Abstract
In mammalian cells, DNA polymerase β (Polβ) functions in base excision repair. We have previously shown that Polβ-deficient mice exhibit extensive neuronal cell death (apoptosis) in the developing nervous system and that the mice die immediately after birth. Here, we studied potential roles in the phenotype for p53, which has been implicated in DNA damage sensing, cell cycle arrest, and apoptosis. We generated Polβ−/− p53−/− double-mutant mice and found that p53 deficiency dramatically rescued neuronal apoptosis associated with Polβ deficiency, indicating that p53 mediates the apoptotic process in the nervous system. Importantly, proliferation and early differentiation of neuronal progenitors in Polβ−/− p53−/− mice appeared normal, but their brains obviously displayed cytoarchitectural abnormalities; moreover, the mice, like Polβ−/− p53+/+ mice, failed to survive after birth. Thus, we strongly suggest a crucial role for Polβ in the differentiation of specific neuronal cell types.
Repair of DNA damage is essential for maintaining the integrity of the genetic information necessary for normal development and physiological consequences (28). DNA polymerase β (Polβ) is a 39-kDa protein of a single polypeptide, consisting of two catalytic, functional domains. The N-terminal 8-kDa domain carries a 5′-deoxyribose phosphate lyase activity, whereas the C-terminal 31-kDa domain carries a polymerase activity that fills a short gap with a 5′-phosphate (26, 40). Polβ is a critical component of the base excision repair (BER) pathway. The BER pathway repairs DNA damage, such as apurinic/apyrimidinic (AP) sites and base modifications, which spontaneously occur or are induced by a variety of endogenous and exogenous agents, including reactive oxygen species and DNA alkylating agents. Biochemical studies have identified two types of BER in mammalian cells: a short-patch pathway involving replacement of one nucleotide and a long-patch pathway involving gap-filling of several nucleotides (46). The BER pathway is generally initiated by a specific DNA glycosylase that recognizes and removes a damaged base to generate an AP site in DNA, followed by incision of the site by an AP endonuclease. In the short-patch BER pathway, Polβ removes the 5′-deoxyribose phosphate and fills the single nucleotide gap, and finally DNA ligase I or a complex of XRCC1 and DNA ligase III ligates the nick. On the other hand, the long-patch BER pathway requires proliferating cell nuclear antigen (PCNA), flap endonuclease 1 (FEN-1), and DNA ligase I to excise a flap-like structure resulting from strand displacement by Polβ and/or Polδ/ɛ and to ligate the nick (6).
We and others previously showed that Polβ-deficient mice exhibit a reduced size and weight and die with a respiratory defect immediately after birth (13, 42). In Polβ-deficient mice, extensive cell death (apoptosis) occurs in postmitotic neurons in the developing central and peripheral nervous systems (42). This neuronal apoptosis is closely associated with the period between the onset and cessation of neurogenesis. Abnormalities in embryonic tissues other than the nervous system have not yet been reported (13, 19, 21). Therefore, we suggested that Polβ plays an essential role specifically in the development of the nervous system (42). However, the cause of this neuronal apoptosis remains entirely unknown. Mouse embryonic fibroblast cells in culture, derived from a Polβ knockout mouse, are viable and show normal growth characteristics (41). Although the mutant cells exhibit BER defects, as evidenced by increased sensitivity to DNA alkylating agents, their cell extracts still retain an activity to repair a damaged base residue in DNA substrate (6), indicating that there are both Polβ-dependent and -independent BER pathways in vivo.
The tumor suppressor protein p53 plays a prominent role in the maintenance of genomic integrity (27). It is activated by different types of DNA damage, including single-strand breaks (SSBs), double-strand breaks (DSBs), and adducts, which are generated by endogenous or exogenous mechanisms. The activated p53 has a choice of cell cycle arrest for repair or apoptosis, depending on the level of damaged DNA; i.e., unless the damage is repaired, p53 leads to apoptosis. Recent studies show that p53 directly interacts with Polβ, stimulating BER activity (37, 50). In the nervous system, it has been shown that p53 regulates neuronal apoptosis after neuronal injury induced by excitotoxins, hypoxia, and ischemia that cause oxidative damage (3, 29, 33, 47). In p53-deficient mice, kainic acid excitotoxicity- or ischemia-induced brain damage is significantly reduced (10, 34). These observations suggest the involvement of p53 in the control of neuronal apoptosis.
A close link between DNA damage and neurodegeneration appears evident from many pathological data and observations with mouse knockouts (8, 38). In mice deficient in DNA ligase IV (Lig4) and XRCC4, the main components of nonhomologous-end-joining apparatus for DSB repair, differentiating neurons undergo massive cell death (4, 14, 17, 22). However, this apoptosis is completely rescued by p53 deficiency (15, 16). It would be important to examine whether neuronal cell death found in Polβ-deficient mice (42) is mediated by p53 activation. Here we study a potential role for p53 in the phenotypes, including neuronal cell death associated with Polβ deficiency. We found that p53 deficiency rescues the neuronal apoptosis in a Polβ-deficient background. However, it should be noticeable that Polβ−/− p53−/− mice still exhibit cytoarchitectural defects in the development of the nervous system and die shortly after birth. These observations strongly suggest that Polβ is crucial for the differentiation process of specific neuronal cell types.
MATERIALS AND METHODS
Mice.
