Abstract
Influenza vaccine effectiveness (IVE) varies over different influenza seasons and virus (sub)types/lineages. To assess the association between IVE and circulating influenza virus (sub)types/lineages, we estimated the overall and (sub)type specific IVE in the Netherlands. We conducted a test-negative case control study among subjects with influenza-like illness or acute respiratory tract infection consulting the Sentinel Practices over 11 influenza seasons (2003/2004 through 2013/2014) in the Netherlands. The adjusted IVE was estimated using generalized linear mixed modelling and multiple logistic regression. In seven seasons vaccine strains did not match the circulating viruses. Overall adjusted IVE was 40% (95% CI 18 to 56%) and 20% (95% CI -5 to 38%) when vaccine (partially)matched and mismatched the circulating viruses, respectively. When A(H3N2) was the predominant virus, IVE was 38% (95% CI 14 to 55%). IVE against infection with former seasonal A(H1N1) virus was 83% (95% CI 52 to 94%), and with B virus 67% (95% CI 55 to 76%). In conclusion IVE estimates were particularly low when vaccine mismatched the circulating viruses and A(H3N2) was the predominant influenza virus subtype. Tremendous effort is required to improve vaccine production procedure and to explore the factors that influence the IVE against A(H3N2) virus.
Introduction
Influenza viruses cause annual epidemics worldwide. Although most infections are relatively mild, in high-risk groups such as people aged 60 years and older the infection can lead to serious complications, hospital admission, and mortality [1]. In the last decades influenza epidemics were caused by type A (subtypes A(H1N1) through 2009, A(H1N1)pdm09 from 2009 onwards and A(H3N2)), and type B influenza viruses (two different genetic lineages: B/Victoria/2/87 (Victoria) and B/Yamagata/16/88 (Yamagata)) [1–2].
According to the World Health Organization (WHO), seasonal influenza vaccination is the main strategy to prevent influenza and its complications [1]. However, due to the changing nature of the influenza virus (antigenic drift), the vaccine composition and the influenza vaccine effectiveness (IVE) can vary each year [3]. To decide about the composition of the influenza vaccine for upcoming Southern or Northern hemisphere season, WHO organizes consultations with an advisory group of experts to analyze the influenza surveillance and the antigenic characteristics data of circulating viruses in order to issue recommendations. The annual recommendations for the Northern hemisphere and Southern hemisphere are given in February and September, respectively [4]. The national and supra-national vaccine regulatory agencies approve the vaccine composition and WHO essential regulatory laboratories and regional pharmaceutical companies then develop and produce the vaccine based on the WHO recommendation, which will take about half a year. Despite all the efforts, the antigenic match between influenza vaccine strains and circulating influenza viruses is sometimes suboptimal, which could consequently affect the IVE [5]. For instance, in influenza season 2014/15 the antigenic mismatch between the vaccine strain and the predominant virus subtype A(H3N2) resulted in a lower IVE and higher excess mortality rate in the Netherlands [6].
Additionally, since IVE can vary from year to year it is important to measure and monitor the IVE annually. It has been shown that even within a same country, IVE estimates have a high fluctuation over different influenza seasons [7–11]. For example, the currently available overall IVE estimates for the Netherlands range from 59% in season 2007/08 to 5% in season 2011/12 [12–13]. Moreover, there are indications that IVE differs per virus type and subtype/lineage [10–11]. In a study conducted in Denmark, IVE for influenza A was −11%; whereas the IVE for influenza B was point estimated at 69% during influenza season 2012/13 [11].
To gain more insight in a possible association between IVE and circulating influenza virus (sub)types/lineages, we conducted a test-negative design case-control study to estimate the overall and (sub)type/lineage specific IVE in preventing laboratory-confirmed influenza in the Netherlands over a series of 11 seasons. Additionally, we explored the relationship between the estimated IVEs with the dominancy of virus (sub)types/lineages and level of vaccine match during these influenza seasons.
Methods
Study design and population
The Sentinel Practices of NIVEL Primary Care Database cover about 0·7% of the Dutch population and being nationally representative by age, population density and regional distribution [14–15]. Each participating sentinel general practitioner (GP) is asked to take a throat swab and nose swab from two patients with influenza-like illness (ILI) symptoms on a weekly basis. If no ILI patient in a week is encountered the GP is asked to swab patients with another acute respiratory tract infection (ARI) [16]. ILI is defined according to the ‘Pel’ criteria [17] as an acute onset of symptoms (prodromal stage ≤ 4 days) including a rectal temperature of at least 38 degrees Celsius and at least one respiratory or systemic symptom (i.e. cough, nasal catarrh, sore throat, frontal headache, retrosternal pain, myalgia). ARI is defined as an acute respiratory illness other than ILI, such as acute sinusitis or pneumonia, and with at least one of the following symptoms; coughing, rhinorrhea or sore throat [18]. Both ILI and ARI patients were included in this study to maximize the power. As required by Dutch legislation, the influenza surveillance is registered in the Personal Data Protection Act Register of the Personal Data Protection Commission (RIVM/EPI-043). No further ethical approval was needed for the IVE study, because only anonymised data was used.
