Abstract
Objective
Determine the feasibility of pulmonary function (PFT) and quality of life (QOL) evaluations in children after Acute Respiratory Distress Syndrome (ARDS).
Design
A prospective follow-up feasibility study
Setting
A tertiary pediatric intensive care unit
Patients
Children <18 year old with ARDS admitted between 2000 and 2005.
Measurements and Main Results
PFTs and QOL questionnaires performed approximately 12-months post-illness were analyzed and correlated to in-hospital clinical parameters. QOL data was compared to published pediatric chronic asthma and general pediatric norms. 180 patients met ARDS criteria; 37 (20%) died, 90 (51%) declined participation, 28 (16%) consented but did not return, and 24 (13%) returned for follow up visit. Twenty-three patients completed QOL testing and 17 completed PFTs. Clinical characteristics of those who returned were no different from those who did not except for age (median age 4.9 vs. 1.8 years). One third had mild to moderate pulmonary function deficits. QOL scores were marginal with general health perception, physical functioning, and behavior being areas of concern. These scores were lower than scores in children with chronic asthma. Parental QOL assessments report lower scores in single parent homes but no differences were noted by race or parental employment status.
Conclusion
Valuable information may be discerned from ARDS patients who return for follow-up evaluation. In this pilot study up to one-third of children with ARDS exhibit pulmonary function deficits and 12-month post-illness QOL scores are lower than in children with chronic asthma. Parental perceptions of post-illness QOL may be negatively impacted by socioeconomic constraints. Long term follow of children with ARDS is feasible and bears further investigation.
Keywords: Acute respiratory distress syndrome, pediatrics, long-term outcomes, pulmonary function testing, quality of life
Introduction
Acute Respiratory Distress Syndrome (ARDS) is noted in approximately 8% of all mechanically ventilated patients and 1.5 to 3% of all admissions to the pediatric intensive care unit (PICU)(1). While the mortality in children with ARDS remains high at approximately 18–35%(2–4), it has been steadily decreasing over the past several years. ARDS is a condition predicated on persistent and profound oxygenation and ventilation deficits, generalized inflammation, dysregulated coagulation and pulmonary fibrosis. It is unsurprising, therefore, to note that both adults and children who survive this illness are discharged from the hospital with neuromuscular weakness, nutritional deficits and persistent pulmonary dysfunction This results in prolonged rehabilitation needs and delayed return to school or work related activities. Currently, pediatric intensivists are ill-equipped to advise general pediatricians or families about what to expect – whether it be physical, emotional, cognitive, or socioeconomic – and, perhaps more importantly, what to do after pediatric ARDS survival.
When appraising the long term consequences of the general PICU admission, where the mortality rate is much lower at 3%, several studies have found resultant deficits in physical function, quality of life, mental health and family function(5–10). Post-traumatic stress disorder (PTSD) has also been found in up to 30% of PICU survivors and appears to be associated with severity of illness, invasive procedures, and length of stay(11–13). It is, therefore, profoundly important to evaluate the long-term physical and psychological consequences in pediatric ARDS survivors. Many clinicians and investigators in the pediatric and adult ARDS environments, have equally noted this imperative(3, 14).
It is our aim to complete this pilot investigation to a) gather preliminary evidence that pediatric ARDS survivors suffer from long term pulmonary function, physical, psychological and quality of life deficits, and b) to assist in determining the feasibility of performing larger, long term, in-person follow up evaluations in PARDS survivors with non-PARDS control groups
Materials and Methods
Patient Selection
All patients admitted to the PICU at UCSF Benioff Children’s Hospital, Oakland between August 2000 and May 2005 were prospectively evaluated and all who were diagnosed with ARDS were approached for study consent. Based on the 1994 American European Consensus Committee definition, ARDS was defined as the presence of bilateral infiltrates on chest radiograph, no evidence of left atrial hypertension, and a PaO2/FiO2 ratio (PF ratio) of < 300(15). Patients were excluded if they were less than 36 weeks corrected gestation age or more than 18 years of age at onset of ARDS, were neonates with infantile respiratory distress syndrome, or had intracardiac mixing lesions. All patients had at least one arterial blood gas supporting the PF ratio requirement for diagnosis. For patients who were not mechanically ventilated, the FiO2 was calculated according to the American Association of Respiratory Care guidelines(16). Radiographic interpretation was confirmed by independent readings from attending radiologists who were blinded to the study. The Institutional Review Board at UCSF Benioff Children’s Hospital, Oakland, approved the study.
Procedures
Demographic and admission data were collected from the medical record including age, gender, race, ethnicity, past medical history, PRISM-III (a severity of illness score)(17) and PELOD score (score of multi-organ dysfunction)(18). Mechanical ventilator parameters were collected throughout admission. Duration of mechanical ventilation was measured as ventilator free days defined as the number of days a patient did not require mechanical ventilation up to 28 days from diagnosis. In this calculation, all patients who died were assigned 0 ventilator free days and all who were never intubated were assigned 28 ventilator free days.
