Abstract
Gram-negative bacteria are inherently impermeable to hydrophobic compounds, due to the synergistic activity of the permeability barrier imposed by the outer membrane and energy dependent efflux systems. The gram-negative, enteric pathogen Vibrio cholerae appears to be deficient in both these activities; the outer membrane is not an effective barrier to hydrophobic permeants, presumably due to the presence of exposed phospholipids on the outer leaflet of the outer membrane, and efflux systems are at best only partially active. When V. cholerae was grown in the presence of bile, entry of hydrophobic compounds into the cells was significantly reduced. No difference was detected in the extent of exposed phospholipids on the outer leaflet of the outer membrane between cells grown in the presence or absence of bile. However, in the presence of energy uncouplers, uptake of hydrophobic probes was comparable between cells grown in the presence or absence of bile, indicating that energy-dependent efflux processes may be involved in restricting the entry of hydrophobic permeants into bile grown cells. Indeed, an efflux system(s) is essential for survival of V. cholerae in the presence of bile. Expression of acrAB, encoding an RND family efflux pump, was significantly increased in V. cholerae cells grown in vitro in the presence of bile and also in cells grown in rabbit intestine.
Vibrio cholerae, a noninvasive enteric bacterium, is the causative agent of the diarrheal disease cholera. Cholera remains a major cause of human mortality in developing countries, where conditions of poor sanitation, war, famine, and malnourishment contribute to regular episodes of cholera epidemics. For successful infection of its human host, V. cholerae must colonize the small intestine and produce copious amounts of cholera toxin (CT), a potent enterotoxin that causes the massive fluid loss characteristic of the disease. In addition to obvious virulence factors like CT, other toxins, toxin-coregulated pilus, hemolysins, and hemagglutinins (factors essential for survival of the bacteria in vivo and evasion of the host defense system) also contribute to the pathogenecity of V. cholerae (for a review, see references 12 and 24).
Enteric pathogens and normal intestinal flora must necessarily survive and colonize the intestine in the presence of bile. Bile salts are surface-active, amphipathic compounds with pronounced detergent-like activity that can cause disaggregation of the lipid bilayer structure of cellular membranes (11). However, gram-negative enteric bacteria are inherently resistant to bile, partly due to the basic, asymmetric structure of their outer membranes (OMs). Although the inner surface of the OM contains phospholipids, a characteristic lipopolysaccharide (LPS) is present on the outer leaflet that significantly retards diffusion of hydrophobic compounds across the OM. The OM thus functions as an effective permeability barrier and gives protection to enteric bacteria from potentially noxious agents present in the intestine, particularly hydrophobic and amphipathic compounds, including bile salts (18). However, the protection provided by the OM is partial; the OM must function in conjunction with energy-dependent efflux systems, which can exclude substrates with a wide range of specificity from the cell (9, 19, 20). Several families of active efflux systems in gram-negative bacteria have been described (13, 17, 22).
Bile affects expression of virulence factors, motility, and production of the OM porins OmpU and OmpT in V. cholerae. Although motility of the cells increase significantly in the presence of bile, expression of the major virulence factors CT and toxin coregulated pilus is drastically reduced (8). Furthermore, bile stimulates production of OmpU with concomitant repression of OmpT synthesis (26).
In this study, we report that unlike the membranes of other gram-negative enteric bacteria, the OM of smooth-type V. cholerae is exceptionally permeable to hydrophobic compounds. However, growth of V. cholerae in the presence of bile restricts entry of hydrophobic compounds, primarily due to the induction of active efflux systems.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
V. cholerae O1 strain O395 of the classical biotype and strain N16961 of the El Tor biotype and the wild-type Escherichia coli strain ZK126 (4) were used in this study. The strains were grown with aeration at 37°C in Luria-Bertani (LB) medium containing 1% tryptone (Difco), 0.5% yeast extract (Difco), and 0.5% NaCl. The bile used was a crude ox bile extract containing sodium salts of taurocholic, glychocholic, deoxycholic, and cholic acids (catalog no. 9875; Sigma). Bile was used at a concentration of 0.4% unless otherwise stated in the text.
