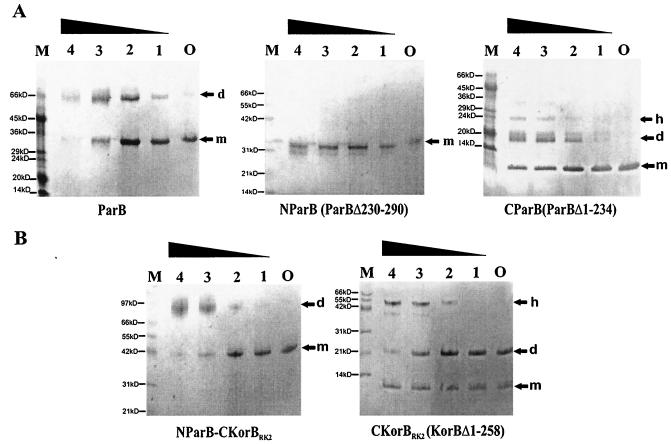
pET28 derivatives with the wild-type parB gene and fragments encoding separately the N-terminal two-thirds of ParB (parBΔ230-290) and the C-terminal 56 aa of ParBPa (parBΔ1-234) were introduced into strain BL21(DE3) to allow overexpression. ParB proteins extended by an N-terminal His6 tag and thrombin cleavage site (an additional N-terminal 23 aa) were purified on Ni-agarose columns and used in cross-linking experiments with glutaraldehyde. Figure 4A shows that intact ParB can be cross-linked to dimers as well as tetramers under such conditions. The purified C-terminal domain of ParBPa was efficiently cross-linked to dimers and higher-order multimers, whereas the N-terminal part of ParBPa without the C-terminal 61 aa was not. The negative result for the N-terminal domain acts as a control that shows that cross-linking of the C-terminal domain is specific. The results indicate that the C-terminal domain of ParBPa is sufficient and necessary for dimerization and confirm the conclusions from the yeast two-hybrid analysis.
FIG. 4.
Cross-linking of purified ParB and its truncated derivatives by glutaraldehyde. Proteins at a concentration of 0.1 mg/ml were incubated with various amounts of glutaraldehyde (GA). The samples were separated on homogeneous gels by SDS-12.5 or 20% PAGE and visualized by Coomassie blue staining (PHAST gel system). Lane O corresponds to the purified protein without GA; lanes 1 to 4 correspond to GA concentrations of 0.001, 0.002, 0.005, and 0.01% (wt/vol), respectively. m, monomer; d, dimer; h, higher-order complex. Lane M contains protein molecular weight markers; different molecular markers were used—one for the gels with ParB and C-ParB and another for the gels with N-ParB, N-ParB-C-KorB, and C-KorB.
As already mentioned, it was previously proposed on the basis of a functional analysis and sequence alignment for KorBRK2 (IncP1α) and KorB from R751 (IncP1β) that the central DNA-binding domain and the C-terminal dimerization domain are separated by a variable linker region (21, 34). The dimeric nature of the C-terminal 62 aa of KorBRK2 was also confirmed by crystallographic studies (8). The similarity of the C termini of the dimerization domains of KorBRK2 and ParBPa prompted us to test whether the dimerization domains could substitute for each other. This was done by joining parBΔ230-290 (N-ParB) with a KorBRK2 fragment (korBΔ1-258) coding for the C-terminal 100 aa (C-KorB) via the BamHI site in the proposed linker region (34). The hybrid protein N-ParBPa-C-KorBRK2 and the C-terminal part of KorBRK2 were overproduced and purified. Cross-linking experiments with glutaraldehyde (Fig. 4B) showed that the CTD of KorBRK2 formed very stable dimers resistant to boiling in the presence of SDS even without glutaraldehyde added (control). The addition of the CTD of KorBRK2 to the N-terminal part of ParB created a hybrid protein with in vitro dimerization ability. We also fused the N-terminal 258 aa of KorBRK2 to the C-terminal 56 aa of ParBPa. The hybrid protein N-KorBRK2-C-ParBPa was less stable but still formed dimers, as indicated by glutaraldehyde cross-linking (data not shown). Thus, the separated C-terminal domain of ParB dimerizes alone, is necessary for dimerization of the whole protein, can drive the dimerization of heterologous N-terminal domains, and is responsible for the interaction with ParA.
An excess of ParBPa causes inhibition of the growth of P. aeruginosa and P. putida strains.
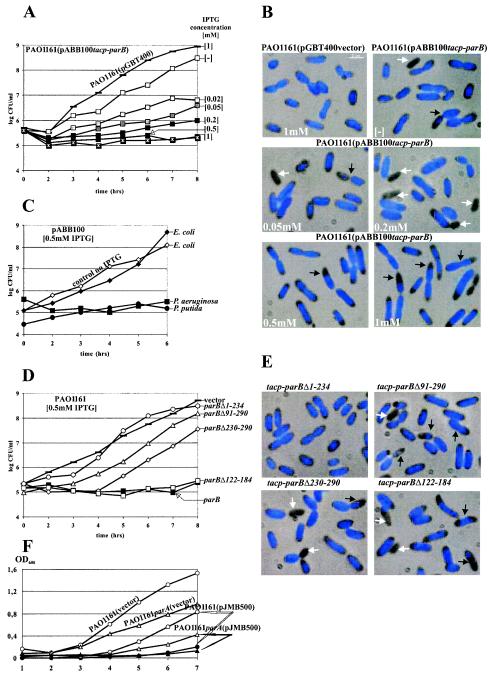
The overproduction of ParBPa had a very strong inhibitory effect on the growth of P. aeruginosa PAO1161. The growth inhibition estimated by CFU per milliliter (Fig. 5A) was directly proportional to the concentration of IPTG used to induce a tacp-parB transcriptional fusion on an IncQ derivative (pABB100). Microscopic observations revealed the appearance of anucleate cells (Fig. 5B) at frequencies of up to 5% in logarithmic phase and up to 9% in stationary phase during growth in the presence of low concentrations of IPTG (0.05 to 0.2 mM). At higher IPTG concentrations, the bacteria increased in size, doubling in size at 1 mM IPTG. In such cells, chromosomes became condensed and mislocated, and many cell appeared to be dividing without proper chromosome separation, so that the DAPI-stained material was guillotined and some progeny cells seemed to have just part of the nucleoid. The internal concentration of ParBPa in the logarithmic culture was estimated by Western analysis to oscillate around 1,000 molecules per cell (data not shown). The presence of pABB100 increased this concentration to 4,000 to 5,000 molecules per cell. Induction by 0.02 mM IPTG for 3 h led to a further three fold increase. Estimation of the amount of ParB produced at higher inducer concentrations (0.2 to 0.5 mM) or with prolonged incubation was complicated by the appearance of degradation products. Prolonged exposure of PAO1161(pABB100) to 1 mM IPTG selected mutants no longer sensitive to IPTG. Representatives of such mutants were studied and shown to carry mutations in plasmid pABB100 either in the parB gene or in tacp. Similar inhibitory effects of ParB overproduction were observed in P. putida strain KT2442(pABB100) but not in E. coli strain DH5α(pABB100) (Fig. 5C). ParB from P. putida and ParB from P. aeruginosa showed 88% similarity at the amino acid levels. Using anti-ParBPa antibodies, we could also detect a signal for the P. putida protein. The amount of ParB protein in logarithmically grown P. putida cells was comparable to that in P. aeruginosa cells. The lack of an effect of ParB overproduction on E. coli cells may correlate with the absence of the par operon on its chromosome or the involvement of different accessory proteins. Although studies with E. coli are often used as the paradigm of cell cycle events, the data accumulated indicate that this species of the Eubacteria differs from the others.