Polβ-deficient mice were described previously (42). p53-deficient mice (C57BL/6J-Trp53 tm1Tyj) were obtained from The Jackson Laboratory (24). PCR genotyping protocol for p53 targeted allele are directed on the website of JAX MICE, The Jackson Laboratory (http://jaxmice.jax.org). Noon of the day on which the vaginal plug was detected in the morning was designated embryonic day 0.5 (E0.5). All mice were maintained in a pathogen-free environment under the guidelines of Kihara Institute for Biological Research, Yokohama City University, for laboratory animals.
Histology, immunohistochemistry, and TUNEL assay.
Embryos were perfused with 4% paraformaldehyde and 7% picric acid in 0.1 M sodium phosphate buffer (pH 7.4); the brain was removed and postfixed in the same fixative for 2 h, equilibrated with 25% sucrose-phosphate-buffered saline, frozen in OCT compound (Sakura Finetechnical Co.) and sectioned on a cryostat (10 μm). The sections were incubated with rabbit anti-p53 polyclonal antibody CM-5 (Novocastra Laboratories, 1:3,000), rabbit anti-cleaved caspase-3 polyclonal antibody (Cell Signaling Technology; 1:100), mouse anti-PCNA monoclonal antibody PC10 (Sigma; 1:100), mouse anti-neuron specific type-III β-tubulin monoclonal antibody Tuj1 (BabCO; 1:1,000), mouse anti-phosphorylated neurofilament SMI31 (Sternberger Monoclonal Antibodies; 1:4,000), and rabbit anti-calbindin/spot 35 polyclonal antibody (a kind gift of T. Yamakuni) (1). Cy3 or horseradish peroxidase-conjugated antibody was used for a secondary antibody to visualize primary antibody. TSA Biotin System (Perkin-Elmer Life Sciences) was applied to anti-p53, anti-calbindin immunohistochemistry. The TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay was performed on cryosections by using 0.12 U of TdTase (Roche)/μl with 0.5 μM biotin-14-dATP (Invitrogen) in 1× TdT buffer (Roche) with 1.5 mM CoCl2. Horseradish peroxidase-conjugated biotin (Jackson Immunoresearch Laboratories) was used for signal detection. Cell nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) for immunofluorescence. Cresyl violet (Sigma) staining was performed to show neuronal architecture.
Western blot analysis.
Cell extracts were prepared from developing telencephalons in E13.5 embryos, electrophoresed in an 8.0% sodium dodecyl sulfate-polyacrylamide gel, and transferred to an Immobilon membrane (Millipore) as described previously (42). The membrane was probed with anti-human phospho-p53 (Ser-15) antibody (Cell Signaling Technology; 1:1,000) and peroxidase-conjugated goat anti-rabbit immunoglobulin G (Chemicon) and detected with enhanced chemiluminescent detection reagents (ECL Plus kit; Amersham).
RESULTS
Polβ deficiency activates the p53-dependent apoptosis pathway.
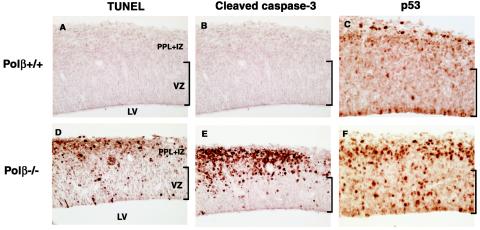
p53 is activated and stabilized after excessive DNA damage generated by endogenous or exogenous mechanisms, and induces apoptosis (27). We examined whether neuronal cell death found in Polβ-deficient mice (42) was induced by such p53 activation. To detect DNA fragmentation in cells undergoing apoptosis, we performed terminal deoxynucleotidyltransferase-mediated dUTP biotin nick-end labeling (TUNEL) assay. In Polβ+/+ (wild-type) mice, TUNEL-positive cells were not detected in developing neocortex at E13.5 (Fig. 1A). In contrast, in Polβ−/− developing neocortex, an extensive number of TUNEL-positive cells were observed in the primordial plexiform layer (PPL) and intermediate zone (IZ), where postmitotic neuronal cells were present; but these cells were less detectable in the ventricular zone (VZ), where proliferating neuronal progenitor cells were present (Fig. 1D).
FIG. 1.
Polβ deficiency induces apoptosis and activates p53 in the developing nervous system. Coronal sections of E13.5 developing neocortices in Polβ+/+ (wild-type) (A to C) and Polβ−/− (D to F) embryos were assayed by TUNEL staining (A and D) or immunohistochemistry with anti-cleaved caspase-3 antibody (B and E) and anti-p53 antibody (C and F). LV, lateral ventricle.
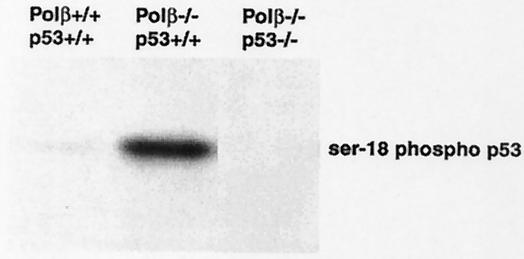
We next examined the expression of cleaved caspase-3 and p53 by immunohistochemistry in E13.5 developing neocortex. Since caspase-3 is activated by proteolytic cleavage of its inactive zymogen by apoptosis (35), cleaved caspase-3 is a useful marker for apoptotic cells. Anti-cleaved caspase-3 antibody could obviously detect apoptotic neuronal cells in Polβ−/− embryos (Fig. 1E), as observed by the above TUNEL assay, whereas wild-type embryos were not stained (Fig. 1B). Importantly, a larger number of cells with higher p53 levels were seen in Polβ−/− embryos than in wild-type (Fig. 1C versus 1F); in the VZ, such p53-stained cells were more frequent than the TUNEL- or cleaved caspase-3-positive cells. It is known that serine-15 of human p53 (equivalent to serine-18 in mouse p53) is phosphorylated in response to DNA damage (39). Therefore, we performed Western blot analysis with anti-phosphorylated serine-15 of human p53 specific antibody with cell extracts from E13.5 developing telencephalons of Polβ+/+ p53+/+ (wild-type), Polβ−/− p53+/+, and Polβ−/− p53−/− embryos (generation of the double-mutant mice will be cited below). We found that the serine-18 of p53 in Polβ−/− p53+/+ extracts was strongly phosphorylated compared to that in wild-type control, with no staining in Polβ−/− p53−/− extracts (Fig. 2). These results suggest an intriguing possibility that neuronal apoptosis observed in Polβ-deficient embryos is induced by p53 activation in response to DNA damage, immediately after final mitosis of the progenitor cells.
