Abstract
The antioxidant activity of naturally occurring stilbene compounds piceatannol (PIC) and isorhapontigenin (ISO) scavenging two free radicals (NO and NO2) were studied using density functional theory (DFT) method. Four reaction mechanisms have been considered: hydrogen atom transfer (HAT), radical adduct formation (RAF), single electron transfer (SET), and sequential proton loss electron transfer (SPLET). The reaction channels in water solution were traced independently, and the respective thermodynamic and kinetic parameters were obtained. We found PIC and ISO scavenge NO mainly through RAF mechanism, and scavenge NO2 through HAT mechanism. The capacity of PIC scavenging NO2 is much higher than ISO, but the reactivity of scavenging NO is lower than ISO.
Introduction
Reactive nitrogen species (RNS) are nitrogen-centered radicals and related species produced by normal cellular metabolism of human body. Nitric oxide radical (NO) is a highly RNS produced by the oxidation of the terminal guanido-nitrogen atoms of L-arginine in organism [1]. In different micro-environment, NO can be converted to various other RNS such as nitrosonium cation (NO+), nitroxyl anion (NO-) and peroxynitrite (ONOO-) [2]. Nitric dioxide radical (NO2) is formed from the reaction of peroxyl radical and NO, polluted air or smoking [3]. Overproduction of RNS is called nitrosative stress [4–5]. Nitrosative stress may initiate the nitrosylation reaction that can change the structures of proteins and so inhibit their normal function. Nitrosative stress can also cause damage to membrane fatty acids, DNA and its repair mechanism. Thus, it is significant to find natural antioxidants that can help in scavenging the excess RNS and then avoid oxidative damage in humans.
Piceatannol (4’,5’,3,5-tetrahydroxystilbene) is a natural occurring stilbene compound widespread in various plant species, such as grape [6], peanut [7], vaccinium berries [8], euphorbia lagascae [9], etc. It has been reported that PIC has numerous beneficial effects on some age-related diseases, such as anti-inflammatory, anticarcinogenic, antiviral, antioxidative, neuroprotective and estrogenic properties [10–16]. As a potential antioxidant, it has been demonstrated that PIC has the ability of scavenging diverse free radicals such as hydroxyl, peroxyl, superoxide and lipid peroxyl radical [17–19]. It has also been reported that PIC can protect against DNA damage caused by hydroxyl radicals in L1210, K562 and HL-60 leukemic cells [20]. Moreover, PIC can suppress reactive oxygen radical generation and increase the GSH/GSSG ratio in B16 melanoma cells [21].
Isorhapontigenin (3,4’,5-trihydroxy-3’-methoxy-stilbene) is also a natural stilbene compound, which can be isolated from Chinese herb Belamcanda chinensis [22] and rhubarb [23]. Wine grapes are main dietary sources of PIC, and recently ISO were also identified from wine grapes [24]. ISO shows potent antioxidant activity in vitro, with activity being higher than that shown by vitamin E [25–26]. It has been reported ISO can inhibit the respiratory burst of PMA-activated rat neutrophils through scavenging oxygen free radicals [27]. ISO could also prevent the oxidation of human LDL and other prooxidant systems in vivo [26, 28].
Except above experimental studies, some theoretical investigation on the radical scavenging activity of PIC and ISO have been completed. Rossi et al. researched the reaction mechanism of PIC and resveratrol (RES) scavenging ·OH and ·OOH by DFT method, they concluded PIC is a more efficient scavenger than RES [29]. Cordova-Gomez et al. investigated the scavenging ability of PIC and RES toward ·OOH in water and lipid solution, using DFT method and SMD solvation model. They found PIC is a better ·OOH scavenger than RES in both media [30]. Perez-Gonzalea et al. evaluated the free radical scavenging activity of a series of polyphenols, PIC is a good antioxidant among them, because having a smaller bond dissociation energy [31]. It is can be found that these research are concerned only with ROS, however RNS as another kind of harmful radical in vivo were ignored. As for as ISO, in previous our team theoretically investigated the antioxidant mechanisms of ISO and PIC scavenging two ROS (·OH and ·OOH) [32], no studies addressed the scavenging activity of ISO toward some specific radicals until quite recently.
Therefore, the aim of the present work is to carry out a theoretical study on the activity of PIC and ISO scavenging two NOS (NO and NO2) by the quantum mechanics-based test for overall free radical scavenging activity (QM-ORSA) protocol. QM-ORSA is a reliable approach to identify the chemical compounds with the highest activity under different conditions [33]. In this study, all possible active sites of PIC and ISO scavenging NO and NO2 have been examined, the thermodynamic and kinetic data of the corresponding channels in water solvent have been obtained to identify the main mechanism of the antioxidant reactions.
Computational Methods
All electronic calculations have been performed with the package of program GAUSSIAN 09 [34], using the DFT M05-2X functional and the 6–311++G(d,p) basis set. M05-2X functional has been chosen as it yields satisfactory overall performance for the thermodynamic and kinetic calculations in organic and biological systems involving free radical reactions [35–37]. Unrestricted wave function was employed for the open-shell system. Geometry optimization and frequency calculation of all stationary points (reactants, complexes, transition states and products) were identified. Intrinsic reaction coordinate (IRC) calculations have been performed to confirm that the transition states (TS) properly connect reactants and products.
Solvent effects were introduced as single point calculations on the optimized gas-phase geometries in the framework of the continuum solvation model based on solute electron density (SMD), which is recommended by Gaussian Manual to compute solvation energy. The calculations of solvent effects have been performed using water as solvent to simulate the internal environments of body.
