Abstract
DNA replication depends critically upon chromatin structure. Little is known about how the replication complex overcomes the nucleosome packages in chromatin during DNA replication. To address this question, we investigate factors that interact in vivo with the principal initiation DNA polymerase, DNA polymerase α (Polα). The catalytic subunit of budding yeast Polα (Pol1p) has been shown to associate in vitro with the Spt16p-Pob3p complex, a component of the nucleosome reorganization system required for both replication and transcription, and with a sister chromatid cohesion factor, Ctf4p. Here, we show that an N-terminal region of Polα (Pol1p) that is evolutionarily conserved among different species interacts with Spt16p-Pob3p and Ctf4p in vivo. A mutation in a glycine residue in this N-terminal region of POL1 compromises the ability of Pol1p to associate with Spt16p and alters the temporal ordered association of Ctf4p with Pol1p. The compromised association between the chromatin-reorganizing factor Spt16p and the initiating DNA polymerase Pol1p delays the Pol1p assembling onto and disassembling from the late-replicating origins and causes a slowdown of S-phase progression. Our results thus suggest that a coordinated temporal and spatial interplay between the conserved N-terminal region of the Polα protein and factors that are involved in reorganization of nucleosomes and promoting establishment of sister chromatin cohesion is required to facilitate S-phase progression.
The DNA polymerase α-primase complex is unique among the eukaryotic replicative DNA polymerases in that it can initiate de novo DNA synthesis at the replication origin and also initiates Okazaki fragment synthesis on the lagging strand throughout S phase (7, 61, 63). Due to this unique property, mutations in the Polα gene that encodes the catalytic subunit of the DNA polymerase α-primase complex have significant effects on many cellular processes. These processes include repair and recombination in both mitotic and meiotic cells (3, 27, 34, 55), epigenetic regulation of transcriptional silencing (43), checkpoint activation (8, 13), telomere length homeostasis (1, 2, 10, 11, 12), and mutation avoidance (21, 31, 33, 36). These findings suggest that proper interactions between Polα and various cellular proteins during replication are important for maintaining cells' genomic integrity.
Several lines of evidence suggest that chromatin structure plays a critical role in the initiation and progression of S phase (4, 5, 28, 29, 35). It remains unclear at the molecular level which cellular factors that modulate chromatin structure are involved in facilitating initiation and progression of S phase. Polα (Pol1p) of budding yeast has been used as an affinity matrix to identify Polα (Pol1p)-associated cellular proteins in vitro, and about six potential binding partners have been identified (42). One of these, the Ctf4p protein, was first identified in a genetic screen for mutants affecting chromosome transmission fidelity (24, 32). CTF4 is not essential for budding yeast viability; however, cells lacking CTF4 are hypersensitive to DNA-damaging agents, such as methyl methanesulfonate, and drugs that inhibit S-phase progression, such as hydroxyurea, and cells with a CTF4 deletion cannot tolerate otherwise nonlethal mutations in DNA replication factor genes (17). Ctf4p and Ctf18p are required for sister chromatid cohesion and are thought to act in association with the replication fork to facilitate sister chromatid cohesion (22, 23, 26, 40, 41, 44, 47). The fission yeast CTF4 homologue is mcl1+, which is essential for fission yeast viability. Fission yeast cells with a mutation in mcl1+ are sensitive to DNA damage and exhibit a chromosome missegregation phenotype, and these mutations display synthetic lethality with mutations in the DNA checkpoint genes rad3 and rad26. Furthermore, overexpression of mcl1+ causes an S-phase delay (66).
Another protein that bound to the Polα (Pol1p) affinity matrices was the product of CDC68/SPT16 (hereafter termed SPT16) (42, 68). SPT16 was previously identified in several budding yeast genetic screens as an essential factor involved in regulation of transcription and promotion of the cell cycle (9, 38, 39, 49, 51, 67). In budding yeast, Spt16p is found in the nucleus as a stable heterodimer with the product of another essential gene, POB3 (9, 14, 69, 72). The Spt16p-Pob3p complex is conserved from yeast to human, with the human homologue being the transcription elongation factor, FACT (45, 46). Human FACT promotes progression of RNA polymerase II through nucleosomes in vitro and interacts with nucleosomes and with histones, supporting a model that suggests that FACT reorganizes nucleosomes to a form that is less inhibitory to the passage of RNA polymerases. Mutations in SPT16 or POB3 in budding yeast cause phenotypes that are consistent with the proposed model. Cells lacking Spt16p-Pob3p function are unable to perform initiation and elongation of transcription normally (38, 49, 67). This finding is thought to be because spt16 or pob3 mutants are unable to reorganize chromatin appropriately (15, 18, 39, 53, 67, 69, 71).
Genetic evidence has also implicated the Spt16p-Pob3p complex in chromosome replication (42, 68, 69). Mutations in either SPT16 or POB3 display genetic interactions with mutations in pol1 and dna2 and with mutations in ctf4 and ctf18 (17, 52, 68, 69). Further, binding of Spt16p-Pob3p to a Polα (Pol1p) affinity matrix was enhanced when extracts lacked Ctf4p, suggesting that Spt16p-Pob3p and Ctf4p compete with one another for binding to Polα (Pol1p) (68). Together, these biochemical and genetic results suggest that the reorganization of nucleosomes promoted by Spt16p-Pob3p is important for both DNA and RNA polymerases acting in replication and transcription (15).
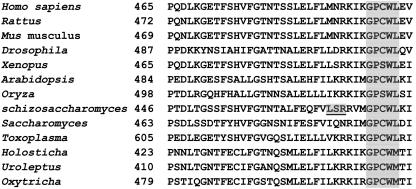
Polα is a member of the B-family (α-like) polymerases. Polα proteins from yeast to humans all contain six highly conserved domains (25, 30, 64). Evidence from mutagenesis and structural analyses has indicated that these six highly conserved domains of Polα are involved in binding the deoxynucleoside triphosphate and the DNA primer-template substrate as well as in binding the metal ion required for polymerase catalytic activity (20, 62, 63). Aside from these catalytic domains, there is a region toward the N terminus of the protein that is highly conserved among Polα homologues from various phyla (Fig. 1). Previous genetic studies of fission yeast polα+ and budding yeast POL1 have shown that mutations in this conserved N-terminal region induce genomic instability manifested in the following ways: (i) a mutator phenotype leading to high frequencies of microsatellite instability, point mutations, single-base frameshifts, and deletions of sequences flanked by short direct repeats (21, 36); (ii) elevated frequencies of chromosome loss (21); and (iii) compromised telomere homeostasis, compromised physical association with the telomerase catalytic subunit (Trt1), and a reduced telomere position effect (11). These findings suggest that this conserved N-terminal region of Polα (Pol1p) is involved in interacting with various cellular factors and that the interaction is important for maintenance of genomic stability.
FIG. 1.
Primary sequence alignment of an N-terminal region of DNA Polα catalytic subunit from various species. Included in this alignment are polymerases from protists (Toxoplasma gondii, Holosticha, Uroleptus, and Oxytricha), fungi (Saccharomyces cerevisiae and Schizosaccharomyces pombe), plants (Arabidopsis thaliana and Oryza), an insect (Drosophila), and vertebrates (Xenopus, Mus musculus, Rattus, and Homo sapiens). Residues shown in shading are a highly conserved amino acid cluster in the region; the three deleted residues in the fission yeast polαts13 allele are underlined and shaded.
To further explore which cellular proteins that interact with this N-terminal conserved region may have an effect on S-phase progression, we generated a panel of pol1 mutants harboring mutations in a cluster of highly conserved residues in this N-terminal region. We show in this study that Spt16p-Pob3p and Ctf4p both interact with this conserved N-terminal region of Polα (Pol1p). Mutation in glycine493 in this cluster of residues compromises the interaction between Polα (Pol1p) and Spt16p-Pob3p, dramatically alters the temporal ordered interaction between Polα (Pol1p) and Ctf4p, and causes a delay in assembling Polα (Pol1p) onto and disassembling it from late-replicating origins, resulting in a slowdown of S-phase progression. Our results thus reveal that robust DNA replication requires a coordinated temporal and spatial interplay between the replication machinery and factors that reorganize nucleosomes and promote establishment of sister chromatin cohesion.