FIG. 2.
Western blot analysis of phosphorylated serine-18 of p53 with cell extracts prepared from E13.5 developing telencephalons of Polβ+/+ p53+/+ (wild-type), Polβ−/− p53+/+, and Polβ−/− p53−/− embryos. The same amounts of protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting with anti-human phospho-p53 (Ser-15) antibody.
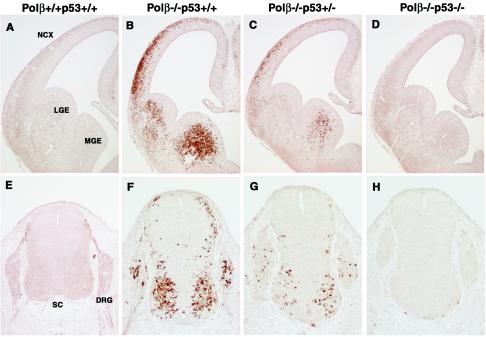
To explore potential physiological interactions between Polβ and p53, we produced mice null for both Polβ and p53. We bred Polβ+/− and p53+/− mice and generated Polβ+/− p53+/− double-heterozygous mice, which exhibited normal development and fertility. When Polβ+/− p53+/− mice were intercrossed, developing embryos with nearly the expected Mendelian ratios of all genotypes were observed at E11.5 to E18.5 (Table 1). However, no offspring with Polβ−/− p53+/+, Polβ−/− p53+/−, and Polβ−/− p53−/− genotypes was detected at weaning (ca. 4 weeks after birth). It should be noted that, like Polβ−/− p53+/+ neonates, all Polβ−/− p53+/− and Polβ−/− p53−/− neonates died at postnatal day 1 (data not shown). With anti-cleaved caspase-3 antibody, we examined neuronal apoptotic phenotypes by staining sections of the developing telencephalon in E13.5 embryos and the spinal cord and dorsal root ganglion in E11.5 embryos of wild-type controls and littermates. In tissues of Polβ−/− 53+/+ mice, a large number of cleaved caspase-3-positive neuronal cells were observed compared to those of control mice (compare Fig. 3A and E with 3B and F). However, these stained cells were dramatically decreased in Polβ−/− p53+/− mice (Fig. 3C and G) and, more importantly, completely disappeared in Polβ−/− p53−/− double-mutant mice (Fig. 3D and H). These observations indicate that p53 deficiency rescues the neuronal apoptosis associated with Polβ deficiency and that p53 haploinsufficiency also substantially does so. These results indicate that the neuronal apoptosis in Polβ-deficient mice is mediated by the p53-dependent apoptosis pathway.
TABLE 1.
Genotypic analysis of Polβ+/− p53+/− intercrosses
| Age | No. of mice with genotype:
|
No. of litters | Total no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polβ+/+ p53
|
Polβ+/− p53
|
Polβ−/− p53
|
|||||||||
| +/+ | +/− | −/− | +/+ | +/− | −/− | +/+ | +/− | −/− | |||
| E11.5 | 5 | 3 | 7 | 10 | 16 | 7 | 3 | 9 | 1 | 8 | 61 |
| E13.5 | 6 | 11 | 2 | 12 | 18 | 10 | 4 | 10 | 4 | 9 | 77 |
| E18.5 | 17 | 21 | 11 | 16 | 25 | 14 | 3 | 20 | 6 | 19 | 133 |
| Weaned (4 wk) | 13 | 16 | 8 | 9 | 14 | 6 | 0 | 0 | 0 | 14 | 66 |
FIG. 3.
Neuronal apoptosis in Polβ-deficient mice is mediated by the p53-dependent pathway. Coronal sections of E13.5 telencephalons (A to D) and E11.5 spinal cords and dorsal root ganglions (E to H) in Polβ+/+ p53+/+ (wild-type) (A and E), Polβ−/− p53+/+ (B and F), Polβ−/− p53+/− (C and G), and Polβ−/− p53−/− (D and H) embryos were stained with anti-cleaved caspase-3 antibody. DRG, dorsal root ganglion; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; NCX, neocortex; SC, spinal cord.
Proliferation and early differentiation of progenitors during neurogenesis appear normal in E13.5 Polβ−/− p53−/− mice.