FIG.5.
Inhibition of host growth by the expression of parB. Overnight bacterial cultures carrying the plasmids indicated and grown under selection were diluted 104-fold into fresh selective medium without or with IPTG over a range of concentrations. Every hour, culture samples were serially diluted, and 20-μl aliquots were spotted on L agar and L agar-streptomycin plates. No plasmid loss was observed. The data shown are based on CFU from L agar plates. After approximately 4 h, samples were stained with DAPI and studied. (A) Effect on the growth of PAO1161 of increasing ParB expression from pABB100. Bacteria with empty vector pGBT400 and grown with 1 mM IPTG were used as the negative control. (B) Fluorescent images of samples from cultures monitored in panel A. White arrows indicate anucleate cells; black arrows indicate dislocalized, condensed chromosomes. (C) Effect of ParB production on the growth of E. coli DH5α and P. putida KT2442 carrying pABB100. PAO1161(pABB100) was used as a control. The data for all cultures grown without IPTG looked very similar and are represented by DH5α(pABB100) as the control (no IPTG). (D) Effect of overproduction of truncated forms of ParB on P. aeruginosa growth. PAO1161(pGBT400) not producing ParB and PAO1161(pABB100) producing wild-type ParB were used as controls. No difference in growth was observed for uninduced cultures. Only data for the induced cultures are presented. Expression plasmids with different derivatives of parB are listed in Table 1. (E) Fluorescent images of PAO1161 transformants overproducing truncated forms of ParB. The cultures were sampled after 4 h of growth in the presence of 0.5 mM IPTG. White arrows indicate anucleate cells; black arrows indicate cells with condensed chromosomes. (F) Effect of ParB overproduction on the growth of the PAO1161 parA::smh strain. The broad-host-range plasmid pBBR1MCS1 and its derivative pJMB500 carrying lacIq and a tacp- parB transcriptional fusion were used to transform PAO1161 parA and PAO1161. The cultures of the transformants were diluted 100-fold into L broth with 0.5 mM IPTG and without IPTG. Growth was monitored as the OD600. The growth of strains with vector pBBR1MCS1 is shown only for cultures with 0.5 mM IPTG.
The parB deletion derivatives were introduced into IncQ overexpression vector pGBT400 to map the region of ParB responsible for the strong inhibitory effect on its host. Strong inhibition of P. aeruginosa growth was still observed with a ParB derivative deprived of the internal 63-aa H-T-H domain (Fig. 5D). This derivative was still able to dimerize (and so it might form heterodimers with wild-type ParB) and to interact with ParA but was not able to bind to parS in vitro (data not shown). When only the C-terminal 56-aa peptide responsible for both of these functions was overproduced (pABB128 tacp-parBΔ1-234), no inhibitory effect was observed. These results indicate that the negative effect is not due to interference with dimerization or the ParA-ParB interaction. The presence of derivatives producing N-terminal parts of ParB, parBΔ230-290 (pABB111) and parBΔ201-290 (pAB114), inhibited the growth of PAO1161, although not to the same extent as wild-type ParB or ParBΔ122-184. Interestingly, all of the cultures overproducing the truncated versions of ParB, with the exception of PAO1161(pABB128), showed an increased number of anucleate cells that apparently started to divide without nucleoid separation. However, in contrast to the strain grown in the presence of excess wild-type ParB, where cells appeared to almost double in size, the nucleate cells from these cultures were of normal size but had slightly condensed chromosomes (Fig. 5E).
The growth inhibition caused by excess Par proteins was observed for other chromosomal systems (14, 26, 38) and was attributed to the disturbances in the relative proportions of Par proteins. The data obtained with the overproduction of truncated versions of ParBPa suggested no role of ParA in this process. To confirm this suggestion, we constructed mutant PAO1161 parA::smh, the properties of which will be described in more detail elsewhere. The introduction of tacp-parB on a pBBR1MCS1 Cmr (28) derivative into this mutant had an inhibitory effect very similar to that seen with the wild-type strain (Fig. 5F).
Stabilization of a segregationally unstable replicon by parAB and a putative parS sequence.