MATERIALS AND METHODS
Yeast strains and methods.
Yeast strains used in this study are listed in Table 1. Pol1p-TAP, Spt16p-13Xmyc and Spt16p-cyan fluorescent protein (CFP), Ctf4p-3XHA, and Rad53p-13Xmyc in their corresponding strains were expressed from their endogenous promoters at their respective chromosomal loci. Expression of these tagged genes did not cause growth defect. Strains used for α-factor arrest experiments contained the bar1Δ mutation, which was introduced by replacing the endogenous BAR1 gene with URA3 followed by removing URA3 by 5-fluoroorotic acid selection. α-Factor arrest was performed by incubating each culture at 25°C for 3 h with 100 ng of pheromone/ml (US Biologicals) and then releasing the cells to fresh yeast extract-peptone-dextrose (YPD) medium at 22°C.
TABLE 1.
Yeast strains
| Strain | Genotype | Source or reference |
|---|---|---|
| DFBP1 | MATa/MATα POL1/pol1 Δ ura3-52/ura3-52 leu2/leu2 | 48 |
| DFBCUp/3d | MATapol1Δ1 ura3-52 leu2+ [Cup91] | 19 |
| DFS4/5a | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 pol1-1 | 19 |
| PGY210 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 | 21 |
| PGY300 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 pol1-17 | 21 |
| ZYJ100 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::POL1-TAP-TRP1 | This study |
| ZYJ200 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::pol1-1-TAP-TRP1 | This study |
| ZYJ101 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 pol1::POL1-TAP-TRP1 rad53::RAD53-13MYC-KAN | This study |
| ZYJ201 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 pol1::pol1-1-TAP-TRP1 rad53::RAD53-13MYC-KAN | This study |
| ZYJ110 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::POL1-TAP-TRP1 spt16::SPT16-13MYC-KAN ctf4::CTF4-3HA-URA3 | This study |
| ZYJ210 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::pol1-1-TAP-TRP1 spt16::SPT16-13MYC-KAN ctf4::CTF4-3HA-URA3 | This study |
| ZYJ120 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::POL1-TAP-TRP1 spt16::SPT16-CFP-KAN | This study |
| ZYJ220 | MATaura3 trp1-1 pep4::HIS3 prb1::LEU2 bar1 pol1::pol1-1-TAP-TRP1 spt16::SPT16-CFP-KAN | This study |
| SKY48/pLacGUS | MATaura3 trp1 his3 6lexAop-LEU23cIop-LYS2 pLacGUS(ura3) | Invitrogen |
Mutations in the N-terminal region of pol1 were constructed by plasmid shuffling with DFBCUp/3d, a segregant from diploid DFBP1 transformed with the plasmid Cup91 (19). DFBCUp/3d has a deletion of POL1 at the chromosomal locus and is sustained by the plasmid Cup91, which contains URA3 and the full-length POL1 gene expressed from its endogenous promoter. DFBCUp/3d was transformed with two DNA fragments; one contains a 462-bp PCR fragment spanning nucleotides 1189 to 1651 of the POL1 open reading frame and carrying a pol1 mutation generated by site-directed mutagenesis. The other is a plasmid, pRS315-POL1 (with LEU2), digested with BspEI to create a gap. In order to create the BspEI site in plasmid pRS315-POL1, a mutation in POL1 was first introduced at nucleotide position 1041 to abolish the endogenous BspEI site, and then mutations at position 1311 and at positions 1521 and 1524 were introduced to create two BspEI sites. Ura+ Leu+ transformants were placed on 5-fluoroorotic acid to select cells that lose Cup91 and contain the insertion of a mutated fragment in the gapped POL1 plasmid which complements the chromosomal deletion of the POL1 gene. Recovery of the pRS315-pol1 plasmids and sequence analysis were then performed to confirm the expected mutation.
Flow cytometry analysis.
Cells (107) grown in YPD medium were harvested and fixed with 70% ethanol overnight. After being washed with 1 ml of 50 mM Tris-HCl (pH 7.5), cells were resuspended in 0.5 ml of Tris-HCl (pH 7.5) containing 1-mg of RNaseA/ml, incubated at 37°C for 2 h, and then further treated with 1 μl of 20-mg/ml proteinase K at 55°C for 1 h. Cells were then washed, resuspended in phosphate-buffered saline buffer with 50 μg of propidium iodide/ml, and analyzed in a Beckman Coulter fluorescence-activated cell sorter (FACS).
Chromatin immunoprecipitation.
Three hundred-milliliter cultures at 2.5 × 107 cells/ml were synchronized by using 100 ng of α-factor/ml for 3 h at 25°C and released into YPD medium at 22°C. Cells (5 × 108) were removed for analysis at 10-min intervals. Chromatin immunoprecipitation (CHIP) was performed essentially as described previously (6), with minor modifications. TAP-tagged Pol1p from POL1 and the pol1-1 mutant were immunoprecipitated with rabbit immunoglobulin G (IgG)-agarose beads (Sigma). The following sequences are those of the primers used for PCR: ARS1-1, 5′-GGTGAAATGGTAAAAGTCAACCCCCTGCG-3′; ARS1-2, 5′-GCTGGTGGACTGACGCCAGAAAATGTT-3′; ARS305-1, 5′-CTCCGTTTTTAGCCCCCCGTG-3′; ARS305-2, 5′-GATTGAGGCCACAGCAAGACCG-3′; ARS501-1, 5′-CTTTTTTAATGAAGATGACATTGCTCC-3′; ARS501-2, 5′-GATGATGATGAGGAGCTCCAATC-3′; ARS603-1, 5′-CTCTTTCCCAGATGATATCTAGATGG-3′; and ARS603-2, 5′-CGAGGCTAAATTAGAATTTTTGAAGTC-3′. PCR products in the 200-to-400-bp size range were then separated on 2% agarose gels and detected by ethidium bromide staining.
Suppressor screen.
A yeast genomic library in YEp24 was transformed into strain DFS4/5a, which carries a pol1-1 mutation (19); cells were then plated onto selective medium at 34°C, which is restrictive for DFS4/5a growth. After several rounds of testing for growth at 34°C, plasmids were rescued from positive transformants, retransformed back to DFS4/5a to confirm the linkage, and sequenced.
Two-hybrid analysis.
Two-hybrid analysis was carried out by using the Invitrogen Hybrid hunter system. The N-terminal fragments of POL1 and pol1-1, from Met1 to Lys550, were amplified by PCR and inserted into the bait vector pHybLex/Zeo, while the full-length SPT16, CTF4, and POB3 genes were individually cloned into prey vector pYESTrp2. The constructed bait plasmids and prey plasmids were transformed pairwise into strain SKY48/pLacGUS selected on yeast minimal defined medium plates containing 200 μg of Zeocin/ml but not tryptophan. The selected transformants were then grown on galactose medium to induce the expression of the β-galactosidase activity and selected for leucine prototrophy. pHybLex/Zeo-Fos2, pYESTrp-Jun, and pYESTrp-RalGDS were used as positive and negative controls for this analysis.
Immunoprecipitation and immunoblotting.
