Abstract
The tumor suppressor BRCA1 contains multiple functional domains that interact with many proteins. After DNA damage, BRCA1 is phosphorylated by CHK2 at serine 988, followed by a change in its intracellular location. To study the functions of CHK2-dependent phosphorylation of BRCA1, we generated a mouse model carrying the mutation S971A (S971 in mouse Brca1 corresponds to S988 in human BRCA1) by gene targeting. Brca1S971A/S971A mice were born at the expected ratio without a developmental defect, unlike previously reported Brca1 mutant mice. However, Brca1S971A/S971A mice suffered a moderately increased risk of spontaneous tumor formation, with a majority of females developing uterus hyperplasia and ovarian abnormalities by 2 years of age. After treatment with DNA-damaging agents, Brca1S971A/S971A mice exhibited several abnormalities, including increased body weight, abnormal hair growth pattern, lymphoma, mammary tumors, and endometrial tumors. In addition, the onset of tumor formation became accelerated, and 80% of the mutant mice had developed tumors by 1 year of age. We demonstrated that the Brca1S971A/S971A cells displayed reduced ability to activate the G2/M cell cycle checkpoint upon γ-irradiation and to stabilize p53 following N-methyl-N′-nitro-N-nitrosoguanidine treatment. These observations suggest that Chk2 phosphorylation of S971 is involved in Brca1 function in modulating the DNA damage response and repressing tumor formation.
Germ line mutations of BRCA1 predispose women to breast cancer and ovarian cancer (1, 7). Although the specific molecular events leading to tumorigenesis remain elusive, several lines of evidence indicate that BRCA1 is involved in genetic stability control, DNA damage repair, centrosome duplication, apoptosis, and cell cycle control (reviewed in references 13, 16, 48, and 56). BRCA1 functions are mediated by several different mechanisms, including phosphorylation. It has been shown that BRCA1 undergoes hyperphosphorylation during late G1 and S phases and is transiently dephosphorylated soon after M phase (42, 47).
During the DNA damage response, BRCA1 is phosphorylated by several protein kinases, such as ATM, ATR, CHK1, CHK2, and MDC1 (10, 31, 58). ATM controls cell cycle arrest in G1 and G2. The control mechanism of G2 arrest by ATM is unclear, but recently CHK2, the mammalian homolog of the budding yeast Rad53 and fission yeast Cds1 checkpoint kinases, was cloned and found to be linked to ATM: in response to ionizing radiation (IR), CHK2 is rapidly phosphorylated and activated in an ATM-dependent manner (18, 21, 33). In Chk2 mutant mouse embryonic stem (ES) cells, maintenance of G2 arrest and reduced Cdc2 kinase activity in response to IR are defective (24).
The first evidence that CHK2 acts as a tumor suppressor came from a finding that a subset of patients with Li-Fraumeni syndrome, which is characterized by multiple tumors at an early age, contained germ line mutations in CHK2 (5). Somatic mutations of CHK2 have also been found in diverse types of sporadic cancer, including carcinomas of the breast (44), colon (5), lung (23), and vulva (41); osteosarcomas (37); and lymphomas (22, 25), with low yet significant frequencies. Most CHK2 mutations in these carcinomas are missense or truncation mutations in functional domains involved in ATM/ATR phosphorylation (the SQ/TQ motif), kinase function, and the forkhead-associated domain (4). An 1100delC mutation that abrogates the kinase activity has been found in 1.1% of sporadic breast cancer and 5.1% of familial breast cancer families that did not carry mutations in BRCA1 or BRCA2, including 13.5% of individuals from families with male breast cancer (34). It is estimated that the 1100delC variant resulted in an ∼2-fold increase of the breast cancer risk in women and a 10-fold increase of the risk in men. One study reported that mutations of CHK2 were found in BRCA1-associated breast cancers at a higher frequency than in sporadic cancers (44), while others found no clear correlation in different study populations (2, 34).
It was shown that CHK2 modulates BRCA1 functions through phosphorylating BRCA1 after DNA damage (28). CHK2 and BRCA1 interact and colocalize within discrete nuclear foci but separate after γ-irradiation. Phosphorylation of BRCA1 at S988 by CHK2 is required for the dissociation and relocalization of BRCA1. This phosphorylation is also important for cell survival after DNA damage. Recent studies have indicated that phosphorylation of BRCA1 S988 is regulated during the cell cycle in response to DNA damage (39) and is required for BRCA1-mediated homologous recombination and suppression of nonhomologous recombination in cultured tumor cells (55). However, the influences of phosphorylation of CHK2 on BRCA1 function in vivo and the progression of breast cancer remain elusive. To investigate this, we mutated the Chk2 phosphorylation site in mouse Brca1 and studied the biological consequences using the mutant mice and cultured mutant cells. Our data indicate that Chk2 phosphorylation of S971 mediates a part of Brca1 function in modulating the DNA damage response, and consequently, the disruption of this site results in increased spontaneous tumor formation, as well as early onset of DNA damage-induced tumors in mutant mice.
MATERIALS AND METHODS
Targeting and generation of mice.
Recombinant phages containing overlapping genomic DNA of the Brca1 locus were isolated from a 129SvEv mouse library (52). To construct the targeting vector for Brca1, a 3.5-kb EcoRV-XhoI fragment 5′ to exon 11 of Brca1 was subcloned into the XbaI and EcoRI sites of a ploxPneo (54). The mutation of S971A in Brca1 was introduced using the PCR primers 5′-GTT TCT CCC ATC AGG GCA TCT ATA AAA ACT G-3′ and 5′-CAG TTT TTA TAG ATG CCC TGA TGG GAG AAA C-3′. (The underlined letters are the introduced mutations). The resulting S971A mutation also generated a new SfaNI site, which can be used to verify the mutation. The PCR product was digested with NheI and NsiI, and the 711-bp fragment was subcloned into the 1.4-kb BamHI fragment and then into the XhoI-XhoI right arm (5.5 kb). The resulting construct was cleaved with XhoI and NotI, followed by insertion of a 5.5-kb XhoI-NotI fragment (the NotI site is from the polylinker of the phage vector). The finished targeting construct was designated ploxPneoBrca1-S971A. TC1 ES cells (11) were transfected with NotI-digested linearized targeting vector DNA and selected with G418 and 2′-fluoro-2′-deoxy-5-iodo-1-β-d-arabinofuranosyluracil (FIAU) as described previously (17). ES cell colonies resistant to double selection were isolated and subjected to Southern blot analysis. Genomic DNAs isolated from the clones and the parental TC1 cell line were digested with HindIII or EcoRV and hybridized with a 5′ probe and an internal probe (PstI and BamH1 fragments, respectively). ES cells heterozygous for the targeted mutation were microinjected into C57BL/6 blastocysts, implanted into the uterus of pseudopregnant Swiss Webster foster mothers, and developed to term. Male chimeras were mated with NIH Black Swiss females. Germ line transmission was confirmed by agouti coat color in F1 animals, and the offspring were genotyped for the Brca1 mutant allele by PCR and Southern blot analyses. Confirmed agouti male chimeras (129SvEv) were directly mated with EIIa-Cre mice (FVB/N) to delete the ploxPneo. Thus, the genetic background of these mice was 50% 129 and 50% FVB.