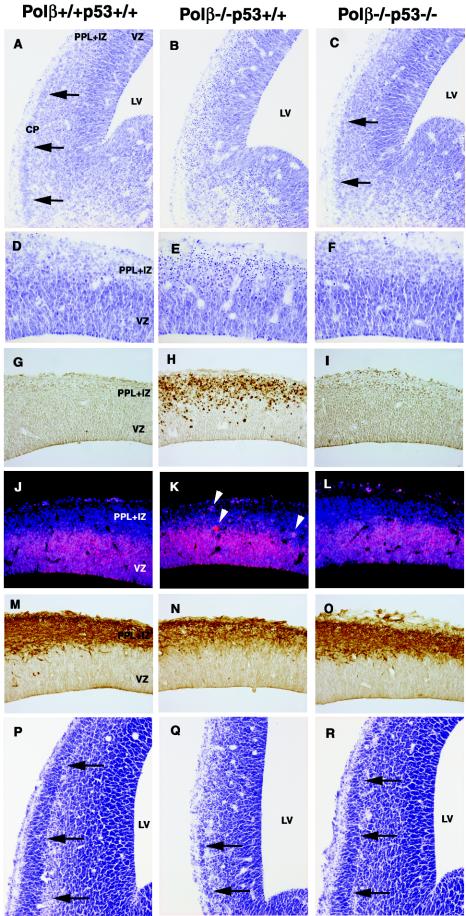
Proliferation and differentiation of progenitors are temporally and spatially controlled during neurogenesis (12). The generation of neurons from the progenitors involves successive steps in commitment and differentiation, which are progressively generating more restricted cell types. These steps include (i) cell type specification, (ii) exit from the cell cycle, (iii) differentiation into distinct cell types, (iv) migration into a correct destination, and (v) production of correct cell-cell contacts through dendritic and axonal processes. We examined effects of p53 deficiency on histogenesis of the neocortex by cresyl violet staining (Fig. 4A to F and 4P to R). In E13.5 wild-type lateral regions of the neocortex, generation of the PPL, IZ, and cortical plate (CP) was clearly observed (Fig. 4A), but the CP in the dorsal region was not (Fig. 4D). The ventrolateral-to-dorsomedial, morphological gradients are evident in the development of the neocortex (5). In contrast, in Polβ−/− p53+/+ neocortices, the CP was not clearly seen due to an extraordinary number of both pyknotic and cleaved caspase 3-positive cells undergoing apoptosis (Fig. 4B, E, and H). However, in Polβ−/− p53−/− neocortices, the CP was almost normally generated (Fig. 4C), parallel with a striking disappearance of apoptotic cells (Fig. 4F and I). With the use of antibody against either PCNA, a proliferating cell marker (Fig. 4J to L), or neuron-specific type III β-tubulin, an early neuron marker (Fig. 4M to O), we observed the state of neuronal cells escaping from apoptosis in E13.5 developing neocortex. In the PPL and IZ of Polβ−/− p53+/+ mice, expression of PCNA appeared in some apoptotic cells (Fig. 4K), whereas expression of type III β-tubulin was reduced with increasing apoptotic cells (compare Fig. 4N and M). Expression of PCNA is regulated by p53 in response to ionizing radiation in neuronal cells (45). Therefore, the PCNA activation in the apoptotic cells of Polβ−/− p53+/+ mice (Fig. 4K) might be due to a response to certain DNA damage. On the other hand, in Polβ−/− p53−/− mice, we found intact expression of both PCNA and type III β-tubulin, similar to wild-type controls (Fig. 4L and O, respectively). Neuronal progenitors expressing PCNA were restricted in the VZ (Fig. 4L); the differentiating neurons present in the PPL and IZ expressed type III β-tubulin at a normal level (Fig. 4O). Finally, at E14.5, formation of the CP in Polβ−/− p53−/− mice was more clearly observed than that in Polβ−/− p53+/+ mice (compare Fig. 4R and Q). Together, it appears that in Polβ−/− p53−/− embryos early differentiation of neuronal cells escaping from apoptosis proceeds normally.
FIG. 4.
Neuronal progenitors in E13.5 Polβ−/− p53−/− embryos appear to normally proliferate and differentiate. Coronal sections of telencephalons in Polβ+/+ p53+/+ (wild-type) (A, D, G, J, M, and P), Polβ−/−p53+/+ (B, E, H, K, N, and Q), and Polβ−/− p53−/− (C, F, I, L, O, and R) embryos at E13.5 (A to O) and E14.5 (P to R) were stained with cresyl violet (A to F and P to R). The sections were also stained with anti-cleaved caspase-3 antibody (G to I), anti-PCNA antibody (red) and DAPI (blue) (J to L), or anti-neuron specific type III β-tubulin antibody TuJ1 (M to O). Arrows in panels A, C, P, Q, and R indicate the CP. Arrowheads in panel K indicate PCNA-activated cells. LV, lateral ventricle.
Formation of the nervous system is incomplete in E18.5 Polβ−/− p53−/− mice.