Logarithmically growing cells (3 × 108 in 20 ml of YPD medium) were harvested, washed with phosphate-buffered saline buffer, and resuspended into 600 μl of prechilled cell extraction buffer (6 mM Na2HPO4, 4 mM NaH2PO4, 1% NP-40, 200 mM NaCl, 2 mM EDTA, 50 mM NaF, 4-μg/ml leupeptin, 0.1 mM Na3VO4, complete protease inhibitors [Roche]). Cells were lysed with 500 μl of glass beads (425 to 600 μm; Sigma) by vortexing using a FASTPREP machine (ThermoSavant) for 40 s three times and then centrifuged at 15,000 × g for 20 min. The supernatant was then incubated with 50-μl rabbit IgG-agarose beads on a rotating platform at 4°C for 2 h to immunoprecipitate the TAP-tagged Pol1p. The immunoprecipitates were collected by centrifugation, washed three times with 1 ml of wash buffer (10 mM Tris-HCl [pH 8.0], 200 mM NaCl, 0.5% NP-40), and boiled in 100 μl of sodium dodecyl sulfate sample buffer, and proteins were then fractionated on 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) and detected with rabbit IgG (Sigma) for TAP-tagged Pol1p or with anti-myc monoclonal antibody (9E10) or anti-green fluorescent protein (anti-GFP; Roche) and antihemagglutinin (anti-HA) monoclonal antibody (12CA5) for coprecipitation of Spt16p-13Xmyc or Spt16p-CFP and Ctf4p-3XHA, respectively.
RESULTS
Mutations in the conserved N-terminal region of Polα (Pol1p) induce genomic instability and affect S-phase progression.
In the conserved N-terminal region of the Polα (Pol1p) protein, there is a cluster of five amino acids, 493-GPCWL-497, that are highly conserved among different species (Fig. 1). Studies of budding yeast have identified a pol1 mutant, the pol1-1 mutant, which exhibits instability of the microsatellite (GT)16 tract, point mutations, deletion of sequences flanked by short direct repeats, and an increased rate of chromosome loss (21). The pol1-1 mutant contains a single missense mutation of Gly493 to Arg in the above cluster of five amino acids in the conserved N-terminal region of the POL1 gene (48). Furthermore, a mutation of Gly493 to Glu in pol1 has been previously identified as a hyperrecombination mutant, hpr3 (3).
Studies of thermosensitive polα mutants in fission yeast have identified the polαts13 mutant, exhibiting a mutator phenotype characterized as base substitution and deletion of sequences flanked by short direct repeats (36). This fission yeast mutant carries a deletion of amino acid residues 470-LSR-472 of the Polα protein (8) (Fig. 1), adjacent to the GPCWL region. In the polαts13 mutant, telomere homeostasis is deregulated, and silencing at telomeric loci is reduced (11). Moreover, mutant Polαts13 protein has a significantly reduced ability to associate with the telomerase catalytic subunit (Trt1). These results suggest that deletions of the three residues within the N-terminal region of the Polα protein in the fission yeast polαts13 mutant induce telomeric complex instability (11). These budding yeast and fission yeast results strongly suggest that this conserved N-terminal noncatalytic region of Polα (Pol1p) interacts with various cellular proteins and that the interactions are essential for maintaining genomic stability.
To test the biological function of the highly conserved cluster of residues in this N-terminal region, we constructed 21 strains with pol1 mutations targeting the conserved GPCWL motif. Eight pol1 mutants harbor mutations of Gly493, to Arg, Glu, Asn, Thr, His, Pro, Met, or Ala; six mutant strains contain mutations of Pro494, to Ala, Gly, His, Thr, Asp, or Asn; one mutant strain has a mutation of Cys495 to Tyr; four mutant strains harbor mutations of Trp496, to Leu, Gly, His, or Ala; and two mutant strains have mutations of Leu497, to Arg or Ser. We tested the viability of these mutants at 25, 30, and 34°C to see if the strains are thermosensitive (Fig. 2A). The DNA content profiles of asynchronous cultures of these mutants were analyzed by flow cytometry at 30°C (Fig. 2B). Interestingly, pol1 mutants harboring a mutation in Gly493 to Arg, Glu, Asn, Thr, Pro, His, or Ala all exhibited different extents of thermosensitivity at 30 and 34°C, whereas the pol1 mutant with the Gly493-to-Met mutation was not thermosensitive at either of these temperatures (Fig. 2A). pol1 mutants containing the mutations in Pro494, with the exception of the mutant harboring the mutation Pro494 to Asp, were not significantly thermosensitive at 34°C. Similarly, pol1 mutants with mutations in Lys495, Trp496, or Leu487 were not overtly thermosensitive (Fig. 2A). The flow cytometry profiles of these mutants correlate with the mutants' thermosensitivities, showing that strains with mutations of Gly493 to Arg, Glu, Asn, Thr, or Pro exhibited an increased population of S/G2 cells, while the pol1 mutant with a mutation of Gly493 to Met exhibited a wild-type-like cell cycle profile at 30°C (Fig. 2B). Notably, a mutation of Gly493 to Met does not affect either the viability or the cell cycle progression of the pol1 mutant, whereas mutants harboring a mutation with similar charge or comparably sized residues, such as Leu, Ile, or Val, exhibit severe growth defects even at 25°C (Fig. 2 and data not shown). Moreover, these mutant Pol1p proteins were all expressed in cells at a comparable, if not identical, level as in cells with wild-type Pol1p (data not shown). These findings suggest that the phenotypes of those pol1 mutants containing a mutation of Gly493 to Arg, Glu, Asn, Thr, or Pro are not due to gross protein structure alterations, protein degradation, or defect in expression caused by the charge or size changes of the mutant residues. More likely is that they are caused by perturbation of protein-protein interactions of mutant Pol1p with other cellular factors. These mutational analyses confirm that residue Gly493 has an important role in cell growth and cell cycle progression.
FIG. 2.
Mutations of Gly493 in the conserved GPCWL motif in POL1 induce thermosensitivity in growth and cell cycle delay. (Left panel) Strain DFB (MATa pol1Δ ura3-52 leu2) carrying plasmid pRS315-POL1 or its mutant derivatives were grown on synthetic medium lacking leucine in 5-fold serial dilutions and incubated at 25, 30, and 34°C. (Right panel) Flow cytometry analyses of pol1 mutants containing mutations in the GPCWL motif. Each asynchronous strain was cultured exponentially at 25°C and then shifted to 30°C for 4 h.
The pol1-1(G493R) mutation (48) has been extensively characterized for its effect on cells' genomic stability (21). We therefore used the pol1-1 mutant as a representative Gly493 mutation to investigate the biological effect of mutations in Gly493.
Overexpression of SPT16 and overexpression of CTF4 have opposite effects on the pol1-1 phenotype.
To investigate which cellular factors interact with this highly conserved cluster of residues in the N-terminal region, we performed a suppressor screen of the pol1-1 mutant with a YEp24 plasmid-based high-copy-number genomic library at 34°C. Five positive suppressors were identified after several rounds of verification. Among the five suppressor plasmids, four contained the full-length POL1 and one contained the full-length SPT16 (Fig. 3A). The finding that overexpression of SPT16 can suppress the thermosensitive growth of the pol1-1 mutant at 34°C suggests that the mutant Pol1p has a defective association with Spt16p.
FIG. 3.
Overexpression of SPT16 and overexpression of CTF4 have opposite effects on the pol1-1 mutant phenotype. (A) Overexpression of SPT16 suppresses the thermosensitivity of the pol1-1 mutant. Suppressor screening with a YEp24 base genomic library reveals that three clones can rescue temperature sensitivity of the pol1-1 mutant, clones 52 and 55 contain full-length POL1, and clone 59 contains full-length SPT16. (B) Overexpression of CTF4 exacerbates the thermosensitivity phenotype of the pol1-1 mutant. Suppression of the pol1-1 mutant by SPT16 is allele specific. Overexpression of either SPT16 or CTF4 does not suppress the thermosensitivity of the pol1-17 mutant, which contains a mutation of Thr1004 to Ile.
