Abstract
It has been suggested that the Schizosaccharomyces pombe Rad50 (Rad50-Rad32-Nbs1) complex is required for the resection of the C-rich strand at telomere ends in taz1-d cells. However, the nuclease-deficient Rad32-D25A mutant can still resect the C-rich strand, suggesting the existence of a nuclease that resects the C-rich strand. Here, we demonstrate that a taz1-d dna2-2C double mutant lost the G-rich overhang at a semipermissive temperature. The amount of G-rich overhang in S phase in the dna2-C2 mutant was lower than that in wild-type cells at the semipermissive temperature. Dna2 bound to telomere DNA in a chromatin immunoprecipitation assay. Moreover, telomere length decreased with each generation after shift of the dna2-2C mutant to the semipermissive temperature. These results suggest that Dna2 is involved in the generation of G-rich overhangs in both wild-type cells and taz1-d cells. The dna2-C2 mutant was not gamma ray sensitive at the semipermissive temperature, suggesting that the ability to process double-strand break (DSB) ends was not affected in the dna2-C2 mutant. Our results reveal that DSB ends and telomere ends are processed by different mechanisms.
Telomeres are essential for the stability of eukaryotic chromosome ends (24). Telomeric DNA comprises tandem repeats of a simple sequence rich in guanine residues. Vertebrate telomeres contain regular repeated TTAGGG motifs. Although the telomeric DNA repeats in Schizosaccharomyces pombe are not completely regular, the most frequent motif can be designated (GGTTACA)n (28). The distal end of telomere DNA has a single-stranded region at the 3′ end, which is called the G-rich overhang. The G-rich overhang is eroded at senescence, suggesting that overhang erosion triggers senescence in cultured primary human cells (51). The G-rich overhangs exist during most of the cell cycle in human cells (35). In contrast, the amount of G-rich overhangs increases in S phase in both Saccharomyces cerevisiae and S. pombe (22, 32, 63). It has been suggested that the G-rich overhang is generated by degradation of the C-rich strand (22, 35). However, the nuclease responsible for this activity has not been identified. Although S. cerevisiae Est2, which is the telomerase catalytic subunit, binds telomeres throughout the cell cycle, Est1 and Cdc13 bind telomeres mainly in S phase (54). Based on these and other studies, it has been proposed that telomerase synthesizes telomere DNA in late S phase (55).
In S. pombe, telomeres are maintained by trt1+, which encodes the catalytic subunit of telomerase (41), and are protected by Taz1, which is an ortholog of human TRF1 and TRF2. Deletion of taz1+ causes massive telomere elongation and a significant increase in the amount of G-rich overhang (19, 59). This overhang is detected in taz1-d trt1-d double mutants, suggesting that the G-rich overhang is produced by the degradation of the C-rich strand. The Rad50 (Rad50-Rad32-Nbs1) complex is required for the generation of G-rich overhangs in taz1-d cells. However, the nuclease activity of Rad32 is not required for the generation of G-rich overhangs in taz1-d cells (59). Therefore, the existence of an additional nuclease that resects the telomere end has been predicted.
The Rad50 complex is also involved in the processing of double-strand break (DSB) ends. Rad50 has ATP-dependent DNA-binding activity and partial DNA-unwinding activity (46, 49). Rad50 stimulates the nuclease activity of Mre11 (45, 60). Mre11 (a homolog of S. pombe Rad32) possesses 3′-to-5′ single- and double-stranded exonuclease and single-stranded endonuclease activities and DNA hairpin-opening activity in vitro (26, 38, 45, 60, 62). Nbs1 (Xrs2) is conserved from S. cerevisiae to humans and is believed to be a regulatory subunit of the Rad50 complex (15, 21, 58, 61).
The S. cerevisiae DNA2 gene was identified as a temperature-sensitive-replication mutant gene (33) and was cloned by the complementation of the dna2ts gene phenotype (11). Dna2 is a flap endonuclease that is essential for cell viability and that is implicated in Okazaki fragment processing by genetic studies of both S. cerevisiae and S. pombe (12, 30). Biochemical reconstitution studies suggested that Dna2 participates in the removal of the RNA-containing segments of Okazaki fragments (5), but the exact function of Dna2, if any, in this step remains unclear (4, 29, 31). Both cell-biological analysis and a chromatin immunoprecipitation (ChIP) assay showed that S. cerevisiae Dna2 is associated with telomeres in G1 phase (18). In S phase, there is a dramatic redistribution of Dna2 from telomeres to sites throughout the replicating chromosome. Dna2 is again localized to telomeres in late S phase. S. cerevisiae Dna2 is also required for de novo telomere addition, suggesting that Dna2 is involved in the tight coordination of the lagging-strand replisome with telomerase activity (18).
In this study, we investigated the detailed roles of S. pombe Dna2 at telomere ends. Our results suggest that Dna2 is involved in the generation of G-rich overhangs in both taz1-d cells and wild-type cells. We also tested the possibility that Dna2 is involved in the processing of DSB ends. However, DSB repair ability was not affected in a dna2-C2 mutant. Although the Rad50 complex is involved in the processing of both telomere ends and DSB ends, our results strongly suggest that telomere ends and DSB ends are processed by different mechanisms.
MATERIALS AND METHODS
Strain construction and growth medium.
Strains used in this report are listed in Table 1. To overexpress the rad2+ gene product, pREP42X-rad2 (ura4+) was constructed by ligation of BamHI-digested rad2+, which was generated from plasmid pREP41X-rad2 (LEU2), with BamHI-digested pREP42X. pREP42X-rad2 suppressed the temperature sensitivity (ts) phenotype of a dna2-C2 mutant (data not shown) (30). To tag the Dna2 protein and Dna2-C2 mutant protein with a Myc epitope tag at their C termini, the plasmid pFA6a-Dna2-13Myc-kanMX6 was constructed as follows. First the dna2+ gene was amplified by PCR with top primer GCATACCCGGGTTTATAAGAAGTGGGAGAAGTTA and bottom primer GCATACCCGGGAAATTCCAGTTGAGGTAAAAT using genomic DNA of wild-type cells (JY741) as a template. Then the SmaI-cut PCR fragment was cloned into SmaI-cut pFA6a-13Myc-kanMX6, resulting in plasmid pFA6a-Dna2-13Myc-kanMX6. pFA6a-13Myc-kanMX6, which contains 13 copies of the Myc epitope and a kanMX6 marker, was provided by John R. Pringle (University of North Carolina) (7). pFA6a-Dna2-13Myc-kanMX6 was linearized with SphI or XbaI and used for transformation of wild-type cells (JY741) or dna2-C2 mutant cells (HK10), respectively. To tag the Trt1 protein with a Myc epitope tag at the C terminus, the plasmid pFA6a-Trt1-13Myc-kanMX6 was constructed as follows. First, the trt1+ gene was amplified by PCR with top primer GATATCCCCGGGACCGAACACCATACCC and bottom primer GATATCCCCGGGATCAGCTATTCTTCTATGTAAAAAT using genomic DNA of wild-type cells (JY741) as a template. Then the SmaI-cut PCR fragment was cloned into SmaI-cut pFA6a-13Myc-kanMX6, resulting in plasmid pFA6a-Trt1-13Myc-kanMX6. pFA6a-Trt1-13Myc-kanMX6 was linearized with AflII and used for transformation of cdc25-22 cells (LSP11) and pku70-d cells in a cdc25-22 background (TK001). Other double or triple mutants were constructed by genetic crosses. Cells were grown in YPAD medium (1% yeast extract, 2% polypeptone, 2% glucose, 20 μg of adenine/ml).