Slovent cage effects was included according to the corrections proposed by Okuno [38], which take into account the free volume theory [39]. In this work the expression used to correct the Gibbs free energy is:
| (1) |
where n represents the molecularity of the reaction. According to the Expression (1), the solvent cage effect causes a decrease of 10.63 kJ/mol in ΔG0 for a bimolecular reaction at 298.15 K.
For the mechanisms involving electronic transfers (ET), the Marcus theory [40–42] was used. Within the transition state formalism, the reaction energy barrier were estimated in terms of the free energy of reaction and the nuclear reorganization energy (λ):
| (2) |
reorganization energy (λ) is calculated as:
| (3) |
Where ΔEET is calculated as the non-adiabatic energy difference between reactants and products, in reactants geometries.
The proton exchange method is applied to predict the pKa of PIC and ISO. We chose cathecol and guaiacol as the reference compounds (HRef), as which structures are similar with that of PIC and ISO, respectively. The calculation of pKa is based on the reaction scheme:
| (4) |
pKa is calculated as:
| (5) |
Where the experimental pKa values of catechol and Guaiacol are 9.25 [43] and 9.80 [44], respectively.
Four mechanisms of stilbene antioxidant (ArOH) scavenging radical (·R) were considered: hydrogen atom transfer (HAT):
| (6) |
radical adduct formation (RAF):
| (7) |
single electron transfer (SET):
| (8a) |
| (8b) |
sequential proton loss electron transfer (SPLET):
| (9a) |
| (9b) |
| (9c) |
The products of ArO· and ArOH-R· are aromatic structures with the odd electron spreading over the entire molecule, resulting in a significant radical stabilization.
Results and Discussion
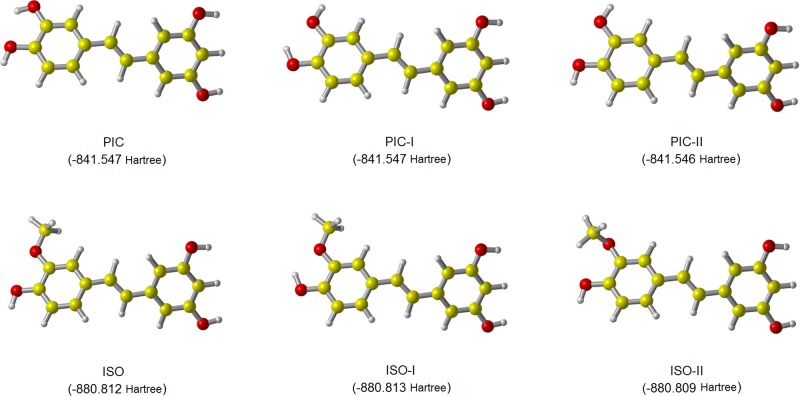
At the M05-2X/6-311++G(d,p) level, the optimized conformers of piceatannol and isorhapontigenin are showed in Fig 1, together with the energies of them. Among the conformers of piceatannol, PIC and PIC-I have relative lower and similar energies in water solution. While among the conformers of isorhapontigenin, ISO and ISO-I have relative lower and similar energies. According to the bond dissociation energy (BDE) obtained by Lu etc., PIC and ISO have lower BDE values and thus have higher reactivity than PIC-I and ISO-I, respectively [32]. Therefore, in this work, PIC and ISO were chosen out from their conformers.
Fig 1. The geometries and energies of PIC’s and ISO’s conformers.
The structures were fully optimized at M05-2X/6-311++G(d,p) level of theory, in water solution.
The pKa value of a molecule determines the amount of protonated and nonprotonated forms at a specific pH. Therefore, the pKa values of PIC and ISO were calculated and reported in Table 1, together with the molar fractions of the neutral and anionic species in aqueous solution, at physiological pH (7.4). To identify which phenolic hydroxy group is involved in the deprotonation, all possible processes were first investigated. The Gibbs free energies of desprotonation in water solution are reported in Table 2, where calculated using ΔGsolv(H+) = -265.9 kcal/mol in water solution based on the recommendation of Camaioni etc [45]. It was found that the most favourable anions of PIC and ISO are the products of deprotonation from A4- and A'4-OH groups, respectively. Therefore, the anion used in this work, when necessary, are deprotonation at these sites. According to the molar fractions, in water (at physiological PH), the dominant forms of PIC and ISO are neutral species, the proportions are 81.7% and 62.9%, respectively.
Table 1. pKa values of PIC and ISO, and molar fractions (f) in water solution at PH = 7.4.
| Piceatannol | Isorhapontigenin | ||
|---|---|---|---|
| pKa | 5.69 | pKa | 7.63 |
| f (PIC) | 0.8170 | f (ISO) | 0.6293 |
| f (PIC-) | 0.1830 | f (ISO-) | 0.3707 |
Table 2. The reaction Gibbs free energies (ΔG0) for all possible deprotonation sites of PIC and ISO in water solution (in kJ/mol).
| PIC | ISO | ||
|---|---|---|---|
| site | ΔG0 | site | ΔG0 |
| A4 | 37.68 | A'4 | 47.97 |
| A5 | 57.60 | ||
| B3 | 56.23 | B'3 | 56.48 |
| B5 | 55.32 | B'5 | 51.71 |
In the light of the optimized geometries of PIC and ISO in Fig 1, both PIC and ISO have an approximately planar structure. At the M05-2X/6-311++G(d,p) level, the dihedral angle between two benzene rings of PIC is 179.95。, which is reasonably consistent with the experimental value of 179.23° [29]. At the same level, the obtained dihedral angle between two benzene rings of ISO is 179.90°.