Previous in vitro studies have shown that both Spt16p and Ctf4p bind to a Pol1p affinity matrix and that deletion of CTF4 from the yeast genome enhances the amount of Spt16p bound to the matrix (42, 68). These in vitro results have suggested that association of Ctf4p and Spt16p with Pol1p may be mutually competitive (68). These in vitro results led us to compare the effects of overexpression of the SPT16 or CTF4 gene on the pol1-1 phenotype. SPT16 and CTF4 were independently placed under the control of the Gal1 promoter. Overexpression of SPT16 suppressed the thermosensitive growth of the pol1-1 mutant at 32°C and partially suppressed pol1-1 mutant growth at 34°C (Fig. 3B). In contrast, CTF4 overexpression exacerbated the thermosensitivity of the pol1-1 mutant (Fig. 3B). The pol1-1 mutant with an overexpressed CTF4 had lower viability than the pol1-1 mutant transformed with the Gal1 vector or the pol1-1 mutant with an overexpressed SPT16 at 25°C. The pol1-1 mutant overexpressing CTF4 was clearly thermosensitive at 30°C, severely thermosensitive at 32°C, and not viable at 34°C (Fig. 3B). It has been shown in a previous study that an increased expression of SPT16 decreases viability of strains with mutations in the catalytic domains of POL1 (68). These results suggest that a critical balance of the interplay between Spt16p, Ctf4p, and Polα (Pol1p) is essential for cell growth.
To test whether the suppression of the pol1-1 mutant by SPT16 is allele specific, we overexpressed SPT16 or CTF4 driven by the Gal1 promoter in another pol1 mutant strain, the pol1-17 mutant, which contains a mutation of Thr1004 to Ile buried in the most conserved domains of B-family (α-like) polymerases (25, 62). Overexpression of SPT16 did not suppress the thermosensitivity of the pol1-17 mutant at 30, 32, and 34°C. This result indicates that the suppression of the pol1-1 mutant by the overexpression of SPT16 is specific for the pol1-1 allele (Fig. 3B, lower panel), whereas overexpression of CTF4 seems to be detrimental to both the pol1-1 and the pol1-17 mutants (Fig. 3B).
These experiments suggest that the in vivo association of Spt16p with Pol1p is through a specific interaction of the conserved N-terminal region of Pol1p. The Gly493 residue in this conserved N-terminal region of Pol1p plays a critical role in the association. The finding that overexpression of CTF4 further exacerbates the thermosensitivity of the pol1-1 mutant suggests that an appropriate level of Spt16p and Ctf4p to associate with the N-terminal region of Pol1p in a coordinated and orderly manner has an effect on cell viability.
Physical interaction between Pol1p and Spt16p is compromised in pol1-1 mutants.
To further ascertain that the interplay of Spt16p and Ctf4p with Pol1p is defective in pol1-1 mutants, we performed a two-hybrid assay. The N-terminal region of Pol1p from the wild type and the pol1-1 mutant from residues 1 to 550 were independently constructed as bait constructs (Fig. 4A). Full-length SPT16, POB3, and CTF4 genes were independently inserted into the prey vector, pYESTrp. Interactions between the N-terminal region of wild-type or mutant Pol1p expressed from the bait vector with Spt16p or Ctf4p from the prey vector will induce the expression of two reporter genes, LEU2 and LacZ, so that such strains can be identified as leucine prototrophs and by expression of β-galactosidase activity. The N-terminal region of wild-type Pol1p was able to interact with Spt16p as well as Ctf4p, as indicated by the production of leucine prototrophs and positive β-galactosidase activity (Fig. 4B and C). This interaction was disrupted by the Gly493-to-Arg mutation, which yielded neither leucine prototrophs nor β-galactosidase activity in parallel tests. These results indicate that the mutant Pol1p in the pol1-1 mutant has a compromised interaction with Spt16p (Fig. 4B and C). The interaction of the N-terminal region of Pol1p with Pob3p, although yielding leucine prototrophic growth, did not induce β-galactosidase activity, suggesting that the interaction of the N-terminal region of Pol1p and Pob3 is weak or indirect, probably through Spt16p.
FIG. 4.
Interaction of the N-terminal region of Pol1p with Spt16p is compromised in the pol1-1 mutant. (A) A schematic diagram of the N-terminal fragments of the wild type and Pol1p in the pol1-1 mutant used as bait. (B) Strain SKY48 was transformed in pairwise combinations of bait and prey vectors as described in Materials and Methods, and the transcriptional activation of LEU2 was scored by determining leucine prototrophy. (C) Transcriptional activation of lacZ was examined with an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overlay assay.
With the expression of CTF4 as prey, the N-terminal regions of Pol1p from either POL1 or mutant pol1-1 were able to yield leucine prototrophs and to induce high levels of β-galactosidase activity (Fig. 4B and C). Results of these two-hybrid assays indicate that the highly conserved N-terminal region of Pol1p is able to physically interact with the chromatin reorganization factor Spt16p and with the sister chromatin cohesion factor Ctf4p in vivo. A mutation of Gly493 to Arg in this conserved N-terminal region of Pol1p perturbs its ability to interact with Spt16p but not its interaction with Ctf4p. The binding sites for these two proteins on Pol1p are therefore likely to be overlapping but not identical.
The temporal ordered association between Pol1p and Ctf4p is altered in the pol1-1 mutant.
Finding that a mutation of Gly493 to Arg in the pol1-1 mutant's Pol1p compromises the association with Spt16p but not the association with Ctf4p by the two-hybrid assay (Fig. 4B and C) led us to investigate the temporal order of the interplay of these three proteins in synchronous cells. Cells were synchronized by α-factor arrest for 3 h at 25°C and then released to grow at 22°C. Cell samples were removed every 10 min after the α-factor release to monitor the S-phase progression by flow cytometry (Fig. 5A). Pol1p was immunoprecipitated from each cell sample, and the Pol1p immunoprecipitates were analyzed for coprecipitation of Spt16p and Ctf4p (Fig. 5B). The flow cytometry analysis indicated that the pol1-1 mutant had an approximately 10-min delay in entering into and progression through S phase compared to wild-type cells (Fig. 5A). After release from the α-factor arrest, Pol1p was present throughout the S phase in wild-type cells with POL1 as well as in pol1-1 mutant cells (Fig. 5B, upper panels). The coprecipitation of the myc-tagged Spt16p and TAP-tagged Pol1p was probed by anti-myc monoclonal antibody (9E10). The rabbit IgG-agarose used for immunoprecipitation of the TAP-tagged Pol1p has a moderate cross-reactivity with the myc epitope tag, thus showing a background protein band in the cell extract with myc-tagged Spt16p from cells that do not contain TAP-tagged Pol1p (Fig. 6B, control lane of the middle panels). However, in POL1 cells, Spt16p levels above the background band were detectable in the Pol1p immunoprecipitates 10 min after α-factor release. The level of Spt16p coprecipitating with Pol1p increased from 70 min to 110 min after α-factor release in late S phase to G2 phase. Consistent with the finding that a mutation of Gly493 to Arg in Pol1p of the pol1-1 mutant compromised the ability of the mutant Pol1p to associate with Spt16p (Fig. 4B and C), lower levels of Spt16p were found to coprecipitate with mutant Pol1p from the pol1-1 mutant (Fig. 5B, middle panels). Furthermore, coprecipitation of Spt16p with the mutant Pol1p seemed to have a slight delay in pol1-1 mutants compared to cells with wild-type Pol1p (Fig. 5B, middle panels). To ensure that the observed lower levels of Spt16p coprecipitating with Pol1 in the pol1-1 mutant were not due to an artifact of cross-reactivity of anti-myc antibody (9E10) with rabbit IgG-agarose, we constructed a strain containing TAP-tagged POL1 and pol1-1 with CFP-tagged SPT16 to confirm the results. Cells were synchronized by α-factor, and TAP-tagged wild-type and mutant Pol1p proteins were immunoprecipitated from each cell sample every 10 min. As shown in Fig. 5B, Pol1p from either the wild type or the pol1-1 mutant was presented throughout S phase (Fig. 5C). Anti-GFP antibody did not exhibit any cross-reactivity with the rabbit IgG-agarose used for precipitation of TAP-tagged Pol1p (Fig. 5C, control lane). Similar to the results shown in Fig. 5B, coprecipitation of Spt16p with Pol1p was detectable 10 min after α-factor release, and increased levels of Spt16p coprecipitated with Pol1p were found as cells approached late S phase and G2 (Fig. 5C, middle panel). Consistent with the finding that mutation in pol1-1 compromised the association between Spt16p and Pol1p (Fig. 4B and C), lower levels of Spt16p were found to coprecipitate with mutant Pol1p when equal amounts of proteins were analyzed (Fig. 5C).