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| JY741 | h−leu1-32 ura4-D18 ade6-M216 | Lab stock |
| JY746 | h+leu1-32 ura4-D18 ade6-M210 | Lab stock |
| KT001 | h−leu1-32 ura4-D18 ade6-M216 taz1::ura4+ | 56 |
| KT110 | h+leu1-32 ura4-D18 ade6-M210 taz1::LEU2 | 56 |
| KT021 | h−leu1-32 ura4-D18 ade6-M216 rad50::LEU2 taz1::ura4+ | 56 |
| KT00g | h−leu1-32 ura4-D18 ade6-M216 exo1::ura4+ | 56 |
| KT01g | h−leu1-32 ura4-D18 ade6-taz1::LEU2 exo1::ura4+ | This work |
| YA132 | h−leu1-32 ura4-D18 rad2::ura4+ | H. Iwasaki |
| KT11f | h+leu1-32 ura4-D18 ade6-M210 taz1::LEU2 rad2::ura4+ | This work |
| HK10 | h−leu1-32 ura4-D18 dna2-C2 | 29 |
| KT010n | h−leu1-32 ura4-D18 taz1::LEU2 dna2-C2 | This work |
| pku70A | h+leu1-32 ura4-D18 ade6-M210 pku70::LEU2::ade6 | 56 |
| KT1105n | h+leu1-32 ura4-D18 ade6-dna2-C2 taz1::LEU2 pku70::LEU2::ade6+ | This work |
| KT120 | h+leu1-32 ura4-D18 ade6-M210 rad50::LEU2 | 56 |
| KT120n | h+leu1-32 ura4-D18 rad50::LEU2 dna2-C2 | This work |
| Rad3D | h−leu1-32 ura4-D18 ade6-704 rad3::ura4+ | 58 |
| KT104 | h+leu1-32 ura4-D18 ade6-M210 rad3::ura4+ | This work |
| KT004n | h−leu1-32 ura4-D18 ade6-M210 dna2-C2 rad3::ura4+ | This work |
| 709 | h+leu1-32 ura4-D18 exo1::ura4+ | 56 |
| KT10gn | h+leu1-32 ura4-D18 dna2-C2 exo1::ura4+ | This work |
| KTtnM | h+leu1-32 ura4-D18 ade6-M210 dna2-myc:kanMX | This work |
| KTtnMM1 | h+leu1-32 ura4-D18 dna2-C2-myc:kanMX | This work |
| TKt7M | h+leu1-32 ura4-D18 ade6-M210 trt1-myc:kanMX | This work |
| KT000n-t7M | h−leu1-32 ura4-D18 dna2-C2 trt1-myc:kanMX | This work |
| LSP11 | h+leu1-32 ura4-D18 cdc25-22 | 31 |
| TK001-t | h+leu1-32 ura4-D18 cdc25-22 trt1-myc:kanMX | This work |
| TK001-t7M | h+leu1-32 ura4-D18 pku70::LEU2 cdc25-22 trt1-myc:kanMX | This work |
| KT146 | h−leu1-32 ura4-D18 ade6-704 rad3::ura4+rad32::ura4+ | This work |
In-gel hybridization.
In-gel hybridization analysis was performed according to the protocol previously published using a G-rich probe, 5′-GATCGGGTTACAAGGTTACGTGGTTACACG-3′, and a C-rich probe, 5′-CGTGTAACCACGTAACCTTGTAACCCGATC-3′ (59). One microgram of genomic DNA was digested with EcoRI or HindIII and separated by electrophoresis on a 0.5% agarose gel in 0.5× Tris-acetate-EDTA (TAE) buffer with 0.01 mg of ethidium bromide/ml. A single-stranded telomeric DNA probe was labeled with [γ-32P]ATP (Amersham Pharmacia Biotech) by using T4 polynucleotide kinase. The gel was hybridized with 10 pmol of probe in hybridization buffer at 37°C overnight. Then the gel was washed and dried. Signals were detected with a Molecular Imager (Bio-Rad). To detect double-stranded telomeric DNA, the gel was treated with denaturing solution (0.5 M NaOH, 150 mM NaCl) for 25 min at room temperature, and then with neutralizing solution (0.5 M Tris-HCl [pH 8.0], 150 mM NaCl) and reprobed with the same probe by in-gel hybridization.
Measurement of telomere length.
Telomere length was measured by Southern hybridization according to the procedure described previously (19) by using an AlkPhos Direct kit module (Amersham Pharmacia Biotech). Briefly, chromosomal DNA, which was digested with ApaI and separated by electrophoresis on a 2% agarose gel, was probed with a 0.3-kb DNA fragment containing telomeric repeat sequences, which was derived from pNSU70 (52).
ChIP.
The ChIP assay described by Takahashi et al. was adopted with a shift modification (56). Cells grown in 100 ml of YPAD culture were fixed with formaldehyde. For immunoprecipitation, the anti-Myc tag 9B11 antibody (Cell Signaling) and protein G-coated Dynabeads (DYNAL) were used. Immunoprecipitated DNA was extracted and suspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA). The telomere DNA and the partial ade6+ DNA were amplified by PCR with [α-32P]CTP (Amersham Pharmacia Biotech) by using mixed primers of telomeric DNA (top, 5′-CGGCTGACGGGTGGGGCCCAATA-3′; bottom, 5′-GTGTGGAATTGAGTATGGTGAA-3′) or partial ade6+ DNA (top, 5′-AGGTATAACGACAACAAACGTTGC-3′; bottom, 5′-CAAGGCATCAGTGTTAATATGCTC-3′). PCR products were separated by electrophoresis on a 0.5% acrylamide gel (Tris-borate-EDTA buffer), and the signals were detected and quantitated with a Molecular Imager (Bio-Rad) or with a transilluminator and National Institutes of Health Image software. All experiments were repeated at least four times with similar results.
DNA damage sensitivity assay.
For the spot assay, 4 μl of 10-fold dilutions of log-phase cells (0.5 × 107 cells/ml) were spotted onto a YPAD (2% agar) plate or a YPAD plate containing bleomycin. For the ionizing radiation survival assay, logarithmically growing cells were irradiated with a 60Co source at a dose rate of 100 to 200 Gy/h. Irradiated cells and unirradiated cells were plated on YPAD medium plates and incubated at 25 or 30°C for 4 days. All experiments were repeated at least twice with similar results.
RESULTS
Dna2 is required for the production of G-rich single-strand overhangs at telomere ends.
It has been suggested that an unknown nuclease resects the C-rich strand at telomere ends in taz1-d cells (59, 61). To identify this nuclease, we first created a taz1-d exo1-d double mutant and a taz1-d rad2-d double mutant because both Exo1 and Rad2 possess 5′-to-3′ exonuclease activity (3, 53). However, these double mutants still contained a significant amount of G-rich overhangs (Fig. 1A, lanes 3 and 4), suggesting that the putative nuclease is neither Exo1 nor Rad2.
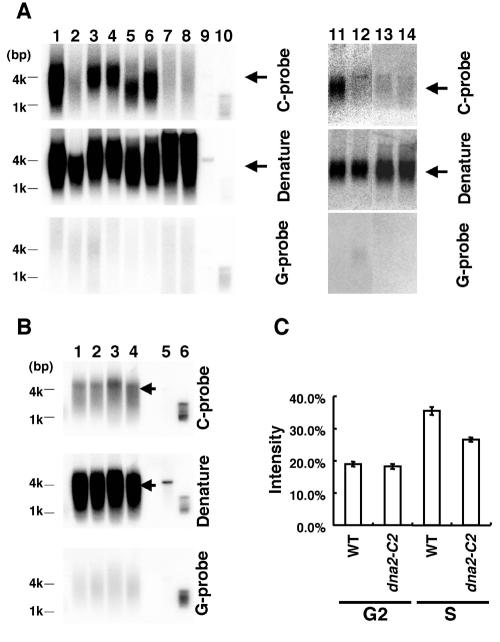
FIG. 1.