Covariates
Data on potential confounding and effect modifiers were collected which included information on date of birth (recoded as age groups 0–4, 5–14, 15–59 and ≥ 60 years at swabbing date for the IVE study dataset), gender, date of symptom onset and swabbing (recoded as delay between both dates as <3 days, 3–5 days and 6–7 days), seasonal influenza vaccination status, respiratory allergy, patient diagnosis (ILI and ARI), influenza season and occurrence of underlying medical conditions (diabetes, cardiovascular disease, chronic pulmonary disease and immunodeficiency).
Study design and outcome
In order to estimate IVE against specific outcome, laboratory confirmed influenza, and partially adjust for differences in health care-seeking behaviour of cases and controls, we performed a test-negative design case-control study [19]. Cases were ILI or ARI patients who tested positive for at least one of the influenza virus subtypes: former seasonal A(H1N1), A(H1N1)pdm09, A(H3N2), or influenza B lineages Victoria and Yamagata, and controls were ILI or ARI patients who tested negative for all the above mentioned virus subtypes/lineages. Both ILI and ARI patients swabbed in the seasons 2003/04 through 2013/14 were included in the analyses. Only patients that were swabbed within the period that swabs tested positive for influenza virus (i.e. influenza virus was circulating) were selected as cases or controls (Table 1). Patients were excluded if (1) the vaccination status was missing; (2) the swabs were collected more than 7 days after symptom onset (since the viral load of the specimens collected 7 days after onset of ILI in general has dropped below the detection limit of the diagnostic test and therefore estimation of the subject status is not reliable); (3) the date of swabbing was before the first of December of each season (since the vaccine campaign in the Netherlands is from half October to half November; the inclusion date was set to 1 December to make sure that vaccination was given at least 14 days before symptom onset); (4) the patient used antiviral medication within the two weeks prior to swabbing (this was done because antiviral medication can influence the viral load and consequently the chance of virus detection); (5) data on ILI and ARI diagnosis, age, or underlying chronic illness was missing [20, 21].
Table 1. Baseline characteristics of laboratory-confirmed influenza cases and test-negative controls, seasons 2003/04 through 2013/14, Netherlands.
| Variable | Cases (Number/Total (%)) | Controls (Number/Total (%)) | P-Value* |
|---|---|---|---|
| Seasonal vaccination received | 197/1422 (14·8) | 754/3410 (22·1) | <0·001 |
| 03/04 | 6/34 (17·6) | 21/79 (26·6) | |
| 04/05 | 22/73 (30·1) | 37/149 (24·8) | |
| 05/06 | 14/110 (12·7) | 44/278 (15·8) | |
| 06/07 | 10/94 (10·6) | 73/309 (23·6) | |
| 07/08 | 13/191 (6·8) | 64/417 (15·3) | |
| 08/09 | 21/197 (10·7) | 78/461 (16·9) | |
| 09/10 | 8/33 (24·2) | 92/289 (31·8) | |
| 10/11 | 27/246 (11·0) | 81/346 (23·4) | |
| 11/12 | 15/81 (18·5) | 91/434 (21·0) | |
| 12/13 | 53/311 (17·0) | 82/332 (24·7) | |
| 13/14 | 8/52 (15·4) | 91/316 (28·8) | |
| Gendera | 0·089 | ||
| Male | 683/1412 (48·4) | 1544/3380 (45·7) | |
| Female | 729/1412 (51·6) | 1836/3380 (54·3) | |
| Age group | <0·001 | ||
| 0–4 years | 128/1422 (9·0) | 503/3410(14·8) | |
| 5–14 years | 263/1422 (18·5) | 364/3410 (10·7) | |
| 15–59 years | 886/1422 (62·3) | 2009/3410 (58·9) | |
| ≥ 60 years | 145/1422 (10·2) | 534/3410 (15·7) | |
| Diagnosis | <0·.001 | ||
| ILI | 1142/1422 (80·3) | 1827/3410 (53·6) | |
| ARI | 280/1422 (19·7) | 1583/3410 (46·4) | |
| Interval from symptom onset to swab date, days | <0·001 | ||
| <3 d | 523/1422 (36·8) | 1193/3410 (35·0) | |
| 3–5 d | 776/1422 (54·6) | 1743/3410 (51·1) | |
| >5 d | 123/1422 (8·6) | 474/3410 (13·9) | |
| Chronic condition by age | 95/1422 (6·7) | 380/3410 (11·1) | <0·001 |
| 0–4 years | 3/95 (3·2) | 14/380 (3·7) | |
| 5–14 years | 5/95 (5·3) | 23/380 (6·0) | |
| 15–59 years | 56/95 (58·9) | 182/380 (47·9) | |
| ≥ 60 years | 31/95 (32·6) | 161/380 (42·4) | |
| Respiratory allerg by ageb | 114/1422 (8·0) | 248/3410 (7·3) | 0·374 |
| 0–4 years | 5/114 (4·4) | 15/248 (6·1) | |
| 5–14 years | 25/114 (21·9) | 38/248 (15·3) | |
| 15–59 years | 72/1114 (63·2) | 159/248 (64·1) | |
| ≥ 60 years | 12/114 (10·5) | 36/248 (14·5) | |
| Seasonal vaccination by age | 198/1430(13·8) | 883/3410 (22·1) | <0·001 |
| 0–4 years | 5/128 (3·9) | 41/566 (7·2) | |
| 5–14 years | 13/264 (4·9) | 28/403 (6·9) | |
| 15–59 years | 96/892 (10·8) | 357/2290 (15·6) | |
| ≥ 60 years | 84/146 (57·5) | 457/622 (73·5) |
*Statistically significant at P < 0·05
a Number of missing = 40
bNumber of missing = 50
The beginning of the ‘positive swab test period’ was determined for each season separately and started if two subsequent weeks had positive test results with the ILI incidence exceeding the baseline activity threshold (51/100,000 population) at national level. The end of the ‘positive swab test period’ was marked when two subsequent weeks had zero positive test results for influenza virus. The influenza vaccine was considered matched with circulating viruses if at least one of the two following criteria was fulfilled: (1) all the vaccine components were antigenically similar to the circulating A subtypes (H1N1 or H1N1pdm09 and H3N2) and B lineages (Victoria or Yamagata); (2) vaccine strain antigenically matched the predominant and one of the non-predominant circulating virus subtypes. An influenza virus type or subtype/lineage was considered as predominant when detected in a proportion of ≥60% among the total influenza virus detections in week 40 through week 39 of the following year [22]. The influenza vaccine was considered to partially matched if: (1) vaccine strain matched the predominant virus subtype but mismatched the non-predominant subtypes. In all other situations the vaccine was considered mismatched with circulating viruses. Antigenic match data, derived using ferret sera raised against vaccine reference strains, were extracted from data published by the Dutch National Influenza Centre [23–33].
