Abstract
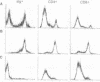
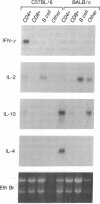
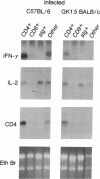
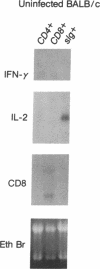
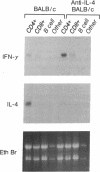
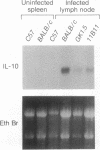
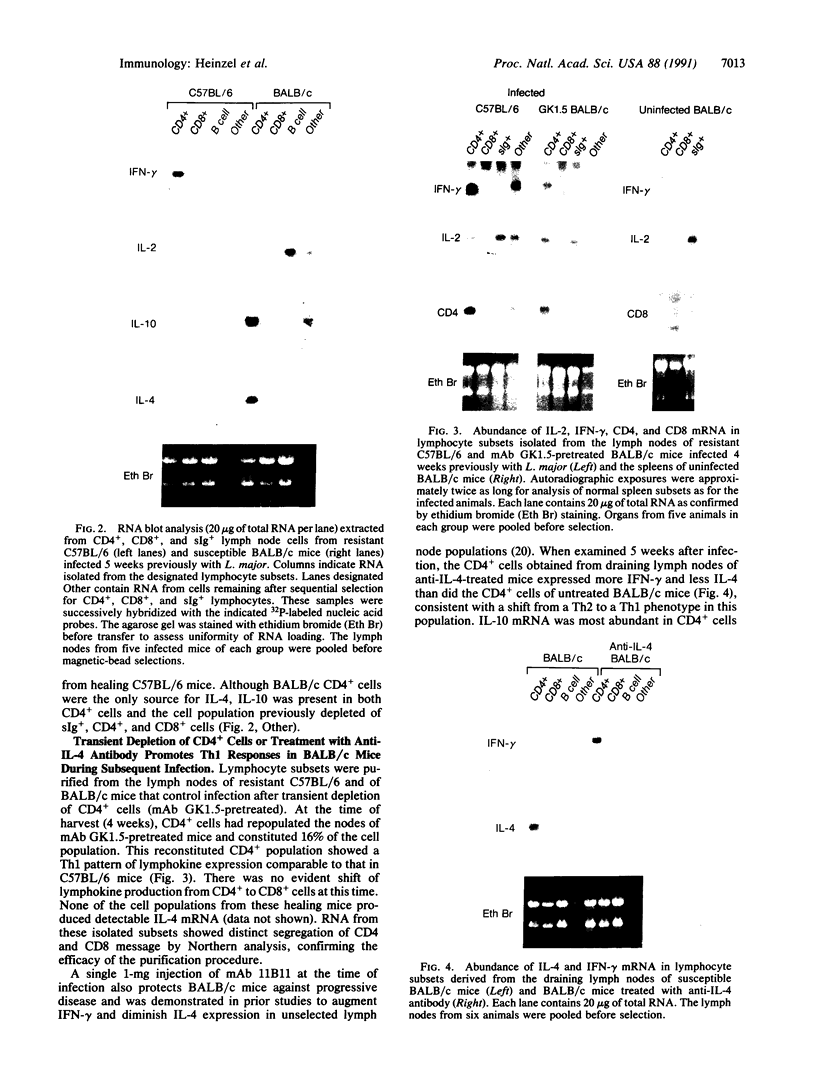
The expression of interleukin (IL) 2, IL-4, IL-10, and interferon gamma (IFN-gamma) by lymphocyte subsets was examined during infection of resistant C57BL/6 and susceptible BALB/c mice with the protozoan parasite Leishmania major. CD4+ and CD8+ T lymphocytes and B lymphocytes were isolated from the lymph nodes draining infectious lesions, and their RNA was examined for lymphokine transcripts. Distinct patterns of CD4+ cell cytokine expression were apparent: C57BL/6 CD4+ cells contained IFN-gamma and IL-2 mRNA, whereas BALB/c CD4+ cells expressed IL-4 and IL-10 message. CD8+ cells contributed little lymphokine expression during disease, but B cells were a major source of IL-2 mRNA in both strains of mice. BALB/c mice made resistant by treatment with anti-CD4 antibody at the time of infection repopulated lymph nodes with CD4+ cells that expressed IL-2 and IFN-gamma. Protective treatment with anti-IL-4 antibody in vivo also resulted in the appearance of CD4+ cells with increased IFN-gamma and diminished IL-4 and IL-10 expression. These data establish CD4+ cells as the primary source of IFN-gamma in healing mice and of IL-4 and IL-10 during progressive infection and confirm that the spectral extremes of this disease are characterized by the presence of CD4+ cells expressing Th1 or Th2 phenotypes in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990 Aug 15;145(4):1127–1136. [PubMed] [Google Scholar]
- Brunt L. M., Portnoy D. A., Unanue E. R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990 Dec 1;145(11):3540–3546. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J. P., Muller I., Louis J. A. A role for Lyt-2+ T cells in resistance to cutaneous leishmaniasis in immunized mice. J Immunol. 1989 Mar 15;142(6):2052–2056. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Gessner A., Moskophidis D., Lehmann-Grube F. Enumeration of single IFN-gamma-producing cells in mice during viral and bacterial infection. J Immunol. 1989 Feb 15;142(4):1293–1298. [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Awwad M., North R. J. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. J Exp Med. 1989 May 1;169(5):1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Gettner S. N. Unusual intron in the immunoglobulin domain of the newly isolated murine CD4 (L3T4) gene. 1987 Jan 29-Feb 4Nature. 325(6103):453–455. doi: 10.1038/325453a0. [DOI] [PubMed] [Google Scholar]
- Lohoff M., Sommer F., Solbach W., Röllinghoff M. Coexistence of antigen-specific TH1 and TH2 cells in genetically susceptible BALB/c mice infected with Leishmania major. Immunobiology. 1989 Oct;179(4-5):412–421. doi: 10.1016/S0171-2985(89)80045-2. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Farrell J. P., Louis J. T-cell responses and immunity to experimental infection with leishmania major. Annu Rev Immunol. 1989;7:561–578. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- Peleman R., Wu J., Fargeas C., Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989 Nov 1;170(5):1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Shigekane V. M., Fisher W. L., Locksley R. M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987 Aug 15;139(4):1303–1309. [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Sher A. B lymphocytes are required for the generation of T cells that mediate healing of cutaneous leishmaniasis. J Immunol. 1986 Aug 1;137(3):1017–1021. [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Taira S., Matsui M., Hayakawa K., Yokoyama T., Nariuchi H. Interleukin secretion by B cell lines and splenic B cells stimulated with calcium ionophore and phorbol ester. J Immunol. 1987 Nov 1;139(9):2957–2964. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Milon G., Marchal G., Vassalli P., Cerottini J. C., Louis J. A. Involvement of specific Lyt-2+ T cells in the immunological control of experimentally induced murine cutaneous leishmaniasis. Eur J Immunol. 1987 Oct;17(10):1429–1433. doi: 10.1002/eji.1830171007. [DOI] [PubMed] [Google Scholar]
- Zwingenberger K., Harms G., Pedrosa C., Omena S., Sandkamp B., Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin Immunol Immunopathol. 1990 Nov;57(2):242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]