Measurement of drug susceptibility.
The MIC of antibiotics was determined by serial twofold dilutions of drugs in LB medium with an inoculum of 5 × 104 exponential-phase cells per ml. Turbidity at 600 nm (optical density at 600 nm) was measured after 16 h of incubation at 37°C with aeration. Optical density at 600 nm of less than 0.05 was considered negative.
Uptake of hydrophobic compounds.
V. cholerae was grown in LB medium or LB medium containing 0.4% bile to the logarithmic phase (2 × 108 to 3 × 108 CFU ml−1), washed with phosphate-buffered saline (PBS), and suspended in an equal volume of PBS. Crystal violet (CV) (5 μg ml−1) was added to the cells, aliquots were removed at regular intervals, and CV remaining in the supernatant was estimated by measuring absorbance at 590 nm. In some experiments, 50 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added prior to the addition of CV. To measure uptake of N-phenyl naphthylamine (NPN), 10 μM NPN was added to exponentially growing cells in PBS or in PBS containing 1 mM KCN, and fluorescence was monitored with a fluorescence spectrophotometer (F-4500; Hitachi) set as follows: excitation, 350 nm; emission, 420 nm; slit widths, 2.5 nm (28). NPN binding by isolated LPS was measured by adding 10 μM NPN to LPS aggregates (400 μg ml−1) in PBS (2).
LPS preparation.
Crude LPS preparations obtained by hot phenol-water extraction (16) were dispersed in 0.05 M MgCl2-0.125 M sodium acetate buffer (pH 7), digested with nucleases, and freeze-dried. Samples were then suspended in 0.01 M CaCl2-0.1 M Tris-HCl (pH 7.3) buffer, digested with proteinase K, dialyzed extensively, freeze-dried, suspended in water, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. To measure the binding of NPN to LPS aggregates, the LPS was thoroughly dispersed by sonication, supplemented with an equal volume of 0.2% sodium deoxycholate in 0.1 M Tris-HCl (pH 8.5), incubated at room temperature for 15 min, and precipitated with 6 volumes of ethanol at −20°C for 18 h. The precipitate was sedimented by centrifugation, washed with ethanol, suspended in water, and dialyzed, and the concentration was adjusted to 400 μg ml−1 (2). NPN binding by LPS aggregates was determined as described for whole cells.
Dansylation of whole cells.
V. cholerae cells grown to the logarithmic phase in the presence or absence of bile were dansylated following the method of Paul et al. (21). Briefly, dansyl chloride-cyclodextrin complex was added to cells suspended in 1 mM MgCl2-50 mM borate buffer (pH 8.5) and stirred in darkness for 60 min at room temperature. Cells were washed repeatedly, and OMs and inner membranes (IMs) were isolated from the treated cells as previously described (8). Phospholipids were extracted from both membranes, separated by thin-layer chromatography (TLC), and visualized under UV light (21, 25).
RNA isolation and RT-PCR.
For isolation of RNA, cells were grown to the logarithmic phase and total RNA was extracted and purified with guanidium isothiocyanate (1). The RNA was treated with RNase free DNase 1 (amplification grade; Gibco-BRL) and reverse transcription-PCR (RT-PCR) was performed with a single-tube RT-PCR kit (Gibco-BRL) in the presence of an RNase inhibitor (RNasin; Gibco-BRL). A total of 200 ng of DNase-treated RNA was used in all reaction mixtures. Amplification was for 25 to 35 cycles (each cycle consisted of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by a 7-min extension at 72°C). Genomic DNA served as a positive control, and DNase-treated RNA that had not been reverse transcribed was used as a negative control. Primers for RT-PCR, corresponding to internal regions of the open reading frames, were designed based on the V. cholerae genome sequence (10). Aliquots removed at 25, 30, and 35 cycles of each PCR were electrophoresed, and the gels were analyzed with a Gel Doc 1000 system (Bio-Rad Laboratories). PCR products were normalized according to the amount of 16S rRNA detected in the same cDNA sample. Each set of experiments was repeated at least thrice. Comparisons between the relative intensities of PCR products were made with the two-sample t test.