Dna2 is involved in the generation of G-rich overhangs. (A) The single-stranded overhangs in various nuclease mutants in a taz1-d background were detected by in-gel hybridization. Lanes 1 and 11, taz1-d cells (KT110) at 30°C; lane 2, rad50-d taz1-d cells (KT021) at 30°C; lane 3, exo1-d taz1-d cells (KT01g) at 30°C; lane 4, taz1-d rad2-d cells (KT11f) at 30°C; lane 5, taz1-d dna2-C2 cells (KT010n) at 25°C; lane 6, taz1-d dna2-C2 pku70-d cells (KT1105n) at 25°C; lane 7, taz1-d dna2-C2 cells (KT010n) at 30°C; lane 8, taz1-d dna2-C2 pku70-d cells (KT1105n) at 30°C; lane 9, double-stranded DNA (dsDNA) control; lane 10, single-stranded DNA (ssDNA) control; lane 12, taz1-d cells (KT110) at 30°C with E. coli exonuclease I; lane 13, taz1-d dna2-C2 cells (KT010n) with pREP42X (empty vector) at 30°C; lane 14, taz1-d dna2-C2 cells (KT010n) with pREP42X-rad2 (overexpression of Rad2) at 30°C. A plasmid containing the telomere repeat sequence derived from pNSU70 was used as dsDNA and ssDNA controls (59). Genomic DNA was digested with EcoRI and separated by electrophoresis. Then the gel was dried and hybridized with a 32P-labeled C-rich (C-probe, top) or G-rich (G-probe; bottom) probe. To detect the double-stranded telomere DNAs, the gel was treated with denaturant and reprobed with the C-rich probe (denature; middle). Arrows, telomeres. (B) The single-stranded overhang in G2 and S phases in wild-type and dna2-C2 mutant cells was detected by in-gel hybridization at a semipermissive temperature (30°C). Lane 1, wild-type cells (JY741) in G2 phase; lane 2, dna2-C2 cells (HK10) in G2 phase; lane 3, wild-type cells (JY741) in S phase; lane 4, dna2-C2 cells (HK10) in S phase; lane 5, dsDNA control; lane 6, ssDNA control. Cells were cultured at 30°C. Then cells in G2 phase and S phase (when the septation index became maximal) were collected from logarithmically growing cells by using an elutriator (2). The septation indexes of wild-type cells and dna2-C2 cells were 34.2 and 33.6%, respectively. Genomic DNA was digested with HindIII, and the single-stranded overhang was detected as described for panel A. Arrows, telomeres. (C) Quantitation of the band intensity of the in-gel hybridization assay shown in panel B. The band intensity was quantitated with a Molecular Imager (Bio-Rad). The signal intensity was calculated as follows. First the nonspecific signal detected with the G-rich probe (G probe) was subtracted from the signal corresponding to the G-rich overhang (C probe) and from the double-strand telomere DNA signal (C probe − G probe and denature − G probe, respectively). Then the signal (C probe − G probe) was divided by (denature − G probe) to adjust for the DNA concentration. Error bars, standard deviations determined from two independent experiments. WT, wild type.
S. cerevisiae Dna2, which possesses nuclease activity, binds to the telomere end (18), and overproduction of Dna2 leads to increased G-rich overhangs (44). Therefore, we next tested whether S. pombe Dna2 is required for the generation of G-rich overhangs in taz1-d cells. For this, we used a dna2-C2 temperature-sensitive mutant, which carries a Leu-to-Ser change at amino acid 1079 (30). This dna2-C2 mutant grows normally at 25 and 28°C (30). We created a taz1-d dna2-C2 double mutant and examined the overhang at both the permissive temperature (25°C) and semipermissive temperature (30°C). The taz1-d dna2-C2 double mutant contained a significant amount of G-rich overhangs at the permissive temperature (25°C) (Fig. 1A, lane 5). In contrast, the taz1-d dna2-C2 double mutant lost the G-rich overhangs following a shift to the semipermissive temperature (30°C) for 1 day (Fig. 1A, lane 7). These results indicate that Dna2 is required for the generation of G-rich overhang in taz1-d cells (59). We also examined the amount of G-rich overhang in taz1-d cells at 25°C and found that the signal is almost identical to that at 30°C (data not shown).
The ts phenotype of the dna2-C2 mutant can be suppressed by overexpression of Cdc1, Cdc27, Cdc17, and Rad2 (30). As each of these gene products plays a role in the elongation or maturation of Okazaki fragments, it is suggested that the dna2-C2 mutant has a defect in Okazaki fragment elongation and maturation. If the defect in generation of the G-rich overhang in the taz1-d dna2-C2 double mutant at the semipermissive temperature is due to a defect in Okazaki fragment maturation, overexpression of Rad2 would suppress the defect in generation of the G-rich overhang in the taz1 dna2-C2 double mutant. To test this possibility, we overexpressed Rad2 in the taz1-d dna2-C2 double mutant at the semipermissive temperature and examined the overhang. Overexpression of Rad2 did not suppress the defect in the generation of the G-rich overhang in the taz1-d dna2-C2 double mutant, suggesting that the telomere phenotype of the dna2-C2 mutant at the semipermissive temperature is not due to a defect in Okazaki fragment maturation (Fig. 1A, lanes 13 and 14). We also confirmed that the single-stranded DNA is present at the termini of the chromosomes in taz1-d cells by incubating genomic DNA in the presence of Escherichia coli exonuclease I (Fig. 1A, lanes 11 and 12).
Although our previous work showed that the Rad50 complex, in addition to Dna2, is required for the generation of G-rich overhangs in taz1-d cells, taz1-d rad50-d pku70-d triple-mutant cells contain the overhang, suggesting that an unknown second nuclease can resect telomere ends without the assistance of the Rad50 complex when both the Taz1 and Ku heterodimers are absent (59). To test the possibility that this second nuclease is also Dna2, we examined the overhang in taz1-d dna2-C2 pku70-d triple-mutant cells at the semipermissive temperature (30°C). The G-rich overhang was detected in the taz1-d dna2-C2 pku70-d triple mutant at 25°C (Fig. 1A, lane 6). However, the G-rich overhang was not detected in the taz1-d dna2-C2 pku70-d triple mutant at 30°C (Fig. 1A, lane 8). These results suggest that Dna2 is the second nuclease that resects telomere ends without the assistance of the Rad50 complex when both the Taz1 and Ku heterodimers are absent.
In wild-type cells, the amount of G-rich overhangs increases in S phase (32). The signal corresponding to the G-rich overhang in S phase disappears following addition of E. coli exonuclease I, indicating that the single-stranded G-rich DNA detected in S phase in wild-type cells is present at the terminus of the telomere (data not shown). The next question we addressed was whether Dna2 is involved in the processing of telomere ends in S phase in wild-type cells. The mechanism of the generation of G-rich overhangs in S phase is not clear, because G-rich overhangs could be generated without nuclease activity at telomere ends that are synthesized by lagging-strand DNA synthesis, simply through failure to complete lagging-strand synthesis. In contrast, telomere ends synthesized by leading-strand DNA synthesis would be blunt and would require a nuclease reaction to produce G-rich overhangs (16). Nonetheless, we next examined the G-rich overhangs in the dna2-C2 mutant in S phase at the semipermissive temperature. Wild-type cells and dna2-C2 mutant cells were grown at 30°C and synchronized by using an elutriator. As shown previously, the G-rich overhangs increased in S phase in wild-type cells (Fig. 1B and C). Although the G-rich overhangs also increased in S phase in dna2-C2 cells at the semipermissive temperature, the signal intensity of the G-rich overhang in S phase in dna2-C2 cells at the semipermissive temperature was lower than that in wild-type cells (Fig. 1B and C). These results suggest that Dna2 is involved in the production of G-rich overhangs not only in taz1-d cells but also in wild-type cells.
Dna2 is required for telomere length maintenance.
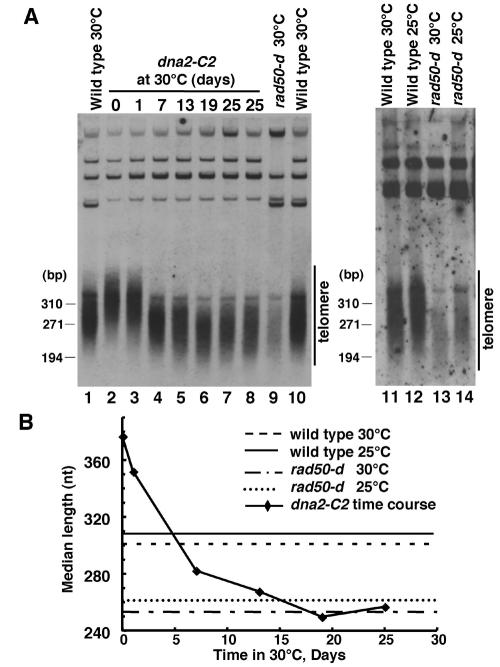
Next we examined the telomere lengths of the dna2-C2 mutant at both the permissive temperature (25°C) and semipermissive temperature (30°C). For an unknown reason, the telomere lengths of wild-type cells and rad50-d cells at 25°C were slightly (about 10 bp) longer than those at 30°C (Fig. 2A and B, lanes 11 to 14). In contrast, the telomere length of the dna2-C2 mutant was significantly (about 70 bp) longer than that of the wild-type cells at 25°C (Fig. 2A and B, lane 2). The telomere length of the dna2-C2 mutant gradually decreased after the temperature shift to 30°C (Fig. 2A, lanes 3 to 7). The telomere length then became stable when it became similar to that of the rad50-d mutant (Fig. 2A and B, lanes 7 and 9). These results indicate that Dna2 is required for telomere length maintenance.