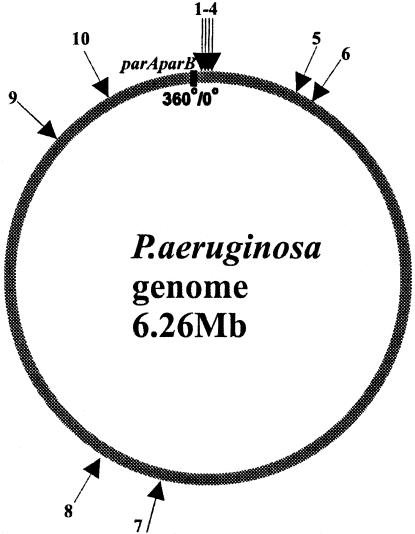
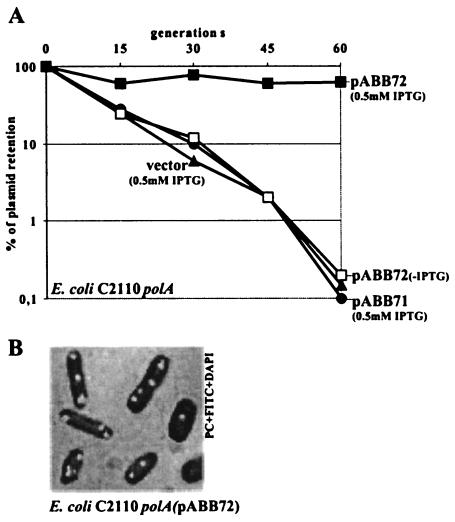
The par systems of both B. subtilis and P. putida have been shown to stabilize unstable plasmid replicons in E. coli (14, 54), and this property has been used to confirm not only the activity of the parAB genes but also the function of the cis-acting centromere-like parS sequence. The whole P. aeruginosa genome sequence was searched for matches to the sequence 5′TGTTNCACGTGAAACA3′ identified in B. subtilis (32). We found 10 repeats of this sequence (with two mismatches allowed); 8 of these localized in the 20% of the genome around oriC of P. aeruginosa, and the remaining 2 localized almost directly opposite oriC (Fig. 6 and Table 2). They were numbered starting from oriC in the clockwise direction. Inspection of the putative parS sequences from the P. aeruginosa chromosome revealed that among the block of four sites located in the proximity of oriC (between nt 3590 and 15496 on the chromosome map), two, designated parS2 and parS3, represent the perfect palindromic sequence 5′TGTTCCACGTGGAACA3′; the two flanking them have only one mismatch (A→C) at position 16 (parS1 and parS4). Other sequences are less conserved. Therefore, for the initial analysis, we chose the 16-nt oligonucleotide that was already mentioned and that corresponds to the perfect palindrome (parS2/3) and cloned it into pOG04 (53), which carries dual pMB1 and P7 replicons deprived of auxiliary stabilization mechanisms. Plasmid pOG04 replicates with a low copy number in a polA E. coli host (C2110) where the high-copy-number pMB1 replicon cannot function; consequently, pOG04 is very unstable, giving rise to a high frequency of plasmid-free cells at cell division. Three derivatives of pOG04 were constructed, with lacIq tacp-parAB (pABB70), with parS2/3 (pABB71), and with both lacIq tacp-parAB and parS2/3 (pABB72). Neither the lacIq tacp-parAB cassette inserted into pOG04 nor parSPa alone had an observable effect on plasmid stability (Fig. 7A). After 20 generations of growth without selection, the percentage of bacteria positive for plasmid pOG04, pABB70, or pABB71 had decreased 10-fold, and after 60 generations, the percentage had decreased by 300-fold. In contrast, the percentage of bacteria with pABB72 had decreased by less than 30% after 60 generations of growth without selection. No increase in the copy numbers of the tested plasmids was observed (data not shown). These results indicate that the P. aeruginosa parABS system works as a stabilization cassette in E. coli.
FIG. 6.
Circular map of P. aeruginosa with localization of the putative ParB-binding sequences. The arrows indicate the putative ParB-binding sites; the numbers correspond to the positions of these sites on the circular map of the chromosome. The black box indicates the position of parAB less than 150 kb counterclockwise from oriC (position 0). The nucleotide sequences and exact locations of identified sites are listed in Table 2.
TABLE 2.
Nucleotide sequences and locations of identified sitesa
| No.b | Sequencec | Location (nt) | Gene- or |
|---|---|---|---|
| 1 | 5′TGTTCCACGTGGAACC3′ | 3590 | recF (DNA- or ATP-binding protein) |
| 2 | 5′TGTTCCACGTGGAACA3′ | 4220 | recF (DNA- or ATP-binding protein) |
| 3 | 5′TGTTCCACGTGGAACA3′ | 14121 | Intergenic |
| 4 | 5′TGTTCCACGTGGAACC3′ | 15481 | Intergenic |
| 5 | 5′TGTTCCATGTAGAACA3′ | 449263 | gshB (glutathione synthetase) |
| 6 | 5′CGTTCCACGTGGAAGA3′ | 552924 | PA0493 (probable biotin-requiring enzyme) |
| 7 | 5′TGTTCCACGAGGAACG3′ | 3444057 | PA3071 (hypothetical protein) |
| 8 | 5′TGTTCCACGAGGCACA3′ | 3748953 | PA3339 (hypothetical protein) |
| 9 | 5′TCTTCCTCGTGGAACA3′ | 5571447 | PA4963 (hypothetical protein) |
| 10 | 5′TGTTCCCTGTGGAACA3′ | 5931301 | PA5266 (hypothetical protein) |
The consensus sequence is 5′tGTTCCacGTgGaAca3′.
Numbers correspond to those shown in Fig. 6.
Underlining indicates a mismatch from the perfect palindromic sequence present in parS2 and parS3.
FIG. 7.
Segregational stabilization of an unstable plasmid in E. coli C2110 polA by parABS of P. aeruginosa. (A) Rate of loss of vector pOG04 and its derivatives with parS2/3 only (pABB71) and with a tacp-parAB transcriptional fusion and parS2/3 (pABB72), determined as described in Material and Methods. The values presented were calculated relative to the plasmids present in the initial overnight cultures. These values were about 30% for pOG04 and all of its derivatives, with the exception of pABB72, for which 50% plasmid retention occured. No effect of IPTG was observed for all plasmids, with the exception of pABB72. To simplify the diagram, data for cultures without IPTG are shown only for pABB72. Data for pABB70, carrying the tacp-parAB transcriptional fusion but lacking a parS site, were the same as for pOG04 and pABB71 (data not shown). (B) E. coli C2110 polA (pABB72 parS tacp-parAB lacIq) from a logarithmic culture grown without selection for 3 h. The cells were stained with DAPI and treated with primary anti-ParB antibodies and secondary FITC-conjugated anti-rabbit IgG antibodies. Only cells with ParB foci are presented.
Purified His6-ParBPa was used to obtain rabbit anti-ParB antibodies. To increase the specificity of the polyclonal antibodies, they were purified on an Affi-Gel column to which ParBPa was attached. Immunofluorescence signals were used to localize ParBPa in C2110(pABB70) and C2110(pABB72). In the absence of parS, the signal from ParB was spread throughout the bacterial cell, whereas when parS was present, there were regularly distributed ParB foci, probably corresponding to ParB bound to plasmid molecules (Fig. 7B).
ParB binds to parS sequences in vitro.