Laboratory analysis
The collected swabs were sent to the National Institute for Public Health and the Environment (RIVM) for diagnostic testing and virus subtype/lineage determination when positive for influenza virus [14–15]. Reverse transcription polymerase chain reaction (RT-PCR) was used for influenza virus detection throughout the study period. Influenza virus positive specimens were further subtyped (type A viruses by hemagglutination inhibition assay (HI) or RT-PCR during seasons 2003/2004 through 2007/2008 and since the 2008/2009 season by RT-PCR only) or the lineage determined (type B viruses by HI; since season 2010/2011 by RT-PCR).
Statistical analysis
The descriptive and clinical characteristics of cases and controls were compared by using Chi-square test and P-value < 0.05 was considered statistically significant. To assess IVE for each influenza season separately, multiple logistic regression model was used. IVE was estimated by using the formula (1 –odds ratio (OR)) × 100%, where OR is the ratio between the odds of vaccination between influenza test-positive cases and influenza test-negative controls.
To assess the overall IVE over the 11 influenza seasons we used generalized linear mixed-effect model (GLMM) with logit link, in which influenza seasons are modelled as a random effect. Moreover, GLMM was used to estimate the overall IVE stratified by influenza virus (sub)types and vaccine match status (Table 1) (S1 File).
To adjust for confounders, the variables that changed the crude OR by more than 5% were included in the GLMM or multiple logistic regression model (S1 File).
The adjusted IVE were calculated for each influenza season separately. In case of low statistical power in the stratified analysis, only unadjusted IVE was estimated. IVE was considered significant when the 95% confidence interval (95% CI) did not contain zero or negative value. Statistical analyses were conducted using SAS software (version 9.4) and SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
Results
Patient characteristics
From the total of 11,199 subjects who were swabbed from 2003/2004 through 2013/2014 influenza seasons, 4,832 (43·15%) ILI and ARI patients met the inclusion criteria (Table 1). A total of 1422 (29%) patients tested positive (cases) and 3410 (71%) tested negative (controls) for influenza virus. Cases and controls were similar with respect to gender and presence of respiratory allergy. However, compared with control subjects, cases presented more frequently with ILI symptoms (80% vs 54%), were more within the age groups of 5–14 years (18% vs 11%) and 15–59 years (62% vs 59%), were less vaccinated (14% vs 22%), and had a lower incidence of chronic medical illness (7% vs 11%) (Table 1).
Overall influenza vaccine effectiveness
Among all the potential confounders, age, chronic medical illness, and influenza season were the only variables that changed the crude OR by more than 5% and therefore were included in the GLMM estimating IVE over all influenza seasons or multiple logistic regressions estimating overall IVE for each influenza season (overall estimate not stratified by subtype).