Mouse treatment and analysis.
For the γ-irradiation study, female mice at 4 to 8 weeks of age were irradiated at 3 Gy (1 Gy/min; Gammacell 40) four times at 1-week intervals. In the 1-methyl-1-nitrosourea (MNU) treatment study, female mice were given a single intraperitoneal injection of MNU at a dose of 50 mg/kg of body weight at 6 weeks of age. The mice were monitored twice a week for possible symptoms related to the treatment and tumor formation. When mice demonstrated a tumor, tumor tissue and adjacent normal tissue were collected to prepare DNA. The remaining tumor and surrounding tissues were further divided, frozen in liquid nitrogen, and stored at −80°C or fixed in 10% buffered formalin and embedded in paraffin for hematoxylin and eosin staining. We also carried out whole-mount staining of mammary glands as described previously (52).
MEFs and analysis.
Mouse embryonic fibroblasts (MEFs) were derived from embryonic day 14.5 embryos generated from intercrosses of Brca1+/S971A and Chk2+/− mice (43). Every comparison between wild type and mutant was performed between the littermates. For G2/M checkpoint analysis, MEFs were plated a day before γ-irradiation. Then, the cells were harvested and fixed in 70% ethanol. After being stained with an antibody that specifically recognizes the phosphorylated form of histone H3 (Upstate Biotechnology), the cells were analyzed by using a FACSCalibur flow cytometer-cell sorter (Becton Dickinson). G1/S and S checkpoint analyses were performed as described before (12, 30, 45, 49). We used at least three embryos from different littermates representing each genotype and obtained similar results.
MNNG treatment.
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) treatments were performed on the MEFs 1 day after plating. Before treatment, the cells were washed with phosphate-buffered saline, and then MNNG was added at the appropriate final concentration dissolved in the serum-free medium. After 30-min exposure of MNNG, the plates were rinsed twice with phosphate-buffered saline and returned to complete growth medium. MNNG was dissolved in 0.1 M sodium acetate (pH 5.0) at a stock concentration of 10 mM and stored at −80°C prior to use.
Immunoblotting and analysis.
Western blot analysis was carried out according to standard procedures using enhanced-chemiluminescence detection (Amersham). The following primary antibodies were used: p53 (Novocastra; Calbiochem), p21 (Pharmingen), and β-actin (Sigma). Horseradish peroxidase-conjugated donkey anti-rabbit or sheep anti-mouse antibodies (Amersham) were used as secondary antibodies.
Microsatellite instability.
Amplification condition of microsatellite locus D4MIT27 is followed by Farber et al. (19). The primers flanking the microsatellite insert were 5′-GCA CGG TAG TTT TTC CAG GA-3′ and 5′-TGG TGG GCA GGC AAT AGT-3′. The PCR products (150 bp) were subjected to electrophoresis in 6% denaturing polyacrylamide gels at 1,500 V for 2.5 h. The gels were dried and exposed to X-ray film at −80°C in the presence of an intensifying screen.
Colony formation in soft agar and tumorigenicity assay.
For colony formation, 5 × 104 tumor cells generated from mammary tumor were suspended in 0.3% agar in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and plated on six-well plates containing a solidified bottom layer (0.5% agar in growth medium). For the tumorigenicity assay, 2 × 106 cells were injected into 6-week-old female nude mice.
Statistical analyses.
Student's t test was used to compare differences in analysis of checkpoints, body weight, and levels of p53 expression between the Brca1-S971A (Brca1SS971A/S971A) mutant and control mice as specified in the text. A P value of ≤0.05 was considered statistically significant.
RESULTS
Targeted disruption of the Chk2 phosphorylation site, S971, in mouse Brca1.
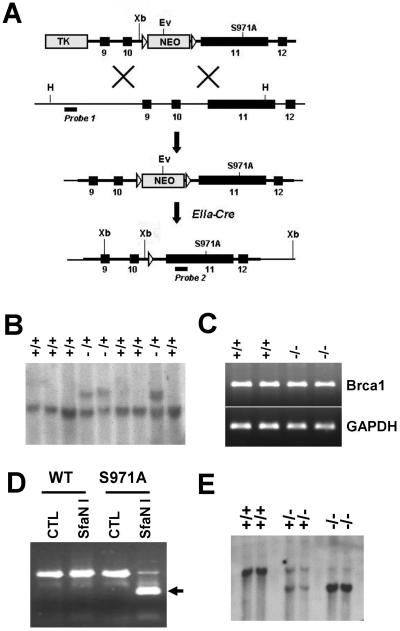
S971 in mouse Brca1 corresponds to the CHK2 phosphorylation site S988 in human BRCA1 (28). To introduce this mutation into mouse Brca1, we replaced S971 with A971 using a cotransfer-type targeting construct (Fig. 1A). Southern blot analysis detected homologous recombination at the Brca1 locus in 14 out of 92 G418-FIAU doubly resistant ES clones (Fig. 1B). After confirming by sequencing that the mutant cells contained an S971A mutation (not shown), three correctly targeted ES clones were injected into blastocysts, and germ line transmission was obtained from all of them. Mice carrying the targeted disruption were further crossed with EIIa-Cre transgenic mice that express Cre in the germ line to delete the ploxPneo gene (Fig. 1A). This is necessary to ensure the normal expression of the mutant allele, based on previous experience (9). After the deletion of ploxPneo, the Brca1-S971A allele is expressed at a level comparable to the wild type, as revealed by reverse transcription-PCR analysis (Fig. 1C).
FIG. 1.