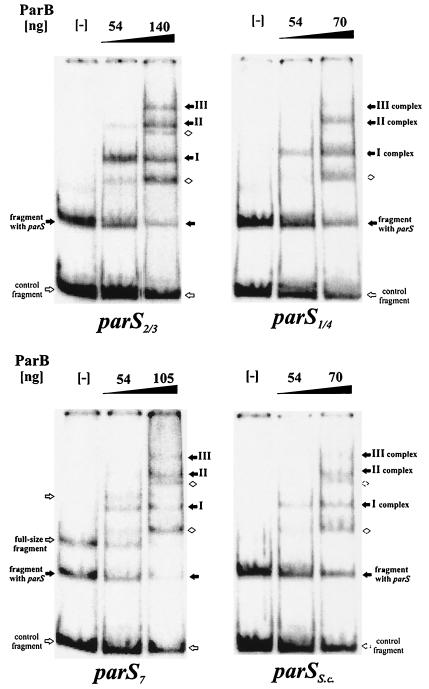
For in vitro studies, we used the oligonucleotide 5′TGTTCCACGTGGAACA3′, corresponding to parS2/3 (the perfect palindrome); the oligonucleotide 5′TGTTCCACGTGGAACC3′, corresponding to parS1/4; the oligonucletide 5′TGTTCCAGGAACG3′, corresponding to parS7 (positioned opposite oriC in the P. aeruginosa chromosome); and the palindromic oligonucleotide 5′TGTTTCACGTGAAACA3′, based on the S. coelicolor parS consensus sequence. All sequences were cloned between the SalI and HindIII sites of pUC18, purified as 282-nt EcoRI-NdeI fragments, radiolabeled, and used in an in vitro mobility shift assay with purified His-tagged ParBPa. The fragments were digested either with PvuI to generate a 191-nt parS-containing fragment or with HaeIII to generate a 76-nt fragment containing parS.
The results obtained with the Pvul digest showed that ParBPa demonstrated the specificity of ParB toward DNA fragment containing parS sequences (Fig. 8). ParBPa preferentially bound DNA fragments with parS and, at increasing concentrations, formed distinct, multiple complexes which we tentatively identified as complexes I, II, and III on the gels, relating newly appearing species to those disappearing. At higher concentrations of ParB, different mobility complexes with nonspecific DNA fragments also became visible (Fig. 8). By comparison of data from many electrophoretic mobility shift assay experiments, we could conclude that the apparent affinity of ParB (estimated experimentally as the concentration of ParB needed to shift 50% of a radioactive fragment) was the highest for the fragment with parS2/3 (120 nM); for the other analyzed oligonucleotides, it was two- to threefold lower (240 to 360 nM). We confirmed that the specific ParB binding was independent of the size of the fragment by using the HaeIII digest of labeled DNA (data not shown). In this experiment, the smaller fragment with parS was retarded before the larger control fragment.
FIG. 8.
DNA-binding activity of ParB. A radioactively labeled 284-nt EcoRI-NdeI DNA fragment of pUC18 with different parS sequences inserted between SalI and HindIII sites was further cut with PvuI to produce 93- and 191-nt fragments, the latter with the putative parS sequence. Purified N-His6-ParB was used in mobility shift experiments at the amounts shown above the lanes. Lanes marked with minus signs indicate DNA in binding buffer, with no protein included. Different retarded species are indicated by arrows: black arrows for complexes formed with parS and white diamonds for nonspecific complexes.
ParB can silence genes adjacent to its parS sequence.
It was demonstrated previously that some plasmid homologues of the ParB family can silence the expression of adjacent genes after binding to the centromere-like sequence and polymerization on DNA (35, 45). This feature of plasmid ParB proteins has been suggested to be vital for their function in the pairing of plasmid molecules prior to separation during active partitioning. No information on the ability of chromosomal ParB family members to silence gene expression is so far available. Plasmid pGB2, which contains a stable pSC101 replicon with its repA gene adjacent to a multiple cloning site, was used previously to test for the ability of plasmid ParB homologues to interact with the inserted fragments and to interfere with pGB2 replication and thus cause loss of the plasmid (7, 15). The presence of putative parS2/3 in pGB2 (pABB811) did not influence plasmid stability during host growth without selection. Competent cells of DH5α(pABB811) were transformed with an empty pGBT30 tacp vector, pKLB1 (tacp-parA), and pKLB2 (tacp-parB) (Table 1), and the expression of the cloned gene(s) was either left at a basal level or induced with 0.5 mM IPTG. In general, all three plasmids gave similar transformation frequencies, except when parA was expressed on its own; in that case, the frequency was slightly reduced, apparently because of the negative effect of ParA on host growth (Table 3). The transformants were selected either for both plasmids or only for the incoming plasmid. Table 3 shows that no effect was observed on retention of the resident plasmid when the incoming plasmid was either the empty vector or carried just parA. However, when the incoming plasmid produced ParB, the resident plasmid was displaced in the vast majority of transformants, as judged by both loss of plasmid-encoded antibiotic resistance and loss of plasmid DNA from standard profile. Resident plasmid loss was accentuated by the addition of IPTG. No displacement was observed when the resident plasmid lacked parS. The results suggest that displacement is dependent on parS in the resident plasmid and parB in the incoming plasmid.
TABLE 3.
Relative transformation frequencies of two recipient strains with plasmids overexpressing various parB allelesa
| Recipient | Plasmid used for transformation | Transformation frequency with the following selection:
|
|||
|---|---|---|---|---|---|
| Pen | PenSm | Pen + IPTG | PenSm + IPTG | ||
| DH5α(pGB2) | pGBT30 (vector) | 1 (2.37 × 103) | 0.89 | 0.84 | 0.89 |
| pKLB1 (ParA) | 1 (1.18 × 103) | 0.93 | 1.08 | 1.06 | |
| pKLB2 (ParB) | 1 (1.75 × 103) | 1.20 | 1.10 | 1.14 | |
| DH5α(pABB811) (parS2/3I) | pGBT30 (vector) | 1 (1.2 × 103) | 0.73 | 1.00 | 0.83 |
| pKLB1 (ParA) | 1 (3.6 × 103) | 1.00 | 0.83 | 0.20 | |
| pKLB2 (ParB) | 1 (1.82 × 103) | 0.03 | 0.83 | <0.001 | |
| pABB311 (ParBΔ230-290) | 1 (2.22 × 104) | 0.82 | 0.84 | 0.74 | |
| pABB323 (ParBΔ1-18) | 1 (1.56 × 104) | 0.08 | 0.88 | <0.001 | |
| pABB324 (ParBΔ1-53) | 1 (1.43 × 104) | 0.84 | 0.92 | 0.73 | |
| pABB326 (ParBΔ1-142) | 1 (1.58 × 104) | 0.98 | 1.04 | 0.93 | |
| pABB328 (ParBΔ1-234) | 1 (1.52 × 104) | 0.78 | 0.67 | 0.78 | |
| pABB330 (ParBΔ121-183) | 1 (1.84 × 104) | 0.90 | 0.77 | 0.69 | |
| pABB316 (N-ParBPa-C-KorBRK2 | 1 (4.2 × 103) | 0.38 | 1.06 | <0.001 | |
Approximately 1 μg of plasmid DNA was used for each transformation. To estimate the amounts of transformants, we prepared 1:10, 1:100, and 1:1,000 dilutions of the initial transformation mixture. Samples of 100 μl of the initial mixture and the subsequent dilutions were plated on each of the selective plates in repetition. Pen, pencillin; PenSm, penicillin and streptomycin. IPTG was added to some plates at a concentration of 0.5 mM. The frequencies presented are relative to the number of transformants (indicated in parentheses) obtained on penicillin plates per 1 μg of plasmid DNA. Frequencies in bold type indicate a significant decrease in transformation frequency.