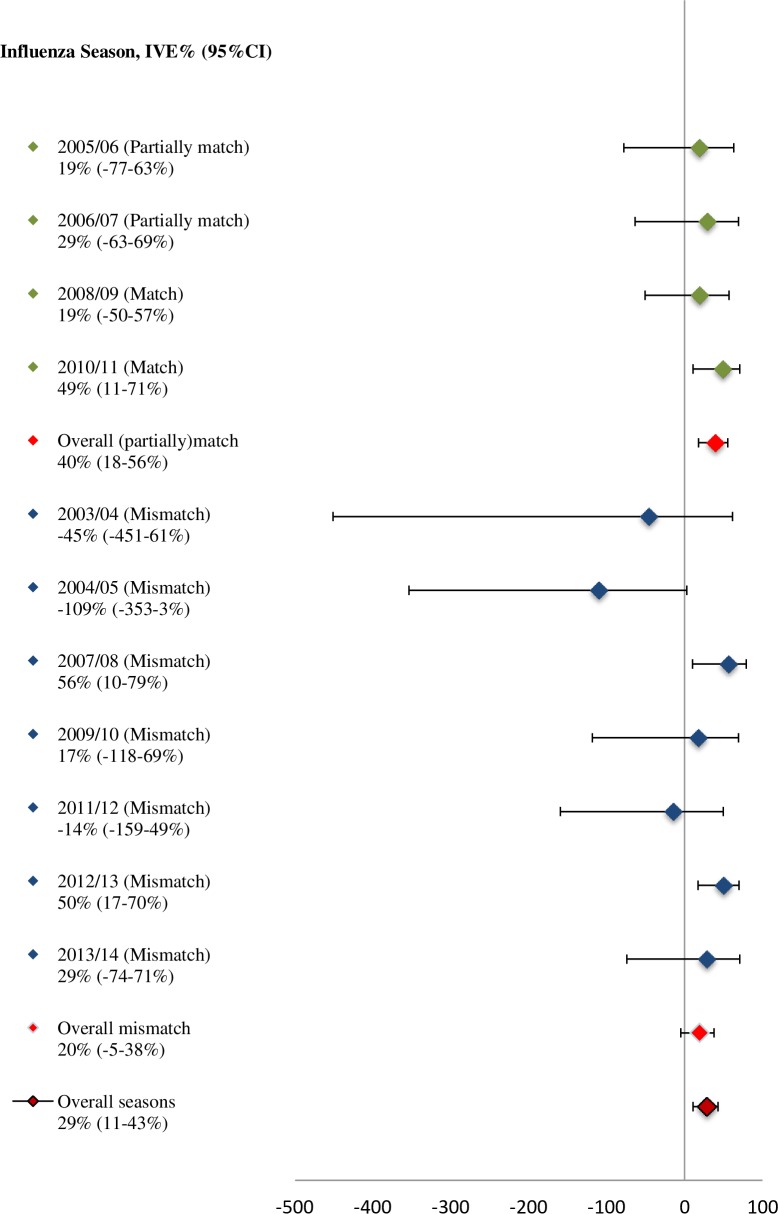
Using GLMM, the overall IVE adjusted for age, chronic medical illness and influenza season was 29% (95% CI 11 to 43%) (Fig 1). Furthermore, the overall adjusted IVE was 40% (95% CI 18 to 56%) when vaccine (partially)matched (influenza seasons 2005/06, 2006/07, 2008/09, and 2010/11) and 20% (95% CI -5 to 38%) when vaccine did not match the circulating viruses (influenza seasons 2003/04, 2004/05, 2007/08, 2009/10, 2011/12, 2012/13, and 2013/14).
Fig 1. Overall adjusted IVE (%) (adjusted for age, chronic medical illness and influenza seasons), and its 95% CI for the (partially)matched and mismatched influenza seasons 2003/04-2013/14.
Using multiple logistic regression for each influenza season, vaccine showed statistically significant effectiveness against laboratory-confirmed influenza for 2010/11 influenza season when vaccine matched (IVE: 49%; 95% CI 11 to 71%) and for influenza seasons 2007/08 and 2012/13 when vaccine did not match the circulating viruses (IVE: 57%; 95% CI 10 to 79% and IVE: 50%; 95% CI 17 to 70%, respectively) (Fig 1).
Influenza vaccine effectiveness by influenza virus type and subtype/lineage
Unadjusted IVE against influenza A(H3N2)
The overall IVE against influenza A(H3N2) (nine seasons) was 20% (95% CI -4 to 38%). Restricting the analysis to the influenza seasons when the A(H3N2) was the predominant virus (2003/04, 2006/07, 2008/09, 2011/12 and 2013/14), IVE increased to 38% (95% CI 14 to 55%). Moreover, IVE was statistically significant against influenza A(H3N2) for season 2006/07; 54% (95% CI 4 to 78%) (Table 2).
Table 2. Overall and (sub)type/lineage specific IVE and the 95% CI for influenza seasons 2003/04 through 2013/14, Netherlands.
| Influenza virus (sub)types/lineages; IVE% (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Influenza Season | Former A(H1N1) | A(H1N1)pdm09 | A(H3N2) | B (total) | B Yamagata | B Victoria |
| 2003/04 | NE | 54% (-48-86%) | NE | |||
| 2004/05 | 66% (-147-96%) | NE | 57% (-53-88%) | |||
| 2005/06 | NE | -6% (-171-58%) | 34% (-65-73%) | |||
| 2006/07 | NE | 54% (4–78%)* | NE | |||
| 2007/08 | 81% (22–96%)* | -10% (-199-59%) | 68% (17–88%)* | |||
| 2008/09 | NE | 31% (-199-60%) | 78% (-16-94%) | |||
| 2009/10 | NE | 25% (-72-68%) | NE | NE | ||
| 2010/11 | NE | 43% (-3-68%) | NE | 73% (47–87%)* | 34% (-205-86%) | 76% (49–89%)* |
| 2011/12 | NE | NE | 11% (-70-53%) | 6% (-351-82%) | NE | |
| 2012/13 | NE | 51% (3–75%)* | -41% (-140-16%) | 64% (44–79%)* | 67% (40–82%)* | |
| 2013/14 | NE | 72% (-12-94%) | 46% (-46-87%) | NE | NE | |
| Overall | 77% (37–92%)* | 47% (22–64%)* | 20% (-4-38%) | 64% (50–74%)* | 59% (30–76%)* | |
*Indicates P < 0·05
IVE: Influenza vaccine effectiveness; CI: confidence interval; NE: Not estimable due to the low number of cases or vaccinated cases.