Ligated rabbit ileal loop model.
In vivo expression of acrA in V. cholerae was assayed by using the ligated rabbit ileal loop model (5, 7). Strain O395 or N16961 (∼5 × 106 CFU) was inoculated into individual ligated rabbit ileal loops; after 16 h, the animals were sacrificed. Bacteria were recovered from each loop, and RNA was isolated and used for RT-PCR. Each bacterial strain was inoculated into at least two loops in each animal, and each strain was tested in at least three individual animals.
RESULTS
Sensitivity of V. cholerae to hydrophobic compounds is reduced when grown in the presence of bile.
V. cholerae is extremely sensitive to a wide variety of compounds, particularly hydrophobic and amphipathic agents (21). It is about 100 times more sensitive than E. coli to novobiocin, a hydrophobic antibiotic that has been used as an indicator of bacterial OM permeability to hydrophobic compounds (Table 1). However, when V. cholerae was grown in the presence of 0.4% bile, the MIC of novobiocin increased by more than 10-fold compared to the novobiocin MIC for cells grown under identical conditions without bile. The presence of up to 5% bile in LB had no effect on the growth rates of strains O395 and N16961. The wild-type E. coli strain ZK126 also grew normally in the presence of bile; but unlike V. cholerae, growth of ZK126 in the presence of 0.4% bile had very little effect on the MIC of novobiocin for E. coli (Table 1).
TABLE 1.
Novobiocin sensitivity of V. cholerae and E. colia
| Strain | Novobiocin MIC (μg ml−1)
|
|
|---|---|---|
| − Bile | + Bile | |
| V. cholerae O395 | 2 | 35 |
| V. cholerae N16961 | 3 | 40 |
| E. coli ZK126 | 300 | 400 |
Experiments were performed with triplicate samples from two independent cultures grown in the absence (−) or presence (+) of 0.4% bile. Standard errors were less than 10% in all cases.
Uptake of hydrophobic compounds into bile-grown V. cholerae cells.
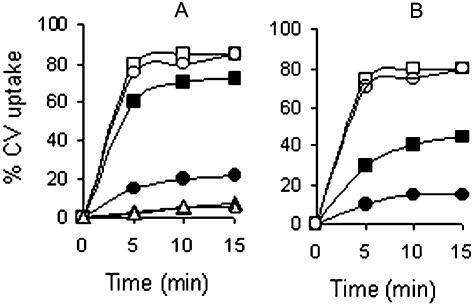
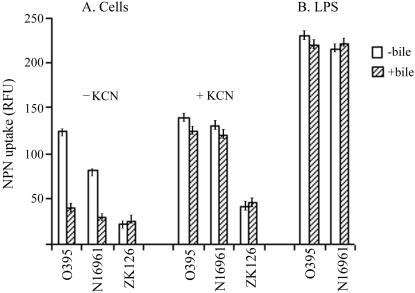
To examine whether growth of V. cholerae in the presence of bile had any effect on permeability to hydrophobic compounds, uptake of the hydrophobic dye CV and the fluorescent hydrophobic probe NPN by cells grown in the presence or absence of bile was estimated. To measure CV uptake, V. cholerae strains O395 and N16961 were grown to the logarithmic phase in LB with or without 0.4% bile, washed, and suspended in buffer containing 5 μg of CV ml−1. At regular time intervals, samples were withdrawn and centrifuged, and the amount of dye in the supernatant was estimated by measuring turbidity at 590 nm. More than 70% of the added CV was taken up in about 10 min by strain O395 grown without bile, although less than 20% CV uptake was observed in cells grown in the presence of the bile (Fig. 1A). In strain N16961 of the El Tor biotype, about 40% CV uptake was observed when the cells were grown without bile, and the uptake was reduced to about 15% in bile-grown cells (Fig. 1B). Fractionation of the cells into membrane and cytoplasmic constituents indicated that the incorporated dye was distributed almost equally between the cell envelope and the cytoplasmic fractions in cells grown in the absence of bile. In bile-grown cells, however, most of the incorporated dye could be detected in the membrane fraction. E. coli cells took up very little CV, and the uptake was similar irrespective of whether the cells were grown in the absence or in the presence of bile (Fig. 1A). These results demonstrate that V. cholerae is unusually permeable to the