DH5α(pABB811) was also transformed with pGBT30 derivatives with different parB alleles linked to tacp to assess which regions were required to cause the segregation. Four N- terminal deletions, one internal deletion, and one C-terminal deletion were chosen. The shortest N-terminal deletion (ParBΔ1-18; pABB323) was still able to displace pABB811, whereas the overproduction of the ParB deletion derivative truncated at the N terminus by 53 aa (pABB324 tacp-parBΔ1-53) or more (pABB326 tacp-parBΔ1-142 and pABB328 tacp- parBΔ1-234) did not cause segregation. Similarly, the parB deletion derivative lacking the putative H-T-H motif (pABB330 tacp-parBΔ122-184) and the ParB derivative truncated at the C terminus and thus lacking the dimerization domain (pABB311 tacp-parBΔ230-290) caused no destabilization. Dimerization could be restored in vitro by linking with the heterologous dimerization domain protein CTD-KorBRK2 (Fig. 4B). The plasmid overproducing the hybrid protein N-ParBPa-C-KorBRK2 (pABB316) was also able to destabilize pABB811, although not as strongly as wild-type ParB. None of the overproducing plasmids used in these experiments displaced pGB2 itself in the absence of the cloned parS sequence. These data suggests for the first time that a chromosomal ParB homologue is able to interfere with the expression of the neighboring genes and that the H-T-H motif, a dimerization domain (even a heterologous dimerization domain), and the N-terminal sequence (although not including the first 18 aa) are all required for this activity.
The silencing phenotype caused by an excess of ParBPa depends on the sequence and orientation of the binding site.
Since there is asymmetry in many parS sequences, it is possible that there is directionality in the action of ParB in the P. aeruginosa chromosome. This possibility prompted us to clone parS1/4 and parS7 sequences in both orientations relative to the promoter of the repApSC101 gene of pGB2. The perfect palindrome (parS2/3) was also cloned in the orientation opposite that in pABB811 to estimate the role of the DNA sequences flanking parS. None of the inserted oligonucleotides influenced the stability of plasmid pGB2. DH5α carrying different pGB2 derivatives was transformed with plasmids pGBT30 (vector) and pKLB2 (tacp-parB) (Table 4). The results clearly showed that the sequences adjacent to parS2/3 had no effect on the silencing of repA by excess ParB (compare the data for pABB811 and pABB812). The loss of the pGB2 derivatives was observed only with parS2/3 in both orientations and parS1/4 in the one specific orientation. Neither parS7 nor parSSc (data not shown) caused any changes in the stability of pGB2 in the presence of excess ParBPa. These results suggest that either the weak binding in vitro is not sufficient to bind in vivo or that not all binding events must result in the formation of complexes influencing the expression of the adjacent genes.
TABLE 4.
Relative transformation frequencies of recipient strains with plasmids overexpressing parBa
| Recipient | Selection | Transformation frequency with the following plasmid used for transformation:
|
|
|---|---|---|---|
| pGBT30 (vector) | pKLB2 (tacp-parB) | ||
| DH5α(pABB811) (parS2/3I) | Pen | 1 (8.68 × 104) | 1 (1.27 × 104) |
| PenSm | 0.75 | 0.002 | |
| Pen + IPTG | 1.00 | 0.94 | |
| PenSm + IPTG | 0.70 | <0.0001 | |
| DH5α(pABB812) (parS2/3II) | Pen | 1 (4.83 × 104) | 1 (1.06 × 104) |
| PenSm | 0.85 | <0.0001 | |
| Pen + IPTG | 0.98 | 0.76 | |
| PenSm + IPTG | 0.6 | <0.0001 | |
| DH5α(pABB821) (parS1/4) | Pen | 1 (6.1 × 104) | 1 (1.4 × 105) |
| PenSm | 0.81 | 0.27 | |
| Pen + IPTG | 1.00 | 1.00 | |
| PenSm + IPTG | 0.64 | 0.25 | |
| DH5α(pABB822) (parS1/4II) | Pen | 1 (3.35 × 104) | 1 (3.38 × 104) |
| PenSm | 0.26 | <0.0001 | |
| Pen + IPTG | 0.94 | 0.95 | |
| PenSm + IPTG | 0.23 | <0.0001 | |
| DH5α(pABB831) (parS7I) | Pen | 1 (9.7 × 104) | 1 (1.58 × 105) |
| PenSm | 0.89 | 0.67 | |
| Pen + IPTG | 1.00 | 0.95 | |
| PenSm + IPTG | 0.82 | 0.63 | |
| DH5α(pABB832) (parS7II) | Pen | 1 (1.58 × 104) | 1 (3.79 × 104) |
| PenSm | 0.55 | 0.21 | |
| Pen + IPTG | 1.38 | 1.00 | |
| PenSm + IPTG | 1.06 | 0.18 | |
Approximately 1 μg of plasmid DNA was used for each transformation, and the number of transformants was calculated as described in Table 3. Pen, penicillin; PenSm, penicillin and streptomycin. IPTG was added to some plates at a concentration of 0.5 mM. Frequencies in bold type indicate significant results.