Unadjusted IVE against influenza A(H1N1)
The IVE against former seasonal A(H1N1) could only be estimated for the 2004/05 and 2007/08 influenza seasons due to absence or low number of cases in the other seasons. The overall IVE against influenza A(H1N1) for these two seasons was 77% (95% CI 37 to 92%). For influenza season 2007/08 IVE against A(H1N1) was statistically significant; although the vaccine did not match the circulating subtype (Table 2).
Unadjusted IVE against influenza A(H1N1)pdm09
The overall IVE against influenza A(H1N1)pdm09 was 47% (95% CI 22 to 64%). The seasonal IVE for the pandemic season (2009/10) for the emerged influenza virus A(H1N1)pdm09, was 25% (95% CI -72 to 68%) though not statistically significant. For the seasons thereafter, the seasonal IVE increased to 43% (95% CI -3 to 68%) in 2010/11, 51% (95% CI 3 to 75%) in 2012/13, which was statistically significant, and 72% (95% CI -12 to 94%) in 2013/14 (Table 2).
Unadjusted IVE against influenza B
The overall IVE against influenza B (total of B Yamagata and B Victoria) was 64% (95% CI 50 to 74%), which was statistically significant. For the influenza seasons 2007/08, 2010/11 and 2012/13 IVE against influenza B was statistically significant (Table 2)
The IVE against influenza virus B Victoria could only be determined for 2010/11 influenza season which was statistically significant with 76% (95% CI 49 to 89%). Except for the influenza season 2012/13, only a limited number of influenza B Yamagata cases were detected in the influenza seasons 2010/11 through 2013/14. The overall IVE against influenza B Yamagata was 59% (95% CI 30 to 76%), which was statistically significant. The season-specific IVE against influenza B Yamagata was only significant for 2012/2013 (IVE: 67%; 95% CI 40 to 82%) (Table 2).
Virological characteristics of influenza seasons
In Table 3, the proportion of influenza virus (sub)types/lineages based on the Netherlands National Influenza Center (NIC) data is presented. The NIC data comes from the Sentinel Practices of NIVEL Primary Care Database and hospital laboratories that submitted influenza virus isolates or clinical specimens with a specimen collection date in week 40 of one year through week 39 of the following year. Based on the NIC data, influenza vaccine strains matched or partially matched the circulating viruses in 2005/06, 2006/07, 2008/09, and 2010/11 influenza seasons (Table 3) [23–33]. Influenza A(H3N2) was predominant in six influenza seasons: 2003/04, 2004/05, 2005/06, 2006/07 and 2008/09 and 2011/12. Among these six seasons, four influenza seasons were characterised by the circulation of a drifted A(H3N2) strain [reference strains 2003/04: A/Fujian/411/02(H3N2), 2004/05: A/California/7/04(H3N2), 2005/06:A/Wisconsin/67/05(H3N2), and 2011/12: A/Victoria/361/11(H3N2)]; and in three seasons (i.e. 2005/06, 2006/07, and 2008/09) influenza vaccine matched the A(H3N2) virus subtype.
Table 3. Proportion of virus (sub)types/lineages (%) and vaccine mismatch per subtype/lineage based on virus isolates and specimens submitted to the NIC in week 40 of one year through week 39 of the following year, seasons 2003/04 through 2013/14, Netherlands.
| Influenza season | |||||||||||
| Virus (sub)type | 2003/04 | 2004/05 | 2005/06 | 2006/07 | 2007/08 | 2008/09 | 2009/2010 | 2010/11 | 2011/12 | 2012/13 | 2013/2014 |
| Proportion of virus (sub) types (%) | |||||||||||
| A | 99 | 80 | 64 | 99 | 49 | 92 | 100 | 60 | 90 | 69 | 94 |
| A(H1N1)1 | 0 | 18 | 4 | 13 | 86 | 1 | 100 | 97 | 1 | 39 | 40 |
| A(H3N2) | 100 | 82 | 96 | 87 | 14 | 99 | 0 | 3 | 99 | 61 | 60 |
| B | 1 | 20 | 36 | 1 | 51 | 8 | 0 | 40 | 10 | 31 | 6 |
| B | Y | Y | Y/V | Y | Y | V | NA | Y/V | Y/V | Y/V | Y/V |
| B/Vic | 91 | 95 | 12 | 6 | 24 | ||||||
| B/Yam | 9 | 5 | 88 | 94 | 76 | ||||||
| Mismatch per subtype | |||||||||||
| A(H3N2) | Yes | Yes | No | No | No | No | NA | No | Yes | Yes | Yes |
| A(H1N1)1 | NA | No | No | Yes | Yes | No | Yes2 | No | No | No | No |
| B/Vic | NA | NA | Yes (Yam in vaccine) | NA | NA | Yes (Yam in vaccine) | NA | No | No | NA | NA |
| B/Yam | Yes (Vic in vaccine) | Yes (Antigenic mismatch) | Yes (Antigenic mismatch) | Yes(Vic in vaccine) | Yes (Vic in vaccine) | NA | NA | Yes (Antigenic mismatch) | Yes (Vic in vaccine) | Yes (Antigenic mismatch) | Yes (Antigenic mismatch) |
| Vaccine mismatch | Mismatch | Mismatch | Partially match | Partially match | Mismatch | Match | Mismatch | Match | Mismatch | Mismatch | Mismatch |
V: B/Victoria/2/87-lineage; Y: B/Yamagata/16/88-lineage; NA: Not applicable.