Nitric oxide radical (NO)
Four reaction mechanisms of PIC and ISO scavenging NO in water solution have been considered: HAT, RAF, SET and SPLET. For HAT mechanism, NO abstracts H atom from the hydroxyl group (‒OH) of PIC/ISO, followed by forming a water molecule and a semiquinone PIC/ISO radical. Therefore, we considered four HAT reaction channels (from sites A4, A5, B3 and B5) for PIC as well as three HAT channels (from sites A'4, B'3 and B'5) for ISO. As far as RAF mechanism, NO adds to either C atom of the carbon-carbon double bond which connects two benzene rings, so we considered two RAF channels (on sites C20 and C21) for PIC as well as two RAF channels (on sites C'20 and C'21) for ISO.
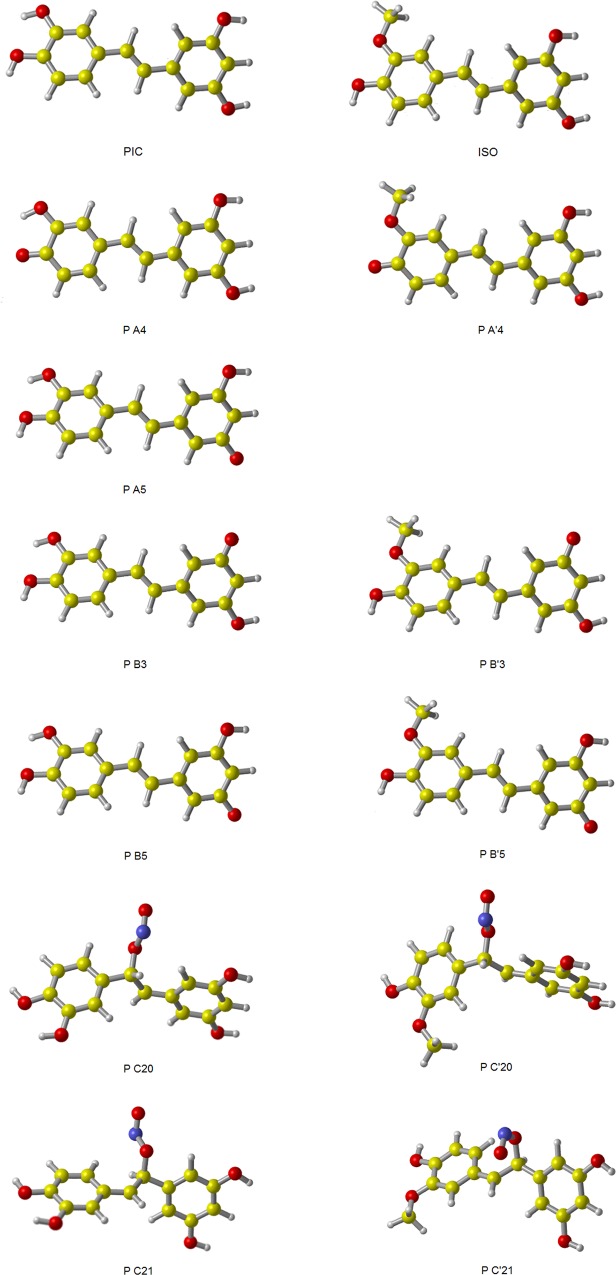
Under the M05-2X/6-311++G(d,p) level, the optimized geometries of all reactants and products for reactions of PIC and ISO with NO are shown in Fig 2, the geometries of TS for HAT and RAF mechanisms are shown in Fig 3. The Gibbs free energy of reaction (ΔG0) in water solution for HAT, RAF, SET and SPLET mechanisms were obtained and gathered in Table 3. It is can be seen, for both PIC and ISO, all channels are very endergonic. Apparently, the reactions of PIC and ISO with NO are less thermodynamical reactivity. This is agreed with the conclusion obtained by Alvarez-Idaboy and his coworkers, that NO itself might not react with any amino acid or even any antioxidant via it is an extremely poor acceptor of both H atoms and electrons, the importance of reaction involved NO is that it is a precursor of other RNS: NO2 and ONOO- [46]. In addition, it is can be found from Table 3 that the endergonic amount of SET mechanism is largest, reached 320.28 kJ/mol for PIC and 310.28 kJ/mol for ISO, indicating that SET mechanism seems irrelevant for PIC and ISO scavenging NO.
Fig 2. The geometries of reactants and products for reactions of PIC and ISO with NO.
The structures were fully optimized at M05-2X/6-311++G(d,p) level of theory, in water solution.