DISCUSSION
In this article we have presented data that support many of the generalizations that are emerging about the ParB family of proteins but that demonstrate a number of unique properties for these proteins. First, we showed that ParB of P. aeruginosa dimerizes and may also form higher-order multimers, an activity which could be vital for its role in driving partitioning. This dimerization function was localized to the C-terminal domain, as found in plasmid-encoded ParB proteins. This region is separated from the rest of ParB by a region that shows less-than-average conservation between ParB homologues, and we have therefore designated this region as a probable variable linker region that may be responsible for providing some of the properties that are unique to each ParB protein. The sequence of the dimerization domain aligns well with the C-terminal domain of KorB from RP4 or KorBRK2 (21), whose crystal structure was recently reported (8). In particular, the leucines or isoleucines and acidic or basic residues, which form a vital part of the dimerization interface, show strong conservation. Removal of 3 aa from the C terminus of KorBRK2 impairs its dimerization phenotype (K. Kostelidou and C. M. Thomas, unpublished data). An identical effect was obtained by the removal of 7 aa from the C terminus of ParBPa. The C-terminal domain of KorBRK2 has an SH3 structure, which seems in eukaryotes to participate particularly in signal transduction or membrane-cytoskeleton interactions (8). This similarity may be significant if the Par complex is associated with the bacterial cytoskeleton.
Despite the fact that CTD-KorB restores dimerization to the N-terminal two-thirds of ParBPa, not all ParBPa properties can be substituted. Silencing with the hybrid protein was less effective than that with intact ParB, indicating either a change in structure or that CTD-ParB carries important determinants of oligomerization needed for silencing. In addition, we do not expect CTD-KorB to be able to restore the interaction with ParAPa, which we mapped to CTD-ParB. This localization of the domain that interacts with ParA is different from that in well-characterized plasmid systems. For example, in P1 (51) and F (27), the N terminus of ParB/SopB is essential for the interaction with ParA, and in the RP4/RK2 system, the central segment of KorB is essential (34). Recent data on ParB of C. crescentus localized the domain of interaction with ParA to the N-terminal 40 aa (9). It contains highly conserved region 1 (the first 16 aa) and the region where chromosomal members of the ParB family show no homology (Fig. 1). These data suggest that the architecture of these complexes with ParA is not universal. Recent work on Spo0J of B. subtilis (2) showed that Spo0J mutants with substitutions in box II, the H-T-H motif, and regions 2, 3, and 4 (Fig. 1) exhibited an abnormal nucleoid structure and lost the ability to form condensed Spo0J foci. The authors suggested that the role of region 3 in DNA binding is to provide a dimerization surface. Our results strongly indicate that region 4 plays the role of the dimerization domain for ParBPa. Similar results were recently reported for ParB of C. crescentus (9). Further mutational analysis of parBPa will be undertaken to understand the function of conserved features of chromosomal members of the ParB family.
In line with other systems, we were able to show that ParB binds specifically to DNA fragments carrying putative centromere-like parS sequence. Although this sequence was identified by similarity to the B. subtilis parS sequence, there are some differences between the P. aeruginosa and B. subtilis consensus sequences, most notably, the highly conserved CG pair at positions −4 and +4 (from the center of dyad symmetry). This difference is also found in the related species P. putida. As with P. putida (14) and B. subtilis (54), we were able to show that the combination of parAB and parS is able to drive plasmid partitioning in a heterologous system (E. coli). It seems that this rule is well established, and yet it is not clear whether the processes of chromosome partitioning and plasmid partitioning are identical. Indeed, the need for the chromosomal par system, as judged by its Par phenotype, is only pronounced in P. putida under specific conditions (14, 31), whereas its ability to stabilize an unstable plasmid occurs throughout exponential phase. The data obtained with E. coli(pABB72) demonstrate that the abilities to promote plasmid stability and form ParB foci depend on ParAPa and the presence of parS sequences. The stability of the tested plasmid and the typical distribution of ParBPa-parS complexes in E. coli suggest either that no accessory proteins are needed for their function or that the accessory elements are so conserved among bacterial species that Par complexes work equally well in heterologous hosts and irrespective of whether the genes are carried on the chromosome or on a plasmid.
The strong growth-inhibitory effect of overproducing full-length ParB not only in P. aeruginosa but also in P. putida may reflect the high degree of conservation of ParB functions in this genus. Mapping of the inhibitory determinant to the N-terminal part of ParB, which does not show interactions with either full-length ParB or ParA, or subsegments thereof, indicates that the inhibition is not the simple result of an imbalance in the concentrations of the ParA and ParB proteins. This conclusion is also supported by the inhibitory effect of intact ParBPa on the PAO1161 parA strain. The presence of ParA is not required for ParB “toxicity.” The results of imaging also indicate that the observed inhibition is not the result of disturbances in ParB function through the formation of heterodimeric forms (wild-type ParB-truncated ParB) (in contrast to ParB from C. crescentus) (9). Mapping of the inhibitory determinant to the N-terminal part of ParB suggests that the N terminus of ParBPa interacts with other cell components important for growth. Thus, ParB overproduction not only would block the interaction of those components with the normal ParA-ParB complex, resulting in anucleate cell formation, but also would interfere with growth. Implicit in this model is the idea that the cell component with which the ParB N terminus interacts is important for its function, although we consider the possibility that its action may be either positive or negative. Indeed, it is possible that it acts both positively and negatively. Such a possibility could explain how the ParABPa system can stabilize an unstable plasmid in E. coli but the ParB protein is not inhibitory for E. coli cells. Although it seems clear that E. coli has an alternative method of chromosome segregation independent of ParA and ParB homologues, the strong conservation of cell division machinery in bacteria makes it likely that the positive action involved in active partitioning depends on the same or equivalent cellular components. If this is the case, then it should be possible to isolate point mutations that prevent the inhibitory effect in Pseudomonas species but that do not inactivate the plasmid stabilization function. These mutations would allow exploration of the link with specific conserved protein motifs in the N-terminal region of ParB (Fig. 1), which includes region 1 in the N tip (the first 16 aa) and ParB box I between aa 65 and 78, according to ParBPa coordinates.