FIG. 2.
Dna2 is required for the telomere length regulation. (A) The telomere length of the dna2-C2 mutant was analyzed by Southern hybridization analysis at the permissive (25°C) and semipermissive (30°C) temperatures. Lanes 1, 10, and 11, wild-type cells at 30°C (JY741); lane 2, dna2-C2 mutant at 25°C (HK10); lanes 3 to 7, dna2-C2 mutant incubated at 30°C (HK10) for the indicated (at the top) numbers of days; lane 8, dna2-C2 mutant incubated at 30°C for 25 days in an independent experiment (HK10); lanes 9 and 13, rad50-d cells at 30°C (KT120); lane 12, wild-type cells at 25°C (JY741); lane 14, rad50-d cells at 25°C (KT120). (B) Time course of the change of telomere length in dna2-C2 mutant after a temperature shift to the semipermissive temperature (30°C). The data shown in panel A were plotted.
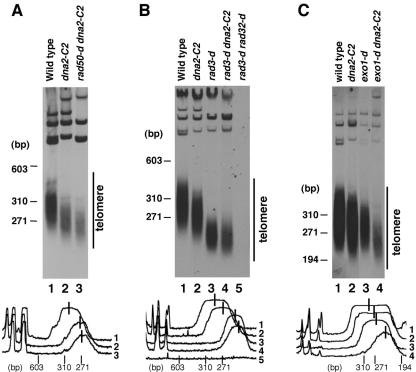
To test whether Rad50 and Dna2 are involved in the same pathway in telomere maintenance, we next examined the telomere length of a rad50-d dna2-C2 double mutant at 30°C. Although the telomere length of the rad50-d dna2-C2 double mutant was similar to that of the dna2-C2 single mutant, they did not match completely (Fig. 3A, lanes 2 and 3). Therefore we could not conclude that Rad50 and Dna2 are involved in the same pathway in telomere length regulation.
FIG. 3.
Epistasis analysis comparing dna2-C2 mutant and rad50-d cells (A), rad3-d cells (B), and exo1-d cells (C) for telomere maintenance at 30°C. (A) Telomere length of the rad50-d dna2-C2 double mutant is slightly shorter than that of the dna2-C2 single mutant. Lane 1, wild-type cells (JY741); lane 2, dna2-C2 mutant (HK10); lane 3, rad50-d dna2-C2 cells (KT120n). (B) Dna2 is not required for telomere maintenance in the absence of Rad3. Lane 1, wild-type cells (JY741); lane 2, dna2-C2 mutant (HK10); lane 3, rad3-d cells (Rad3D); lane 4, rad3-d dna2-C2 cells (KT004n); lane 5, rad3-d rad32-d cells (KT146). (C) Dna2 and Exo1 function independently for telomere length regulation. Lane 1, wild-type cells (JY741); lane 2, dna2-C2 mutant (HK10); lane 3, exo1-d cells (KT00g); lane 4, exo1-d dna2-C2 cells (KT10gn). Telomere length was studied as in Fig. 2A. Because of the phenotypic lag, cells were incubated for 10 days after the temperature shift to the semipermissive temperature (30°C). Peaks and distributions of the telomeric-DNA-derived bands analyzed with National Institutes of Health Image, version 1.62, software are shown below. Telomere peaks are indicated by lines.
The deletion of any of the rad32+, rad50+, or nbs1+ genes in combination with deletion of the rad3+ gene, which is required for the response to DNA damage, causes catastrophic loss of telomeres, resulting in chromosomal end fusions (8, 15, 42). To test whether Dna2 and the Rad50 complex function in the same pathway for telomere maintenance in the absence of Rad3, we examined the telomere length of a rad3-d dna2-C2 double mutant. Although the rad3-d rad32-d double mutant lost the telomere repeats completely, the rad3-d dna2-C2 double mutant did not lose the telomere repeats (Fig. 3B, lanes 4 and 5). This result suggests that Dna2 and Rad50 have different roles in telomere maintenance in the absence of Rad3.
Since neither deletion of the exo1+ gene in taz1-d cells nor deletion of the exo1+ gene in taz1-d rad50-d ku70-d triple-mutant cells affects the amount of G-rich overhang, Exo1 seemed to have no roles in telomere maintenance in S. pombe (Fig. 1A, lane 3) (59). However, we found that the telomere length of an exo1-d dna2-C2 double mutant was shorter than that of each single mutant (Fig. 3C, lane 4). These results suggest that Exo1 plays an important role at telomere ends and that Dna2 and Exo1 function independently at telomere ends.
Dna2 binds telomeres preferentially, and binding is severely impaired by a temperature shift to the semipermissive temperature.
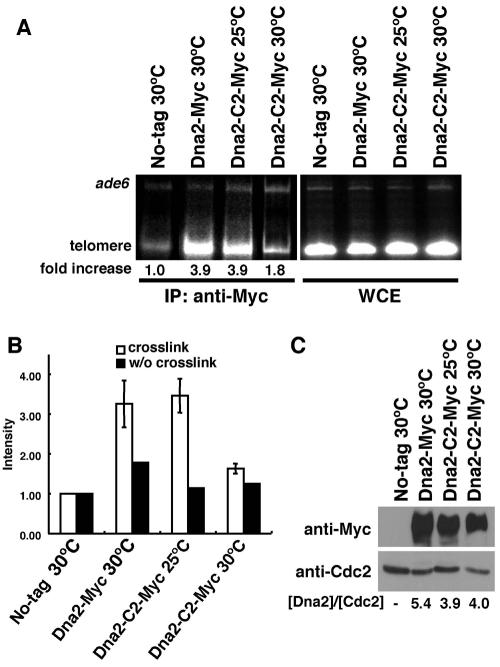
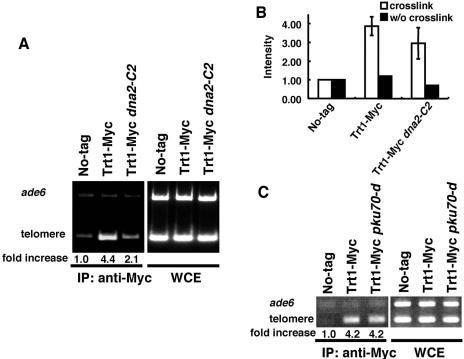
We next performed ChIP assays to examine whether Dna2 binds to telomere ends. We created strains in which the only wild-type copy of dna2+ was replaced by either dna2-myc or dna2-C2-myc (7). In both the wild-type cells and dna2-C2 mutant cells, tagging of Dna2 did not affect the growth rates or the methyl methanesulfonate (MMS) and hydroxyurea sensitivities (data not shown). We found that the Dna2-Myc protein was bound preferentially to telomeric DNA in a ChIP assay (Fig. 4A and B). Next, we examined the telomere binding of the Dna2-C2 mutant protein. The Dna2-C2-Myc mutant protein bound to telomeres at the permissive temperature. However, the telomere binding was severely impaired after a shift to the semipermissive temperature (Fig. 4A and B). The reduced telomere binding at the semipermissive temperature was not due to reduction of protein level, because the protein level in the dna2-C2 mutant at the permissive temperature was not different from that at the semipermissive temperature (Fig. 4C). Our results suggest that telomere shortening and loss of G-rich overhangs in the dna2-C2 mutant at the semipermissive temperature are due to loss of the telomere-binding ability of Dna2 protein.
FIG. 4.