FIG. 5.
Cell cycle-regulated interaction of Pol1p with Spt16p and Ctf4p. Cells harboring POL1-TAP, SPT16-13Xmyc, and CTF4-3XHA were synchronized in G1 with α-factor and then released into YPD medium at 22°C. Cell samples were removed at the indicated times for FACS analysis and immunoprecipitation. (A) Flow cytometry profiles of cells at indicated times. (B) Immunoprecipitations of POL1-TAP were performed from cell extracts of the wild type and the pol1-1 mutant with rabbit IgG-agarose at the indicated times (in minutes). Coprecipitation of Spt16p-13Xmyc and Ctf4p-3XHA with Pol1p-TAP were detected with anti-myc and anti-HA antibodies, respectively. Control lanes, Western blot immunoprecipitates with rabbit IgG-agarose from extracts of cells that do not harbor the TAP-tagged POL1 but contain SPT16-13Xmyc or CTF4-3XHA with anti-myc (9E10) or anti-HA (12CA5). (C) Immunoprecipitations of TAP-tagged Pol1p were performed from cell extracts of the wild type and the pol1-1 mutant by rabbit IgG-agarose at the indicated times, as in panel B. Coprecipitation of Spt16p-CFP with Pol1p-TAP was detected with anti-GFP. Control lanes show Western blot immunoprecipitates with rabbit IgG-agarose from extracts of cells that do not harbor the TAP-tagged POL1 or pol1-1 genes but that contain SPT16-CFP with anti-GFP. Protein loading control was performed by Western blotting immunoprecipitates with anti-IgG.
FIG. 6.
Assembly of Pol1p onto late-replicating origin is delayed in the pol1-1 mutant. (A) Flow cytometry profile of the asynchronous wild type and the pol1-1 mutant harboring POL1-TAP or pol1-1-TAP at different temperatures. (B) Assembly of wild-type and mutant Pol1p-TAP onto replication origins (ARS). Wild-type and pol1-1 mutant cells containing the TAP-tagged POL1 or pol1-1 were synchronized by α-factor arrest and then released into YPD medium at 22°C. The cells were withdrawn from the culture every 10 min for FACS analysis and CHIP assay with rabbit IgG-agarose. A PCR was performed on the immunoprecipitates and on whole-cell extract as input control at each time point. (C) Checkpoint kinase Rad53p is not activated in the pol1-1 mutant. Wild-type cells harboring POL1-TAP RAD53-13Xmyc or mutant cells harboring pol1-1-TAP RAD53-13Xmyc were grown at 22 and 25°C with or without 0.2 M hydroxyurea. The whole-cell extract was then subjected to electrophoresis fractionation and Western blotting with anti-myc antibody to detect the mobility of Rad53p phosphorylation.
Coprecipitation of Ctf4p and wild-type Pol1p was detected 10 min after release from α-factor arrest in POL1 cells (Fig. 5B, lower panels). A progressive increase of Ctf4p coprecipitated with wild-type Pol1p was observed from 40 to 60 min after α-factor release. After 70 min, when cells entered into late S phase or G2, the coprecipitation of Ctf4p and Pol1p in POL1 cells gradually diminished. These results indicate that the association of Ctf4p with Pol1p is cell cycle regulated (Fig. 6B, lower panels). Interestingly, in the pol1-1 mutant, no detectable Ctf4p was coprecipitated with the mutant Pol1p until 40 min after release from α-factor arrest. Furthermore, the coprecipitation of Ctf4p and mutant Pol1p from the pol1-1 mutant was detected at a constant level throughout S phase to G2 (from 50 to 110 min after α-factor release). Hence, the temporal order of interaction between Ctf4p and mutant Pol1p is severely perturbed in the pol1-1 mutant.
Since the mutation of Gly493 to Arg of Pol1p compromises only the association with Spt16p and not Ctf4p, these results indicate that a perturbed association of Pol1p with the chromatin reorganization factors, Spt16p-Pob3p, may have a profound effect on the cell cycle-regulated interaction between Pol1p and the sister chromatin cohesion factor Ctf4p.
The assembly and disassembly of complexes that contain Pol1p at late-replicating autonomously replicating sequence (ARS) elements are delayed in the pol1-1 mutant.
To investigate whether a compromised association of Spt16p with Pol1p in the pol1-1 mutant could have an effect on the initiation and progression of S phase, we first analyzed the flow cytometry profile of the pol1-1 mutant at 22, 25, 30, and 34°C (Fig. 6A). Surprisingly, even at the permissive temperature of 22°C, the pol1-1 mutant exhibited an increased population of S/G2 cells, and this result was more apparent as temperatures progressively increased.
To further analyze how the compromised interaction of Spt16p with Pol1p in the pol1-1 mutant could cause a delay in S-phase progression, we analyzed the kinetics of Pol1p assembling onto early-replicating origin at ARS1 and ARS305 and compared it to that of the late-replicating origin at ARS501 and ARS603 by a CHIP assay of the wild type and the pol1-1 mutant. Strains carrying POL1 and pol1-1 were synchronized by α-factor arrest and release into a permissive temperature of 22°C. CHIP assays were performed as described previously (6) with minor modification, as described in Materials and Methods. With equal amounts of input of early-replicating origins ARS1 and ARS305, wild-type Pol1p assembled onto both of the early-firing ARSs 30 min after release from α-factor arrest and disassembled from the early-replicating ARSs after 70 min (Fig. 6B). Pol1p of the pol1-1 mutant assembled onto the early ARSs with kinetics similar to those of wild-type Pol1p 30 min after release from α-factor arrest. Seventy minutes after release from α-factor arrest, a majority of the mutant Pol1p disassembled from the early-firing ARSs, similar to wild-type Pol1p (Fig. 6B). Analysis of the assembling Pol1p onto the late-replicating ARSs indicated that the wild-type Pol1p began to assemble onto ARS501 and ARS603 40 min after release from α-factor arrest and disassembled from the late-replicating ARSs after 90 min, when cells completed S phase (Fig. 6B). In striking contrast, mutant Pol1p in the pol1-1 mutant assembled onto the late-replicating ARSs 60 min after release from α-factor arrest. While wild-type cells exhibited a peak of Pol1p assembly at 60 min after release from α-factor arrest, the assembly of replication complex containing mutant Pol1p onto late ARS in the pol1-1 mutant peaked at 70 min after α-factor release, showing a 10-min delay. Moreover, mutant Pol1p persistently associated with ARS501 and ARS603 up to 120 min after release from α-factor arrest without disassembling the mutant initiation complex from the late-replicating origins (Fig. 6B).
These CHIP assay results are consistent with the flow cytometry profile of the pol1-1 mutant shown at 22°C (Fig. 6B), indicating that an overt delay of mutant Pol1p assembly onto and disassembly from the late-replicating ARSs correlates with a slowdown of late S-phase progression.
Perturbation in S phase should induce activation of replication checkpoint kinase Rad53p to stabilize the replication fork and prevent the replication fork progression from the early-replicating ARSs and the firing of the late-replicating ARSs (37, 58). To test whether the delay of mutant Pol1p assembling onto and disassembling from the late-replicating ARSs in the pol1-1 mutant is due to activation of the replication checkpoint kinase Rad53p, we constructed myc-tagged RAD53 into the POL1 and pol1-1 strains. At 22°C, Rad53p kinase was not activated in either the POL1 or the pol1-1 strains, since no phosphorylation of Rad53p, shown as slow mobility protein, was detected by gel analysis (Fig. 6C). To ensure that the replication checkpoint is intact in both the POL1 and pol1-1 strains, cells with POL1 and pol1-1 were treated with hydroxyurea to induce Rad53p kinase activation. Phosphorylation of Rad53p, shown as a slow mobility protein species, was observed, indicating that the replication checkpoint is intact in both the wild type and the pol1-1 mutant that were used for the CHIP assay (Fig. 6C). These results indicate that the perturbation of Pol1p assembling onto and disassembling from the late-replicating ARSs in the pol1-1 mutant is not caused by the activation of replication checkpoint kinase Rad53p to delay the cell cycle transition.