Once discharged, patients were followed at 6-month intervals for 2 years. All subjects were reminded about follow up visits frequently by both telephone discussions and regularly mailed reminders. At each visit, acute health status was reviewed to ensure appropriateness for pulmonary function testing. Both the parent and the child, where developmentally appropriate, were asked to complete the Child Health Questionnaire (CHQ, healthactchq.com). The CHQ is a generic quality of life instrument that is commercially available and scored. It has been used widely in the pediatric population, including those with complex and chronic diseases, in both clinical and research settings as it has strong validity in assessing both physical and psychosocial concepts(19). There is currently no disease specific quality of life instrument for ARDS, either in adults or in children. The CHQ concepts include general health, physical functioning, emotional and behavioral role/social limitations, physical role/social limitations, bodily pain/discomfort, general behavior, global behavior, mental health, self-esteem and general health perception. Individual CHQ questionnaire results were coded, and final scores were calculated by computing the mean of the items completed. These raw scale scores were then transformed to a 0–100 score (100 being the bets possible score) for each concept. These data were also compared to National Normative and Asthma Clinical Trials CHQ results(19) available in the public domain.
Pulmonary function tests were obtained on infants up to 18 months of age and in children ages 3 years or older. Standard procedures for both infant and child pulmonary function testing were applied(20, 21). For the infant patients, the infant was sedated with chloral hydrate during PFT testing. Once asleep, the infant was placed on 100% oxygen via face mask and nitrogen washout technique was used to obtain functional residual capacity measurements. Thereafter, they were placed in an inflatable plastic vest (the “baby hugger”), arms outside the vest, and the rapid chest compression technique was applied to obtain partial expiratory flow volume loops. Rapid chest compressions occurred at the end of tidal inspiration. Flow was measured at the mouth by a pneumotachynograph attached to the facemask. Occluding the expiratory port of the facemask at end-inspiration for 200–400ms and allowing the infant to expire passively tested passive mechanics. Airway reactivity was tested by repeating the “baby hugger” technique 10 minutes after nebulized albuterol administration.
For children >3 years of age and with appropriate developmental ability, pulmonary function testing included spirometry and body plethysmography. Spirometry data was recorded for forced vital capacity and forced expiratory flows. Plethysmograph values included tidal volumes, functional residual capacity, residual volume, and diffusion capacity. All children underwent standardized nebulized albuterol challenges to identify patients with airway reactivity, and thus possible undiagnosed obstructive disease. All PFTs were interpreted by a pediatric pulmonologist who was blinded to any patient data, including past medical history and ARDS course. Obstructive lung disease was defined as FEV1/FVC <0.8, or as FEF25 of <70% predicted in patients with a normal FVC, and restrictive lung disease was defined as a FVC <80% predicted with a normal FEV1/FVC ratio. Mild obstructive lung disease was defined as an FEV1 equal to 70–79% predicted, moderate as 50–69% predicted, and severe as <49% predicted. A diffusion defect was defined as a DLCO <80% predicted. A mild diffusion abnormality was defined as 60–79% predicted, moderate as 40–59% predicted, and severe as <40% predicted(21, 22).
Statistical Analysis
This is a preliminary study for the purposes of determining feasibility of long term follow up examinations and pulmonary function testing in children who have recently been critically ill, rather than determining statistically significant correlations between severity of ARDS and long term pulmonary function. Therefore, p-values were not intended for interpretation as they would be in randomized, controlled clinical trials. Summary statistics were calculated for all who consented to long term follow up and for all who actually returned for follow up. Comparison of demographic data between those who did and did not return for follow up assessment were performed by Fisher Exact χ2 testing between categorical variables and Wilcoxon rank-sum or Kruskal-Wallis tests for continuous variables. PF ratio, PELOD score and ventilator parameters recorded 24 hours after ARDS diagnosis were used and compared by spearman correlation to PFT results. Due to few patients returning for more than one follow up evaluation, we chose to use the PFT and CHQ data closest to the 12-month post hospital admission for those with multiple visits. We used Student’s t-tests to compare parent and child CHQ scores, and CHQ scores from the present study with normative and chronic asthma samples. We examined relationships between CHQ scores and demographic variables using Spearman correlations (for continuous variables such as education level and parent age) and analysis of variance or Student’s t-test (for categorical variables such as single vs. double parent home, parental employment status, or race).” All analyses were performed using STATA statistical software, version 13.1 (StataCorp, College Station, Texas).
Results
During the enrollment period, 180 children met AECC definitions for Acute Lung Injury or Acute Respiratory Distress Syndrome. Of this cohort, 37(20%) died, 91(51%) refused to participate in long term follow up, 28 (16%) consented but did not return despite numerous appointment reminders, and 24 (13%) returned for pulmonary function testing and to answer CHQ quality of life surveys. Of these 24, only 17 were able to successfully complete some, or all, of the pulmonary function test portion. Seven patients and 23 parents completed the CHQ questionnaire.
There were no significant differences in gender, ethnicity, duration of mechanical ventilation, PRISM-III score, PF ratio or length of stay between those who returned for follow up and those who did not, or between those who returned for follow up and all ARDS survivors within the cohort. Patients who returned for follow up were older than those who did not, with a median age of 5.3 years (IQR 3 to 12) for those who returned for follow up evaluation compared to a median age of 1.8 years (IQR 0.2 to 5) for those who did not return (p = 0.03). The mean time to follow-up was 10.7 ± 3.9 months. Table 1 shows the demographic data of all eligible patients, those who did not consent, those who consented for follow up but did not return and those who consented and completed follow up evaluation.
Table 1.