Binding of Dna2-C2 mutant protein to telomere DNA is severely impaired by a temperature shift to the semipermissive temperature. (A) ChIP assay of the Dna2 protein. Untagged wild-type control cells (JY741), dna2-myc (KTtnM) cells, and dna2-C2-myc (KTtnMM1) cells were cultured at the indicated temperatures. PCRs were performed on whole-cell extract (WCE; input) and on chromatin immunoprecipitates (IP: anti-Myc) with primers to amplify telomere DNA (telomere) and DNA from the ade6+ gene (ade6). The relative enrichment of precipitated telomere DNA is shown underneath each lane. Ratios of telomere signals to ade6 signals were used to calculate relative precipitation enrichment. (B) Relative precipitation enrichment determined in the ChIP assay shown in panel A. Error bars, standard deviations determined from four independent experiments. As a control, the ChIP assay was performed without (w/o) cross-linking. (C) Protein expression level is not affected in the dna2-C2 mutant at the permissive temperature versus the semipermissive temperature. The Dna2-Myc protein from dna2-myc cells (KTtnM) and the Dna2-C2-Myc protein from dna2-C2-myc cells (KTtnMM1) were detected by Western blotting with the anti-Myc 9B11 antibody (Cell Signaling). As a control, Cdc2 was also detected with an anti-Cdc2 antibody (PSTAIRE). The relative amounts of Dna2 or Dna2-C2 are shown underneath each lane. Ratios of Dna2 or Dna2-C2 signals to Cdc2 signals were calculated to express the relative amounts of Dna2 and Dna2-C2.
Binding of telomerase to telomeric DNA is reduced in the dna2-C2 mutant.
We next asked whether the mutation of dna2+ affects the binding of the telomerase catalytic subunit, Trt1, to telomeric DNA. As shown in Fig. 5A and B, Myc-tagged Trt1 bound to telomeric DNA. In contrast, the binding of Trt1 to telomeric DNA was reduced in the dna2-C2 mutant at the semipermissive temperature (Fig. 5A and B). The expression level of Trt1-Myc was not affected in the dna2-C2 mutant, indicating that the reduced binding was not due to a reduced protein level (data not shown). As the dna2-C2 mutant has short telomeres, the reduced telomere binding of telomerase might have been due to telomere shortening. To test this possibility, we examined the telomere binding of Trt1 in pku70-d cells, which have short telomeres. The binding of Trt1 to telomeric DNA was not affected in pku70-d cells, suggesting that the reduced telomere binding of Trt1 in the dna2-C2 mutant is not due to reduced telomere length (Fig. 5C).
FIG. 5.
Telomere binding of Trt1 is affected in the dna2-C2 mutant at 30°C. (A) ChIP assay of the Trt1 protein in the dna2-C2 mutant. Untagged wild-type control cells (JY741), trt1-myc (TKt7 M) cells, and trt1-myc dna2-C2 (KT000n-t7 M) cells were cultured at 30°C. PCRs were performed as for Fig. 4A. WCE, whole-cell extracts; IP, immunoprecipitation. (B) Relative precipitation enrichment determined in the ChIP assay shown in panel A. Error bars, standard deviations determined from four independent experiments. As a control, the ChIP assay was performed without (w/o) cross-linking. (C) ChIP assay of the Trt1 protein in pku70-d cells. Untagged wild-type control cells (LSP11), trt1-myc (TK001-t) cells, and trt1-myc pku70-d (TK001-t7 M) cells were cultured at 25°C. PCRs were performed as for Fig. 4A.
DSB repair ability is not affected in the dna2-C2 mutant.
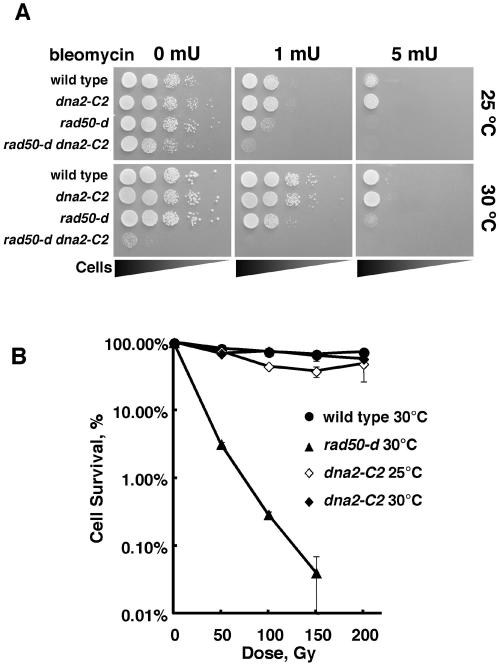
Our results suggest that Dna2 is involved in the production of G-rich overhangs at telomere ends. The next question is whether Dna2 is involved in the processing of DSB ends. To address this, we examined the sensitivity of the dna2-C2 mutant to bleomycin and gamma rays at 25 and 30°C. The sensitivities to bleomycin and gamma rays at 30°C were not affected by the mutation of dna2+ (Fig. 6). These results suggest that DSB repair ability, including the ability to process DSB ends, is not affected by the dna2-C2 mutation.
FIG. 6.
The dna2-C2 mutant is not sensitive to bleomycin or gamma rays at 25 and 30°C. (A) Bleomycin sensitivities of wild-type (JY741), rad50-d (JY120), dna2-C2 (HK10), and rad50-d dna2-C2 (KT120n) cells were assayed by a spot test at 25 and 30°C. (B) Sensitivities to gamma rays of wild-type (JY741), rad50-d (KT120), and dna2-C2 (HK10) cells at 25 and at 30°C. The percentages of survival of the indicated strains were plotted versus the gamma ray doses. Error bars, standard deviations.
DISCUSSION
Dna2 is required for generation of G-rich overhangs in taz1-d cells.
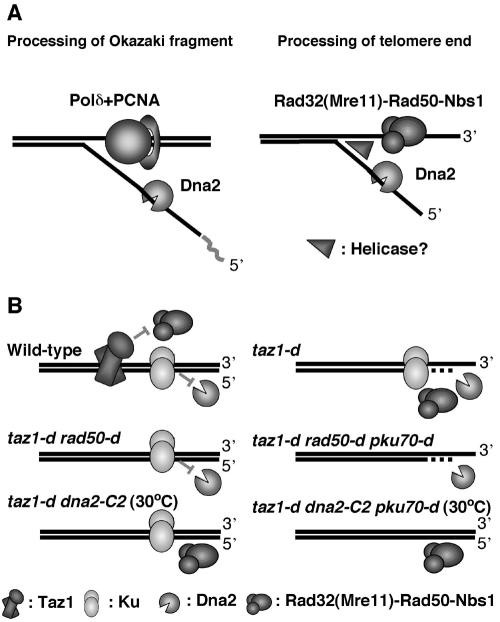
We found that a dna2-C2 taz1-d double mutant lost the G-rich overhang at the semipermissive temperature (30°C), indicating that Dna2 is required for generation of G-rich overhangs in taz1-d cells (Fig. 1A). This was not due to a defect in Okazaki fragment maturation, because a rad2-d taz1-d double mutant did not lose the G-rich overhangs (Fig. 1A). How does Dna2 contribute to the generation of G-rich overhangs in taz1-d cells? S. cerevisiae Dna2 possesses endonuclease activity and can remove 5′ flap DNA likely to be generated during Okazaki fragment processing in vitro (5). We have also found that purified S. pombe Dna2 has nuclease activity (data not shown). Therefore, we propose a model in which Dna2 is required for degradation of the C-rich strand in taz1-d cells, similar to the mechanism of removal of the 5′ flap DNA during Okazaki fragment processing (Fig. 7A). In this model, telomere ends would be unwound by the Rad50 complex and/or an unknown helicase. Then, Dna2 would remove 5′ flap DNA by using its endonuclease activity. S. cerevisiae Dna2 possesses helicase activity. In contrast, purified S. pombe Dna2 lacks any detectable ATPase activity, suggesting that S. pombe Dna2 has no helicase activity (6, 13, 57). However, it is possible that the helicase domain of Dna2 might have some role in DNA unwinding, because the human Rad50 complex has DNA-unwinding activity in the absence of ATP (46). Therefore, we do not exclude the possibility that the DNA-unwinding activity of Dna2, but not a nuclease activity, is required for the processing of telomeric DNA.
FIG. 7.