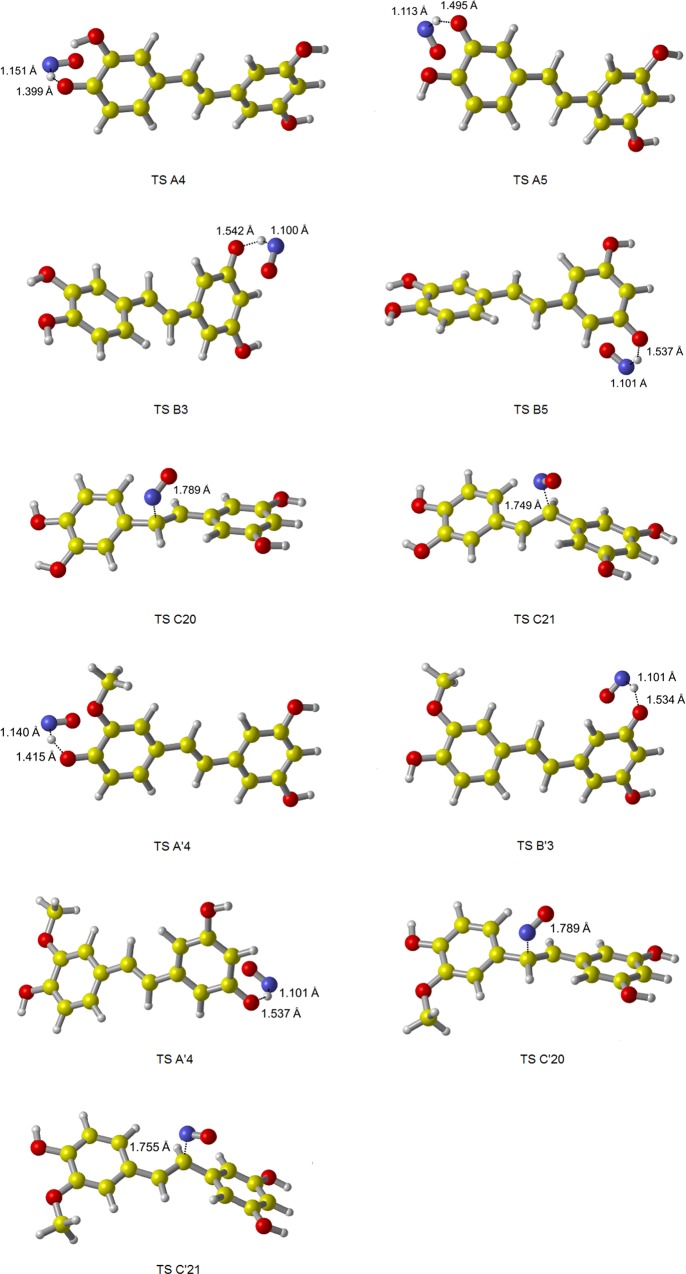
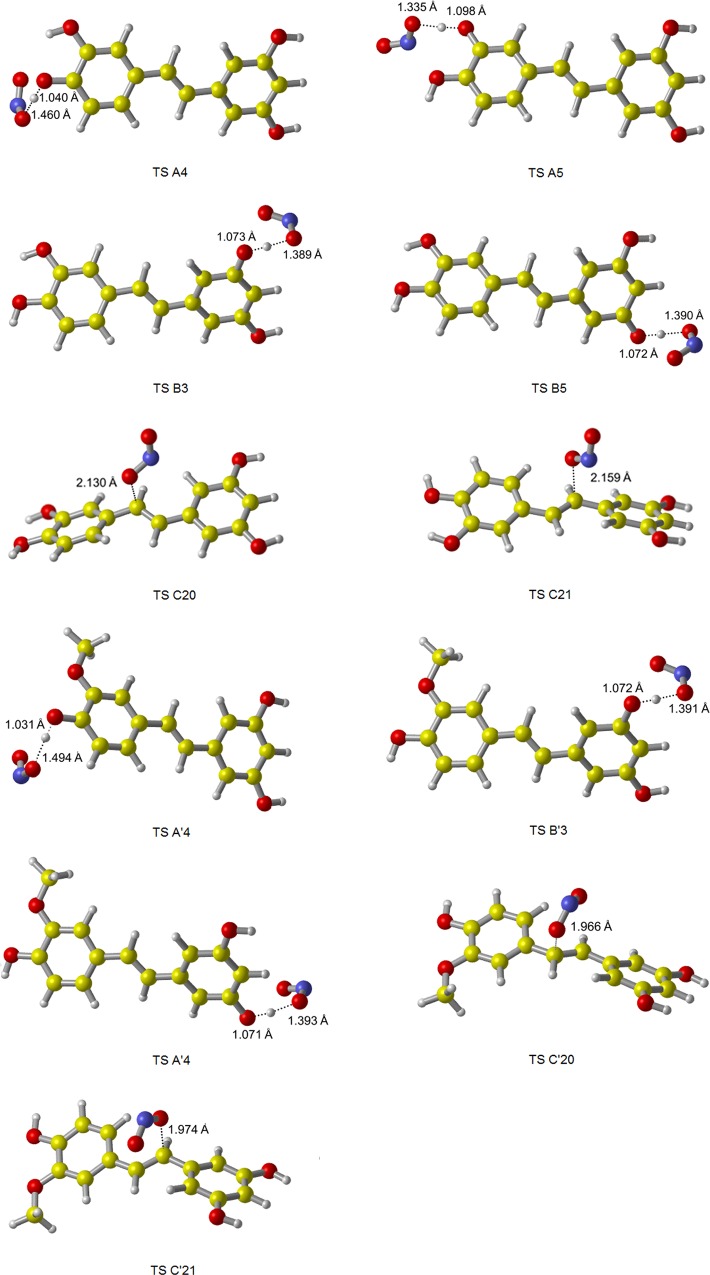
Fig 3. The geometries of TS for PIC and ISO scavenging NO, fully optimized at M05-2X/6-311++G(d,p) level of theory.
All TS have only one imaginary frequency which corresponds to the expected motion along the reaction coordinate.
Table 3. The Gibbs free energies of reaction (ΔG0) and reaction energy barrier (ΔG≠) for PIC and ISO scavenging NO in water solution, at 298 K (in kJ/mol).
| PIC+NO | ISO+NO | ||||
|---|---|---|---|---|---|
| Mechanism | ΔG0 | ΔG≠ | Mechanism | ΔG0 | ΔG≠ |
| HAT-A4 | 97.60 | 125.37 | HAT-A'4 | 103.48 | 121.41 |
| HAT-A5 | 129.02 | 141.08 | |||
| HAT-B3 | 251.19 | 159.17 | HAT-B'3 | 251.47 | 158.30 |
| HAT-B5 | 253.79 | 158.60 | HAT-B'5 | 250.51 | 158.39 |
| RAF-C20 | 100.92 | 80.68 | RAF-C'20 | 95.85 | 80.49 |
| RAF-C21 | 94.60 | 70.15 | RAF-C'21 | 96.10 | 64.73 |
| SET | 320.28 | 533.76 | SET | 310.28 | 483.82 |
| SPLET | 223.97 | 306.39 | SPLET | 214.82 | 286.98 |
The energy barriers (ΔG≠) of four mechanisms for PIC and ISO scavenging NO in water solution were obtained and listed in Table 3. As the electron transfer process occurs without passing through a saddle point, the ΔG≠ values for SET mechanism were calculated in terms of Eq (2). For SPLET mechanism, the the ΔG≠ values for step (9b) have also been calculated according to Eq (2). As once the PIC- is formed at the proper pH conditions, the energy cost for the rest of the reaction to proceed is associated to the electron transfer (9b) from this species.