hydrophobic dye CV, but growth of the cells in the presence of bile restricts entry of CV into the cells. Similar results were obtained with NPN. Quantum yield of the fluorescent probe NPN increases upon transfer from a hydrophilic to a hydrophobic environment (28). When NPN was added to cells grown to the logarithmic phase, only a small increase in fluorescence was observed with the wild-type E. coli strain ZK126. A significantly higher increase was observed with both the V. cholerae strains O395 and N16961, although NPN uptake by strain O395 was higher than that of strain N16961. However, when the V. cholerae strains were grown in the presence of 0.4% bile, NPN uptake was significantly reduced to similar levels in both strains (P = 0.02) (Fig. 2A).
FIG. 1.
CV uptake by V. cholerae O395 and E. coli ZK126 (A) and V. cholerae N16961 (B). Cells were grown to the logarithmic phase in the absence or presence of 0.4% bile and suspended in buffer containing CV (5 μg ml−1) with or without 50 μM CCCP. CV uptake was measured at regular time intervals and expressed as a percentage of added CV taken up by 108 CFU of each strain. Results represent the average of five independent experiments. Symbols: V. cholerae strains (solid squares), V. cholerae plus CCCP (open squares), V. cholerae plus bile (solid circles), V. cholerae plus bile plus CCCP (open circles), E. coli ZK126 (solid triangles), E. coli ZK126 plus bile (open triangles).
FIG. 2.
NPN uptake. (A) V. cholerae strains O395 and N16961 and E. coli ZK126 were grown without or with 0.4% bile, and NPN uptake was measured in the presence or absence of KCN. (B) NPN binding by LPS aggregates isolated from V. cholerae strains O395 and N16961 grown in the presence or absence of 0.4% bile. The results represent the average of three independent experiments, and error bars indicate standard errors of the means.
Taken together, these results clearly indicated that although the OM of V. cholerae did not constitute an effective permeability barrier against hydrophobic compounds, entry of these compounds into the cells was significantly reduced when the cells were grown in the presence of bile. No statistically significant difference (P = 0.25) was detected in the partitioning of NPN into LPS aggregates isolated from V. cholerae strains O395 and N16961 grown with or without bile (Fig. 2B). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis also did not reveal any difference between LPS isolated from cells grown with or without bile (data not shown).
Presence of exposed phospholipids.
It has been reported that unlike most gram-negative bacteria, V. cholerae contains phospholipids on the outer surface of the OM (21). Labeling of intact cells with the membrane impermeant probe dansyl chloride-cyclodextrin complex was used to examine whether exposed phospholipids were present in the OMs of cells grown in the presence of bile. In intact cells, the probe binds to the amino group of phosphatidylethanolamine only when phosphatidylethanolamine is exposed on the outer leaflet of the OM (25). When phospholipids were isolated from the OMs and IMs of dansylated cells of strains O395 and N16961 and analyzed by TLC, similar fluorescent spots were detected in phospholipids isolated from the OMs of cells grown in the absence or presence of 0.4% bile (Fig. 3). Phospholipids from the IM were not dansylated, confirming the impermeant nature of the probe. Thus, the extent of phospholipid bilayer zones in the OM is similar in cells grown in the presence or absence of bile.
FIG. 3.