Demographic and ICU data of Eligible, Consented and Non-consented ARDS patientsa.
| Eligible Patients/Survivors (n=142) | Did not consent (n = 90) | Returned for Follow Up (n = 24) | Consented but did not follow up (n = 28) | |
|---|---|---|---|---|
| Age in months | 21 (2.4, 109) | 13 (1.7, 88) | 64 (38, 145) | 21 (2.5, 63) |
| Male (%) | 86 (61) | 54 (60) | 13 (54) | 17 (55) |
| Race (%) | ||||
| Caucasian | 61 (43) | 37 (41) | 12 (50) | 12 (43) |
| African American | 22 (16) | 14 (16) | 5 (21) | 3 (11) |
| Hispanic/Latino | 42 (30) | 27 (30) | 6 (25) | 9 (32) |
| Asian/Pacific Islander | 11 (8) | 10 (11) | 0 (0) | 1 (4) |
| Other | 6 (5) | 2 (2) | 1 (4) | 3 (11) |
| Any Prior Comorbidity (%) | 69 (49) | 46 (51) | 10 (42) | 13 (46) |
| Chronic Pulmonary Disease (%) | 31 (22) | 20 (22) | 3 (13) | 8 (29) |
| Cancer History (%) | 5 (4) | 3 (3) | 2 (8) | 0 (0) |
| ARDS Etiology | ||||
| Pneumonia (%) | 60 (42) | 37 (41) | 9 (38) | 14 (50) |
| Aspiration (%) | 29 (13) | 15 (17) | 1 (4) | 3 (11) |
| Sepsis (%) | 17 (12) | 11 (12) | 4 (17) | 2 (7) |
| Near Drowning (%) | 6 (4) | 2 (2) | 3 (12) | 1 (4) |
| Trauma (%) | 9 (6) | 4 (4) | 2 (8) | 3 (11) |
| Other | 31 (22) | 21 (23) | 5 (21) | 5 (18) |
| PaO2:FiO2 at 24 hours of illness | 148 (93, 231) | 153 (122, 262) | 167 (113, 297) | 240 (182, 280) |
| Unadjusted PRISM III score | 5 (2, 10) | 5 (2, 11) | 5 (4,9) | 4.5 (1.5, 10) |
| Ventilator Free Days | 23 (15, 26) | 23 (17, 26) | 22 (13, 26) | 20 (13, 27) |
| ICU Length of Stay | 9 (5, 19) | 8 (5, 19) | 9 (5,17) | 10 (6, 18) |
| Hospital Length of Stay | 20 (10, 36) | 18 (11, 28) | 11 (8, 28) | 12 (8, 28) |
Units are median (IQR) or n (%)
Of the 24 patients who did return for follow up, two (8 %) had prior diagnoses of reactive airway disease, no other patient reported a history of chronic pulmonary or neuromuscular conditions. The most common etiology of ARDS was pneumonia (n = 9, 38%) followed in frequency by sepsis (n = 4, 17%) and near-drowning (n = 3, 12%) with aspiration, trauma, pulmonary hemorrhage or transfusion related acute lung injury all compromising less than 10% each. Four did not require intubation and mechanical ventilation, four high frequency oscillation, one extracorporeal life support, and the remainder used conventional mechanical ventilation for a period during their PICU admission. The median number of ventilator free days up to 28 days from diagnosis was 22 days (IQR 13, 26).
Pulmonary Function Test Results
Because the mean time to follow up was 10.7 months and because most participants returned only once, the PFTs performed nearest to the 12-month post-ARDS mark were analyzed. Of the 17 who completed PFTs, 14 had standard child and 3 had infant testing. The testing found that 11 (64%) had normal PFTs; 2 (12%) had mild obstruction, 2 (12%) had moderate obstruction, and 2 (12%) had abnormal diffusion capacity. None exhibited restrictive disease. Of note, one of the patients with moderate obstruction and one with a diffusion capacity deficit had reported reactive airway disease prior to admission but had been compliant with controller medications. No patient reported an emergency room visit or hospitalization for an intercurrent respiratory illness between the time of ICU discharge and PFT testing. Table 2 reports the pulmonary function results for these 17 patients.
Table 2.