Introduction of the S971A mutation into mouse Brca1 locus. (A) Targeting vector and final structure of the mutant allele. The point mutation S971A in exon 11 was cotransferred with ploxPneo through homologous recombination in ES cells. Once germ line transmission was established, mice heterozygous for the mutation (+/S971A-neo) were crossed with EIIa-Cre transgenic mice to delete ploxPneo. H, HindIII; Ev, EcoRV; X, XhoI Xb, XbaI TK, thymidine kinase; open triangles, loxp sites. The positions of the probes used for Southern blot analyses and the sizes of the endogenous and targeted DNA fragments recognized by these probes are shown. (B) Southern analysis of ES cell DNA digested with HindIII to identify the targeting event that is characterized by a fragment shift from 10 to 12 kb due to the presence of neo. Same samples digested with HindIII and EcoRV were subjected to Southern analysis with an internal BamHI probe (data not shown). (C) Reverse transcription-PCR analysis to check the proper expression of Brca1 was performed in wild-type and Brca1S971A/S971A mutant MEFs by using primers against exon 11 of Brca1 with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control. (D) DNA fragments containing the S971A mutation were amplified by PCR, followed by digestion with SfaNI. The PCR product (500 bp) from S971A was cleaved by SfaNI (the cleaved fragment [250 bp] is indicated by an arrow). The presence of the mutation was also confirmed by sequencing of the PCR product (data not shown). WT, wild type. (E) Southern blot analysis of XbaI-digested tail DNA isolated from wild-type, heterozygous (+/S971A), and homozygous (S971A/S971A) mice after neo excision. The presence of a 7-kb fragment by Southern blotting represented the removal of neo.
Mice carrying a heterozygous mutation of Brca1-S971A (Brca1+/S971A) were phenotypically normal and were further crossed to produce Brca1S971A/S971A mice (Fig. 1D and E). Our data indicated that Brca1S971A/S971A mice were present at expected rates at weaning (data not shown). Our analysis of Brca1S971A/S971A mice younger than 12 months of age did not detect any obvious abnormalities in appearance, activity, or fertility (data not shown). This observation indicated that the loss of the Chk2 phosphorylation site of Brca1 did not cause any developmental defects.
Uterus hyperplasia and spontaneous tumor formation in aging population of mutant mice.
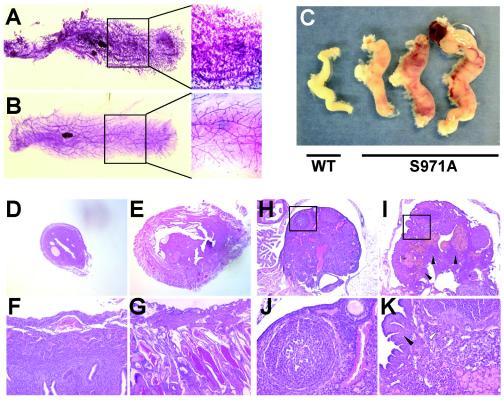
Brca1Δ11/Δ11 mutant mice survive to adulthood in a p53+/− background and exhibit premature aging before 1 year of age (8). Therefore, we asked whether Brca1S971A/S971A mice would have a similar phenotype. Our study revealed no sign of premature aging in a study population of eight mice. However of six female Brca1S971A/S971A mice examined at 2years of age, four exhibited very dense branches in mammary glands with small hyperplasic foci (Fig. 2A). Interestingly, seven out of eight mutant females showed significantly enlarged uteri and invasion by thick blood vessels (Fig. 2C). Histological sections confirmed the vascular nature of the mutant uteri and the existence of a lot of polyps (Fig. 2E and G). Three of the mutant mice completely lost their ovaries with blood aggregates (Fig. 2C), while the ovaries of others exhibited abnormal structures (Fig. 2I and K). One of these mice also developed a hepatoma, and one developed a lipoma. These observations indicated that Brca1S971A/S971A mice were susceptible to spontaneous tumor formation.
FIG. 2.
Phenotypes in Brca1S971A/S971A mice. Whole-mount staining of mammary glands from Brca1S971A/S971A (A) and age-matched wild-type (B) mice. The boxed areas are amplified. (C) Uteri and ovaries from wild-type (WT) and Brca1S971A/S971A mice. Examples of histological sections of uterus (D to G) and ovary (H to K) from wild-type (D, F, H, and J) and Brca1S971A/S971A (E, G, I, and K) mice. J and K represent the magnification of boxed areas of H and I, respectively.
Abnormalities induced by DNA-damaging agents.
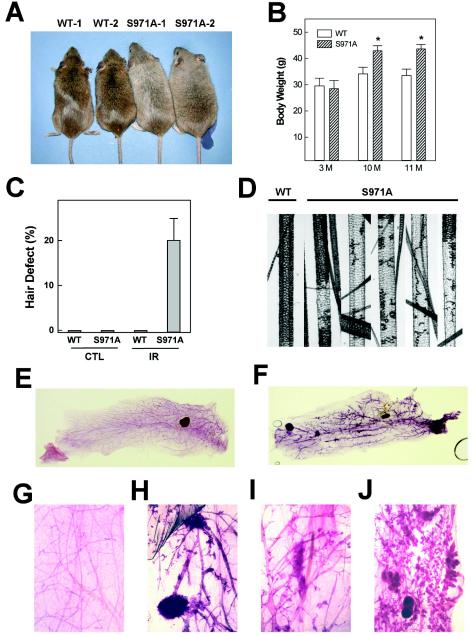
Phosphorylation of BRCA1 by CHK2 occurs after DNA damage, suggesting that this event is important for BRCA1 function in the DNA damage response. To determine the significance of Chk2 phosphorylation of Brca1 in vivo, we treated the mice with a sublethal dose of γ-irradiation (3 Gy) four times at 1-week intervals and studied their responses. We found that Brca1S971A/S971A mice were normal when they were young; however, the mutant mice showed increased body weights compared with the wild-type controls when they were 10 and 11 months of age (Fig. 3A). At both time points, the average body weights of mutant mice were ∼25% more than those of control mice (Fig. 3B).
FIG. 3.
Phenotypes in Brca1S971A/S971A mouse response upon γ-irradiation. (A) Wild-type (WT) and Brca1S971A/S971A mice 10 months of age after irradiation. (B) Body weights of wild-type (n = 14 at 10 and 11 months [M]) and Brca1S971A/S971A mice (n = 5 at 10 months; n = 3 at 11 months). (C) Percentages of abnormal awl-type hairs determined under the microscope. The error bars indicate standard deviations. (D) Microscopic image of normal and defective hairs from wild-type and Brca1S971A/S971A mice, respectively. (E and F) Whole-mount staining of mammary glands from wild-type (E) and Brca1S971A/S971A (F) mice 13 months after irradiation. (G to J) More highly magnified whole-mount images of wild type (G) and Brca1S971A/S971A (H to J).