TLC of phospholipids isolated from dansylated V. cholerae strains O395 (lanes a and b) and N16961 (lanes c and d) grown in the absence (lanes a and c) or presence (lanes b and d) of 0.4% bile.
Accumulation of hydrophobic compounds in de-energized cells.
To examine if the lower accumulation of hydrophobic compounds in bile-grown cells is due to the activation of energy-dependent efflux systems, V. cholerae strains O395 and N16961 grown in the absence or presence of 0.4% bile were treated with NPN in a buffer containing KCN, which inhibits respiration and prevents active efflux of NPN. Although in the absence of KCN, the rate of NPN binding to bile-grown cells was about threefold lower than the binding to cells grown without bile, in the presence of KCN, no difference in NPN uptake was observed between cells grown in the presence or absence of bile (Fig. 2A).
Furthermore, when the cellular electrochemical proton gradient, which energizes efflux processes, is dissipated by the addition of CCCP, rapid accumulation of CV occurred even in bile-grown V. cholerae. In the presence of CCCP, no difference in CV accumulation was observed between cells of either biotype grown in the presence or absence of bile (Fig. 1).
These results indicate that energy-dependent active efflux is required for restricting the entry of hydrophobic compounds into bile-grown V. cholerae.
V. cholerae is extremely sensitive to CCCP in the presence of bile.
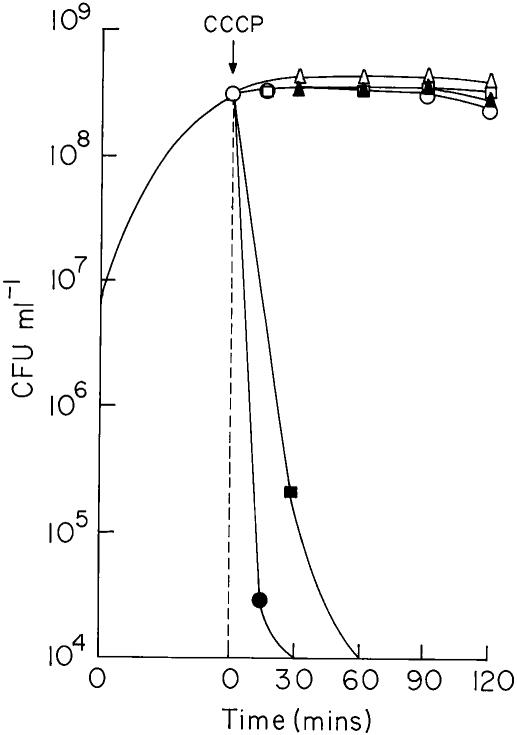
V. cholerae strains O395 and N16961 were grown in LB in the presence or absence of 0.8% bile; when the cultures reached logarithmic phase (2 × 108 to 3 × 108 CFU ml−1), 75 μM CCCP was added, and viability was assayed at regular intervals. When CCCP was added to cells grown in LB without bile, viability of the culture remained almost constant during the 2 h it was examined (Fig. 4). Interestingly, a drastic reduction in the number of CFU was observed when CCCP was added to V. cholerae cultures in LB containing bile. Within 15 min of addition of CCCP, the viability of the strain O395 decreased 104 fold. Strain N16961 was relatively less sensitive to CCCP in the presence of bile, and the number of CFU decreased about 104 fold in 1 h (Fig. 4). However, when up to 100 μM CCCP was added to cultures of E. coli ZK126, although cell growth was inhibited, no loss of viability was observed for up to 2 h, irrespective of whether or not the cultures contained 0.8% bile (Fig. 4).
FIG. 4.
Cell growth in the presence of CCCP. V. cholerae O395 (circles), V. cholerae N16961 (squares), and E. coli ZK126 (triangles) were grown to the logarithmic phase in the absence (open symbols) or presence (solid symbols) of 0.8% bile. CCCP was added, and viability was assayed at regular time intervals. The time of addition of CCCP is indicated.