DISCUSSION
In this study, we described an interplay between a nucleosome reorganization factor, Spt16p, a sister chromatid cohesion factor, Ctf4, and the Polα (Pol1p) protein in the replication complex. Our data suggest that the interplay between these three proteins plays a role in facilitating chromosome replication. We demonstrate here that a glycine residue, Gly493, in a conserved N-terminal noncatalytic region of Polα (Pol1p) is involved in the interplay. A mutation of Gly493 to Arg results in a reduced ability of the mutant Pol1p to associate with Spt16p and an alteration of the cell cycle-regulated association and dissociation between the mutant Pol1p with Ctf4p. The compromised interplay of these three proteins also causes a delay of the replication complex in assembling onto and disassembling from the late-replicating origins, resulting in a delay of S-phase progression, particularly in the late S-phase progression. These findings led us to propose that during normal and robust S-phase progression, cells require a coordinated temporal orderly interplay between Spt16p, Ctf4p, and an N-terminal noncatalytic region of Polα (Pol1p) in the replication complex. We discuss how the interplay of these three proteins is required to facilitate S-phase progression and how a compromised interplay could enhance genomic instability in cells.
How might the association of Spt16p with Pol1p affect S-phase progression?
Nucleosome position has been shown to have a positive as well as a negative impact on replication and transcription of genomic DNA (4, 5, 35, 54, 69, 70). Nucleosome position over cis-acting DNA elements correlates with loss of origin function in yeast (54, 60), and chromatin remodeling affects the simian virus 40 origin-dependent DNA replication in vitro (5). These studies imply that nucleosome occupancy may prevent cis-acting elements from interacting with initiation factors for replication and suggest that nucleosome position exerts a negative effect on initiation of replication. The positioning of a nucleosome has also been shown to have a positive effect on replication (35, 69). Origin recognition complex (ORC) has been shown to be a primary determinant of the nucleosome positioning at replication origins in vivo and in vitro. Alteration of the ORC-dependent nucleosomal patterns in ARS1 has a significant negative effect on replication initiation. These results suggest that ORC-dependent nucleosome positioning may facilitate prereplication complex formation (35). Together, these studies indicate that nucleosome positioning could have a dual role in replication.
Studies of budding yeast have suggested that the high-mobility group protein Nhp6 binds to nucleosomes and that the Nhp6-nucleosome complex recruits Spt16p-Pob3p to modify the nucleosome structure during transcription and replication (16). In vitro, Spt16p-Pob3p binds to Pol1p affinity matrix (42, 68). SPT16 has a strong genetic interaction with replication genes POL1 and DNA2, which is a gene encoding a nuclease and helicase essential for Okazaki fragment maturation (17). These studies suggest that nucleosome modification might have a positive role in replication. In this study, we show at the molecular level that Spt16p associates with a highly conserved N-terminal noncatalytic region of Pol1p in vivo (Fig. 4 and 5). This result suggests that the association of Spt16p with Polα (Pol1p) has a physiological role in promoting reorganization of nucleosome structures during initiation of replication.
We demonstrate here that Spt16p associates with Pol1p throughout the cell cycle. However, a higher level of Spt16p associating with Pol1p was observed 80 to 100 min after release from α-factor arrest during late S phase to G2 (Fig. 5B and C). High-resolution structural analysis of yeast chromatin has shown that heterochromatins at specific loci are maintained in a unique nucleosomal configuration (50, 65). Late-firing replication origins often localize in the heterochromatin regions. For replisome to progress through the heterochromatin regions would require substantial nucleosome modification and chromatin reorganization. The Pol1p of the pol1-1 mutant has a reduced ability to associate with Spt16p (Fig. 4 and 5B and C). It is possible that pol1-1 mutant cells, having lower levels of Spt16p associated with Pol1p, may not have sufficient ability to reorganize and modify the nucleosomes packaged in the heterochromatin region. This result may cause a compromised reorganization of the heterochromatin region in the late-firing origins, resulting in a delay in assembling replication complex onto the late-firing ARS (Fig. 6B). The finding that assembly of mutant Pol1p onto early-replication origins has kinetics similar to wild-type Pol1p (Fig. 6B) supports the premise that reorganization of nucleosome positioning in the late origin heterochromatin may require some appropriate levels of Spt16p associating with Pol1p in the replication complex. Therefore, a compromised association between Spt16p and Pol1p in the pol1-1 mutant has a particularly negative impact on the chromatin configuration in the late origins, causing a perturbed timing in assembly and/or disassembly of the replication complex onto the late origin, resulting in a delay of S-phase progression. Our results thus support the notion that the reorganization of nucleosome position in the heterochromatin region has a positive role in replication.
Although the exact physiological role of Ctf4p in cohesion is not yet clear, the deletion of CTF4 in yeast causes some nonlethal defects in sister chromatid cohesion and a spindle assembly checkpoint MAD2-dependent preanaphase delay (23, 32, 41, 57). A recent study has shown that CTF4, CTF8, and a helicase, CHL1, are all required for efficient sister chromatid cohesion in active cycling mitotic cells (47). That the overexpression of Ctf4p and Spt16p have opposite effects on the pol1-1 phenotype (Fig. 3B) supports the notion that the binding of Ctf4p and Spt16p to Pol1p is competitive and perhaps mutually exclusive (68). Ctf4p interacts with Pol1p in vivo by two-hybrid assay (Fig. 4) and coprecipitation (Fig. 5B), indicating the requirement of a tight coupling of replication and cohesion during S phase. Ctf4p in mutant pol1-1 cells exhibits a dramatic change in its temporal orderly association and dissociation with mutant Pol1p (Fig. 5B). It is not clear whether a compromised nucleosome position in pol1-1 would have an impact on coupling the sister chromatid cohesion establishment with initiation of replication and the timing of sister chromatin cohesion separation. Given the competitive nature of the binding of Spt16p and Ctf4p to Pol1p in vitro (68) and the opposite effects of overexpression of these two proteins on the pol1-1 phenotype (Fig. 3B), it is possible that a compromised association between Spt16p and Pol1p might have an influence on the temporal order of association and dissociation between Ctf4p and Pol1p. Moreover, sister chromatid cohesion is closely connected with spindle integrity and spindle assembly checkpoint (56); this might also contribute to a delay of the cell cycle progression seen in late S phase and G2.
Together, our results demonstrate that an orderly temporal coordinated interplay of chromatin reorganization factors, sister chromatin cohesion establishment factor, and Pol1p in the replication machinery is required for facilitating normal and robust S-phase progression.
How might a compromised association of Spt16p with Pol1p enhance genomic instability in cells?
Previous studies have shown that a mutation of Gly493 to Glu induces a hyperrecombination phenotype (3) and that a mutation of Gly493 to Arg induces chromosome loss, microsatellite (GT)16-tract instability, and a mutator phenotype of base substitution and deletion of genomic sequences (21). A deletion of three residues adjacent to Gly493 in the fission yeast Polα mutant also induces a similar dramatic mutator phenotype (36) and has a significant effect on the maintenance of telomere length homeostasis and telomeric complex stability (11). These budding yeast and fission yeast results indicate that the proper interaction of cellular proteins with the conserved N-terminal noncatalytic region of Polα (Pol1p) is important for maintaining genomic stability.
Here, we show that a mutation of Gly493 to Arg in the conserved N-terminal region of Polα (Pol1p) affects its ability to interact with Spt16p. As discussed above, suboptimum levels of chromatin modification factors associating with Polα (Pol1p) in the replication complex may not be sufficient to properly reorganize the chromatin structure, especially in the heterochromatin regions, such as the telomere regions. This effect could manifest as a decrease of the telomere position effect, cause inappropriate coordination of the G/C-strand synthesis, and destabilize the coupling between the lagging-strand replication complex and telomeric complex seen in the fission yeast polαts13 mutant (11).