Pulmonary function test results of ARDS patients who returned for follow up
| Patient IDa |
Age at ARDS (months) |
Infant PFTs | Child PFTs | Spirometry Interpretation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Infant FRC (mL/kg) |
Infant V50 |
FVC, Lb | FEV1, % |
|
FEF 25– 75, L/secb |
FEV1 change after bronchodilatorc |
TLC, Lb |
|
DLCO, mL/mmHg/ minb |
DLCO/VA mL/mHg/ min/Lb |
Raw, cmH2O/L/sb |
|||
| 1 | 0.5 | 7.5 | 72 | no | normal | |||||||||
| 2 | 2 | 15 | 54 | yes | normal | |||||||||
| 3 | 17 | 24 | 130 | yes | normal | |||||||||
| 4 | 40 | 1.3 (95) | 1.3 (103) | 0.96 | 2.3 (140) | no data | 1.8 (96) | 25 | 22.8 (425) | normal | ||||
| 5Ŧ | 59 | 1.2 (84) | 0.9 (70) | 0.74 | 0.9 (55) | yes | 1.9 (105) | 39 | 11.1 (60) | 7.4 (142) | 7.8 (145) | mild obstruction | ||
| 6* | 130 | 2.7 (87) | 2.0 (69) | 0.73 | 1.5 (45) | yes | 4.2 (107) | 37 | 22.2 (83) | 5.8 (113) | 5.3 (174) | moderate obstruction | ||
| 7 | 89 | 1.4 (92) | 1.1 (80) | 0.79 | 1.1 (65) | no | 1.7 (90) | 21 | 11.5 (74) | 6.9 (132) | 17.5 (351) | mild diffusion abnormality | ||
| 8 | 126 | 3.0 (116) | 2.6 (106) | 0.87 | 3.1 (66) | no | 3.6 (105) | 14 | 20.7 (96) | 6.2 (121) | 4.9 (146) | normal | ||
| 9 | 55 | 1.0 (89) | 98 | 0.96 | 1.1 (76) | no | 1.5 (107) | 38 | 10.1 (158) | normal | ||||
| 10 | 133 | 2.4 (88) | 1.9 (77) | 0.8 | 1.9 (66) | no | 3.0 (87) | 21 | 21 (85) | 6.6 (131) | 23.9 (710) | normal | ||
| 11 | 85 | 0.8 (81) | 0.5 (57) | 0.62 | 0.2 (16) | yes | 1.3 (89) | 32 | 11.5 (179) | moderate obstruction | ||||
| 12 | 195 | 4.6 (125) | 4.1 (122) | 0.9 | 5.2 (139) | no | 6.0 (127) | 19 | 23.5 (80) | 4.4 (90) | 4.2 (159) | normal | ||
| 13 | 39 | 0.7 (79) | 0.6 (79) | 0.85 | 1.0 (86) | no data | mild obstruction | |||||||
| 14Ŧ | 156 | 3.3 (88) | 2.9 (81) | 0.88 | 3.2 (80) | no | 4.1 (83) | 17 | 19.9 (79) | 4.9 (99) | 3.3 (129) | normal | ||
| 15Ŧ | 167 | 3.9 (107) | 3.3 (94) | 0.84 | 3.1 (81) | no | 5.0 (105) | 22 | 25 (107) | 5.5 (94) | 3.4 (130) | normal | ||
| 16Ŧ,* | 176 | 2.8 (83) | 2.2 (72) | 0.8 | 197 (58) | no | 3.7 (87) | 23 | 18.6 (59) | 5.4 (110) | 7.1 (247) | diffusion abnormality | ||
| 17 | 37 | 0.5 (108) | 0.5 (130) | 0.92 | 0.7 (90) | yes | normal | |||||||
Patient ID in table 3 correlates to patient ID in table 2.
Value is parentheses represent the % predicted.
Significant change was defined as an increase in FEV1 of 12% or in FEF25–75 by 25%
patient was not intubated during ARDS course,
Patient has known reactive airway disease
FRC = functional residual capacity, FVC = forced vital capacity, FEV1 = forced expiratory volume(1 sec), FEF25–75 = forced expiratory flowFEF25–75 = forced expiratory flow TLC = total lung capacity, RV/TLC = residual volume as % of TLC, DLCO = diffusion capacity, DLCO/VA = diffusion capacity corrected for alveolar volume, Raw = airway resistance
When evaluating for any correlations between ICU events, mechanical ventilation parameters and PFT results, we found that airway resistance (Raw) correlated negatively with PF ratio calculated on day 1 of illness (n = 10, rho −0.66, p 0.004) such that as PF ratio worsened (smaller value) so did the airway resistance measured on PFTs performed after discharge. Additionally, PICU length of stay appeared to have a potential correlation with increased airway resistance on post-illness PFTs (n = 12, rho 0.5, p 0.08). No other correlations between PFT results and mechanical ventilation parameters (incl. PIP, PEEP, MAP, tidal volume), PRISM-III, PELOD, duration of mechanical ventilation, or length of hospital or ICU stay were found to be significant. Because the number of patients returning for pulmonary function testing is small, and because such testing may be impacted by a patient’s prior comorbidities or by respiratory illnesses during the interval between ARDS and follow up appointment, we provide such details in a descriptive Table 3 of the 17 patients who returned for PFT testing.
Table 3.