Induction of acrAB expression in bile-grown V. cholerae.
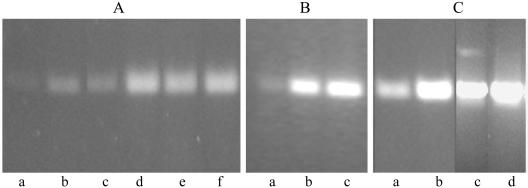
In view of the fact that the acrAB efflux system has been shown to be necessary for efflux of hydrophobic compounds in E. coli (14), expression of acrAB in V. cholerae was examined. RT-PCR analysis of RNA extracted from strain O395 grown to the exponential phase in the absence or presence of 0.4% bile indicated a three- to fourfold increase in acrAB expression in bile-grown cells compared to cells grown without bile (Fig. 5A). A progressive increase in acrAB expression occurred with increasing bile concentrations, and a maximum induction of six- to eightfold was observed in the presence of 1.6% bile (Fig. 5B). With El Tor biotype strain N16961, the basal level of acrAB expression was higher than in strain O395, and a further 1.5- to 2-fold induction was observed in the presence of bile (Fig. 5A). Induction of acrAB was also observed in V. cholerae grown in vivo in rabbit intestine. The expression of acrAB in strain O395 grown in rabbit ileal loops was comparable to that in cells grown in the presence of 0.4% bile and about three- to fourfold higher than that in cells grown in vitro in LB without bile (Fig. 5C). In strain N16961, in vivo expression of acrAB was about 1.5-fold higher than expression in vitro (Fig. 5C). The relatively low levels of acrAB induction by bile in strain N16961 were similar to the bile salt-dependent induction of acrAB expression in E. coli (23).
FIG. 5.
Effect of bile on acrA expression. (A) RT-PCR was performed for 25 (lanes a and b) and 30 (lanes c to f) cycles with RNA isolated from strain O395 (lanes a to d) or strain N16961 (lanes e and f) grown without (lanes a, c, and e) or with (lanes b, d, and f) 0.4% bile for estimation of acrAB mRNA. (B) RNA was isolated from V. cholerae O395 grown in the absence of bile (lane a) or in the presence of 0.8% bile (lane b) or 1.6% bile (lane c). RT-PCR was performed for estimation of acrAB mRNA. (C) RT-PCR analysis of acrAB expression in V. cholerae strains O395 (lanes a and b) and N16961 (lanes c and d) grown in LB (lanes a and c) or in rabbit intestine (lanes b and d). RT-PCR for estimation of 16S rRNA and PCR with DNase-treated RNA samples that had not been reverse transcribed were used as controls.
DISCUSSION
The ability to restrict the entry of hydrophobic compounds through the OM is crucial for enteric bacteria that must necessarily survive in the intestine in the presence of bile salts and other noxious hydrophobic agents. OM impermeability is generally considered to be a characteristic feature of enteric bacteria. The enteropathogen V. cholerae appears to be a curious exception, since its OM is relatively permeable to hydrophobic agents. Levels of novobiocin sensitivity and accumulation of the hydrophobic probes CV and NPN in V. cholerae were significantly higher than in E. coli (Table 1;, Fig. 1 and 2) and were similar to those reported for Brucella, a pathogen that uses a nonenteric route for infection (15). This observation raises fundamental questions about how V. cholerae survives in the intestine. We report here that when V. cholerae was grown in the presence of bile at concentrations similar to those present in the intestine, accumulation of hydrophobic probes in the cells was very significantly reduced. The lower level of accumulation of hydrophobic compounds in bile-grown V. cholerae was not due to alterations in the passive diffusion across the exposed phospholipid bilayer zones present in the OM (Fig. 3) but to the bile-dependent induction of an active energy-requiring efflux process.