12003/2004 through 2008/2009 season former seasonal A(H1N1); 2009/2010 through 2013/2014 season A(H1N1)pdm09.
2Seasonal A(H1N1) was used in the vaccine; A(H1N1)pdm09 monovalent vaccine was available late in the 2009/10 influenza season.
In general the activity of the former seasonal A(H1N1) influenza subtype was low and A(H1N1) or A(H1N1)pdm09 mainly co-circulated with influenza B virus (2007/08, 2012/13, 2013/14) (Table 2). In 2009/10 influenza season, the pandemic influenza virus A(H1N1)pdm09 emerged which was antigenically different from the seasonal influenza vaccine [i.e. circulating virus A/California/7/2009(H1N1)pdm09 vs. vaccine strain A/Brisbane/59/2007(H1N1)].
In five influenza seasons, both influenza B/Yamagata and B/Victoria lineages were circulating; in three (2011/12, 2012/13 and 2013/14) the proportion of influenza B/Yamagata lineage was higher (i.e. > 60%). In six seasons (2003/04, 2005/06, 2006/07, 2007/08, 2008/09 and 2011/12) circulating influenza B virus belonged to the opposite lineage of the vaccine.
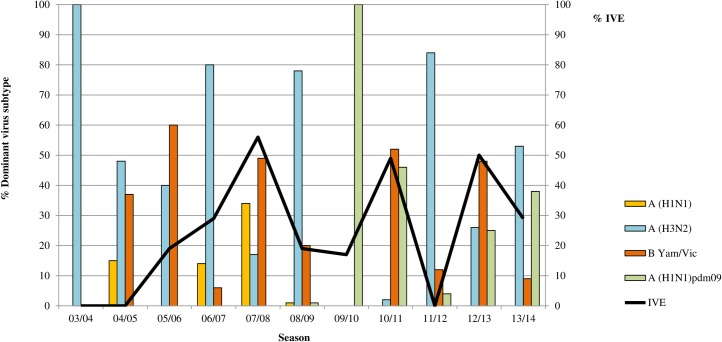
Fig 2 presents the distribution of circulating influenza virus (sub)types according to data from the Sentinel Practices of NIVEL Primary Care Database for each of the seasons. Remarkebly, in the six seasons in which the IVE was low (i.e. < 30%), the proportion of influenza virus A(H3N2) virus was high (i.e. 48–100%), while in the three seasons in which the IVE was moderate to high (i.e. > 50%), the proportion of influenza virus A(H3N2) was much lower (i.e. 2–26%).
Fig 2. IVE for the seasons 2003/04-2013/14 and distribution of circulating influenza virus (sub)types according to data from Sentinel Practices of NIVEL Primary Care Database.
IVE: influenza vaccine effectiveness. IVE estimates are relatively low (i.e. <30%), during influenza seasons with H3N2 as the predominant virus subtype (i.e. 2003/04, 2004/05, 2006/07, 2008/09, 2011/12, and 2013/14).
In general the activity of the former seasonal A(H1N1) influenza subtype was low and A(H1N1) or A(H1N1)pdm09 mainly co-circulated with influenza B virus (2007/08, 2012/13, 2013/14) (Table 2). In 2009/10 influenza season, the pandemic influenza virus A(H1N1)pdm09 emerged which was antigenically different from the seasonal influenza vaccine.
In five influenza seasons, both influenza B/Yamagata and B/Victoria lineages were circulating; in three (2011/12, 2012/13 and 2013/14) the proportion of influenza B/Yamagata lineage was higher (i.e. > 60%). In six seasons (2003/04, 2005/06, 2006/07, 2007/08, 2008/09 and 2011/12) circulating influenza B virus belonged to the opposite lineage of the vaccine.
Finally, considering the distribution of circulating influenza virueses during each influenza season, the vaccine showed lower effectiveness (i.e. < 30%) during influenza seasons with relatively high influenza A(H3N2) virus activity (i.e. the proportion of A(H3N2) virus ranged from 48% to 100%) (2003/04, 2004/05, 2006/07, 2008/09, 2011/12, 2013/14), while in the three seasons in which the IVE was moderate to high (i.e. > 50%) (i.e. 2007/08, 2010/11, 2012/13), the proportion of influenza virus A(H3N2) was much lower (i.e. 2–26%).
Discussion
In the present study, seasonal influenza vaccine strains did not match perfectly the circulating viruses in many influenza seasons (seven vs. four seasons). However, influenza vaccine showed moderate effectiveness when vaccine (partially) matched circulating viruses (four seasons) (IVE: 40%; 95% CI 18 to 56%) and low effectiveness when vaccine did not match (seven seasons) (IVE: 20%; 95% CI -5 to 38%). Moreover, the overall IVE as well as the (sub)type/lineage specific IVE showed extreme variability from season to season.