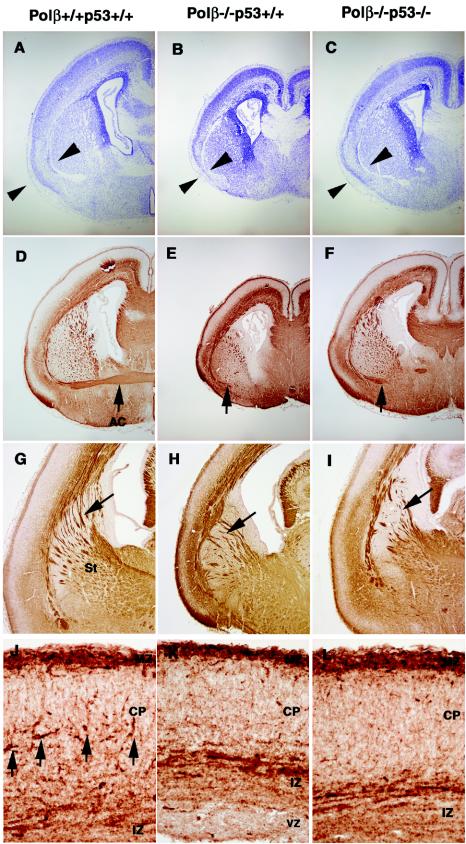
p53 deficiency dramatically rescued neuronal apoptosis in Polβ-deficient mice (Fig. 3). As mentioned above, early neuronal differentiation appeared to proceed normally in E13.5 Polβ−/− p53−/− embryos (Fig. 4). As shown in Table 1, Polβ−/− p53−/− embryos could survive during gestation and died at postnatal day 1. Therefore, we examined the development of their brains at E18.5 by immunohistochemical analysis (Fig. 5). We observed slight but significant, neuronal defects in the telencephalon of Polβ−/− p53−/− embryos. Cresyl violet staining revealed that, compared to Polβ−/− p53+/+ mice, the size of the telencephalon and its cytoarchitecture were moderately recovered in Polβ−/− p53−/− mice (compare Fig. 5B and C). We analyzed the axonal tract formation by staining phosphorylated neurofilaments. In Polβ−/− p53+/+ brains, the major axonal tract, anterior commissure did not cross at the midline (Fig. 5E); notably, this defect could not be rescued by p53 deficiency (Fig. 5F). We also observed more aberrant axonal tracts in the striatum of Polβ−/− p53+/+ brains (Fig. 5H) than in that of wild-type controls (Fig. 5G), and similar aberrations were observed in Polβ−/− p53−/− brains (Fig. 5I). These results indicate that, at least in some areas, the brain development in Polβ−/− p53−/− embryos was not complete. Recently identified is a cell type migrating from the lateral ganglionic eminence and the medial ganglionic eminence of the basal ganglia to the neocortex of telencephalons (2, 32). The tangentially migrating cells become interneurons synthesizing inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the CP of the neocortex. A great number of neuronal apoptotic cells were observed in both the lateral ganglionic eminence and the medial ganglionic eminence in Polβ−/− p53+/+ embryos at E13.5 (compare Fig. 3A and B). We therefore examined whether GABAergic interneurons existed in the E18.5 neocortex. The interneurons in developing neocortex are known to express calbindin D28k (Calbindin), an intracellular calcium-binding protein (2, 32). In Polβ−/− p53+/+ embryos (Fig. 5K), we found a significant decrease in the number of calbindin-positive cells in the CP compared to wild-type embryos (Fig. 5J). More importantly, this decrease was not rescued by p53 deficiency in Polβ−/− p53−/− embryos (Fig. 5L). Taken together, these data, shown in Fig. 5, indicate that p53 deficiency does not completely rescue developmental defects in the central nervous system, associated with Polβ deficiency. These phenotypes are in sharp contrast to those of Lig4−/− p53−/− and XRCC4−/− p53−/− mice, which can be alive for several weeks after birth. These results suggest a crucial role for Polβ in the formation of the intact neuronal circuit and migration of certain neuronal cell types.
FIG. 5.
Development of the brain is incomplete in E18.5 Polβ−/− p53−/− embryos. Coronal sections of telencephalons in Polβ+/+ p53+/+ (wild-type) (A, D, G, and J), Polβ−/− p53+/+ (B, E, H, and K), and Polβ−/−p53−/− (C, F, I, and L) embryos at E18.5 were stained with cresyl violet (A to C), anti-phosphorylated neurofilament antibody SMI31 (D to I), and anticalbindin antibody (J to L). Photographs of the sections in each genotype were taken with the same magnification. The arrowheads in panels A, B, and C indicate part of the cytoarchitecture recovered moderately in Polβ−/− p53−/− mice compared to Polβ−/− p53+/+. The arrows in panels D, E, and F indicate the anterior commissure (AC). The arrows in panels G, H, and I indicate aberrant axonal tracts in the striatum (St). The arrows in panel J indicate calbindin-positive cells. MZ, marginal zone.
DISCUSSION
We have shown here that p53 deficiency rescues neuronal apoptosis in Polβ-deficient mice and that haploinsufficiency substantially does so as well (Fig. 3). We have also shown that p53 is activated by its serine-18 phosphorylation in developing telencephalons of Polβ-deficient mice (Fig. 2). These results indicate that the neuronal apoptosis associated with Polβ deficiency is mediated by the p53-dependent pathway. In addition, it should be noted that, like Polβ−/− p53+/+ neonates, Polβ−/− p53−/− neonates die shortly after birth.
The onset of p53-dependent repair or apoptosis is determined by the level of accumulated damaged DNA (36). There is ample biochemical evidence for functioning of Polβ mainly in the short-patch BER pathway to repair SSBs, which are mostly generated as intermediates of damaged bases metabolized by DNA glycosylase and AP endonuclease (46). Mouse embryonic fibroblasts defective in Polβ are highly sensitive to DNA alkylating agents but not to X-ray radiation (41, 42), supporting in vivo that Polβ deficiency leads to defects in SSB repair but not in DSB repair. Thus, Polβ deficiency should result in increased levels of SSBs, even if the Polβ-independent long-patch BER is able to partially substitute for the short-patch BER. The increased SSB levels would stabilize and activate p53, leading to apoptosis during neuronal differentiation in Polβ−/− mice. In mice defective in Lig4, XRCC4, Ku70/80, or XRCC2, which all function in DSB repair, differentiating neurons undergo massive apoptosis (4, 11, 14, 17, 22). Therefore, in these mice, unrepaired DSBs are thought to be the cause of the apoptosis (4, 11, 14, 17, 22). The apoptosis in Lig4−/− and XRCC4−/− embryos is rescued by p53 deficiency (15, 16). Lig4−/− p53−/− and XRCC4−/− p53−/− neonates can survive several weeks after birth without behavioral or neurological abnormalities. This is in sharp contrast with our observation that Polβ−/− p53−/− neonates die shortly after birth. The degree and quality of rescue by p53 deficiency in repair-deficient mice appear to vary depending on the type and level of DNA damage. As discussed above, in Polβ−/− mice, the damage is most likely SSBs, but the possibility that these SSBs are subsequently converted into DSBs in the final DNA replication of neuronal progenitor cells cannot be ruled out.