In gram-negative bacteria, efflux pumps complement the barrier function of the OM (13, 17, 22). The efflux systems, which can pump out a variety of structurally diverse hydrophobic and amphiphilic compounds, are generally energized by an electrochemical proton gradient. In V. cholerae grown in the presence of bile, when the proton gradient was dissipated by the addition of CCCP, hydrophobic permeants accumulated to high levels, comparable to the accumulation in cells grown without bile (Fig. 1 and 2) and suggesting that the activity of the energy-dependent efflux system is solely responsible for the restricted entry of hydrophobic compounds into bile-grown V. cholerae (27). Indeed, active efflux systems are essential for survival of V. cholerae in the presence of bile. Addition of 75 μM CCCP to cells in the presence of bile causes a drastic reduction in viability. Under identical conditions, no loss of viability was observed for a wild-type E. coli strain (Fig. 4). It is likely that even 100 μM CCCP cannot completely dissipate the proton motive force in E. coli, due to the intrinsically lower OM permeability and relatively high constitutive levels of expression of efflux pumps in E. coli compared to V. cholerae.
Although V. cholerae strain N16961 of the El Tor biotype takes up less CV and NPN than strain O395 of the classical biotype, uptake of the probes was similar in both strains when treated with CCCP or KCN (Fig. 1 and 2), suggesting that the relatively lower accumulation of the hydrophobic probes in the El Tor biotype strain N16961 was due to higher basal activity of efflux pumps than in strain O395 of the classical biotype. Indeed, RT-PCR analysis indicated that the basal level of expression of the acrAB efflux pump in strain N16961 was higher than in strain O395 (Fig. 5). In strain O395, the acrAB efflux system was strongly induced when cells were grown in vitro in the presence of bile and also in cells grown in vivo in rabbit intestine; under these conditions, acrAB expression in strains O395 and N16961 was similar (Fig. 5). The modest induction of acrAB in the presence of bile in strain N16961 is similar to the pattern observed with E. coli (23).
It would be relevant to mention in this context that TolC, which functions as an OM pore protein for several RND family efflux systems, is necessary for the survival of V. cholerae in the presence of bile (3), suggesting that a member(s) of the TolC-dependent RND family of efflux systems is essential for bile resistance in V. cholerae. Also, a TolC-independent efflux system, VceAB, an EmrAB homolog, has been shown to contribute modestly to the resistance of V. cholerae to deoxycholate (6).
In conclusion, it may be stated that the OMs of smooth-type V. cholerae of both the classical and El Tor biotypes are not effective barriers to hydrophobic permeants, probably due to the presence of exposed phospholipids on the outer surface of the OM. Also, efflux pumps are not active in the classical biotype, since their inactivation by the addition of CCCP caused only a marginal increase in the permeability of hydrophobic compounds. The basal activity of efflux pumps is higher in strain N16961 of the El Tor biotype, which accounts for the somewhat lower accumulation of hydrophobic compounds in strain N16961 than in strain O395. When V. cholerae was grown in the presence of bile, entry of hydrophobic compounds into the cells was very significantly reduced, not from alterations in the OM barrier function per se but from the induction of efflux systems. Although induction of efflux pumps in the presence of bile decreases the accumulation of hydrophobic compounds in V. cholerae, even bile-grown V. cholerae does not acquire the high level of resistance to hydrophobic drugs observed with E. coli (Table 1; Fig. 1 and 2), where optimum coordination between efflux pump activity and OM barrier function appears to have been achieved.
Acknowledgments
We thank I. Guha Thakurta and P. Majumdar for excellent technical support and all members of the Biophysics Division for cooperation and helpful discussions during the study.
The work was supported by research grant BT/PRO/411/Med/09/086/96 from the Department of Biotechnology and grant 61/2/2000-BMS from the Indian Council of Medical Research, Government of India. A.C. and S.C. are grateful to the Council of Scientific and Industrial Research for research fellowships.