Of note, Brca1S971A/S971A mice gradually showed abnormalities in their hair and appeared gray after γ-irradiation. Mice normally have four types of hair: monotrich, awl, auchene, and zigzag (43). Microscopic examination of hairs revealed that ∼20% of the awl type of hairs in mutant mice had defects in pigmentation after γ-irradiation (Fig. 3C and D). Because of a gradually increasing percentage of gray hair, mutant mice looked gray after 6 months of γ-irradiation (Fig. 3A). The cause and the consequence of gray hair in Brca1S971A/S971A mice upon irradiation are not clear. However, gray hair was also observed in ATM-deficient mice upon irradiation (3). Furthermore, it has been demonstrated recently that targeted disruption of Per2 results in tumor development and hair graying (20).
We next studied mammary gland development in mutant mice. Whole-mount staining of mammary glands isolated from control and Brca1S971A/S971A mice 13 months after irradiation revealed that the mutant glands showed an increased branch morphogenesis. Hyperplasic foci in mammary glands were also detected in four out of five mice (Fig. 3E to J). Examination of six mice that were younger than 300 days revealed no focus formation (not shown).
Accelerated tumorigenesis in the Brca1S971A/S971A mice induced by DNA-damaging agents.
The most significant abnormality in mutant mice was tumorigenesis induced by γ-irradiation, which started at ∼3 months of age. At ∼1 year of age, 80% of mutant mice had developed tumors (Fig. 4A and C). In contrast, only 15% of wild-type mice developed tumors during the same period (Fig. 4A). The majority types of tumors were lymphomas in both mutant and control mice. However, mutant mice had a significantly higher frequency than control mice (Fig. 4C). Three mutant mice developed mammary tumors (Fig. 4D to G), and one developed colon and uterus tumors (Fig. 4H and I). We also treated five compound mutant mice (Brca1KO/S971A) under the same conditions, and all of them developed tumors, including lymphomas, mammary tumors, and colon tumors (Fig. 4C).
FIG. 4.
Reduced survival with high tumor incidence in Brca1S971A/S971A mice treated with IR or MNU. (A) Kaplan-Meier survival curve of wild-type (open circles; n = 19) and Brca1 mutant (filled circles; Brca1KO/S971A, n = 5; Brca1S971A/S971A, n = 19) mice after irradiation. The data are combined from two separate experiments. (B) Kaplan-Meier survival curve of wild-type (n = 15) and Brca1S971A/S971A (n = 15) mice after injection of MNU (50 mg/kg) at 6 weeks of age. Red and blue circles in the curves represent the mammary tumors and colon-uterus tumors, respectively. (C) Tumor spectrum of mice after treatment. Parentheses represent the number of mice that died without detectable tumors. *, three mice had tumors in more than one organ. WT, wild type. (D to I) Brca1S971A/S971A mutants develop a variety of tumors after DNA damage. (D) A Brca1S971A/S971A mouse bearing a mammary tumor (arrow). Histological sections from mammary (E to G), colon (H), and uterus (I) tumors.
The increased tumor incidence after DNA damage has also been found in Brca1S971A/S971A mice treated with MNU, which alkylates DNA, yielding O6-methylguanine and O4-methylthymine. Our data indicated that the Brca1S971A/S971A mice were much more prone to MNU-induced tumorigenesis than wild-type control mice (Fig. 4B). Of 15 mice studied, 13 developed tumors, including 7 (47%) lymphomas, 1 (7%) mammary tumor, 3 (20%) colon cancers, and 3 (20%) uterus tumors.
Previous investigations showed that mammary tumors derived from Brca1 conditional mutant mice exhibited a high degree of genetic instability (6, 52). To see if this was the case in tumors developed from Brca1S971A/S971A mice, we established cell lines from mammary tumors of the mice. These tumor cells grew well in vitro and form colonies in soft agar (Fig. 5A). They also formed tumors after implantation into nude mice (Fig. 5B) with histopathologies similar to those of the primary tumors from which they were derived (Fig. 5C). Our analysis of the chromosomes of two mammary tumor cell lines revealed significant variations in chromosome numbers, with an average of 55 and 75 for each line (Fig. 5D). We also detected microsatellite instability in two out of nine primary tumors analyzed (Fig. 5E). Thus, similar to Brca1-null or Brca1 full-length isoform mutations, the disruption of Brca1-S971A also results in genetic instability.
FIG. 5.
Characterization of tumors from Brca1S971A/S971A mice. (A) Soft-agar colony formation assay of three different clones (94A, 94B, and 94C) of V94 mammary tumor. (B) Tumorigenicity assay of mammary tumor cells in nude mice. Tumors (arrows) were observed 3 weeks after injection of 94A, 94B, and 94C. (C) Hematoxylin- and eosin-stained sections of original mammary tumor from V94 and three different tumors from injection of clones into nude mice. (D) Chromosome spreads from V94 and V296 tumors. (E) Microsatellite analysis of tumors of Brca1S971A/S971A mice. T and N represent the DNAs from tumor and normal tissue, respectively.
Brca1S971A/S971A cells displayed partial loss of the G2/M cell cycle checkpoint upon γ-irradiation.
Increased tumorigenesis in Brca1S971A/S971A mice after DNA damage suggests that the S971A mutation may impair the DNA damage checkpoint response of Brca1. To assess the possible impacts of the Brca1-S971A mutation on DNA damage checkpoint control, we derived MEFs from Brca1S971A/S971A and wild-type embryos. The Brca1S971A/S971A and wild-type cells were irradiated and analyzed by fluorescence-activated cell sorter analysis after being labeled with an antibody to phosphohistone H3, a specific marker for mitotic cells. Our analysis detected a sharp reduction in the mitotic index in both wild-type and Brca1S971A/S971A cells that received 10 Gy of γ-irradiation (Fig. 6A). However, the Brca1S971A/S971A cells had significantly more cells in mitotic phase at the multiple time points analyzed, ranging from 1 through 4 h after the treatment (Fig. 6A and D). The γ-irradiation-induced difference between control and mutant cells in the mitotic population was more obvious in the presence of nocodazole (100 ng/ml), which prevents cells from leaving M phase (Fig. 6B). We had also treated cells with nocodazole only and found it did not cause any differences between mutant and control cells (Fig. 6C), suggesting that the differential accumulation in the M phase population of these cells is directly related to DNA damage.