Model for the processing of telomere ends. (A) Comparison of models for the processing of Okazaki fragments and telomere ends. Dna2 can specifically cut the 5′ end during Okazaki fragment processing. Similarly, we assume that Dna2 specifically cuts C-rich DNA after the telomere end is unwound by the Rad50 complex. Additional helicase activity may be required for unwinding of telomere ends. (B) Summary of the roles of Dna2, the Rad50 complex, and the Ku heterodimer in telomere resectioning in taz1-d cells. In taz1-d cells, G-rich overhangs are significantly increased in a Rad50 complex-dependent manner, suggesting that Taz1 inhibits the Rad50 complex from performing the resection (59). The taz1-d dna2-C2 double mutant has no overhang, suggesting that Dna2 is required for the resectioning. The taz1-d rad50-d pku70-d triple mutant has the overhang, but the taz1-d dna2-C2 ku70-d triple mutant does not have it, suggesting that Dna2 resects telomere ends without the assistance of the Rad50 complex in the absence of both the Taz1 and Ku heterodimers. Our results suggest that the Ku heterodimer inhibits Dna2 from performing the resectioning but that the Rad50 complex allows Dna2 to resect telomere ends in the presence of the Ku heterodimer.
Another possible explanation for the lack of a G-rich overhang in the taz1-d dna2-C2 double mutant at the semipermissive temperature is that Dna2 protects G-rich overhangs from degradation. If this were true, G-rich overhangs would not be detected in S phase in the dna2-C2 mutant at the semipermissive temperature. However, the G-rich overhang was still detected in S phase in the dna2-C2 mutant (Fig. 1B and C), making this explanation is unlikely.
Consistent with our two-step model, the binding of the Dna2-Myc protein to the telomeres in a ChIP assay was not affected in the rad32-d mutant (data not shown). Moreover, the Rad32-Myc protein bound to telomeric DNA in a dna2-C2 mutant background at both 25 and 30°C in a ChIP assay (data not shown). These results indicate that Dna2 and the Rad50 complex bind to telomeres independently. We also tested the interaction between Dna2 and the Rad50 complex by coimmunoprecipitation experiments and found that the Dna2-Myc protein was not coimmunoprecipitated with the Rad50-TAP protein, suggesting that Dna2 does not stably interact with the Rad50 complex in vivo (data not shown). Although we assume that the nuclease activity of Dna2 is involved in telomere processing, it remains unclear whether the Dna2-C2 mutant protein has a defect in nuclease activity at the semipermissive temperature. Further biochemical studies are required to confirm our model.
As a taz1-d rad50-d pku70-d triple mutant possesses the G-rich overhangs, the existence of a second nuclease that resects telomere ends without assistance from the Rad50 complex in the absence of the Taz1 and Ku heterodimers has been suggested (Fig. 7B) (59). We found that a taz1-d dna2-C2 pku70-d triple mutant did not have the G-rich overhangs at the semipermissive temperature, suggesting that this second nuclease is also Dna2 (Fig. 1A and 7B). These results suggest that the Rad50 complex allows Dna2 to resect telomere ends in the presence of the Ku heterodimer but that, in the absence of the Ku heterodimer, Dna2 can resect telomere ends without assistance from the Rad50 complex (Fig. 7B). How does the Rad50 complex allow Dna2 to resect telomere ends in the presence of the Ku heterodimer? In our model, telomere ends must be unwound by a helicase activity. As the Ku heterodimer binds and protects DNA ends from the activity of enzymes, such as nucleases, Ku might inhibit DNA unwinding at telomere ends. In that case, the Rad50 complex might be required for DNA unwinding in the presence of the Ku heterodimer.
Mutation in dna2+ affects the generation of G-rich overhangs in S phase in wild-type cells.
Although the above model was substantiated based on the study using a taz1-d background, this model might be applicable to the generation of G-rich overhangs in S phase in wild-type cells, because Taz1 inhibits telomerase activity and Taz1 might be detached from telomeric DNA or inactivated in S phase to allow telomerase to access telomere ends. To test this possibility, we examined the effect of the dna2-C2 mutation on the G-rich overhang in S phase in a wild-type background. In wild-type cells, the intensity of the signal corresponding to the G-rich overhang increased 16.50% in S phase compared to G2 phase. In contrast, in the dna2-C2 mutant, the signal intensity increased only 8.36% in S phase compared to G2 phase at the semipermissive temperature (Fig. 1B and C). We assume that this reduction in G-rich overhangs in S phase in the dna2-C2 mutant can be attributed to the defect in the degradation of the C-rich strand by Dna2. Although G-rich overhangs were still detected in the dna2-C2 mutant in S phase, these G-rich overhangs could have been produced without nuclease activity at telomere ends that were synthesized by lagging-strand DNA synthesis. Therefore we assume that the G-rich overhang detected in the dna2-C2 mutant in S phase is produced without nuclease activity. At this time, however, it is impossible to distinguish the telomere ends synthesized by leading-strand DNA synthesis from those generated by lagging-strand synthesis. Further studies will be required to elucidate the detailed roles of Dna2 at telomere ends in S phase.
We found that the telomere length of a dna2-C2 exo1-d double mutant was shorter than that of each single mutant at 30°C (Fig. 3C). These results indicate that Dna2 and Exo1 function independently at telomere ends. In S. cerevisiae, Exo1 is required for the production of G-rich overhangs in a yku70 mutant (36). Similarly, S. pombe Exo1 might be able to produce the G-rich overhangs in the dna2-C2 mutant via its exonuclease activity. These facts further support our model that Dna2 is required for the production of the G-rich overhangs in wild-type cells.
Role of Dna2 in the recruitment of telomerase to telomere DNA.
We found that the binding of Trt1 was reduced in the dna2-C2 mutant at the semipermissive temperature (Fig. 5). Our results suggest that Dna2 is involved in the production of G-rich overhangs in wild-type cells (Fig. 1B and C). Telomerase binds to G-rich overhangs to elongate telomeric DNA. These facts suggest that the reduced telomere binding of telomerase in the dna2-C2 mutant is due to reduced G-rich overhangs. If Trt1 binding to the telomere depends on the length of the single-stranded overhang, Trt1 might bind to the telomere more tightly in pku70-d cells than in wild-type cells, because pku70-d cells have longer overhangs than wild-type cells (32). However, the telomere binding of telomerase did not increase in pku70-d cells. The possible explanation for this is that telomerase binding might be saturated in the wild-type cells and hence longer overhangs in pku70-d cells might not lead to increased Trt1 binding.
The other explanation for the reduced telomere binding of telomerase in the dna2-C2 mutant is that Dna2 is required for the recruitment of telomerase through a protein-protein interaction. S. cerevisiae Dna2 binds to replication protein A (RPA). RPA also binds to telomere DNA in both S. pombe and S. cerevisiae (43, 50). Moreover, S. cerevisiae RPA is required for loading Est1p onto telomeres during S phase (43, 50). These facts imply that S. pombe Dna2 might be involved in the loading of the telomerase complex onto the telomere.
Telomere shortening of dna2-C2 mutant cells at the semipermissive temperature can be explained by a defect in telomere end processing and/or insufficient recruitment of telomerase to telomeric DNA. However, these dna2-C2 mutant cells had longer telomeres than wild-type cells at the permissive temperature (Fig. 2A and B). These results suggest that the dna2-C2 mutant has opposite defects in telomere length regulation at these two different temperatures. In S. cerevisiae, mutations in DNA polymerase alpha and other replication proteins, including Dna2, also cause telomere elongation (1, 14, 20, 25). It has been suggested that this elongation is due to defects in the coordination of DNA polymerase alpha with telomerase activity (17, 37, 47, 48). Similarly, at the permissive temperature, the S. pombe dna2-C2 mutant might have a defect in the coordination of DNA polymerase alpha with telomerase activity, which might allow uncontrolled telomere elongation.
The defect in telomere end processing is not related to the defect in telomere maintenance in the absence of Rad3.