Pertinent Patient History, ICU Care and Intercurrent Illnesses by Pulmonary Function Abnormality
| Patient IDa | Age at ARDS (months) | Prior Comorbidities | Not Intubated | Required HFOV or ECLS | Ventilator Data at time of diagnosisb
|
Intercurrent Respiratory ED visit or hospitalization | |||
|---|---|---|---|---|---|---|---|---|---|
| PIPc | MAPc | TV (mL/kg) | PaO2:FiO2 | ||||||
| Obstructive Disease on Pulmonary Function Testing | |||||||||
| 5 | 59 | None | X | NA | NA | NA | 300 | None | |
| 6 | 130 | Reactive Airway Disease | 24 | 6.7 | 5.8 | 158 | None | ||
| 11 | 85 | Premature (no lung disease) | 24 | 8 | 7.1 | 271 | None | ||
| 13 | 39 | None | X | 37 | 211 | None | |||
| Diffusion Capacity Abnormality | |||||||||
| 7 | 89 | Bone Marrow Transplant | X | 27 | 18 | 8.7 | 116 | fever unknown origin | |
| 16 | 176 | Reactive Airway Disease, Sickle Cell | X | NA | NA | NA | 56 | None | |
| Normal Pulmonary Function Test | |||||||||
| 1 | 0.5 | Corrected Cardiac Defect | 34 | 9.6 | 9.4 | 68 | No data | ||
| 2 | 2 | Trisomy 21, corrected cardiac defect | 40 | 10.6 | 10.6 | 93 | None | ||
| 3 | 17 | None | 24 | 11 | 6.2 | 135 | No data | ||
| 4± | 40 | None | 267 | None | |||||
| 8 | 126 | None | 25 | 7.6 | 6 | 200 | None | ||
| 9 | 55 | Seizure disorder | 19 | 9.7 | 6.4 | 71 | None | ||
| 10 | 133 | None | 39 | 10 | 7 | 167 | None | ||
| 12 | 195 | Leukemia (not immunocompromised) | X | 25 | 37 | 7.1 | 240 | None | |
| 14 | 156 | None | X | NA | NA | NA | 167 | None | |
| 15 | 167 | None | X | NA | NA | NA | 293 | None | |
| 17§ | 37 | None | NA | NA | NA | 117 | None | ||
Patients with no details were not requiring invasive mechanical ventilation at the time;
Unit of measurement is cmH2O
Patient intubated en route to hospital but extubated within a few hours of admission;
Patient not intubated until 72 hours of ARDS
Abbreviations: HFOV = high frequency oscillatory ventilation; ECLS = Extracorporeal Life Support; PIP = Peak inspiratory pressure; PEEP = Positive end expiratory pressure; MAP = Mean airway pressure; TV = Tidal Volume
Quality of Life Assessment
Parent and Child CHQ results
Parent General Health Perceptions were notably low (mean 46.5 ± 17.6 out of 100) indicating parental belief that the child’s health is poor and likely to get worse. Parents also rated their child’s health as worse now than one year ago (General Health mean = 63 ± 22). Parent scores for their children on the Physical functioning (mean 77 ± 22) and General Behavior (mean = 75 ± 14) scales were in the moderate range. Parents rated children as highest on Role/Social Limitations - Behavior (mean = 88 ± 22) and Self-Esteem (mean = 86 ± 14). Figure 1 demonstrates all parents’ responses as well as matched parent and patient (n = 7) responses on the CHQ assessment. Although there is insufficient number of comparisons to make statistical inferences in these seven parent-patient responses we found that, except for Social Limitations – Physical and Behavior, children generally reported better long-term health across many domains (General Health, Bodily Pain, Mental Health and General Health Perceptions) compared with their parents.
Figure 1. Child Health Questionnaire (CHQ) responses for all parents and matched parent-patient pairs.
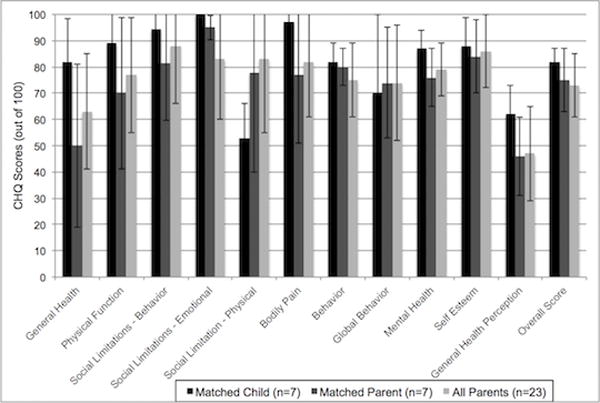
Seven children (black bars) and their parents (dark grey bars) completed CHQ questionnaires 10–12 months after ARDS. The light grey bars represent all 24 parents who completed the CHQ at this time point.
Comparison to National Asthma Clinical Trials and National Normative CHQ cohorts
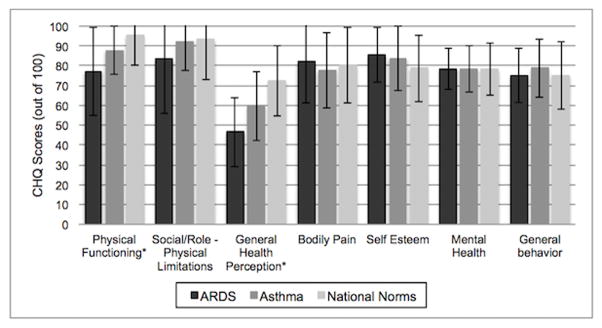
Parent CHQ ratings for their children who were pediatric ARDS survivors in the present study reported significantly decreased Physical Functioning, Role Physical and worse General Health Perceptions (p< 0.05) when compared to both Asthma Clinical Trial and National Normative samples of U.S. children whose parents completed the CHQ (figure 2). Remaining scales (Behavior, Mental Health, Bodily Pain, Self-Esteem) were on par across all cohorts compared.
Figure 2. Comparison of Children Health Questionnaire (CHQ) scores for children after ARDS to children with asthma and with national baseline data.
* Represents p<0.05 when comparing ARDS score to that of patients with asthma.
Relationships between CHQ Scores and Demographics
Parental reports indicated diminished Mental Health (−0.78, p=0.01) and a trend toward diminished Role/Social Limitations - Emotional (−0.60, p=0.07) scores in children of increasing grade level. Parents with a higher level of education reported lower Bodily Pain in their child (rho −0.44, p=0.03). Single parenting was also associated with a trend toward higher ratings of General Behavior (p=0.07). Social determinants of health were, for the most part, not associated with parent ratings of their children’s health related quality of life on the CHQ. No associations were identified between any of the CHQ domains tested and race, gender and parent employment outside of the home.
