Abstract
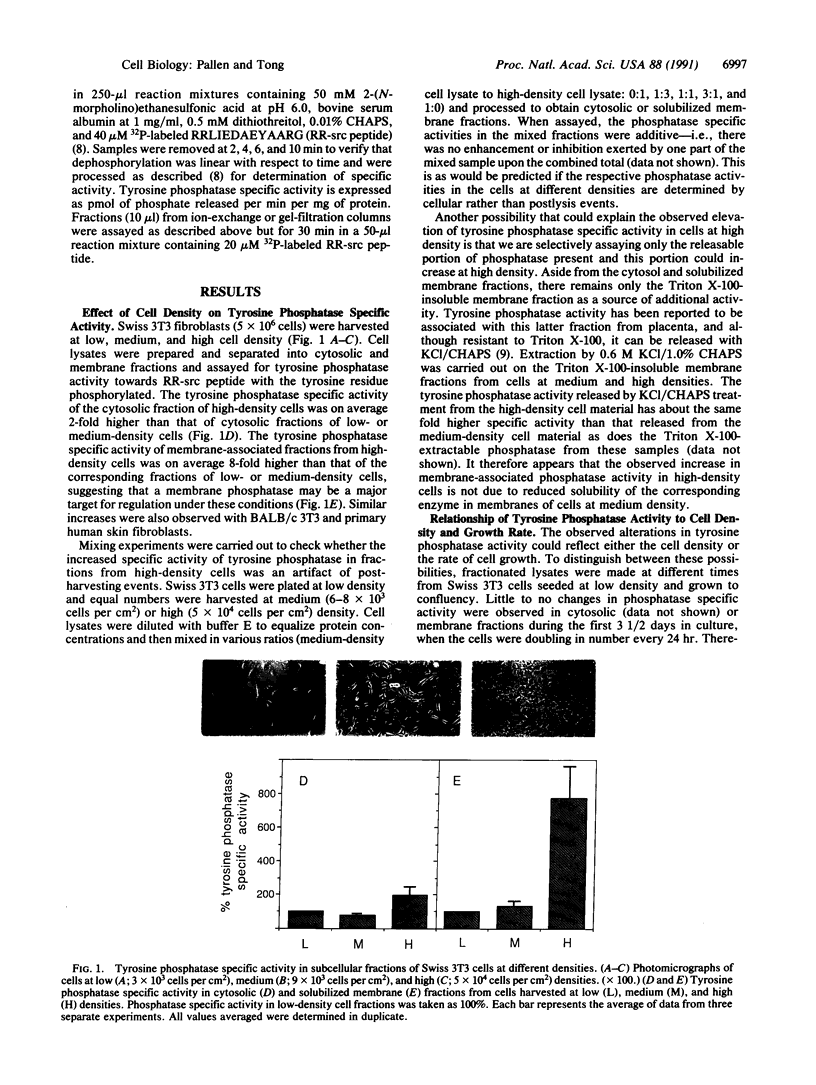
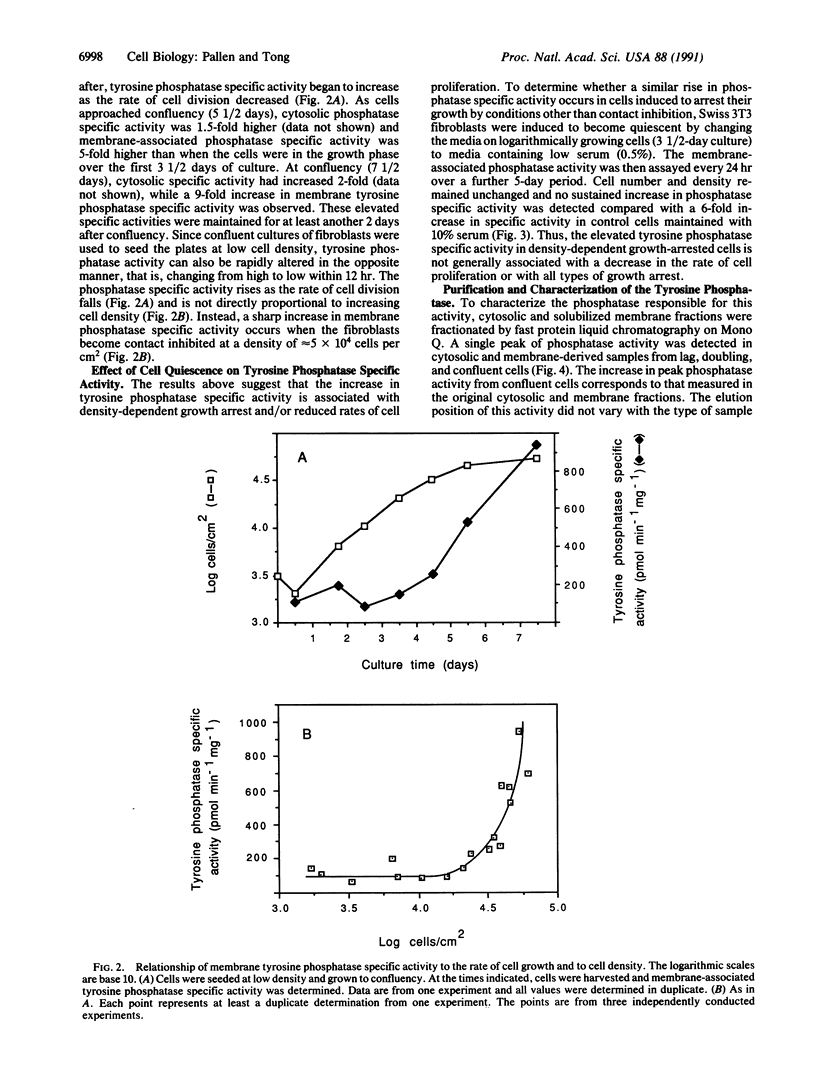
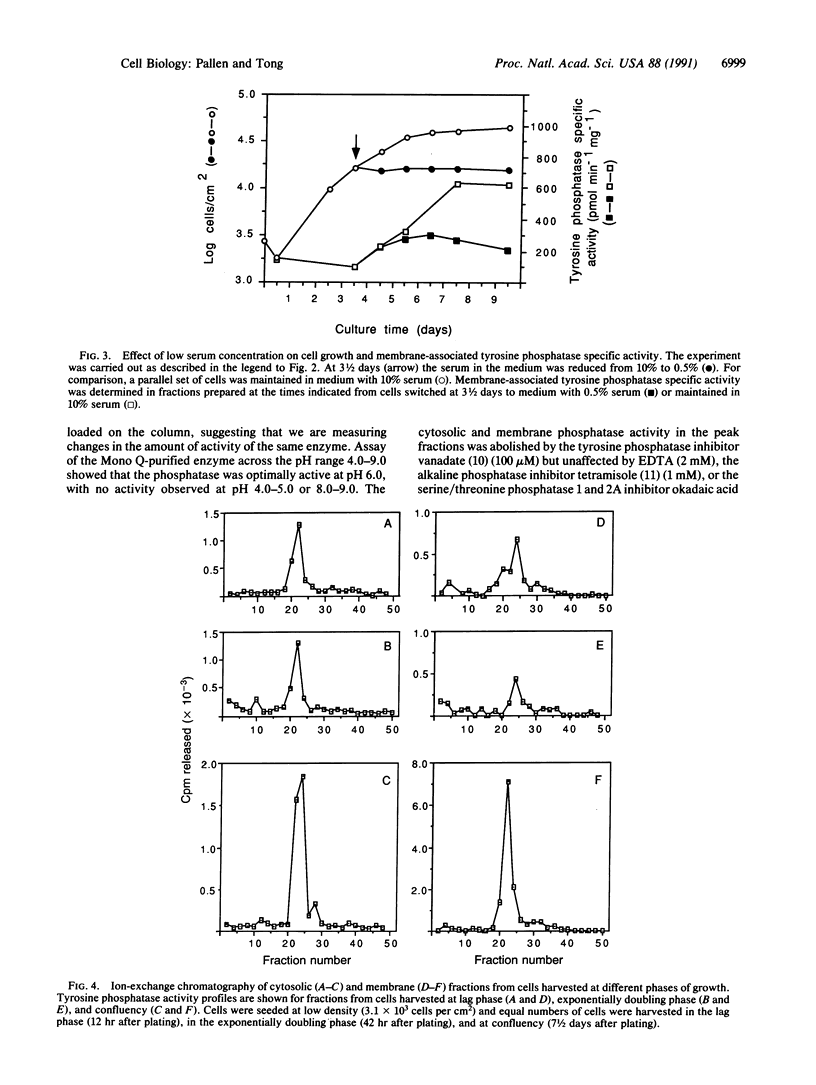
Swiss 3T3 cells harvested at high density contain a membrane protein-tyrosine-phosphatase (EC 3.1.3.48) whose specific activity is on average 8-fold higher than that of cells harvested at low or medium densities. Investigation of the conditions affecting this elevation of specific activity suggests that it is associated with density-dependent growth arrest. Fibroblasts in the exponentially doubling phase have a relatively low level of membrane phosphatase specific activity, which rises only as the rate of cell proliferation decreases and is maximal when cell growth is contact inhibited. These observations have been extended to BALB/c 3T3 fibroblasts and normal human diploid fibroblasts. The increase in membrane tyrosine phosphatase activity is coupled to density arrest and not to cellular quiescence in general, as no increase in phosphatase specific activity is detected when non-contact-inhibited cells are induced to arrest their growth through serum deprivation. The observed alterations in specific activity are attributable to a tyrosine phosphatase of Mr 37,000 that was purified and characterized from solubilized membrane fractions of Swiss 3T3 cells. A regulatory mechanism controlling tyrosine phosphatase activity may play a role in cell proliferation and growth arrest caused by cell contact.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Pallen C. J., Sahlin L., Panayotou G., Waterfield M. D. Purification and characterization of placental membrane phosphotyrosine phosphatases. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):447–454. doi: 10.1101/sqb.1988.053.01.052. [DOI] [PubMed] [Google Scholar]
- Roome J., O'Hare T., Pilch P. F., Brautigan D. L. Protein phosphotyrosine phosphatase purified from the particulate fraction of human placenta dephosphorylates insulin and growth-factor receptors. Biochem J. 1988 Dec 1;256(2):493–500. doi: 10.1042/bj2560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Subrahmanyam G. Purification and characterization of a protein-phosphotyrosine phosphatase from rat spleen which dephosphorylates and inactivates a tyrosine-specific protein kinase. J Biol Chem. 1989 May 15;264(14):7801–7808. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]