The estimated IVE for former A(H1N1) for the two influenza seasons 2004/05 and 2007/08 appeared to be consistently high (> 65%). The high IVE for influenza A(H1N1)pmd09 for the seasons 2010/11 onwards, corresponded well with the virological data that showed no drift variants and no vaccine mismatch for this subtype. The vaccine did not show statistically significant effectiveness against influenza virus B (total) for influenza seasons 2004/05, 2005/06, 2008/09 and 2011/12 that could be explained by vaccine mismatch with the circulating influenza B virus. The high IVE for influenza virus B Victoria lineage in influenza seasons 2010/11 also corresponded well with the absence of a vaccine mismatch for this lineage. On the other hand, the high IVE for influenza B virus (total) in 2007/08 (when Victoria lineage was in the vaccine) and for influenza virus B Yamagata lineage in 2012/13 (when there was an antigenic mismatch and the majority of the reported B/Yamagata/16/88 viruses were antigenically more closely related to B/Massachusetts/2/2012-like) [31] contradicted the fact that in those seasons vaccine did not match the circulating influenza B virus. Despite that, as discussed by a similar Canadian study, the high IVE for influenza B virus in 2007/08 influenza season could partly be explained by the possibility of cross-protection between the lineages [33].
Overall, IVE was low for most of the seasons in which influenza virus A(H3N2) dominated. Moreover, there was an inconsistency between the IVE estimates and vaccine match status for several influenza seasons. In two out of three influenza seasons (2005/06 and 2007/08) in which vaccine did not show effectiveness against influenza A(H3N2), vaccine strain matched the circulating virus subtype. Additionally, in three out of six seasons in which some degree of vaccine effectiveness was found (2003/04, 2011/12 and 2013/14) influenza vaccine strain mismatched the circulating influenza virus A(H3N2) and in two seasons (2003/04 and 2013/14) there was an antigenic drift compared to the previous influenza seasons.
Similarly, other studies in Europe, Canada and the USA have also observed a lower IVE for the influenza subtype A(H3N2), although vaccine matched the circulating virus subtype [10,34–36].
The inconsistency between the vaccine antigenic match and vaccine effectiveness is complex and has been reported in both observational studies and clinical trials [37,38]. Virological as well as epidemiological factors could contribute to this unexplained inconsistency. From the virological perspective, a recently conducted study has shown that ferret post-infection antisera responds differently than human post-vaccination sera to influenza A(H3N2) virus [39]. This finding indicates that new techniques such as human serologic testing should be used to improve vaccine virus strains selection. From the epidemiological perspective, it has been shown that the global circulation of influenza virus types and subtypes differs substantially [40]. For instance, influenza A(H3N2) has a faster antigenic evolution rate, occur more frequently, and result in more infection among adult population [40]. Hence, these epidemiological factors could result in different proportion of cases in each influenza season and consequently affect the IVE [5].
This is the first in depth multiple season virus type and subtype specific test-negative case-control study conducted among the general population in the Netherlands. Data provided by the Sentinel Practices of NIVEL Primary Care Database and laboratory diagnoses allowed estimating IVE over 11 influenza seasons (2003/04 through 2013/14). One of the strengths of this study is using datasets over a large number of influenza seasons which enabled us to not only assess the overall and season specific IVE, but also to study the potential association between the influenza virus types and subtypes/lineages.
Though, in order to interpret the results, it is important to note the study limitations. Firstly, due to the observational nature of the study design, it is unlikely that included ILI and ARI cases in the current study are a random sample of all individuals with ILI and ARI in the population. Moreover, although it is expected that GPs select the ILI and ARI patients randomly; in reality collecting a swab from a patient may partly depend on the GP discretion and patients’ consent. Secondly, due to the low power especially in some influenza seasons, we either could not estimate the IVE within influenza types/subtypes or we found rather wide confidence intervals. Besides, due to the low power, the adjusted IVE for influenza subtypes could not be calculated and subgroup analysis of VE based on baseline characteristics of the patients (e.g. age and presence of chronic diseases) for each influenza season could not be performed. Thirdly, the distribution of the ILI and ARI patients as well as the health care-seeking behavior of the two patient groups may differ in the influenza season, which could consequently lead to misclassification bias. However, since we did not find any confounding effect for the diagnosis variable (ILI and ARI), it is unlikely that it could influence our estimates. Finally, in the current study in order to estimate IVE, test-negative design case-control study as the most accurate observational study design has been used [35,41]. However, similar to other observational study designs, test-negative design has methodological limitations, which could bias the IVE estimates. Although the design partially controls for the bias due to the similar health care-seeking behavior among cases and controls, it still lacks randomization of influenza vaccination. For example, elderly population and patients with chronic medical conditions are more likely to get vaccinated due to the higher risk of developing severe influenza complications. As a result, patients among these high-risk groups could be more frequently selected for influenza virus infection diagnosis [42].
Conclusions
In conclusion, the Sentinel Practices of NIVEL Primary Care database provides a suitable data source to measure IVE in the Netherlands. Based on our results, influenza vaccine had the highest protective effect against A(H1N1), A(H1N1)pdm09 and B influenza viruses. The overall IVE was particularly low during the seasons with A(H3N2) as the predominant influenza virus subtype. Moreover, vaccine showed moderate effectiveness when matched and low effectiveness when mismatched the circulating viruses. Therefore, efforts should be renewed to improve vaccine production procedures and to explore the potential factors that especially influence IVE against different virus subtypes in each influenza season such as age and presence of chronic medical conditions.
Supporting Information
(DOCX)
(PDF)
(DOCX)
Acknowledgments
We thank all the GPs from the Dutch Sentinel General Practice Network and their patients for taking swabs and supplying associated data used in this study.