FIG. 6.
Response to γ-irradiation and MNNG in Brca1S971A/S971A MEFs and tumor cells. (A to D) Partially defective G2/M checkpoint activation in Brca1S971A/S971A MEFs. (A) Mitotic index of irradiated or unirradiated wild-type (WT) and Brca1S971A/S971A MEFs was determined at the indicated times after 10 Gy of IR. The mitotic index of irradiated (B) or unirradiated (C) wild-type and Brca1S971A/S971A MEFs was determined in the presence of nocodazole (100 ng/ml). Means ± standard errors of the mean from at least three different littermate experiments are given. Significant differences are indicated by asterisks. (D) Representative fluorescence-activated cell sorter plots of wild-type and Brca1S971A/S971A MEFs 4 h after 0 or 10 Gy of irradiation. For each analysis, 10,000 cells of each genotype were analyzed. (E) DNA synthesis was measured 1 h after the indicated dose of irradiation in wild-type and Brca1S971A/S971A MEFs. (F) Expression patterns of p53 and p21 after treatment with γ-irradiation (10 Gy). C and IR, unirradiated and irradiated MEFs, respectively. (G) p53 expression levels in wild-type (W) and Brca1S971A/S971A (M) cells in the absence (CTL) and presence (MNNG) of MNNG for 12 h were measured and compared as described in Materials and Methods. (H) p53 levels in wild-type and Brca1S971A/S971A MEFs from 0 to 12 h after MNNG (250 μM) treatment. (I) Levels of p53 after treatment with MNNG in wild-type and Chk2−/− MEFs generated independently from two different littermates.
Because Brca1S971A/S971A cells exhibited partial loss of the ability to prevent cells from entering M phase upon γ-irradiation while Brca1Δ11/Δ11 cells were defective in the G2/M checkpoint (53), we conclude that S971 of Brca1 mediates a part of the Brca1 function in this checkpoint. Our further analyses also showed that Brca1S971A/S971A cells exhibited no obvious defects in cell proliferation, G1/S cell cycle checkpoint, and S checkpoint (Fig. 6E and data not shown).
We next tested whether the Brca1S971A mutation would cause accumulation of DNA damage in mutant cells due to their impaired DNA damage repair ability. To address this, we stained cells with antibodies to Brca1; γ-H2AX, which is a DNA damage sensor (40); and RAD 51, which displayed diminished focus formation upon γ-irradiation in Brca1Δ11/Δ11 cells (26). Our analysis revealed similar patterns of focus formation in both wild-type and Brca1S971A/S971A cells upon γ-irradiation, indicating that the irradiation resulted in similar damage in both cells (unpublished data). It also suggests that the Brca1S971A mutation does not have a major impact on DNA damage repair. Consistently, we have accessed the efficiency of microhomology-mediated end joining of double-strand breaks in Brca1S971A/S971A and control cells and detected no differences (unpublished data).
Previous Brca1 mutant mouse models have shown that deletion of Brca1 significantly increased the accumulation of p53 upon DNA damage (8). Thus, we next examined the levels of p53 after treatment of cells with γ-irradiation or MNNG (an MNU analogue that appears to be more potent than MNU in vitro). We found that both control and Brca1S971A/S971A MEFs showed similar p53 levels in response to γ-irradiation (Fig. 6F). However, the mutant cells exhibited lower levels (63%) of p53 induction after MNNG treatment, while p21 expression remained similar in MEFs (Fig. 6G). We carefully further analyzed the accumulation of p53 at several time points after MNNG treatment. As shown in Fig. 6H, levels of p53 in the mutant cells appeared lower at all the time points. These results suggest that Brca1-S971 may be involved in the regulation of p53 stabilization upon treatment with MNNG.
It has been shown that the absence of Brca1 or Chk2 attenuates p53 accumulation upon γ-irradiation (45, 51, 52), suggesting an interplay between Brca1 and Chk2 in maintaining p53 stability. However, in Brca1S971A/S971A MEFs, we observed attenuated p53 only after MNNG treatment but not after γ-irradiation. It is difficult to conclude whether this is Chk2 dependent or independent, because of the lack of information about the effect of MNNG treatment on p53 stability in Chk2 mutant cells. Therefore, we next performed a direct comparison of p53 accumulation in Chk2−/− and Brca1S971A/S971A MEFs upon MNNG treatment. We showed that p53 levels were significantly reduced in Chk2−/− MEFs after MNNG treatment in comparison with those of the wild type (Fig. 6I). The extent of reduction in the Chk2 mutant (46% of the wild-type level) appeared more significant than that in Brca1S971A/S971A mice (63% of the wild-type level). These results support the hypothesis that there is a genetic interplay between Brca1 and Chk2 in p53 stability after DNA damage which may vary depending on the type of DNA damage. Specifically, Brca1S971 may mediate the Chk2-dependent pathway only upon MNNG treatment but not irradiation.
DISCUSSION
CHK2 has been considered a low-penetrance tumor suppressor gene in multiple types of tumors (34, 46). Because CHK2 phosphorylates BRCA1 and affects the interaction and localization of BRCA1 after DNA damage, it has been suggested that part of its tumor suppressor function is mediated through its interaction with BRCA1 (28). However, the impact of phosphorylation of CHK2 on BRCA1 functions in vivo has not been determined. Because CHK2 phosphorylates BRCA1 on a unique site (S988 in humans or S971 in mice), we were able to address this question by mutating S971 of Brca1 and studying the biological consequences in mutant mice. We showed that the Brca1S971A/S971A mice were developmentally normal and survived for at least 1.5 years without obvious abnormalities, indicating that loss of this phosphorylation site does not interfere with mouse development. Previous gene-targeting studies showed that a majority of Brca1 mutant mice died during embryonic developmen, except for two mutant strains that survive to adulthood under special circumstances (reviewed in reference 14). In one case, a mutant strain of mice carrying a truncation at the 3′ half of Brca1 exon 11 (Brca1tr/tr) died in an inbred 129 background but survived to adulthood in a 129/B6 background (32). The second strain of mice, which carries a targeted deletion of exon 11 (Brca1Δ11/Δ11), died at later stages of gestation but could survive to adulthood in a p53+/− or p53−/− background (51). Nearly all the mutant mice of both strains developed tumors in multiple organs at later stages of development.