In Table 3, the barrier heights of SET mechanism are extremely high for both of PIC (533.76 kJ/mol) and ISO (483.82 kJ/mol), further confirmed that SET mechanism is irrelevant to the NO scavenging activity of PIC and ISO. Besides, the barrier heights of SPLET reactions of PIC (306.39 kJ/mol) and ISO (286.98 kJ/mol) are also much higher than that of HAT and RAF mechanisms, thus HAT and RAF are relative feasible mechanisms in kinetics.
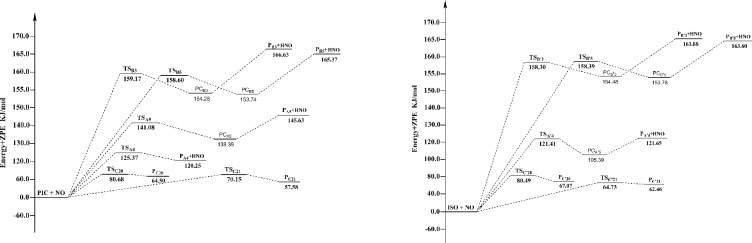
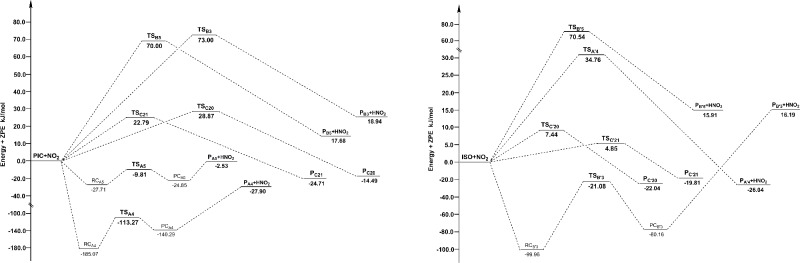
The potential energy surface profiles for HAT and RAF channels of PIC and ISO are gathered in Fig 4, showing the relative energies of TS to reactants, and products to reactants. From Fig 4, it is easily to find that RAF-C21 channel has the lowest barrier height among all channels of PIC with NO, and RAF-C'21 channel has the lowest barrier height among all channels of ISO with NO. In addition, the barrier heights of all RAF channels are much lower than that of HAT channels for both PIC and ISO. Thus, we can conclude that RAF is the main mechanism of PIC and ISO scavenging NO, and the most efficient sites are C21 and C'21 atom on the carbon-carbon double bond. The barrier heights of channel RAF-C'21 (64.73 kJ/mol) are lower than that of channel RAF-C21 (70.15 kJ/mol in water), proved that ISO is more reactive than PIC in scavenging NO.
Fig 4. The potential energy surface profiles for HAT and RAF reactions of PIC and ISO with NO.
The relative energies (in kJ/mol) were calculated at the M05-2X/6-311++G(d,p) + ZPE level. To facilitate the comparison, the energy of the reactants are set to zero.
As far as the HAT process is concerned, the activity order in terms of barrier height is HAT-A4 (125.37 kJ/mol) > HAT-A5 (141.08 kJ/mol) > HAT-B5 (158.60 kJ/mol) > HAT-B3 (159.17 kJ/mol) for PIC with NO; HAT-A'4 (121.41 kJ/mol) > HAT-B'3 (158.30 kJ/mol) > HAT-B'5 (158.39 kJ/mol) for ISO with NO. We can find the reactivity of sites from A-ring are higher than that of from B-ring, showing that A-ring is more efficient for PIC and ISO scavenging NO. Among HAT mechanism, the most reactive sites are A4‒ and A'4‒OH groups, both of them have another ‒OH group on their ortho-positions (A5 and A'5). Therefore, it is avail to state that the existence of the adjacent ‒OH group may have a promoting effect on the radical scavenging activity of ‒OH group in PIC and ISO.
Nitrogen dioxide radical (NO2)
Similar with scavenging NO, the HAT, RAF, SET and SPLET mechanisms of PIC and ISO scavenging NO2 in water solution were investigated. Four HAT channels (A4, A5, B3 and B5) and two RAF channels (C20 and C21) for PIC with NO2 were denoted, together with three HAT channels (A'4, B'3 and B'5) and two RAF channels (C'20 and C'21) for ISO with NO2.
The optimized geometries of all reactants and products for reactions of PIC and ISO with NO2 are shown in Fig 5, and the geometries of TS for HAT and RAF mechanisms are collected in Fig 6. The calculation results of ΔG0, ΔG≠ in water solution for four mechanisms are listed in Table 4. According to Table 4, HAT, SET and SPLET mechanisms are thermodynamically feasible for both PIC and ISO. Among them, SPLET is the most exergonic mechanism for PIC (-99.90 kJ/mol) and ISO (-109.05 kJ/mol). Thus, SPLET mechanism is most thermodynamically feasible for PIC and ISO scavenging NO2 in water solution.
Fig 5. The geometries of reactants and products for reactions of PIC and ISO with NO2.