Both Dna2 and the Rad50 complex are required for the generation of G-rich overhangs in taz1-d cells. (Fig. 2A). This fact suggests that Dna2 and Rad50 are epistatic in telomere end resection. Although the rad3-d rad50-d double mutant and the rad3-d rad32-d double mutant lost telomere DNA completely (Fig. 3B) (42), the rad3-d dna2-C2 double mutant did not lose telomere DNA at the semipermissive temperature (Fig. 3B). These results indicate that Dna2 and Rad50 are not epistatic in telomere maintenance in the absence of Rad3. It has been suggested that Tel1 and Rad50 function in the same pathway for telomere maintenance in the absence of Rad3 (39, 40, 42). Therefore, we tested whether Tel1 is required for generation of G-rich overhangs in taz1-d cells. However, we found that deletion of tel1+ in the taz1-d mutant did not affect the G-rich overhang (data not shown). This result indicates that Tel1 is not epistatic to Rad50 in the generation of G-rich overhang in taz1-d cells. Our results allow us to dissect telomere end resection in taz1-d cells and telomere maintenance in the absence of Rad3. Rad50 is required for both processes, whereas Dna2 is involved only in the former and Tel1 is involved only in the latter.
Role of Dna2 in DNA repair.
The dna2-C2 mutant is MMS and hydroxyurea sensitive, suggesting that dna2+ is involved in the repair of DNA damage generated by alkylating agents (30). Surprisingly, the dna2-C2 mutant was not gamma ray sensitive, suggesting that dna2+ is not involved in repair of DNA damage generated by gamma rays. MMS stalls the replication fork and is thought to collapse the replication fork in S. pombe (27, 59). In contrast, gamma rays cause DNA DSBs mostly in G2 phase, and these DSBs are repaired in G2 phase. These facts suggest that Dna2 is specifically required for DNA damage generated at a stalled or collapsed replication fork. Similar to the dna2-C2 mutant, S. pombe mus81-d cells are MMS sensitive, but not gamma ray sensitive. It has also been suggested that Mus81 is involved in the processing of collapsed replication forks (9, 23).
Difference between DSB ends and telomere ends.
Although the Rad50 complex is involved in the processing of both telomere ends and DSB ends, our results revealed that DSB ends and telomere ends are processed differently. Our results suggest that Dna2 is involved in the processing of telomere ends. However, DSB repair ability (probably including DSB end processing ability) was normal in the dna2-C2 mutant at the semipermissive temperature, suggesting that Dna2 is not involved in the processing of DSB ends (Fig. 6) (59). Consistent with these data, S. cerevisiae dna2 mutants that are sensitive to X rays are not defective in mitotic recombination, suggesting that S. cerevisiae Dna2 is not involved in the processing of DSB ends (10). The mechanism of processing of DSB ends is not fully understood. It is clear that the Rad50 complex is involved in this process. However the importance of the nuclease domain in Rad32 remains unclear. The S. pombe rad32-D25A mutant is DNA damage sensitive, but the complex formed between Rad32 and Rad50 is unstable in the rad32-D25A mutant (59). Therefore, the DNA damage sensitivity of the rad32-D25A mutant might be due to defective formation of the complex between Rad32 and Rad50. In S. cerevisiae, the nuclease-deficient mre11 mutant is not as DNA damage sensitive as the mre11 null mutant. Based on these and other data, the existence of an unknown nuclease that resects DSB ends has been suggested (34). Although our results suggest that Dna2 is not involved in the processing of DSB ends, we have not excluded the possibility that other dna2 mutant alleles might have a defect in DSB end processing. Isolation of additional dna2 mutant alleles will provide useful information for elucidating the roles of Dna2 in telomere maintenance and other aspects of DNA metabolism.
Finally, our results and previous results indicate that S. pombe and S. cerevisiae Dna2 proteins play important roles at telomere ends (18). Dna2 is conserved from yeast to higher eukaryotes and thus the function of Dna2 at telomere DNA might be conserved in higher eukaryotes.
Acknowledgments
We are grateful to Judith Campbell for suggesting that Dna2 might be involved in the processing of telomere ends. We thank Hiroyuki Araki for help with the elutriation; Takeshi Saito, Shinji Yasuhira, and Hiroshi Utsumi for help with the gamma ray irradiation; Hiroshi Iwasaki and Akira Matsuura for providing strains; and John R. Pringle for providing plasmids. We also thank all the members of our laboratory for their valuable discussions and help with revision and Julia Cooper for allowing Kazunori Tomita to do some experiments for revision at her laboratory in Cancer Research UK.
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan to Masaru Ueno and by a grant from the Yokohama City Collaboration of Regional Entities for the Advancement of Technological Excellence, JST, to Masaru Ueno. This work was supported by the NIB Cooperative Research Program (2004-8).
REFERENCES
- 1.Adams, A. K., and C. Holm. 1996. Specific DNA replication mutations affect telomere length in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Alleva, J. L., and P. W. Doetsch. 1998. Characterization of Schizosaccharomyces pombe Rad2 protein, a FEN-1 homolog. Nucleic Acids Res. 26:3645-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayyagari, R., X. V. Gomes, D. A. Gordenin, and P. M. Burgers. 2003. Okazaki fragment maturation in yeast. I. Distribution of functions between Fen1 and Dna2. J. Biol. Chem. 278:1618-1625. [DOI] [PubMed] [Google Scholar]
- 5.Bae, S. H., K. H. Bae, J. A. Kim, and Y. S. Seo. 2001. RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature 412:456-461. [DOI] [PubMed] [Google Scholar]
- 6.Bae, S. H., J. A. Kim, E. Choi, K. H. Lee, H. Y. Kang, H. D. Kim, J. H. Kim, K. H. Bae, Y. Cho, C. Park, and Y. S. Seo. 2001. Tripartite structure of Saccharomyces cerevisiae Dna2 helicase/endonuclease. Nucleic Acids Res. 29:3069-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 8.Bentley, N. J., D. A. Holtzman, G. Flaggs, K. S. Keegan, A. DeMaggio, J. C. Ford, M. Hoekstra, and A. M. Carr. 1996. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 15:6641-6651. [PMC free article] [PubMed] [Google Scholar]
- 9.Boddy, M. N., A. Lopez-Girona, P. Shanahan, H. Interthal, W. D. Heyer, and P. Russell. 2000. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol. Cell. Biol. 20:8758-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budd, M. E., and J. L. Campbell. 2000. The pattern of sensitivity of yeast dna2 mutants to DNA damaging agents suggests a role in DSB and postreplication repair pathways. Mutat. Res. 459:173-186. [DOI] [PubMed] [Google Scholar]
- 11.Budd, M. E., and J. L. Campbell. 1995. A yeast gene required for DNA replication encodes a protein with homology to DNA helicases. Proc. Natl. Acad. Sci. USA 92:7642-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budd, M. E., and J. L. Campbell. 1997. A yeast replicative helicase, Dna2 helicase, interacts with yeast FEN-1 nuclease in carrying out its essential function. Mol. Cell. Biol. 17:2136-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budd, M. E., W. C. Choe, and J. L. Campbell. 1995. DNA2 encodes a DNA helicase essential for replication of eukaryotic chromosomes. J. Biol. Chem. 270:26766-26769. [DOI] [PubMed] [Google Scholar]
- 14.Carson, M. J., and L. Hartwell. 1985. CDC17: an essential gene that prevents telomere elongation in yeast. Cell 42:249-257. [DOI] [PubMed] [Google Scholar]
- 15.Chahwan, C., T. M. Nakamura, S. Sivakumar, P. Russell, and N. Rhind. 2003. The fission yeast Rad32 (Mre11)-Rad50-Nbs1 complex is required for the S-phase DNA damage checkpoint. Mol. Cell. Biol. 23:6564-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakhparonian, M., and R. J. Wellinger. 2003. Telomere maintenance and DNA replication: how closely are these two connected? Trends Genet. 19:439-446. [DOI] [PubMed] [Google Scholar]
- 17.Chandra, A., T. R. Hughes, C. I. Nugent, and V. Lundblad. 2001. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15:404-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe, W., M. Budd, O. Imamura, L. Hoopes, and J. L. Campbell. 2002. Dynamic localization of an Okazaki fragment processing protein suggests a novel role in telomere replication. Mol. Cell. Biol. 22:4202-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper, J. P., E. R. Nimmo, R. C. Allshire, and T. R. Cech. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385:744-747. [DOI] [PubMed] [Google Scholar]