A comparison between Brca1S971A/S971A and Brca1Δ11/Δ11 cells may provide useful clues to biological functions that Brca1-S971 may perform. Our studies indicated that Brca1Δ11/Δ11 cells aredefective in the G2/M cell cycle checkpoint (53). An essential function of this checkpoint is to arrest cells containing damaged DNA in the G2 phase to prevent the segregation of unrepaired DNA to daughter cells. Therefore, loss of Brca1 leads to genetic instability, which might be the basis for the chromosome abnormalities associated with Brca1 mutations. Our analysis of Brca1S971A/S971A mutant cells revealed that this checkpoint is partially lost, suggesting that G2/M checkpoint regulation of Brca1 is partly modulated by Chk2 phosphorylation, but other pathways clearly exist, such as ATM, ATR, and Chk1.
The partial loss of these DNA damage checkpoint controls may account for the spontaneous tumor formation observed in Brca1S971A/S971A mutant mice. The frequencies of tumorigenesis increased to 79 and 93%, respectively, after treatment with γ-irradiation and MNU. It has been shown in cultured HCC1397 cells, a human breast cancer cell line carrying truncated BRCA1, that BRCA1 plays an essential role in the S checkpoint (49). Our analysis of Brca1S971A/S971A cells did not detect an obvious defect in the S checkpoint, suggesting that other domains rather than exon 11 are important for activation of this checkpoint. This is consistent with an earlier study demonstrating that phosphorylation of S1387 in Brca1 is specifically required for the ATM-mediated S checkpoint after ionizing irradiation (50). S1387 of Brca1 is located in exon 12 (27, 36), which is not altered in Brca1Δ11/Δ11 and Brca1S971A/S971A cells.
Notably, our data revealed that disruption of Brca1 phosphorylation by Chk2 increases the chance of tumor formation. A majority of Brca1S971A/S971A females spontaneously exhibited uterus hyperplasia and ovary abnormalities at ∼2 years of age. After treatment with DNA-damaging agents, Brca1S971A/S971A mice became highly susceptible to tumorigenesis. Brca1S971A/S971A females did not form mammary tumors spontaneously, while four out of six female mice examined at 2 years of age exhibited very dense branches in the mammary glands with small hyperplasic foci. Mammary tumor formation in Brca1S971A/S971A mice dramatically increased after γ-irradiation. We found that 3 out of 19 Brca1S971A/S971A mice and 1 out of 5 Brca1KO/S971A mice developed mammary tumors, while no control mice did (n = 19). Hyperplasic foci in mammary glands were also detected in four out of five mice >300 days after γ-irradiation, suggesting that focus formation in the mammary gland is also accelerated by γ-irradiation.
An interesting finding of these studies is endometrial tumor formation in Brca1S971A/S971A female mice. The predominant abnormalities of mutant mice are uterus hyperplasia and ovary abnormalities. In addition, 6 out of 15 MNU-treated mice developed uterus and colon cancers. These phenotypes were not extensively studied in previously generated Brca1 mutant mouse models, although 3 out of 103 tumors were formed in the uterus and colon in Brca1tr/tr mice (32). One possibility to account for the higher incidence of endometrial tumor formation in Brca1S971A/S971A mice than in other mice is that other Brca1 mutant mice did not survive long enough for various reasons (14) while Brca1S971A/S971A mice have a virtually normal life span, which allows the development of these phenotypes. The role of BRCA1 in endometrial carcinoma has been investigated; however, different studies reached different conclusions. Niederacher et al. (38) examined 113 archival endometrial cancer samples and found that 18.1% carried BRCA1 mutations. Moreover, loss of heterozygosity of BRCA1 correlated significantly with a decreased overall survival rate of patients (38). In contrast, Levine et al. (29) investigated 199 Ashkenazi Jewish patients with endometrial cancers by screening three BRCA founder mutations (185delAG and 5382insC in BRCA1 and 6174delT in BRCA2) and found that only 3 of the 199 patients (1.5%) had BRCA1 or BRCA2 mutations (29).
Recently, Meijers-Heijboer et al. (35) defined a subset of families with hereditary breast cancer characterized by the presence of colorectal cancer cases (HBCC). In these families, disruption of the CHK2 pathway by 1100delC was present in 18% of 55 families with HBCC compared with 4% of 380 families with breast cancer but without colorectal cancer (35). Our studies of Brca1S971A/S971A mice indicate that mutant mice displayed spontaneous hyperplasia in their uteri and developed mammary tumors and endometrial tumors upon treatment with DNA-damaging agents. However, the risk of endometrial cancer in Brca1S971A/S971A mice varies under different conditions, appearing higher after MNU (38%) than γ-irradiation (7%) treatment, while mammary tumors arise only after γ-irradiation. Thus, these studies suggested that the influence of BRCA1 mutations on endometrial cancer vary significantly in different genetic and environmental backgrounds.
In summary, Brca1 is a large protein with multiple functional domains that interact with many proteins (15). In our continuous efforts to address the biological functions of Brca1, we specifically mutated S971, which is a unique site phosphorylated by Chk2. Our data indicate that S971-Brca1 might play a specific role in the G2/M cell cycle checkpoint but not in the S and G1/S cell cycle checkpoints. We also detected a specific role of Brca1 S971 in mediating p53 accumulation after treatment with some types of DNA-damaging agents, such as MNNG, but not others, such as γ-irradiation. Thus, the impact of the Brca1S971A/S971A mutation is much milder than those in any other BRCA1 mutant mice reported so far (14). With long latency, Brca1S971A/S971A mice exhibited uterus hyperplasia and were predisposed to spontaneous tumorigenesis. The mutant mice are also highly susceptible to tumorigenesis after carcinogen treatment. This model should be useful for studies of functional modulation of Brca1 by Chk2 upon DNA damage response and for studies of HBCC.
Acknowledgments
We thank H. Westphal for EIIa-Cre mice, N. Motoyama for Chk2 mutant mice, and S. Lee and members of the Deng laboratory for critically reading the manuscript.