In vitro studies demonstrated that ParB is a general DNA-binding protein. It exhibited specificity for parS sequences, with the highest affinity for a perfect palindrome (5′TGTTCCACGTGGAACA3′) present twice in the chromosome close to oriC (parS2 and parS3) and between two palindromes with one mismatch at position 16 (parS1 and parS4). One of the properties associated with plasmid partitioning proteins and relevant to cellular levels of the proteins is the ability to silence genetic functions adjacent to parS sites (35, 45). This activity is thought to occur either by spreading along the DNA or compartmentalization of parS-ParB nucleoprotein complexes away from RNA polymerase. We have shown that ParBPa can achieve the same effect of silencing and that this effect is dependent on a putative H-T-H motif, the ability of ParB to dimerize, and the N terminus. The N-terminal 18 aa seem not to be necessary for ParB functioning in the silencing process, whereas further deletion up to 53 aa changes ParB properties drastically, although the protein should still be able to form higher-order complexes, bind to DNA, and interact with ParA. In our experiments, the putative polymerization of ParB was initiated only from particular parS sequences and, in the case of the imperfect palindromes (parS1 and parS4), proceeded only in one direction. A comparison of these data with oriC region organization, indicated the possibility of unidirectional spreading of ParB from parS4 toward oriC and then beyond parS1 in the direction of the parAB operon. How far such spreading may proceed depends on how many molecules of ParB are available for binding, which other parS sequences function in the same way, and what role (if any) ParA plays in this process. Neither parS7 nor parS of C. crescentus allowed ParB to exert its negative effect, although in vitro experiments confirmed the ability of ParB to bind to them; therefore, we can assume that only the sequences closest to oriC are involved. Recent data on Spo0J (29) suggest that it may be directly or indirectly involved as the negative regulator in the initiation of chromosome replication in B. subtilis. The questions of whether ParBPa helps to silence the oriC region between the rounds of replication and how this process may be regulated still remain open.
In conclusion, while ParBPa clearly can perform the same functions as other members of the family, it has unique properties that may allow further dissection of the function of ParB proteins. Its ability to bring about gene silencing suggests that it may switch off the oriC region between rounds of replication and that it is necessary to remove this effect during stationary phase so that oriC is ready for reactivation when nutritional conditions improve. These observations may provide the basis for designing the key experiments that will reveal the role of ParB and its link to the intracellular architecture in the active partitioning of chromosomes.
Acknowledgments
We thank Wei Zeng for instruction in fluorescence microscopy and technical assistance.
This work was funded by Wellcome Trust Collaborative Research Initiative grant 056022/Z/98/Z and in part by an EMBO short-term fellowship awarded to A.A.B. and BBSRC grant 6/G10277.
REFERENCES
- 1.Arnold, F. H. 1991. Metal-affinity separations: a new dimension in protein processing. Bio/Technology 9:151-156. [DOI] [PubMed] [Google Scholar]
- 2.Autret, S., R. Nair, and J. Errington. 2001. Genetic analysis of the chromosome segregation protein Spo0J of Bacillus subtilis: evidence for separate domains involved in DNA binding and interactions with Soj protein. Mol. Microbiol. 41:743-755. [DOI] [PubMed] [Google Scholar]
- 3.Bignell, C. R., A. S. Haines, D. Khare, and C. M. Thomas. 1999. Effect of growth rate and incC mutation on symmetric plasmid distribution by the IncP-1 partitioning apparatus. Mol. Microbiol. 34:205-216. [DOI] [PubMed] [Google Scholar]
- 4.Bignell, C. R., and C. M. Thomas. 2001. The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91:1-34. [DOI] [PubMed] [Google Scholar]
- 5.Bouet, J. V., and B. E. Funnell. 1999. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 18:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervin, M. A., G. B. Spiegelman, B. Raether, K. Ohlsen, M. Perego, and J. A. Hoch. 1998. A negative regulator linking chromosome segregation to developmental transcription in Bacillus subtilis. Mol. Microbiol. 29:85-95. [DOI] [PubMed] [Google Scholar]
- 7.Churchward, G., D. Belin, and Y. Nagamine. 1984. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31:165-171. [DOI] [PubMed] [Google Scholar]
- 8.Delbruck, H., G. Ziegelin, E. Lanka, and U. Heinemann. 2002. An Src homology 3-like domain is responsible for dimerization of the repressor protein KorB encoded by the promiscuous IncP plasmid RP4. J. Biol. Chem. 277:4191-4198. [DOI] [PubMed] [Google Scholar]
- 9.Figge, R. M., J. Easter, and J. W. Gober. 2003. Productive interaction between the chromosome partitioning proteins, ParA and ParB, is required for the progression of the cell cycle in Caulobacter crescentus. Mol. Microbiol. 47:1225-1237. [DOI] [PubMed] [Google Scholar]
- 10.Friedman, S. A., and S. J. Austin. 1988. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid 19:103-112. [DOI] [PubMed] [Google Scholar]
- 11.Gal-Mor, O., I. Brorvok, Y. Av-Gay, G. Cohen, and Y. Aharonowitz. 1998. Gene organization in the trxA/B-oriC region of the Streptomyces coelicolor chromosome and comparison with other eubacteria. Gene 217:83-90. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes, K., J. Møller-Jensen, and R. Bugge Jensen. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455-466. [DOI] [PubMed] [Google Scholar]
- 13.Glaser, P., M. E. Sharpe, B. Raether, M. Perego, K. Ohlsen, and J. Errington. 1997. Dynamic, mitotic-like behaviour of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 11:1160-1168. [DOI] [PubMed] [Google Scholar]
- 14.Godfrin-Estevenon, A.-M., F. Pasta, and D. Lane. 2002. The parAB gene products of Pseudomonas putida exhibit partition activity in both P. putida and Escherichia coli. Mol. Microbiol. 43:39-49. [DOI] [PubMed] [Google Scholar]
- 15.Grigoriev, P. S., and M. B. Łobocka. 2001. Determinants of segregational stability of the linear plasmid-prophage N15 of Escherichia coli. Mol. Microbiol. 42:355-368. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, M., H. Mori, T. Onoqi, M. Yamazoe, H. Niki, T. Ogura, and S. Hiraga. 1998. Autoregulation of the partition genes of the mini-F plasmid and the intracellular localization of their products in Escherichia coli. Mol. Gen. Genet. 257:392-403. [DOI] [PubMed] [Google Scholar]
- 17.Irani, V. R., and J. J. Rowe. 1997. Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. BioTechniques 22:54-56. [DOI] [PubMed] [Google Scholar]