In Polβ−/− p53−/− mice at E13.5, early steps of neuronal differentiation seem to proceed normally, as judged by immunohistochemical analysis (Fig. 4). However, at E18.5, these and Polβ−/− p53+/+ mice displayed serious cytoarchitectural defects in the major axonal tract (Fig. 5E and F) (with more aberrant axonal tracts in the striatum [Fig. 5H and I]) and the migration in GABAergic interneurons (Fig. 5K and L). These results suggest that, although p53 deficiency indeed rescues neuronal apoptosis, these neurons are still incomplete as mature ones, implying that the deficiency cannot fully restore the neuronal development of at least certain cell types. The brain is composed of remarkably complex neuronal cell types and networks. In the development of the brain, cell migration, axon growth, and pathfinding are fundamental processes (12). Recent studies with knockout mice have identified a number of molecules responsible for such processes (30, 32). Loss of these molecules severely affects the brain development and is critical for survival. The abnormal development of the nervous system observed in both Polβ−/− p53−/− and Polβ−/− p53+/+ mice at E18.5 may be responsible for death shortly after birth. In Lig4−/− p53−/− or XRCC4−/− p53−/− mice, severe defects in lymphogenesis are never recovered by p53 deficiency, implying that Lig4 or XRCC4 is a critical factor for lymphogenesis (15, 16). Similarly, our finding that the neuronal differentiation in Polβ−/− mice is not completely rescued by p53 deficiency strongly suggests that Polβ is a critical factor for neurogenesis; that is, Polβ may absolutely be required for neuronal differentiation.
The reason why Polβ is required for neuronal differentiation remains obscure. One possibility is that in neuronal differentiation, a large amount of damaged bases and SSBs are generated by reactive oxygen species, which might occur particularly in some neuronal cell types actively undergoing migration and/or axon pathfinding. Recently, the Polβ-dependent pathway was shown to be induced in response to oxidative base damage (7). Polβ might specifically be required to repair those damaged bases and SSBs. Thus, Polβ deficiency would lead to increased levels of DNA damage and activation of p53, eventually resulting in apoptosis. A second possibility is that Polβ is involved in chromatin remodeling and transcription in neuronal differentiation. When neuronal progenitor cells become postmitotic neurons, they exit cell cycle and drastically alter the pattern of gene expression from immature to mature neurons (12). Transcriptional activation of a gene involves recruitment of not only a sequence-specific DNA-binding protein but also a coactivator complex, including proteins with chromatin-modifying activity. For example, DNA topoisomerase IIβ alters DNA topology and forms complexes with proteins involved in chromatin remodeling and transcription (25, 44). The enzyme-deficient mice show defects in the laminar organization of the neocortex and motor axon growth, resulting in a breathing impairment and death of the pups shortly after birth (31, 49). This finding suggests that the control of chromatin reorganization is indispensable for neuronal differentiation. Interestingly, we note that transcriptional coactivator p300 forms a physical and functional interaction with Polβ (23). p300 integrates a diverse signaling pathway for a number of sequence-specific transcription factors and activates transcription through chromatin remodeling via intrinsic histone acetyltransferase activity (20). Therefore, in association with p300 or related proteins, Polβ might function to maintain the integrity of genes being, or to be, expressed in certain neuronal cell types. A third possibility is that during neuronal differentiation, a genomic rearrangement factor(s) is expressed and generates a certain type of DNA damage (repairable by Polβ) to initiate a specific differentiation. In the immune system, the molecular mechanism of diversity by rearrangement of the immunoglobulin or T-cell receptor gene clusters is well understood (43). In V(D)J recombination, the lymphocyte-specific endonucleases RAG1 and RAG2 initially cleave specific recognition sequences in immunoglobulin loci, followed by completion of rearrangements through DSB repair by the action of nonhomologous-end-joining factors (18). Similarly, in the nervous system, neuronal diversity might be created by such genomic rearrangement (9, 48). If this is the case, DNA repair by Polβ would be an essential part of the diversity mechanism.
In conclusion, our studies show that p53 deficiency dramatically rescues neuronal apoptosis associated with Polβ deficiency, indicating that p53 mediates the apoptotic process in the nervous system. However, p53 deficiency cannot restore complete differentiation of neuronal progenitors and leads to lethality shortly after birth. These observations suggest a crucial role for Polβ in differentiation of specific neuronal cell types. In addition, it is evident that in neuronal differentiation, p53 acts as a gatekeeper to maintain genomic stability against various types of DNA damage (27). Further studies will be needed to elucidate the precise role of Polβ in neurogenesis.
Acknowledgments
We thank T. Yamakuni (Tohoku University) for the gift of the anti-calbindin antibody, Y. Tanabe (Mitsubishi Kagaku Institute of Life Sciences) for helpful discussion, and N. Adachi (Yokohama City University) for critical reading of the manuscript. We also thank C. Nishigaki for technical support and F. Oonuma for animal care.