Our previous studies have shown that among all of the replication mutators analyzed, the fission yeast polαts13 mutant and the budding yeast pol1-1 mutant, both containing mutations in the conserved N-terminal region of the polα+ (POL1) gene, exhibit a much more severe mutator phenotype than those polα (pol1) mutants harboring a mutation outside of the conserved N-terminal region (21, 36). A compromised chromatin reorganization due to suboptimal levels of Spt16p associating with Pol1p in the replication complex could give rise to potentially mutagenic chromatin structures, leading to chromosome loss and microsatellite tract instability found in the pol1-1 mutant (21), and a more severe mutator phenotype seen in the fission yeast polαts13 and budding yeast pol1-1 mutants (21, 36).
CTF4 was first identified by a genetic screen for mutations affecting chromosome transmission fidelity (32). A specific replisome configuration is thought to be required for recruiting cohesion complexes and establishing cohesion (59). A compromised association between Pol1p and Spt16p could induce a perturbation in the replisome configuration, resulting in an aberrant cohesion establishment and the potential of having mutagenic chromatin structure. Together, our results from previous studies and this study indicate that an inappropriate association of chromatin reorganization factor Spt16p with Pol1p could have a profound effect on the overall chromosomal status during replication, which could enhance the potential of genomic instability.
Results of this study indicate that a compromised association between replication machinery and factors involved in chromatin reorganization could have broad implications for chromosome replication. Our results in this study underscore the importance of having appropriate levels of nucleosome modification factors associating with Polα in the initiation complex to modulate the chromatin in proper condition for robust replication.
Acknowledgments
We thank members of our lab for helpful discussion, Rose Borbely for her excellent technical help, Ekaterina Schwartz for helpful advice in budding yeast work, and Oscar Aparicio for myc-tagged RAD53 and helpful advice on the CHIP assay.
This work was supported by grant CA14835 from the National Cancer Institute of the National Institutes of Health.
REFERENCES
- 1.Adams, A. K., and C. Holm. 1996. Specific DNA replication mutations affect telomere length in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams-Martin, A., I. Dionne, R. J. Wellinger, and C. Holm. 2000. The function of DNA polymerase α at telomeric G tails is important for telomere homeostasis. Mol. Cell. Biol. 20:786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilera, A., and H. L. Klein. 1988. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics 119:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexiadis, V., L. Halmer, and C. Gruss. 1997. Influence of core histone acetylation on SV40 minichromosome replication in vitro. Chromosoma 105:324-331. [DOI] [PubMed] [Google Scholar]
- 5.Alexiadis, V., P. D. Varga-Weisz, E. Bonte, P. B. Becker, and C. Gruss. 1998. In vitro chromatin remodelling by chromatin accessibility complex (CHRAC) at the SV40 origin of DNA replication. EMBO J. 17:3428-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aparicio, O. M., D. M. Weinstein, and S. P. Bell. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: Redistribution of MCM proteins and Cdc45p during S phase. Cell 91:59-69. [DOI] [PubMed] [Google Scholar]
- 7.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 8.Bhaumik, D., and T. S.-F. Wang. 1998. Mutational effect of fission yeast Polα on cell cycle events. Mol. Biol. Cell 9:2107-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewster, N. K., G. C. Johnston, and R. A. Singer. 1998. Characterization of the CP complex, an abundant dimer of Cdc68 and Pob3 proteins that regulates yeast transcriptional activation and chromatin repression. J. Biol. Chem. 273:21972-21979. [DOI] [PubMed] [Google Scholar]
- 10.Carson, M. J., and L. Hartwell. 1985. CDC17: an essential gene that prevents telomere elongation in yeast. Cell 42:249-257. [DOI] [PubMed] [Google Scholar]
- 11.Dahlen, M., P. Sunnerhagen, and T. S. Wang. 2003. Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol. Cell. Biol. 23:3031-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diede, S. J., and D. E. Gottschling. 1999. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerase α and δ. Cell 99:723-733. [DOI] [PubMed] [Google Scholar]
- 13.D'Urso, G., B. Grallert, and P. Nurse. 1995. DNA polymerase alpha, a component of the replication initiation complex, is essential for the checkpoint coupling S phase to mitosis in fission yeast. J. Cell Sci. 108:3109-3118. [DOI] [PubMed] [Google Scholar]
- 14.Evans, D. R., N. K. Brewster, Q. Xu, A. Rowley, B. A. Altheim, G. C. Johnston, and R. A. Singer. 1998. The yeast protein complex containing cdc68 and pob3 mediates core-promoter repression through the cdc68 N-terminal domain. Genetics 150:1393-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formosa, T. 2003. Changing the DNA landscape: putting a SPN on chromatin. Curr. Top. Microbiol. Immunol. 274:171-201. [DOI] [PubMed] [Google Scholar]
- 16.Formosa, T., P. Eriksson, J. Wittmeyer, J. Ginn, Y. Yu, and D. J. Stillman. 2001. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 20:3506-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Formosa, T., and T. Nittis. 1999. Dna2 mutants reveal interaction with DNA polymerase α and Ctf4, a Pol α accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics 151:1459-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formosa, T., S. Ruone, M. D. Adams, A. E. Olsen, P. Eriksson, Y. Yu, A. R. Rhoades, P. D. Kaufman, and D. J. Stillman. 2002. Defects in SPT16 or POB3 (yFACT) in Saccharomyces cerevisiae cause dependence on the Hir/Hpc pathway: polymerase passage may degrade chromatin structure. Genetics 162:1557-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francesconi, S., W. C. Copeland, and T. S.-F. Wang. 1993. In vivo species specificity of DNA polymerase α. Mol. Gen. Genet. 241:457-466. [DOI] [PubMed] [Google Scholar]
- 20.Franklin, M. C., J. Wang, and T. A. Steitz. 2001. Structure of the replicating complex of a Pol alpha family DNA polymerase. Cell 105:657-667. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez, P. J., and T. S.-F. Wang. 2003. Genomic instability induced by mutations in Saccharomyces cerevisiae POL1. Genetics 165:65-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haering, C. H., and K. Nasmyth. 2003. Building and breaking bridges between sister chromatids. Bioessays 25:1178-1191. [DOI] [PubMed] [Google Scholar]
- 23.Hanna, J. S., E. S. Kroll, V. Lundblad, and F. A. Spencer. 2001. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell. Biol. 21:3144-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris, S. D., and J. E. Hamer. 1995. sepB: an Aspergillus nidulans gene involved in chromosome segregation and the initiation of cytokinesis. EMBO J. 14:5244-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heringa, J., and P. Argos. 1994. Evolution of viruses as recorded by their polymerase sequences, p. 87-103. In S. S. Morse (ed.), The evolutionary biology of viruses. Raven Press, Ltd., New York, N.Y.