REFERENCES
- 1.Ausbel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 2.Bengoechea, J. A., R. Diaz, and I. Moriyon. 1998. Yersinia pseudotuberculosis and Yersinia pestis show increased outer membrane permeability to hydrophobic agents which correlates with lipopolysaccharide acyl-chain fluidity. Microbiology 144:1517-1526. [DOI] [PubMed] [Google Scholar]
- 3.Bina, J. E., and J. J. Mekalanos. 2002. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 69:4681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of σ70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty, S., N. Sengupta, and R. Chowdhury. 1999. Role of DnaK in in vitro and in vivo expression of virulence factors of Vibrio cholerae. Infect. Immun. 67:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colmer, J. A., J. A. Fralick, and A. N. Hamood. 1998. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol. Microbiol. 27:63-72. [DOI] [PubMed] [Google Scholar]
- 7.De, S. N., and S. N. Chatterjee. 1953. An experimental study of the mechanisms of action of Vibrio cholerae on the intestinal mucous membrane. J. Pathol. Bacteriol. 46:559-562. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 65:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock, R. E. W. 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5:37-42. [DOI] [PubMed] [Google Scholar]
- 10.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, O. H. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann, A. F. 1998. Bile secretion and the enterohepatic circulation of bile acids, p. 937-948. In M. Feldman, B. F. Scharchmidt, and M. H. Sleisenger (ed.), Sleisenger and Fordtran's gastrointestinal and liver disease. W. B. Saunders Co., Philadelphia, Pa.
- 12.Krukonis, E. S., and V. J. DiRita. 2003. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Curr. Opin. Microbiol. 6:186-190. [DOI] [PubMed] [Google Scholar]
- 13.Ma, D., D. N. Cook, J. E. Hearst, and H. Nikaido. 1994. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 14.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 15.Martinez de Tejada, G., and I. Moriyon. 1993. The outer membranes of Brucella spp. are not barriers to hydrophobic permeants. J. Bacteriol. 175:5273-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer, H., R. N. Thurahathan, and J. Weckesser. 1985. Analysis of lipo-polysaccharides of gram-negative bacteria. Methods Microbiol. 18:157-207. [Google Scholar]
- 17.Middlemiss, J. K., and K. Poole. 2004. Differential impact of MexB mutations on substrate selectivity of the MexAB-OprM multidrug efflux pump of Pseudomonas aeruginosa. J. Bacteriol. 186:1258-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido, H., and M. Vaara. 1985. Molecular basis of outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido, H. 2003. Molecular basis of outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul, S., K. Chaudhuri, A. N. Chatterjee, and J. Das. 1992. Presence of exposed phospholipids in the outer membrane of Vibrio cholerae. J. Gen. Microbiol. 138:755-761. [DOI] [PubMed] [Google Scholar]
- 22.Rojas, A., A. Segura, M. E. Guazzaroni, W. Teran, A. Hurtado, M. T. Gallegos, and J. L. Ramos. 2003. In vivo and in vitro evidence that TtgV is the specific regulator of the TtgGHI multidrug and solvent efflux pump of Pseudomonas putida. J. Bacteriol. 185:4755-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory proteins. Mol. Microbiol. 48:1609-1619. [DOI] [PubMed] [Google Scholar]
- 24.Sack, D. A., R. B Sack, G. B Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-333. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Ullrich, R., H. Knufermann, and D. F. H. Wallach. 1973. The reaction of 1-dimethylaminonaphthalene-5-sulfonyl chloride (DANSC1) with erythrocyte membranes. A new look at “vectorial” membrane probes. Biochim. Biophys. Acta 307:352-365. [DOI] [PubMed] [Google Scholar]
- 26.Schuhmacher, D. A., and K. K. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tikhonova, E. B., Q. Wang, and H. I. Zgurskaya. 2002. Chimeric analysis of the multicomponent multidrug efflux transporters from gram-negative bacteria. J. Bacteriol. 184:6499-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trauble, H., and P. Overath. 1973. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim. Biophys. Acta 307:491-512. [DOI] [PubMed] [Google Scholar]