Discussion
Several adult studies have shown deficits in diffusion capacity, airflow obstruction, restrictive lung disease or persistent hypoxemia even 6 to 12 months after hospital discharge(23–25). The few studies that have been published evaluating the pulmonary function in children after ARDS are small series studies (n=7, n=9, n=5, n=11), all performed before lung protective mechanical ventilation strategies were utilized and, therefore, most children included in the prior research reports were exposed to much higher ventilator settings than are currently preferred(26–31). From these early publications, it appears that ARDS in children leads to similar pulmonary deficits as seen in adults with ARDS.
Although a pilot investigation, our study of 24 patients represents one of the largest cohorts of pediatric ARDS survivors with long term follow up evaluations to date. While one third of our patients exhibited deficits on pulmonary function testing, most did not have pulmonary sequelae of grave nature. It is interesting that no patient was noted to have restrictive lung disease given ARDS is, for the most part, a parenchymal disease. Several adults studies note a similar finding and report obstructive disease and diffusion capacity deficits as often as restrictive disease(23–25), begging the question if prolonged pulmonary dysfunction is from the disease itself or from the use of mechanical ventilation in addition to the presence of diseased lung parenchyma. Two of the six patients with abnormal PFT results had prior pulmonary disease that may have contributed to their risk of developing ARDS and to their prolonged pulmonary dysfunction. No patient reported intercurrent pulmonary illnesses or pulmonary related hospitalizations in the period between ARDS hospitalization and time of testing. While the number of patients in our study makes it difficult to provide strict conclusions between ARDS and prolonged pulmonary dysfunction, it is important for the primary care providers and outpatient pulmonologists to be aware of the high potential of prolonged pulmonary function deficits after ARDS, particularly in those with chronic risk factors.
Aside from prolonged pulmonary dysfunction, there is significant evidence that ARDS results in long term consequences in adult survivors, including global cognitive delay, anxiety, depression, and post-traumatic stress disorder (PTSD)(32–36). While long term quality of life outcomes have recently become an area of focus in the general PICU population, there have been no studies solely focusing on the long term effect of ARDS on children’s neurocognitive outcome. Knoester and colleagues reported only 31% of PICU survivors having normal Pediatric Overall Performance Category (POPC) at 3 months post discharge, highlighting substantial physical sequelae in these survivors of critical illness(9, 37). In 2009, Colville and colleagues documented that PICU survivors again reported lower physical function scores, but no significant association between severity of PICU illness and quality of life scores at 1 year(38). Focusing solely on the impact of ARDS on aspects of quality of life, a multicenter trial of prone positioning in children with ARDS revealed that the POPC scale worsened from admission to discharge in 22% of survivors while 17% of survivors had worsened Pediatric Cerebral Performance Category (PCPC) scales(39, 40). This study, however, did not evaluate performance or quality of life outcomes after discharge. Our quality of life testing indicates that after hospitalization for ARDS, children describe a remarkable resilience of spirit, yet parents and caregivers note a significant decrease in overall physical functioning and concerning global health discrepancies compared to their peers. Since our study did not have a control group of critically ill children admitted to the PICU for non-ARDS diagnoses, further investigation will be needed to discern quality of life burdens secondary to ARDS and prolonged mechanical ventilation compared to critical illness alone. Long-term neurocognitive outcome data from large multi-center studies like RESTORE Cog (https://clinicaltrials.gov/show/NCT02225041), focusing particularly on mechanically ventilated pediatric ARDS and non-ARDS patients, will no doubt shed important light on this finding and lead the next therapeutic, diagnostic and research initiatives in this area.
Our findings reveal that social stressors may impact aspects of quality of life after ARDS. Parents with a lower level of education and those in single parent families reported greater bodily pain in their child compared to their peers. This association is certainly not unique to ARDS and has been reported in several disease entities after prolonged ICU stay. In a systematic review of quality of life assessments of children after ICU admission, they found significant ongoing morbidity after discharge with key determinants of poor quality of life including reason for admission (i.e. severity of illness), prior comorbidities, need for invasive therapy, but also parental characteristics, particularly low socioeconomic status and parent education level(5). Some of our data is consistent with this systematic review, and supports the recently published Pediatric Acute Lung Injury Consensus Conference (PALICC) encouraging assessment of long term morbidity after ARDS. Furthermore, it reinforces the importance of counseling to, and by, the primary care providers of children with ARDS, or any critical illness, that long term physical and quality of life deficits are to be monitored closely and addressed, especially in high risk populations such as those with chronic illness or with parents with significant socioeconomic strain.
While our study population is small, with the number of patients who returned for follow up being far less than the number who survived their ARDS, we do believe this data presented is novel and the results important for future long term outcome study design. This finding of poor return for follow up plagues most long term outcomes research. However, in our study there was no statistical differences between patients eligible for follow up evaluation, patients who consented for the study and those who actually completed the study in the categories of gender, ethnicity, length of stay, ventilator duration, severity of illness, or presence of pre-ARDS comorbidities. While this does not eliminate all selection biases it does show that it might be possible to generalize “in person” follow up data to those who could not return in this and in future studies. As such, a potential future, novel approach to long term follow up studies could be utilized, with a two pronged approach – one “traditional limb” where patients return for in person evaluation and an “alternate” limb where patients are assessed via video or teleconference at home or the primary care provider’s office, as long as privacy can be maintained.