N.S. is a recipient of Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists. This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Abe, H., O. Amano, T. Yamakuni, Y. Takahashi, and H. Kondo. 1990. Localization of spot 35-calbindin (rat cerebellar calbindin) in the anterior pituitary of the rat: developmental and sexual differences. Arch. Histol. Cytol. 53:585-591. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S. A., D. D. Eisenstat, L. Shi, and J. L. Rubenstein. 1997. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278:474-476. [DOI] [PubMed] [Google Scholar]
- 3.Banasiak, K. J., and G. G. Haddad. 1998. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 797:295-304. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, D. E., G. Stamp, I. Rosewell, A. Denzel, and T. Lindahl. 1998. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr. Biol. 8:1395-1398. [DOI] [PubMed] [Google Scholar]
- 5.Bayer, S. A., and J. Altman. 1991. Neocortical development. Raven Press, New York, N.Y.
- 6.Biade, S., R. W. Sobol, S. H. Wilson, and Y. Matsumoto. 1998. Impairment of proliferating cell nuclear antigen-dependent apurinic/apyrimidinic site repair on linear DNA. J. Biol. Chem. 273:898-902. [DOI] [PubMed] [Google Scholar]
- 7.Cabelof, D. C., J. J. Raffoul, S. Yanamadala, Z. Guo, and A. R. Heydari. 2002. Induction of DNA polymerase beta-dependent base excision repair in response to oxidative stress in vivo. Carcinogenesis 23:1419-1425. [DOI] [PubMed] [Google Scholar]
- 8.Caldecott, K. W. 2003. DNA single-strand break repair and spinocerebellar ataxia. Cell 112:7-10. [DOI] [PubMed] [Google Scholar]
- 9.Chun, J., and D. G. Schatz. 1999. Rearranging views on neurogenesis: neuronal death in the absence of DNA end-joining proteins. Neuron 22:7-10. [DOI] [PubMed] [Google Scholar]
- 10.Crumrine, R. C., A. L. Thomas, and P. F. Morgan. 1994. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J. Cereb. Blood Flow Metab. 14:887-891. [DOI] [PubMed] [Google Scholar]
- 11.Deans, B., C. S. Griffin, M. Maconochie, and J. Thacker. 2000. Xrcc2 is required for genetic stability, embryonic neurogenesis, and viability in mice. EMBO J. 19:6675-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edlund, T., and T. M. Jessell. 1999. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96:211-224. [DOI] [PubMed] [Google Scholar]
- 13.Esposito, G., G. Texido, U. A. Betz, H. Gu, W. Muller, U. Klein, and K. Rajewsky. 2000. Mice reconstituted with DNA polymerase beta-deficient fetal liver cells are able to mount a T cell-dependent immune response and mutate their Ig genes normally. Proc. Natl. Acad. Sci. USA 97:1166-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank, K. M., J. M. Sekiguchi, K. J. Seidl, W. Swat, G. A. Rathbun, H. L. Cheng, L. Davidson, L. Kangaloo, and F. W. Alt. 1998. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature 396:173-177. [DOI] [PubMed] [Google Scholar]
- 15.Frank, K. M., N. E. Sharpless, Y. Gao, J. M. Sekiguchi, D. O. Ferguson, C. Zhu, J. P. Manis, J. Horner, R. A. DePinho, and F. W. Alt. 2000. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 5:993-1002. [DOI] [PubMed] [Google Scholar]
- 16.Gao, Y., D. O. Ferguson, W. Xie, J. P. Manis, J. Sekiguchi, K. M. Frank, J. Chaudhuri, J. Horner, R. A. DePinho, and F. W. Alt. 2000. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 404:897-900. [DOI] [PubMed] [Google Scholar]
- 17.Gao, Y., Y. Sun, K. M. Frank, P. Dikkes, Y. Fujiwara, K. J. Seidl, J. M. Sekiguchi, G. A. Rathbun, W. Swat, J. Wang, R. T. Bronson, B. A. Malynn, M. Bryans, C. Zhu, J. Chaudhuri, L. Davidson, R. Ferrini, T. Stamato, S. H. Orkin, M. E. Greenberg, and F. W. Alt. 1998. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell 95:891-902. [DOI] [PubMed] [Google Scholar]
- 18.Gellert, M. 2002. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71:101-132. [DOI] [PubMed] [Google Scholar]
- 19.Gonda, H., M. Sugai, T. Katakai, N. Sugo, Y. Aratani, H. Koyama, K. J. Mori, and A. Shimizu. 2001. DNA polymerase beta is not essential for the formation of palindromic (P) region of T-cell receptor gene. Immunol. Lett. 78:45-49. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 21.Gu, H., J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky. 1994. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 22.Gu, Y., J. Sekiguchi, Y. Gao, P. Dikkes, K. Frank, D. Ferguson, P. Hasty, J. Chun, and F. W. Alt. 2000. Defective embryonic neurogenesis in Ku-deficient but not DNA-dependent protein kinase catalytic subunit-deficient mice. Proc. Natl. Acad. Sci. USA 97:2668-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan, S., N. El-Andaloussi, U. Hardeland, P. O. Hassa, C. Burki, R. Imhof, P. Schar, and M. O. Hottiger. 2002. Acetylation regulates the DNA end-trimming activity of DNA polymerase beta. Mol. Cell 10:1213-1222. [DOI] [PubMed] [Google Scholar]