The structures were fully optimized at M05-2X/6-311++G(d,p) level of theory, in water solution.
Fig 6. The geometries of TS for PIC and ISO scavenging NO2, fully optimized at M05-2X/6-311++G(d,p) level of theory.
All TS have only one imaginary frequency which corresponds to the expected motion along the reaction coordinate.
Table 4. The Gibbs free energies of reaction (ΔG0) and reaction energy barrier (ΔG≠) for PIC and ISO scavenging NO2 in water solution, at 298 K (in kJ/mol).
| PIC+NO2 | ISO+NO2 | ||||
|---|---|---|---|---|---|
| Mechanism | ΔG0 | ΔG≠ | Mechanism | ΔG0 | ΔG≠ |
| HAT-A4 | -47.78 | -113.27 | HAT-A'4 | -41.90 | 34.76 |
| HAT-A5 | -16.36 | -9.81 | |||
| HAT-B3 | 105.80 | 73.00 | HAT-B'3 | 106.09 | -21.08 |
| HAT-B5 | 108.41 | 70.00 | HAT-B'5 | 105.13 | 70.54 |
| RAF-C20 | 20.84 | 28.87 | RAF-C'20 | 19.00 | 7.44 |
| RAF-C21 | 17.83 | 22.79 | RAF-C'21 | 20.01 | 4.85 |
| SET | -3.59 | 31.11 | SET | -12.87 | 28.29 |
| SPLET | -99.90 | 1.78 | SPLET | -109.05 | 0.90 |
In terms of the energy barrier values, the channel HAT-A4 has the lowest barrier height among all reactions of PIC with NO2, and thus it is the main channel of total reaction. The channel HAT-B'3 has the lowest barrier height among all reactions of ISO with NO2, therefore HAT-B'3 is the kinetic superiority channel. In summary, HAT is the main mechanism of PIC and ISO scavenging NO2.
The potential energy surface profiles for HAT and RAF channels for PIC and ISO are shown in Fig 7. Making comparison of the activity between PIC and ISO, ΔG≠ value of the major channel of PIC (HAT-A4, -113.27 kJ/mol) is much lower than that of ISO (HAT-B'3, -21.08 kJ/mol). Moreover, the exergonic amounts of the channel HAT-A4 (-47.78 kJ/mol) are higher than that of the channel HAT-B'3 (-41.90 kJ/mol). From a general view, PIC is much efficient than ISO in scavenging NO2 in vivo. It is probably because the two adjacent ‒OH groups of PIC can form a intramolecular hydrogen bond after the H atom have been abstracted, which increases the stability of the product.
Fig 7. The potential energy surface profiles for HAT and RAF reactions of PIC and ISO with NO2.
The relative energies (in kJ/mol) were calculated at the M05-2X/6-311++G(d,p) + ZPE level. To facilitate the comparison, the energy of the reactants are set to zero.
The above results are consistent with the previous theoretical study [47] on the reactions of β-carotene with NO2, they discovered that the ET reaction is thermodynamically favorable in polar solvents. RAF reaction was endergonic in water solution. The reaction mechanism is controlled by HAT and the lowest energy barrier in water solution is 22.18 kJ/mol. Hence, it seems that the NO2 scavenging capacity of PIC and ISO are better than β-carotene.
Features of scavenging radicals
In the previous study, the radical scavenging activity of PIC and ISO toward ·OH and ·OOH were theoretically investigated using the same method of M05-2X/6-311++G(d,p), and the reaction energy barriers with ZPE corrections for HAT and RAF mechanisms were also obtained [32]. We gathered ΔG≠sol values of the major channels in water solution of PIC and ISO scavenging ·OH, ·OOH, NO and NO2 in Table 5, in order to research the features of PIC and ISO scavenging radicals in vivo.
Table 5. The reaction energy barriers (ΔG≠) for the reactions of PIC and ISO scavenging ·OH, ·OOH, NO and NO2 in water solution (in kJ/mol).
| PIC | ISO | |||
|---|---|---|---|---|
| radical | major channel | ΔG≠ | major channel | ΔG≠ |
| ·OH | A4 | -58.86 | A'4 | -56.45 |
| ·OOH | A5 | 30.36 | A'5 | 15.70 |
| NO | C21 | 70.15 | C'21 | 64.73 |
| NO2 | A4 | -113.27 | B'3 | -21.08 |
Table 5 shows that except scavenging NO, PIC and ISO scavenging other radicals (·OH, ·OOH and NO2) are mainly through HAT mechanism. While for scavenging NO, RAF is the main mechanism for both PIC and ISO. A-ring is the most efficient part of PIC scavenging most of radicals studied here. As far as ISO, the most reactive site is differ to different radical, covered several parts including A'-ring, B'-ring, and C' atom of carbon-carbon double bond.
In addition, in vivo environment, PIC and ISO are more sensitive to ·OH and NO2 via the energy barriers of them are relative lower. This is reasonable, since the activity of these radicals are different. However, it is interestingly to find that for scavenging radicals ·OH and NO2, PIC is more efficient than ISO; while for radicals ·OOH and NO, ISO is more efficient.
Conclusions
For NO scavenging activity of PIC and ISO, RAF mechanism is favored with respect to HAT mechanism in water solution, while SET and SPLET are negligible mechanisms. The C atom linked with B/B'-ring is the most active site. For scavenging NO2, HAT reactions from sites A4 and B'3 are the major channels of PIC and ISO, respectively. Within the HAT processes of PIC scavenging NO and NO2, A4‒OH group in A-ring with an ortho‒OH group is more reactive, showing that the introducing of the adjacent ‒OH group could improve the radical scavenging activity of PIC.