Our feasibility study identified other limitations as well. Pulmonary function testing is a lengthy process and requires patients to be developmentally appropriate to follow commands. As such, patients with cognitive impairments and of pre-school age were unable to participate. Newer methods of pulmonary function testing, such as impulse oscillometry(41, 42) are more suited to children of preschool age and may provide more options in future studies. Additionally, patients with chronic pulmonary or neuromuscular comorbidities may have had some pre-ARDS pulmonary function abnormalities and interpreting the PFT results and the impact of ARDS on long term pulmonary function must take such comorbidities into consideration. With regard to our CHQ testing, there is no published quality of life assessment tool specific for the pediatric ARDS cohort. Further, although the CHQ format was quite extensive, it did not assess for PTSD or include methods to assess physical functioning, such as a 6-minute walk. Additionally, with there being no validated quality of life assessment tool for pediatric critical illness or ARDS, we chose the CHQ format because of its validation of chronic pulmonary diseases, such as asthma, and of children after prolonged hospitalization for any illness. A potential strategy to reduce this limitation in the future is to ask parents to complete the questionnaire at time of admission to the ICU and again at follow up appointments so that matched comparisons can be analyzed and reduce pre-hospitalization factors.
Overall this feasibility study describes the challenge of having a majority of survivors of pediatric ARDS return to their tertiary center of referral for extensive long term outcome testing; yet despite incomplete follow up, valuable information may be obtained from those who return for post-discharge evaluation. In this cohort of 24 children who returned for follow up after hospitalization for ARDS, one third exhibited mild to moderate impairments in pulmonary function approximately 10 months after ARDS diagnosis, and abnormal PFTs were observed in both children who required mechanical ventilation and those who did not. Additionally, at first glance, it appears that the ARDS patients do fairly well after their admission; however in comparison to national norms and other children with impaired lung health (asthma), it is clear that pediatric survivors of ARDS are impacted across many quality of life domains, particularly general health perception, physical functioning and behavior. More research and information in this area is imperative to inform healthcare providers who can help direct and authorize additional services and treatments to enable as much recovery as possible and to engage and empower the families of these children that must deal with the socioeconomic concerns and stressors these additional morbidities yield.
Acknowledgments
Supported by National Institutes of Health grants 5T32HD049303-08 (Ward and Spicer), NCRR RR15543 (Flori) and CTSI NIH RR 01271 (Flori)
We thank Isabello Elisan and his team in the PFT lab at UCSF Benioff Children’s Hospital, Oakland for the completion of PFT evaluations and their dedication to the study.
References
- 1.Cornfield DN. Acute respiratory distress syndrome in children: physiology and management. Curr Opin Pediatr. 2013;25:338–43. doi: 10.1097/MOP.0b013e328360bbe7. [DOI] [PubMed] [Google Scholar]
- 2.Flori H, Glidden D, Rutherford G, et al. Pediatric acute lung injury prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 3.Khemani R, Smith LS, Zimmerman JJ, et al. Pediatric Acute Respiratory Distress Syndrome: Consensus Recommendations From the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–39. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand—A prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–23. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 5.Aspesberro F, Mangione-Smith R, Zimmerman J. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015;41:1235–46. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahim S, Singh S, Hutchison JS, et al. Adaptive Behavior, Functional Outcomes, and Quality of Life Outcomes of Children Requiring Urgent ICU Admission. Pediatr Crit Care Med. 2013;14:10–18. doi: 10.1097/PCC.0b013e31825b64b3. [DOI] [PubMed] [Google Scholar]
- 7.Conlon NP, Breatnach C, O’Hare BP, et al. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med. 2009;10:41–44. doi: 10.1097/PCC.0b013e31819371f6. [DOI] [PubMed] [Google Scholar]
- 8.Namachivayam P, Taylor A, Montague T, et al. Long-stay children in intensive care: Long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr Crit Care Med. 2012;13:520–8. doi: 10.1097/PCC.0b013e31824fb989. [DOI] [PubMed] [Google Scholar]
- 9.Knoester H, Bronner M, Bos A. Surviving pediatric intensive care: physical outcome after 3 months. Intensive Care Med. 2008;34:1076–1082. doi: 10.1007/s00134-008-1061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polic B, Mestrovic J, Markic J, et al. Long-term quality of life of patients treated in paediatric intensive care unit. Eur J Pediatr. 2013;172:85–90. doi: 10.1007/s00431-012-1843-0. [DOI] [PubMed] [Google Scholar]
- 11.Rennick J, Johnston C, Dougherty G, et al. Children’s Psychological Responses After Critical Illness and Exposure to Invasive Technology. J Dev Behav Pediatr. 2002;23:133–44. doi: 10.1097/00004703-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Rees G, Gledhill J, Garralda ME, et al. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30:1607–1614. doi: 10.1007/s00134-004-2310-9. [DOI] [PubMed] [Google Scholar]