- 24.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, C. A., K. Padget, C. A. Austin, and B. M. Turner. 2001. Deacetylase activity associates with topoisomerase II and is necessary for etoposide-induced apoptosis. J. Biol. Chem. 276:4539-4542. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, A., J. Abbotts, E. M. Karawya, and S. H. Wilson. 1990. Identification and properties of the catalytic domain of mammalian DNA polymerase beta. Biochemistry 29:7156-7159. [DOI] [PubMed] [Google Scholar]
- 27.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 28.Lindahl, T., and R. D. Wood. 1999. Quality control by DNA repair. Science 286:1897-1905. [DOI] [PubMed] [Google Scholar]
- 29.Liu, P. K., C. Y. Hsu, M. Dizdaroglu, R. A. Floyd, Y. W. Kow, A. Karakaya, L. E. Rabow, and J. K. Cui. 1996. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J. Neurosci. 16:6795-6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Bendito, G., and Z. Molnar. 2003. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4:276-289. [DOI] [PubMed] [Google Scholar]
- 31.Lyu, Y. L., and J. C. Wang. 2003. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIβ. Proc. Natl. Acad. Sci. USA 100:7123-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin, O., and J. L. Rubenstein. 2003. Cell migration in the forebrain. Annu. Rev. Neurosci. 26:447-483. [DOI] [PubMed] [Google Scholar]
- 33.McGahan, L., A. M. Hakim, and G. S. Robertson. 1998. Hippocampal Myc and p53 expression following transient global ischemia. Brain Res. Mol. Brain Res. 56:133-145. [DOI] [PubMed] [Google Scholar]
- 34.Morrison, R. S., H. J. Wenzel, Y. Kinoshita, C. A. Robbins, L. A. Donehower, and P. A. Schwartzkroin. 1996. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J. Neurosci. 16:1337-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson, D. W., A. Ali, N. A. Thornberry, J. P. Vaillancourt, C. K. Ding, M. Gallant, Y. Gareau, P. R. Griffin, M. Labelle, Y. A. Lazebnik, et al. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37-43. [DOI] [PubMed] [Google Scholar]
- 36.Offer, H., N. Erez, I. Zurer, X. Tang, M. Milyavsky, N. Goldfinger, and V. Rotter. 2002. The onset of p53-dependent DNA repair or apoptosis is determined by the level of accumulated damaged DNA. Carcinogenesis 23:1025-1032. [DOI] [PubMed] [Google Scholar]
- 37.Offer, H., I. Zurer, G. Banfalvi, M. Reha'k, A. Falcovitz, M. Milyavsky, N. Goldfinger, and V. Rotter. 2001. p53 modulates base excision repair activity in a cell cycle-specific manner after genotoxic stress. Cancer Res. 61:88-96. [PubMed] [Google Scholar]
- 38.Rolig, R. L., and P. J. McKinnon. 2000. Linking DNA damage and neurodegeneration. Trends Neurosci. 23:417-424. [DOI] [PubMed] [Google Scholar]
- 39.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325-334. [DOI] [PubMed] [Google Scholar]
- 40.Singhal, R. K., and S. H. Wilson. 1993. Short gap-filling synthesis by DNA polymerase beta is processive. J. Biol. Chem. 268:15906-15911. [PubMed] [Google Scholar]
- 41.Sobol, R. W., J. K. Horton, R. Kuhn, H. Gu, R. K. Singhal, R. Prasad, K. Rajewsky, and S. H. Wilson. 1996. Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature 379:183-186. (Errata, 379:848 and 383:457.) [DOI] [PubMed] [Google Scholar]
- 42.Sugo, N., Y. Aratani, Y. Nagashima, Y. Kubota, and H. Koyama. 2000. Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. EMBO J. 19:1397-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonegawa, S. 1983. Somatic generation of antibody diversity. Nature 302:575-581. [DOI] [PubMed] [Google Scholar]
- 44.Tsai, S. C., N. Valkov, W. M. Yang, J. Gump, D. Sullivan, and E. Seto. 2000. Histone deacetylase interacts directly with DNA topoisomerase II. Nat. Genet. 26:349-353. [DOI] [PubMed] [Google Scholar]
- 45.Uberti, D., L. Piccioni, M. Cadei, P. Grigolato, V. Rotter, and M. Memo. 2001. p53 is dispensable for apoptosis but controls neurogenesis of mouse dentate gyrus cells following gamma-irradiation. Brain Res. Mol. Brain Res. 93:81-89. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, S. H., R. W. Sobol, W. A. Beard, J. K. Horton, R. Prasad, and B. J. Vande Berg. 2000. DNA polymerase beta and mammalian base excision repair. Cold Spring Harbor Symp. Quant. Biol. 65:143-155. [DOI] [PubMed] [Google Scholar]
- 47.Xiang, H., D. W. Hochman, H. Saya, T. Fujiwara, P. A. Schwartzkroin, and R. S. Morrison. 1996. Evidence for p53-mediated modulation of neuronal viability. J. Neurosci. 16:6753-6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yagi, T. 2003. Diversity of the cadherin-related neuronal receptor/protocadherin family and possible DNA rearrangement in the brain. Genes Cells 8:1-8. [DOI] [PubMed] [Google Scholar]
- 49.Yang, X., W. Li, E. D. Prescott, S. J. Burden, and J. C. Wang. 2000. DNA topoisomerase IIβ and neural development. Science 287:131-134. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, J., J. Ahn, S. H. Wilson, and C. Prives. 2001. A role for p53 in base excision repair. EMBO J. 20:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]