- 26.Hirano, T. 2000. Chromosome cohesion, condensation, and separation. Annu. Rev. Biochem. 69:115-144. [DOI] [PubMed] [Google Scholar]
- 27.Holmes, A. M., and J. E. Haber. 1999. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell 96:415-424. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka, M., and B. Stillman. 1999. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 274:23027-23034. [DOI] [PubMed] [Google Scholar]
- 29.Ishimi, Y., S. Ichinose, A. Omori, K. Sato, and H. Kimura. 1996. Binding of human minichromosome maintenance proteins with histone H3. J. Biol. Chem. 271:24115-24122. [DOI] [PubMed] [Google Scholar]
- 30.Ito, J., and D. K. Braithwaite. 1991. Compilation and alignment of DNA polymerase sequences. Nucleic Acids Res. 19:4045-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kai, M., and T. S.-F. Wang. 2003. Checkpoint activation regulates mutagenic translesion synthesis. Genes Dev. 1:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouprina, N., E. Kroll, V. Bannikov, V. Bliskovsky, R. Gizatullin, A. Kirillov, V. Zakharyev, P. Hieter, F. Spencer, and V. Larionov. 1992. CTF4 (CHL15) mutants exhibit defective DNA metabolism in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 12:5736-5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunkel, T. A., and K. Bebenek. 2000. DNA replication fidelity. Annu. Rev. Biochem. 69:497-529. [DOI] [PubMed] [Google Scholar]
- 34.Lindahl, T., and R. D. Wood. 1999. Quality control by DNA repair. Science 286:1897-1904. [DOI] [PubMed] [Google Scholar]
- 35.Lipford, J. R., and S. P. Bell. 2001. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 7:21-30. [DOI] [PubMed] [Google Scholar]
- 36.Liu, V. F., D. Bhaumik, and T. S.-F. Wang. 1999. Mutator phenotype induced by aberrant replication. Mol. Cell. Biol. 19:1126-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani, M. Muzi-Falconi, C. S. Newlon, and M. Foiani. 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557-561. [DOI] [PubMed] [Google Scholar]
- 38.Lycan, D., G. Mikesell, M. Bunger, and L. Breeden. 1994. Differential effects of Cdc68 on cell cycle-regulated promoters in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:7455-7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malone, E. A., C. D. Clark, A. C. Chiang, and F. Winston. 1991. Mutations in SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:5710-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer, M. L., I. Pot, M. Chang, H. Xu, V. Aneliunas, T. Kwok, R. Newitt, R. Aebersold, C. Boone, G. W. Brown, and P. Hieter. 2004. Identification of protein complexes required for efficient sister chromatid cohesion. Mol. Biol. Cell 15:1736-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer, M. L., S. P. Gygi, R. Aebersold, and P. Hieter. 2001. Identification of RFC(Ctf18p, ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol. Cell 7:950-970. [DOI] [PubMed] [Google Scholar]
- 42.Miles, J., and T. Formosa. 1992. Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. Proc. Natl. Acad. Sci. USA 89:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama, J., R. C. Allshire, A. J. Klar, and S. I. Grewal. 2001. A role for DNA polymerase α in epigenetic control of transcriptional silencing in fission yeast. EMBO J. 20:2857-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasmyth, K. 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35:673-745. [DOI] [PubMed] [Google Scholar]
- 45.Orphanides, G., G. LeRoy, C. H. Chang, D. S. Luse, and D. Reinberg. 1998. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92:105-116. [DOI] [PubMed] [Google Scholar]
- 46.Orphanides, G., W. H. Wu, W. S. Lane, M. Hampsey, and D. Reinberg. 1999. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature 400:284-288. [DOI] [PubMed] [Google Scholar]
- 47.Petronczki, M., B. Chwalla, M. F. Siomos, S. Yokobayashi, W. Helmhart, A. M. Deutschbauer, R. W. Davis, Y. Watanabe, and K. Nasmyth. 2004. Sister-chromatid cohesion mediated by the alternative RF-CCtf18/Dcc1/Ctf8, the helicase Chl1 and the polymerase-alpha-associated protein Ctf4 is essential for chromatid disjunction during meiosis II. J. Cell Sci. 117:3547-3559. [DOI] [PubMed] [Google Scholar]
- 48.Pizzagalli, A., P. Valsasnini, P. Plevani, and G. Luccini. 1988. DNA polymerase I gene of Saccharomyces cerevisiae: nucleotide sequence, mapping of a temperature-sensitive mutation, and protein homology with other DNA polymerases. Proc. Natl. Acad. Sci. USA 85:3772-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prendergast, J. A., L. E. Murray, A. Rowley, D. R. Carruthers, R. A. Singer, and G. C. Johnston. 1990. Size selection identifies new genes that regulate Saccharomyces cerevisiae cell proliferation. Genetics 124:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravindra, A., K. Weiss, and R. T. Simpson. 1999. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating-type locus HMRa. Mol. Cell. Biol. 19:7944-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowley, A., R. A. Singer, and G. C. Johnston. 1991. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol. Cell. Biol. 11:5718-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlesinger, M. B., and T. Formosa. 2000. POB3 is required for both transcription and replication in the yeast Saccharomyces cerevisiae. Genetics 155:1593-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnell, R., L. D'Ari, M. Foss, D. Goodman, and J. Rine. 1989. Genetic and molecular characterization of suppressors of SIR4 mutations in Saccharomyces cerevisiae. Genetics 122:29-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson, R. T. 1990. Nucleosome positioning can affect the function of a cis-acting DNA element in vivo. Nature 343:387-389. [DOI] [PubMed] [Google Scholar]
- 55.Singh, J., and A. J. Klar. 1993. DNA polymerase-alpha is essential for mating-type switching in fission yeast. Nature 361:271-273. [DOI] [PubMed] [Google Scholar]
- 56.Skibbens, R. V., L. B. Corson, D. Koshland, and P. Hieter. 1999. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 13:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spencer, F., S. L. Gerring, C. Connelly, and P. Hieter. 1990. Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 124:237-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tercero, J. A., and J. F. Diffley. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412:553-557. [DOI] [PubMed] [Google Scholar]
- 59.Uhlmann, F., and K. Nasmyth. 1998. Cohesion between sister chromatids must be established during DNA replication. Curr. Biol. 8:1095-1101. [DOI] [PubMed] [Google Scholar]
- 60.Venditti, P., G. Costanzo, R. Negri, and G. Camilloni. 1994. ABFI contributes to the chromatin organization of Saccharomyces cerevisiae ARS1 B-domain. Biochim. Biophys. Acta 1219:677-689. [DOI] [PubMed] [Google Scholar]
- 61.Waga, S., and B. Stillman. 1998. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67:721-751. [DOI] [PubMed] [Google Scholar]
- 62.Wang, J., A. K. M. A. Sattar, C. C. Wang, J. D. Karam, W. H. Konigsberg, and T. A. Steitz. 1997. Crystal structure of a pol α family replication DNA polymerase from bacteriophage RB69. Cell 89:1087-1099. [DOI] [PubMed] [Google Scholar]
- 63.Wang, T. S.-F. 1996. Cellular DNA polymerases, p. 461-493. In M. L. DePamphilis (ed.), DNA replication in eukaryotic cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 64.Wang, T. S.-F. 1991. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 60:513-552. [DOI] [PubMed] [Google Scholar]
- 65.Weiss, K., and R. T. Simpson. 1998. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HMLα. Mol. Cell. Biol. 18:5392-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams, D. R., and J. R. McIntosh. 2002. mcl1+, the Schizosaccharomyces pombe homologue of CTF4, is important for chromosome replication, cohesion, and segregation. Eukaryot. Cell 1:758-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winston, F., D. T. Chaleff, B. Valent, and G. R. Fink. 1984. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107:179-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wittmeyer, J., and T. Formosa. 1997. The Saccharomyces cerevisiae DNA polymerase α catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol. Cell. Biol. 17:4178-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wittmeyer, J., L. Joss, and T. Formosa. 1999. Spt16 and Pob3 of Saccharomyces cerevisiae form an essential, abundant heterodimer that is nuclear, chromatin-associated, and copurifies with DNA polymerase alpha. Biochemistry 38:8961-8971. [DOI] [PubMed] [Google Scholar]
- 70.Wolffe, A. P., and H. Kurumizaka. 1998. The nucleosome: a powerful regulator of transcription. Prog. Nucleic Acid Res. Mol. Biol. 61:379-422. [DOI] [PubMed] [Google Scholar]
- 71.Xu, Q., G. C. Johnston, and R. A. Singer. 1993. The Saccharomyces cerevisiae Cdc68 transcription activator is antagonized by San1, a protein implicated in transcriptional silencing. Mol. Cell. Biol. 13:7553-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu, Q., R. A. Singer, and G. C. Johnston. 1995. Sug1 modulates yeast transcription activation by Cdc68. Mol. Cell. Biol. 15:6025-6035. [DOI] [PMC free article] [PubMed] [Google Scholar]