REFERENCES
- 1.Alberg, A. J., and K. J. Helzlsouer. 1997. Epidemiology, prevention, and early detection of breast cancer. Curr. Opin. Oncol. 9:505-511. [DOI] [PubMed] [Google Scholar]
- 2.Allinen, M., P. Huusko, S. Mantyniemi, V. Launonen, and R. Winqvist. 2001. Mutation analysis of the CHK2 gene in families with hereditary breast cancer. Br. J. Cancer 85:209-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow, C., M. A. Eckhaus, A. A. Schaffer, and A. Wynshaw-Boris. 1999. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat. Genet. 21:359-360. [DOI] [PubMed] [Google Scholar]
- 4.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. [DOI] [PubMed] [Google Scholar]
- 5.Bell, D. W., J. M. Varley, T. E. Szydlo, D. H. Kang, D. C. Wahrer, K. E. Shannon, M. Lubratovich, S. J. Verselis, K. J. Isselbacher, J. F. Fraumeni, J. M. Birch, F. P. Li, J. E. Garber, and D. A. Haber. 1999. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286:2528-2531. [DOI] [PubMed] [Google Scholar]
- 6.Brodie, S. G., X. Xu, W. Qiao, W. M. Li, L. Cao, and C. X. Deng. 2001. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene 20:7514-7523. [DOI] [PubMed] [Google Scholar]
- 7.Brody, L. C., and B. B. Biesecker. 1998. Breast cancer susceptibility genes. BRCA1 and BRCA2. Medicine 77:208-226. [DOI] [PubMed] [Google Scholar]
- 8.Cao, L., W. Li, S. Kim, B. G. Brodie, and C. X. Deng. 2003. Senescence, ageing and malignant transformation mediated by p53 in mice lacking Brca1 exon 11 isoform. Genes Dev. 17:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., R. Adar, X. Yang, E. O. Monsonego, C. Li, P. V. Hauschka, A. Yayon, and C. X. Deng. 1999. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J. Clin. Investig. 104:1517-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortez, D., Y. Wang, J. Qin, and S. J. Elledge. 1999. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286:1162-1166. [DOI] [PubMed] [Google Scholar]
- 11.Deng, C., A. Wynshaw-Boris, F. Zhou, A. Kuo, and P. Leder. 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911-921. [DOI] [PubMed] [Google Scholar]
- 12.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675-684. [DOI] [PubMed] [Google Scholar]
- 13.Deng, C. X. 2002. Roles of BRCA1 in centrosome duplication. Oncogene 21:6222-6227. [DOI] [PubMed] [Google Scholar]
- 14.Deng, C. X. 2002. Tumor formation in Brca1 conditional mutant mice. Environ. Mol. Mutagen. 39:171-177. [DOI] [PubMed] [Google Scholar]
- 15.Deng, C. X., and S. G. Brodie. 2000. Roles of BRCA1 and its interacting proteins. Bioessays 22:728-737. [DOI] [PubMed] [Google Scholar]
- 16.Deng, C. X., and R. H. Wang. 2003. Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum. Mol. Genet. 12:R113-R123. [DOI] [PubMed] [Google Scholar]
- 17.Deng, C. X., A. Wynshaw-Boris, M. M. Shen, C. Daugherty, D. M. Ornitz, and P. Leder. 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 8:3045-3057. [DOI] [PubMed] [Google Scholar]
- 18.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 19.Farber, R. A., T. D. Petes, M. Dominska, S. S. Hudgens, and R. M. Liskay. 1994. Instability of simple sequence repeats in a mammalian cell line. Hum. Mol. Genet. 3:253-256. [DOI] [PubMed] [Google Scholar]
- 20.Fu, L., H. Pelicano, J. Liu, P. Huang, and C. Lee. 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111:41-50. [DOI] [PubMed] [Google Scholar]
- 21.Gatei, M., D. Young, K. M. Cerosaletti, A. Desai-Mehta, K. Spring, S. Kozlov, M. F. Lavin, R. A. Gatti, P. Concannon, and K. Khanna. 2000. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Genet. 25:115-119. [DOI] [PubMed] [Google Scholar]
- 22.Hangaishi, A., S. Ogawa, Y. Qiao, L. Wang, N. Hosoya, K. Yuji, Y. Imai, K. Takeuchi, S. Miyawaki, and H. Hirai. 2002. Mutations of Chk2 in primary hematopoietic neoplasms. Blood 99:3075-3077. [DOI] [PubMed] [Google Scholar]
- 23.Haruki, N., H. Saito, Y. Tatematsu, H. Konishi, T. Harano, A. Masuda, H. Osada, Y. Fujii, and T. Takahashi. 2000. Histological type-selective, tumor-predominant expression of a novel CHK1 isoform and infrequent in vivo somatic CHK2 mutation in small cell lung cancer. Cancer Res. 60:4689-4692. [PubMed] [Google Scholar]
- 24.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287:1824-1827. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann, W. K., C. W. Miller, K. Tsukasaki, S. Tavor, T. Ikezoe, D. Hoelzer, S. Takeuchi, and H. P. Koeffler. 2001. Mutation analysis of the DNA-damage checkpoint gene CHK2 in myelodysplastic syndromes and acute myeloid leukemias. Leukoc. Res. 25:333-338. [DOI] [PubMed] [Google Scholar]
- 26.Huber, L. J., T. W. Yang, C. J. Sarkisian, S. R. Master, C. X. Deng, and L. A. Chodosh. 2001. Impaired DNA damage response in cells expressing an exon 11-deleted murine Brca1 variant that localizes to nuclear foci. Mol. Cell. Biol. 21:4005-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane, T. F., C. Deng, A. Elson, M. S. Lyu, C. A. Kozak, and P. Leder. 1995. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 9:2712-2722. (Erratum, 10:365, 1996.) [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. S., K. M. Collins, A. L. Brown, C. H. Lee, and J. H. Chung. 2000. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404:201-204. [DOI] [PubMed] [Google Scholar]
- 29.Levine, D. A., O. Lin, R. R. Barakat, M. E. Robson, D. McDermott, L. Cohen, J. Satagopan, K. Offit, and J. Boyd. 2001. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol. Oncol. 80:395-398. [DOI] [PubMed] [Google Scholar]
- 30.Linke, S. P., K. C. Clarkin, and G. M. Wahl. 1997. p53 mediates permanent arrest over multiple cell cycles in response to gamma-irradiation. Cancer Res. 57:1171-1179. [PubMed] [Google Scholar]