- 18.Ireton, K., N. W. T. Gunther, and A. D. Grossman. 1994. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176:5320-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagura-Burdzy, G., J. P. Ibbotson, and C. M. Thomas. 1991. The korF region of broad-host-range plasmid RK2 encodes two polypeptides with transcriptional repressor activity. J. Bacteriol. 173:826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagura-Burdzy, G., K. Kostelidou, J. Pole, D. Khare, A. Jones, D. R. Williams, and C. M. Thomas. 1999. IncC of broad-host-range plasmid RK2 modulates KorB transcriptional repressor activity in vivo and operator binding in vitro. J. Bacteriol. 181:2807-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagura-Burdzy, G., D. P. Macartney, M. Zatyka, L. Cunliffe, G. D. Cooke, C. Huggins, F. Khanim, and C. M. Thomas. 1999. Repression at a distance by the global regulator KorB of promiscuous IncP plasmids. Mol. Microbiol. 32:519-532. [DOI] [PubMed] [Google Scholar]
- 22.Jagura-Burdzy, G., and C. M. Thomas. 1994. KorA protein of promiscuous plasmid RK2 controls a transcriptional switch between divergent operons for plasmid replication and conjugative transfer. Proc. Natl. Acad. Sci. USA 91:10571-10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagura-Burdzy, G., and C. M. Thomas. 1995. Purification of KorA protein from broad host range plasmid RK2: definition of a hierarchy of KorA operators. J. Mol. Biol. 253:39-50. [DOI] [PubMed] [Google Scholar]
- 24.Jensen, R. B., and K. Gerdes. 1997. Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR-parC complex. J. Mol. Biol. 269:505-513. [DOI] [PubMed] [Google Scholar]
- 25.Kahn, M. R., R. Kolter, C. M. Thomas, D. Figurski, R. Meyer, E. Remault, and D. R. Helinski. 1979. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 68:268-280. [DOI] [PubMed] [Google Scholar]
- 26.Kim, H. J., M. J. Calcutt, F. J. Schmidt, and K. F. Chater. 2000. Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J. Bacteriol. 182:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, S.-K., and J. Shim. 1999. Interaction between F plasmid partition proteins SopA and SopB. Biochem. Biophys. Res. Commun. 263:113-117. [DOI] [PubMed] [Google Scholar]
- 28.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 29.Lee, P. S., D. C.-H. Lin, S. Moriya, and A. D. Grossman. 2003. Effects of the chromosome partitioning protein Spo0J (ParB) on oriC positioning and replication initiation in Bacillus subtilis. J. Bacteriol. 185:1326-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis, P. J., and J. Errington. 1997. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the Spo0J partitioning protein. Mol. Microbiol. 25:945-954. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, R. A., C. R. Bignell, W. Zeng, A. C. Jones, and C. M. Thomas. 2002. Chromosome loss from par mutants of Pseudomonas putida depends on growth medium and phase of growth. Microbiology 148:537-548. [DOI] [PubMed] [Google Scholar]
- 32.Lin, D. C.-H., and A. D. Grossman. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675-685. [DOI] [PubMed] [Google Scholar]
- 33.Lin, D. C.-H., P. A. Levin, and A. D. Grossman. 1997. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 94:4721-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Łukaszewicz, M., K. Kostelidou, A. A. Bartosik, G. D. Cooke, C. M. Thomas, and G. Jagura-Burdzy. 2002. Functional dissection of the ParB homologue (KorB) from IncP-1 plasmid RK2. Nucleic Acids Res. 30:1046-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch, A. S., and J. C. Wang. 1995. SopB protein-mediated silencing of genes linked to the sopC locus of Escherichia coli F-plasmid. Proc. Natl. Acad. Sci. USA 92:1896-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma, J., and M. Ptashne. 1987. A new class of yeast transcriptional activators. Cell 51:113-119. [DOI] [PubMed] [Google Scholar]
- 37.Mohl, D. A., J. Easter, Jr., and J. W. Gober. 2001. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol. Microbiol. 42:741-755. [DOI] [PubMed] [Google Scholar]
- 38.Mohl, D. A., and J. W. Gober. 1997. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell 88:675-684. [DOI] [PubMed] [Google Scholar]
- 39.Mori, H., Y. Mori, C. Ichinose, H. Niki, T. Ogura, A. Kato, and S. Hiraga. 1989. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J. Biol. Chem. 264:15535-15541. [PubMed] [Google Scholar]
- 40.Mullis, K., F. Faloona, S. Scharf, R. Saiki, G. Horn, and H. Erlich. 1986. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. 51:263-273. [DOI] [PubMed] [Google Scholar]
- 41.Ogasawara, N., and H. Yoshikawa. 1992. Genes and their organization in the replication origin of the bacterial chromosome. Mol. Microbiol. 6:629-634. [DOI] [PubMed] [Google Scholar]
- 42.Quisel, J. D., and A. D. Grossman. 2000. Control of sporulation gene expression in Bacillus subtilis by the chromosome partitioning proteins Soj (ParA) and Spo0J (ParB). J. Bacteriol. 182:3446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quisel, J. D., D. C. Lin, and A. D. Grossman. 1999. Control of development by altered localization of a transcription factor in B. subtilis. Mol. Cell 4:665-672. [DOI] [PubMed] [Google Scholar]
- 44.Reznekov, O., S. Alper, and R. Losick. 1996. Subcellular localization of proteins governing the proteolytic activation of a developmental transcription factor in Bacillus subtilis. Genes Cells 1:529-542. [DOI] [PubMed] [Google Scholar]
- 45.Rodionov, O., M. Łobocka, and M. Yarmolinsky. 1999. Silencing of genes flanking the P1 plasmid centromere. Science 283:546-549. [DOI] [PubMed] [Google Scholar]
- 46.Rosche, T. M., A. Siddique, M. H. Larsen, and D. H. Figurski. 2000. Incompatibility protein IncC and global regulator KorB interact in active partition of promiscuous plasmid RK2. J. Bacteriol. 182:6014-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Sharpe, M. E., and J. Errington. 1996. The Bacillus subtilis soj-spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol. Microbiol. 21:501-509. [DOI] [PubMed] [Google Scholar]
- 49.Sharpe, M. E., and J. Errington. 1998. A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol. Microbiol. 28:981-990. [DOI] [PubMed] [Google Scholar]
- 50.Studier, F. W., and B. A. Moffatt. 1981. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 51.Surtees, J. A., and B. E. Funnell. 1999. P1 ParB domain structure includes two independent multimerization domains. J. Bacteriol. 181:5898-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb, C. D., A. Teleman, S. Gordon, A. Straight, A. Belmont, D. C.-H. Lin, A. D. Grossman, A. Wright, and R. Losick. 1997. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell 88:667-674. [DOI] [PubMed] [Google Scholar]
- 53.Williams, D. R., D. P. Macartney, and C. M. Thomas. 1998. The partitioning activity of the RK2 central control region requires only incC, korB and KorB-binding site O(B)3 but other KorB-binding sites form destabilizing complexes in the absence of O(B)3. Microbiology 144:3369-3378. [DOI] [PubMed] [Google Scholar]
- 54.Yamaichi, Y., and H. Niki. 2000. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:14656-14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]