- 20.Dahlen, M., P. Sunnerhagen, and T. S. Wang. 2003. Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol. Cell. Biol. 23:3031-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Amours, D., and S. P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3:317-327. [DOI] [PubMed] [Google Scholar]
- 22.Dionne, I., and R. J. Wellinger. 1996. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl. Acad. Sci. USA 93:13902-13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doe, C. L., J. S. Ahn, J. Dixon, and M. C. Whitby. 2002. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 277:32753-32759. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira, M. G., K. M. Miller, and J. P. Cooper. 2004. Indecent exposure: when telomeres become uncapped. Mol. Cell 13:7-18. [DOI] [PubMed] [Google Scholar]
- 25.Formosa, T., and T. Nittis. 1999. Dna2 mutants reveal interactions with Dna polymerase alpha and Ctf4, a Pol alpha accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics 151:1459-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuse, M., Y. Nagase, H. Tsubouchi, K. Murakami-Murofushi, T. Shibata, and K. Ohta. 1998. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 17:6412-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartsuiker, E., E. Vaessen, A. M. Carr, and J. Kohli. 2001. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 20:6660-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiraoka, Y., E. Henderson, and E. H. Blackburn. 1998. Not so peculiar: fission yeast telomere repeats. Trends Biochem. Sci. 23:126. [DOI] [PubMed] [Google Scholar]
- 29.Jin, Y. H., R. Ayyagari, M. A. Resnick, D. A. Gordenin, and P. M. Burgers. 2003. Okazaki fragment maturation in yeast. II. Cooperation between the polymerase and 3′-5′-exonuclease activities of Pol delta in the creation of a ligatable nick. J. Biol. Chem. 278:1626-1633. [DOI] [PubMed] [Google Scholar]
- 30.Kang, H. Y., E. Choi, S. H. Bae, K. H. Lee, B. S. Gim, H. D. Kim, C. Park, S. A. MacNeill, and Y. S. Seo. 2000. Genetic analyses of Schizosaccharomyces pombe dna2+ reveal that Dna2 plays an essential role in Okazaki fragment metabolism. Genetics 155:1055-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao, H. I., J. Veeraraghavan, P. Polaczek, J. L. Campbell, and R. A. Bambara. 2004. On the roles of Saccharomyces cerevisiae Dna2p and FEN1 in Okazaki fragment processing. J. Biol. Chem. 179:15014-15024. [DOI] [PubMed] [Google Scholar]
- 32.Kibe, T., K. Tomita, A. Matsuura, D. Izawa, T. Kodaira, T. Ushimaru, M. Uritani, and M. Ueno. 2003. Fission yeast Rhp51 is required for the maintenance of telomere structure in the absence of the Ku heterodimer. Nucleic Acids Res. 31:5054-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo, C., H. Nuang, and J. L. Campbell. 1983. Isolation of yeast DNA replication mutants in permeabilized cells. Proc. Natl. Acad. Sci. USA 80:6465-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. E., D. A. Bressan, J. H. Petrini, and J. E. Haber. 2002. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair 1:27-40. [DOI] [PubMed] [Google Scholar]
- 35.Makarov, V. L., Y. Hirose, and J. P. Langmore. 1997. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88:657-666. [DOI] [PubMed] [Google Scholar]
- 36.Maringele, L., and D. Lydall. 2002. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Δ mutants. Genes Dev. 16:1919-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, A. A., I. Dionne, R. J. Wellinger, and C. Holm. 2000. The function of DNA polymerase alpha at telomeric G tails is important for telomere homeostasis. Mol. Cell. Biol. 20:786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreau, S., J. R. Ferguson, and L. S. Symington. 1999. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 19:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naito, T., A. Matsuura, and F. Ishikawa. 1998. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat. Genet. 20:203-206. [DOI] [PubMed] [Google Scholar]
- 40.Nakada, D., K. Matsumoto, and K. Sugimoto. 2003. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 17:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, T. M., B. A. Moser, and P. Russell. 2002. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161:1437-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono, Y., K. Tomita, A. Matsuura, T. Nakagawa, H. Masukata, M. Uritani, T. Ushimaru, and M. Ueno. 2003. A novel allele of fission yeast rad11 that causes defects in DNA repair and telomere length regulation. Nucleic Acids Res. 31:7141-7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parenteau, J., and R. J. Wellinger. 1999. Accumulation of single-stranded DNA and destabilization of telomeric repeats in yeast mutant strains carrying a deletion of RAD27. Mol. Cell. Biol. 19:4143-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paull, T. T., and M. Gellert. 1998. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol. Cell 1:969-979. [DOI] [PubMed] [Google Scholar]
- 46.Paull, T. T., and M. Gellert. 1999. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 13:1276-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi, H., and V. A. Zakian. 2000. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev. 14:1777-1788. [PMC free article] [PubMed] [Google Scholar]
- 48.Ray, S., Z. Karamysheva, L. Wang, D. E. Shippen, and C. M. Price. 2002. Interactions between telomerase and primase physically link the telomere and chromosome replication machinery. Mol. Cell. Biol. 22:5859-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond, W. E., and N. Kleckner. 1993. RAD50 protein of S. cerevisiae exhibits ATP-dependent DNA binding. Nucleic Acids Res. 21:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schramke, V., P. Luciano, V. Brevet, S. Guillot, Y. Corda, M. P. Longhese, E. Gilson, and V. Geli. 2004. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nat. Genet. 36:46-54. [DOI] [PubMed] [Google Scholar]
- 51.Stewart, S. A., I. Ben-Porath, V. J. Carey, B. F. O'Connor, W. C. Hahn, and R. A. Weinberg. 2003. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 33:492-496. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara, N. 1988. DNA sequences at the telomeres of the fission yeast S. pombe. Ph.D. thesis. Harvard University, Cambridge, Mass.
- 53.Szankasi, P., and G. R. Smith. 1995. A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction. Science 267:1166-1169. [DOI] [PubMed] [Google Scholar]
- 54.Taggart, A. K., S. C. Teng, and V. A. Zakian. 2002. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297:1023-1026. [DOI] [PubMed] [Google Scholar]
- 55.Taggart, A. K., and V. A. Zakian. 2003. Telomerase: what are the Est proteins doing? Curr. Opin. Cell Biol. 15:275-280. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi, K., S. Saitoh, and M. Yanagida. 2000. Application of the chromatin immunoprecipitation method to identify in vivo protein-DNA associations in fission yeast. Sci. STKE 2000:PL1. [Online.] http://www.stke.org. [DOI] [PubMed]
- 57.Tanaka, H., G. H. Ryu, Y. S. Seo, K. Tanaka, H. Okayama, S. A. MacNeill, and Y. Yuasa. 2002. The fission yeast pfh1+ gene encodes an essential 5′ to3′ DNA helicase required for the completion of S-phase. Nucleic Acids Res. 30:4728-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tauchi, H., S. Matsuura, J. Kobayashi, S. Sakamoto, and K. Komatsu. 2002. Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene 21:8967-8980. [DOI] [PubMed] [Google Scholar]
- 59.Tomita, K., A. Matsuura, T. Caspari, A. M. Carr, Y. Akamatsu, H. Iwasaki, K. I. Mizuno, K. Ohta, M. Uritani, T. Ushimaru, K. Yoshinaga, and M. Ueno. 2003. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol. Cell. Biol. 23:5186-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trujillo, K. M., S. S. Yuan, E. Y. Lee, and P. Sung. 1998. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem. 273:21447-21450. [DOI] [PubMed] [Google Scholar]
- 61.Ueno, M., T. Nakazaki, Y. Akamatsu, K. Watanabe, K. Tomita, H. D. Lindsay, H. Shinagawa, and H. Iwasaki. 2003. Molecular characterization of the Schizosaccharomyces pombe nbs1+ gene involved in DNA repair and telomere maintenance. Mol. Cell. Biol. 23:6553-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usui, T., T. Ohta, H. Oshiumi, J. Tomizawa, H. Ogawa, and T. Ogawa. 1998. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95:705-716. [DOI] [PubMed] [Google Scholar]
- 63.Wellinger, R. J., A. J. Wolf, and V. A. Zakian. 1993. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell 72:51-60. [DOI] [PubMed] [Google Scholar]