- 31.Lou, Z., C. C. Chini, K. Minter-Dykhouse, and J. Chen. 2003. Mediator of DNA damage checkpoint protein 1 regulates BRCA1 localization and phosphorylation in DNA damage checkpoint control. J. Biol. Chem. 278:13599-13602. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig, T., P. Fisher, S. Ganesan, and A. Efstratiadis. 2001. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 15:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijers-Heijboer, H., A. van den Ouweland, J. Klijn, M. Wasielewski, A. de Snoo, R. Oldenburg, A. Hollestelle, M. Houben, E. Crepin, M. van Veghel-Plandsoen, F. Elstrodt, C. van Duijn, C. Bartels, C. Meijers, M. Schutte, L. McGuffog, D. Thompson, D. Easton, N. Sodha, S. Seal, R. Barfoot, J. Mangion, J. Chang-Claude, D. Eccles, R. Eeles, D. G. Evans, R. Houlston, V. Murday, S. Narod, T. Peretz, J. Peto, C. Phelan, H. X. Zhang, C. Szabo, P. Devilee, D. Goldgar, P. A. Futreal, K. L. Nathanson, B. Weber, N. Rahman, and M. R. Stratton. 2002. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat. Genet. 31:55-59. [DOI] [PubMed] [Google Scholar]
- 35.Meijers-Heijboer, H., J. Wijnen, H. Vasen, M. Wasielewski, A. Wagner, A. Hollestelle, F. Elstrodt, R. van den Bos, A. de Snoo, G. T. Fat, C. Brekelmans, S. Jagmohan, P. Franken, P. Verkuijlen, A. van den Ouweland, P. Chapman, C. Tops, G. Moslein, J. Burn, H. Lynch, J. Klijn, R. Fodde, and M. Schutte. 2003. The CHEK2 1100delC mutation identifies families with a hereditary breast and colorectal cancer phenotype. Am. J. Hum. Genet. 72:1308-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miki, Y., J. Swensen, D. Shattuck-Eidens, P. A. Futreal, K. Harshman, S. Tavtigian, Q. Liu, C. Cochran, L. M. Bennett, W. Ding, et al. 1994. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66-71. [DOI] [PubMed] [Google Scholar]
- 37.Miller, C. W., T. Ikezoe, U. Krug, W. K. Hofmann, S. Tavor, V. Vegesna, K. Tsukasaki, S. Takeuchi, and H. P. Koeffler. 2002. Mutations of the CHK2 gene are found in some osteosarcomas, but are rare in breast, lung, and ovarian tumors. Genes Chromosomes Cancer 33:17-21. [DOI] [PubMed] [Google Scholar]
- 38.Niederacher, D., H. X. An, S. Camrath, S. I. Dominik, U. J. Gohring, A. Oertel, M. Grass, P. Hantschmann, M. R. Lordnejad, and M. W. Beckmann. 1998. Loss of heterozygosity of BRCA1, TP53 and TCRD markers analysed in sporadic endometrial cancer. Eur. J. Cancer 34:1770-1776. [DOI] [PubMed] [Google Scholar]
- 39.Okada, S., and T. Ouchi. 2003. Cell cycle differences in DNA damage-induced BRCA1 phosphorylation affect its subcellular localization. J. Biol. Chem. 278:2015-2020. [DOI] [PubMed] [Google Scholar]
- 40.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 41.Reddy, A., M. Yuille, A. Sullivan, C. Repellin, A. Bell, J. A. Tidy, D. J. Evans, P. J. Farrell, B. Gusterson, M. Gasco, and T. Crook. 2002. Analysis of CHK2 in vulval neoplasia. Br. J. Cancer. 86:756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruffner, H., and I. M. Verma. 1997. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc. Natl. Acad. Sci. USA 94:7138-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silvers, W. K. 1979. The coat colors of mice. Springer-Verlag, New York, N.Y.
- 44.Sullivan, A., M. Yuille, C. Repellin, A. Reddy, O. Reelfs, A. Bell, B. Dunne, B. A. Gusterson, P. Osin, P. J. Farrell, I. Yulug, A. Evans, T. Ozcelik, M. Gasco, and T. Crook. 2002. Concomitant inactivation of p53 and Chk2 in breast cancer. Oncogene 21:1316-1324. [DOI] [PubMed] [Google Scholar]
- 45.Takai, H., K. Naka, Y. Okada, M. Watanabe, N. Harada, S. Saito, C. W. Anderson, E. Appella, M. Nakanishi, H. Suzuki, K. Nagashima, H. Sawa, K. Ikeda, and N. Motoyama. 2002. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21:5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varley, J., and D. A. Haber. 2003. Familial breast cancer and the hCHK2 1100delC mutation: assessing cancer risk. Breast Cancer Res. 5:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaughn, J. P., P. L. Davis, M. D. Jarboe, G. Huper, A. C. Evans, R. W. Wiseman, A. Berchuck, J. D. Iglehart, P. A. Futreal, and J. R. Marks. 1996. BRCA1 expression is induced before DNA synthesis in both normal and tumor-derived breast cells. Cell Growth Differ. 7:711-715. [PubMed] [Google Scholar]
- 48.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 49.Xu, B., S. Kim, and M. B. Kastan. 2001. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, B., A. H. O'Donnell, S. T. Kim, and M. B. Kastan. 2002. Phosphorylation of serine 1387 in Brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res. 62:4588-4591. [PubMed] [Google Scholar]
- 51.Xu, X., W. Qiao, S. P. Linke, L. Cao, W. M. Li, P. A. Furth, C. C. Harris, and C. X. Deng. 2001. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat. Genet. 28:266-271. [DOI] [PubMed] [Google Scholar]
- 52.Xu, X., K. U. Wagner, D. Larson, Z. Weaver, C. Li, T. Ried, L. Hennighausen, A. Wynshaw-Boris, and C. X. Deng. 1999. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22:37-43. [DOI] [PubMed] [Google Scholar]
- 53.Xu, X., Z. Weaver, S. P. Linke, C. Li, J. Gotay, X. W. Wang, C. C. Harris, T. Ried, and C. X. Deng. 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3:389-395. [DOI] [PubMed] [Google Scholar]
- 54.Yang, X., J. J. Letterio, R. J. Lechleider, L. Chen, R. Hayman, H. Gu, A. B. Roberts, and C. Deng. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 18:1280-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, J., H. Willers, Z. Feng, J. C. Ghosh, S. Kim, D. T. Weaver, J. H. Chung, S. N. Powell, and F. Xia. 2004. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 24:708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng, L., S. Li, T. G. Boyer, and W. H. Lee. 2000. Lessons learned from BRCA1 and BRCA2. Oncogene 19:6159-6175. [DOI] [PubMed] [Google Scholar]
- 57.Zhong, Q., C. F. Chen, P. L. Chen, and W. H. Lee. 2002. BRCA1 facilitates microhomology-mediated end joining of DNA double strand breaks. J. Biol. Chem. 277:28641-28647. [DOI] [PubMed] [Google Scholar]
- 58.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]