- 13.Rennick JE, Rashotte J. Psychological outcomes in children following pediatric intensive care unit hospitalization: a systematic review of the research. J Child Heal Care. 2009;13:128–149. doi: 10.1177/1367493509102472. [DOI] [PubMed] [Google Scholar]
- 14.Pearmain L, Herridge M. Outcomes after ARDS: a distinct group in the spectrum of disability after complex and protracted critical illness. Minerva Anestesiol. 2013;79:793–803. [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Committee on ARDS: Definitions, Mechanisms, Relevant Outcomes, and Clinical Trial Coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro B. Clinical Application of Blood Gases. Chicago: Year Book Medical Publishers Inc; 1976. [Google Scholar]
- 17.Pollack MM, Patel KM, Ruttimann UE. The pediatric risk of mortality III— Acute physiology score (PRISM III-APS): A method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 18.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–7. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 19.Landgraf J, Abetz L, Ware J. The CHQ User’s Manual, 2nd Printing. Boston: Health Act; 1999. [Google Scholar]
- 20.Taussig L, Chernick V, Wood R, et al. Standardization of lung function testing in children. Pediatrics. 1980;97:668–676. doi: 10.1016/s0022-3476(80)80039-4. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Standardization of spirometry--1987 update. Statement of the American Thoracic Society. The American review of respiratory disease. 1987;136(5):1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 22.Francisco B, Ner Z, Ge B, et al. Sensitivity of different spirometric tests for detecting airway obstruction in childhood asthma. J Asthma. 2015;52:505–511. doi: 10.3109/02770903.2014.984842. [DOI] [PubMed] [Google Scholar]
- 23.Herridge MSM, Cheung AAM, Tansey CM, et al. One-Year Outcome in Survivors of the Acute Respiratory Distress Syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 24.Orme J, Romney JS, Hopkins RO, et al. Pulmonary Function and Health-related Quality of Life in Survivors of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2003;167:690–694. doi: 10.1164/rccm.200206-542OC. [DOI] [PubMed] [Google Scholar]
- 25.Yahav J, Lieberman P, Molho M. Pulmonary function following the adult respiratory distress syndrome. CHEST J. 1978;74:247–250. doi: 10.1378/chest.74.3.247. [DOI] [PubMed] [Google Scholar]
- 26.Fanconi S, Kraemer R, Weber J, et al. Long-term sequelae in children surviving adult respiratory distress syndrome. J Pediatr. 1985;106:218–22. doi: 10.1016/s0022-3476(85)80290-0. [DOI] [PubMed] [Google Scholar]
- 27.Golder N, Lane R, Tasker R. Timing of recovery of lung function after severe hypoxemic respiraory failure in children. Intensive Care Med. 1998;24:530–533. doi: 10.1007/s001340050607. [DOI] [PubMed] [Google Scholar]
- 28.Ben Abraham R, Weinbroun AA, Roizin H, et al. Long-term assessment of pulmonary function tests in pediatric survivors of acute respiratory distress syndrome. MedSciMonit. 2002;8:CR153–158. [PubMed] [Google Scholar]
- 29.Effman E, Merten D, Kirks D, et al. Adult Respiratory distress syndrome in children. Radiology. 1985;157:69–74. doi: 10.1148/radiology.157.1.4034980. [DOI] [PubMed] [Google Scholar]
- 30.Weiss I, Ushay H, DeBruin W, et al. Respiratory and cardiac function in children after acute hypoxemic respiratory failure. Crit Care Med. 1996;24:148–154. doi: 10.1097/00003246-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Dahlem P, De Jongh FHC, Griffioen RW, et al. Respiratory sequelae after acute hypoxemic respiratory failure in children with meningococcal septic shock. 2004;7:20–26. [Google Scholar]
- 32.Herridge MS, Tansey CM, Matté A, et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 33.Herridge M, Cameron JI. Disability after Critical Illness. N Engl J Med. 2013;369:1367–1369. doi: 10.1056/NEJMe1309482. [DOI] [PubMed] [Google Scholar]
- 34.Hodgson CL, Hayes K, Everard T, et al. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit Care. 2012;16:R202–R202. doi: 10.1186/cc11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masclans JR, Roca O, Muñoz X, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest. 2011;139:1340–6. doi: 10.1378/chest.10-2438. [DOI] [PubMed] [Google Scholar]
- 36.Needham DM, Wozniak AW, Hough CL, et al. Risk Factors for Physical Impairment after Acute Lung Injury in a National, Multicenter Study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knoester H, Bronner MB, Bos AP, et al. Quality of life in children three and nine months after discharge from a paediatric intensive care unit: a prospective cohort study. Health Qual Life Outcomes. 2008;6:21. doi: 10.1186/1477-7525-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colville GA, Pierce CM. Children’s Self-Reported Quality of Life After Intensive Care Treatment. Pediatr Crit Care Med. 2013:14. doi: 10.1097/PCC.0b013e3182712997. [DOI] [PubMed] [Google Scholar]
- 39.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 40.Curley M, Hibberd P, Fineman L, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellinckx J, Cauberghs M, De Boeck K, et al. Evaluation of impulse oscillation system: comparison with forced oscillation technique and body plethysmography. Eur Respir J. 2001;18:564–570. doi: 10.1183/09031936.01.00046401. [DOI] [PubMed] [Google Scholar]
- 42.Dencker M, Malmberg LP, Valind S, et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006;26:247–250. doi: 10.1111/j.1475-097X.2006.00682.x. [DOI] [PubMed] [Google Scholar]