Taking together four kinds of radicals ·OH, ·OOH, NO and NO2, A/A'-ring is the most important active position for PIC and ISO scavenging these radicals. In vivo, PIC is the best scavenger for NO2, ISO is the best scavenger for ·OH. Above results could provide some valuable theoretical data for designing and recognizing medical antioxidant.
Supporting Information
(DOC)
Acknowledgments
We thanks the grid computing server provided by the Chinese Academy of Sciences.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Palmer RMJ, Rees DD, Ashton DS, Moncada S (1988) L-Arginine is the physiological precursor for the formation of nitric oxide in endo-thelium dependent relaxation. Biochemical and Biophysical Research Communications 153: 1251–1256. [DOI] [PubMed] [Google Scholar]
- 2.Stamler JS, Single D, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi N, Niki E (1999) Chemistry of active oxygen species and antioxidants Antioxidant status, diet, nutrition, and health. Boca Raton, Fla.: CRC Press: 3–20. [Google Scholar]
- 4.Klatt P, Lamas S (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. European Journal Of Biochemistry 267: 4928–4944. [DOI] [PubMed] [Google Scholar]
- 5.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, et al. (2004) The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biological Chemistry 385: 1–10. 10.1515/BC.2004.001 [DOI] [PubMed] [Google Scholar]
- 6.Bavaresco L, Fregoni M, Trevisan M, Mattivi F, Vrhovsek U, Falchetti R (2002) The occurrence of the stilbene piceatannol in grapes.Vitis 41: 133–136. [Google Scholar]
- 7.Ku KL, Chang PS, Cheng YC, Lien CY (2005) Production of stilbenoids from the callusof arachis hypogaea: A novel source of the anticancer compound piceatannol. Journal of Agricultural and Food Chemistry 5: 33877–3881. [DOI] [PubMed] [Google Scholar]
- 8.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR (2004) Resveratrol, pterostilbene, and piceatannol in vaccinium berries. Journal of Agricultural and Food Chemistry 52: 4713–4719. 10.1021/jf040095e [DOI] [PubMed] [Google Scholar]
- 9.Ferrigni NR, McLaughlin JL, Powell RG, Smith CR (1984) Use of potato disc and brine shrimp bioas-says to detect activity and isolation of piceatannol as the antileukemic principle from the seeds of euphorbia lagascae. Journal of Natural Products 47: 347–352. [DOI] [PubMed] [Google Scholar]
- 10.Kimura Y, Okuda H, Arichi S (1985) Effects of stilbenes on arachidonate metabolism in leukocytes. Biochimica et Biophysica Acta 834: 275–278. [PubMed] [Google Scholar]
- 11.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, et al. (1997) Cancer chemo-preventive activity of resveratrol, a natural product derived from grapes. Science 275: 218–220. [DOI] [PubMed] [Google Scholar]
- 12.Docherty JJ, Fu MMH, Stiffler BS, Limperos RJ, Pokabla CM, Delucia AL (1999) Resveratrol inhibition of herpes simplex virus replication. Antiviral Research 43: 135–145. [DOI] [PubMed] [Google Scholar]
- 13.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, et al. (2001) Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. Journal of Biological Chemistry 276: 22586–22594. 10.1074/jbc.M101846200 [DOI] [PubMed] [Google Scholar]
- 14.King RE, Bomser JA, Min DB (2006) Bioactivity of resveratrol. Comprehensive Reviews in Food Science and Food Safety 5: 65–69. [Google Scholar]
- 15.Djoko B, Chiou RYY, Shee JJ, Liu YW (2007) Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW264.7 macrophages. Journal of Agricultural and Food Chemistry 55: 2376–2383. 10.1021/jf062741a [DOI] [PubMed] [Google Scholar]
- 16.Bastianetto S, Dumont Y, Han Y, Quirion R (2009) Comparative neuroprotective properties of stilbene and catechin analogs: action via a plasma membrane receptor site? CNS Neuroscience & Therapeutics 15: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi M, Caruso F, Opazo C, Salciccioli J, (2008) Crystal and molecular structure of piceatannol; scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals and docking with transthyretin. Journal of Agricultural and Food Chemistry 56: 10557–10566. 10.1021/jf801923j [DOI] [PubMed] [Google Scholar]
- 18.Murias M, Jager W, Handler N, Erker T, Horvath Z, Szekeres T, et al. (2005) Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure‒activity relationship. Biochemical Pharmacology 69: 903–912. 10.1016/j.bcp.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Rhayem Y, Therond P, Camont L, Couturier M, Beaudeux JL, Legrand A, et al. (2008) Chain-breaking activity of resveratrol and piceatannol in a linoleate micellar model. Chemistry and Physics of Lipids 155: 48–56. 10.1016/j.chemphyslip.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Ovesna Z, Kozics K, Bader Y, Saiko P, Handler N, Erker T, et al. (2006) Antioxidant activity of resveratrol, piceatannol and 3,3’,4,4’,5,5’-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncology Reports 16: 617–624. [PubMed] [Google Scholar]
- 21.Yokozawa T, Kim YJ (2007) Piceatannol inhibits melanogenesis by its antioxidative actions. Biological and Pharmaceutical Bulletin 30: 2007–2011. [DOI] [PubMed] [Google Scholar]
- 22.Li JB, Lin M, Li SZ, Song WZ (1991) Studies on the structure of gnetifolin A of Gnetum parvifolium (Warb.) C.Y.Cheng. Acta pharmaceutica Sinica 26: 437–441. [PubMed] [Google Scholar]
- 23.Matsuda H, Morikawa T, Toguchida I, Park JY, Harima S, Yoshikawa M (2001) Antioxidant constituents from rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorganic and Medicinal Chemistry 9: 41–50. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Marin MI, Guerrero RF, Garcia-Parrilla MC, Puertas B, Richard T, Rodriguez-Werner MA, et al. (2012) Isorhapontigenin: a novel bioactive stilbene from wine grapes. Food Chemistry 135: 1353–1359. 10.1016/j.foodchem.2012.05.086 [DOI] [PubMed] [Google Scholar]
- 25.Iliya I, Ali Z, Tanaka T, Iinuma M, Furusawa M, Nakaya KI, et al. (2003) Stilbene derivatives from Gnetum gnemon Linn. Phytochemistry 62: 601–606. [DOI] [PubMed] [Google Scholar]
- 26.Wang QL, Lin M, Liu GT (2001) Antioxidative activity of natural isorhapontigenin. The Japanese Journal of Pharmacology 87: 61–66. [DOI] [PubMed] [Google Scholar]
- 27.Fang YN, Liu GT (2002) Effect of isorhapontigenin on respiratory burst of rat neutrophils. Phytomedicine 9: 734–738. [DOI] [PubMed] [Google Scholar]
- 28.Stojanovic S, Sprinz H, Brede O (2001) Efficiency and mechanismof the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Archives of Biochemistry and Biophysics 391: 79–89. 10.1006/abbi.2001.2388 [DOI] [PubMed] [Google Scholar]
- 29.Rossi M, Caruso F, Opazo C, Salciccioli J (2008) Crystal and molecular structure of piceatannol; scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals and docking with transthyretin. Journal of Agricultural and Food Chemistry 56: 10557–10566. 10.1021/jf801923j [DOI] [PubMed] [Google Scholar]
- 30.Cordova-Gomez M, Galano A, Alvarez-Idaboy JR (2013) Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Advances 3: 20209–20218. [Google Scholar]
- 31.Perez-González A, Rebollar-Zepeda AM, Leon-Carmona JR, Galano A (2012) Reactivity indexes and O-H bond dissociation energies of a large series of polyphenols: Implications for their free radical scavenging activity. Journal of the Mexican Chemical Society 56: 241–249. [Google Scholar]
- 32.Lu Y, Wang A, Shi P, Zhang H, Li Z (2015) Quantum Chemical Study on the Antioxidation Mechanism of Piceatannol and Isorhapontigenin toward Hydroxyl and Hydroperoxyl Radicals. PloS one, 10(7), e0133259 10.1371/journal.pone.0133259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galano A, Alvarez-Idaboy JR (2013) A computational Methodology for Accurate Predictions of Rate Constants in Solution: Application to the Assessment of Primary Antioxidant Activity. Journal of computational chemistry, 34(28): 2430–2445. 10.1002/jcc.23409 [DOI] [PubMed] [Google Scholar]
- 34.Gaussian 09, Revision A. 02: Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Fox DJGaussian, Inc., Wallingford CT, 2009. [Google Scholar]
- 35.Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) OH radical gas phase reactions with aliphatic ethers: a variational transition State Theory Study. The Journal of Physical Chemistry A 113: 13913–13920. 10.1021/jp906144d [DOI] [PubMed] [Google Scholar]
- 36.Galano A, Macías-Ruvalcaba NA, Campos ONM, Pedraza-Chaverri J (2010) Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: a combined theoretical and experimental study. The Journal of Physical Chemistry B 114: 6625–6635. 10.1021/jp912001c [DOI] [PubMed] [Google Scholar]
- 37.Iuga C, Alvarez-Idaboy JR, Vivier-Bunge A (2011) ROS initiated oxidation of dopamine under oxidative stress conditions in aqueous and lipidic environments. The Journal of Physical Chemistry B 115: 12234–12246. 10.1021/jp206347u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuno Y (1997) Theoretical investigation of the mechanism of the baeyer-villiger reaction in nonpolar solvents. Chemistry-A European Journal 3: 212–218. [DOI] [PubMed] [Google Scholar]
- 39.Benson SW (1982) The Foundations of Chemical Kinetics. Krieger, Malabar, FL. [Google Scholar]
- 40.Marcus RA (1997) Transfer reactions in chemistry theory and experiment. Pure and applied chemistry 69(1): 13–30. [Google Scholar]
- 41.Marcus RA (1964) Chemical and electrochemical electron-transfer theory. Annual Review of Physical Chemistry 15(1): 155–196. [Google Scholar]
- 42.Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Reviews of Modern Physics 65(3): 599. [Google Scholar]
- 43.Schweigert N, Zehnder AJB, Eggen RIL (2001) Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environmental Microbiology 3(2): 81–91. [DOI] [PubMed] [Google Scholar]
- 44.Douglas WH (1978) The metal oxide/eugenol cements. I. The chelating power of the eugenol type molecule. Journal of dental research 57(7): 800–804. [DOI] [PubMed] [Google Scholar]
- 45.Camaioni DM, Schwerdtfeger CA (2005) Comment on “accurate experimental values for the free energies of hydration of H+, OH-, and H3O+”. The Journal of Physical Chemistry A 109(47): 10795–10797. 10.1021/jp054088k [DOI] [PubMed] [Google Scholar]
- 46.Pérez-González A, Muñoz-Rugeles L, Alvarez-Idaboy JR (2016) Tryptophan versus nitric oxide, nitrogen dioxide and carbonate radicals: differences in reactivity and implications for oxidative damage to proteins. Theoretical Chemistry Accounts 135: 155, [Google Scholar]
- 47.Cerón-Carrasco JP, Bastida A, Requena A, Zúñiga J, Miguel B (2010) A theoretical study of the reaction of β-carotene with the nitrogen dioxide radical in solution. The Journal of Physical Chemistry B 114(12): 4366–4372. 10.1021/jp911846h [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.