Data Availability
Related data will be made available to interested researchers upon request under a Data Transfer Agreement restricting onward transfer of the data to third parties without prior agreement with the National Institute of Public Health and the Environment (RIVM), Netherlands. A request should initially be submitted to Dr. Adam Meijer (adam.meijer@rivm.nl).
Funding Statement
The study was partly funded by the Ministry of Health, Welfare and Sport (VWS). GAD received funding from VWS for organization of the Sentinel Practices of NIVEL Primary Care Database. AM, FD and MMAdL received funding from VWS for data collection and laboratory testing. VWS had no role in the study design, data analysis, writing or decision to publish this manuscript.
References
- 1.The World Health Organization. Influenza (Seasonal) Fact sheet N 211. 2009. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/ Accessed 02 September 2015.
- 2.Meijer A, Rimmelzwaan G.F, Dijkstra F, Donker G.A. Actuele ontwikkelingen betreffende influenza; griepspotters in actie (Article in Dutch). Tijdschrift voor infectieziekten 2009;4(5):176–184. [Google Scholar]
- 3.The World Health Organization. WHO Global Influenza Programme. Geneva: WHO. 2009. Position paper on Influenza vaccines. Weekly epidemiological record. 2005; 33: 279–287.
- 4.The World Health Organization. WHO Consultation and Information Meeting on the Composition of Influenza Virus Vaccines for the Northern Hemisphere 2013–2014. February 2013. Available at: http://www.who.int/influenza/vaccines/virus/recommendations/consultation201302/en/index.html Accessed 02 September 2015.
- 5.Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 2014;14(12):1228–1239. 10.1016/S1473-3099(14)70960-0 [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport (RIVM). Annual report surveillance of influenza and other respiratory infections in the Netherlands: winter 2014/2015. Available at: http://www.rivm.nl/dsresource?objectid=rivmp:292759&type=org&disposition=inline&ns_nc=1 Accessed 24 November 2015.
- 7.Kelly H, Carville K, Grant K, Jacoby P, Tran T, Barr I. Estimation of influenza vaccine effectiveness from routine surveillance data. PloS One. 2009;4(3):e5079 10.1371/journal.pone.0005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skowronski DM, Janjua NZ, De Serres G, Dickinson JA, Winter AL, Mahmud SM, et al. Interim estimates of influenza vaccine effectiveness in 2012/13 from Canada’s sentinel surveillance network, January 2013. Euro Surveill. 2013;18(5). [DOI] [PubMed] [Google Scholar]
- 9.Fielding JE, Grant KA, Papadakis G, Kelly HA. Estimation of type- and subtype-specific influenza vaccine effectiveness in Victoria, Australia using a test negative case control method, 2007–2008. BMC Infect Dis. 2011;11:170 10.1186/1471-2334-11-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowronski DM, Masaro C, Kwindt TL, Mak A, Petric M, Li Y, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine. 2007;25(15):2842–51. 10.1016/j.vaccine.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Bragstad K, Emborg H, Fischer TK, Voldstedlund M, Gubbels S, Andersen B, et al. Low vaccine effectiveness against influenza A(H3N2) virus among elderly people in Denmark in 2012/13—a rapid epidemiological and virological assessment. Euro Surveill. 2013;18(6). [PubMed] [Google Scholar]
- 12.van der Hoek W, Dijkstra F, de Lange MM, Donker GA, Meijer A, van der Sande MA. Euro Surveillance. Letter to the editor: Influenza vaccine effectiveness: heterogeneity in estimates for the 2012/13 season. Euro Surveill. 2013;18(7):5 [PubMed] [Google Scholar]
- 13.Steens A, van der Hoek W, Dijkstra F, van der Sande M. Influenza vaccine effectiveness, 2010/11. Euro Surveill. 2011;16(15). [PubMed] [Google Scholar]
- 14.Netherlands institute for health services research. Measure flu: how? (Article in Dutch) Available at: https://www.nivel.nl/en/node/2440 Accessed 02 September 2015.
- 15.Netherlands institute for health services research. Continuous Morbidity Registration Dutch Sentinel General Practice Network 2012. Available at: http://www.nivel.nl/sites/default/files/bestanden/Rapport-continuous-morbidity-registration-dutch-sentinel-2012.pdf? Accessed 02 September 2015.
- 16.Netherlands institute for health services research. Influenza(-like illness) (Article in Dutch). Available at: https://www.nivel.nl/NZR/influenza-achtig-ziektebeeld Accessed 02 September 2015.
- 17.Pel J.Z.S. 1965 Proefonderzoek naar de frequentie en de aetiologie van griepachtige ziekten in de winter 1963–1964. Huisarts en Wetenschap. 1965;86:321. [Google Scholar]
- 18.van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 2005;41(4):490–497. 10.1086/431982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;19;31(17):2165–8. 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control. Protocol for case-control studies to measure influenza vaccine effectiveness in the European Union and European Economic Area Member States (2009). Available at: http://ecdc.europa.eu/en/publications/Publications/0907_TED_Influenza_AH1N1_Measuring_Influenza_Vaccine_Effectiveness_Protocol_Case_Control_Studies.pdf Accessed 02 September 2015.
- 21.Stichting Nationaal Programma Grieppreventie. Vaccination period (in Dutch). Available at: https://www.nhg.org/sites/default/files/content/nhg_org/uploads/nhg_snpg_handleiding_influenzavaccinatie_2015_def.pdf Accessed 23 November 2015.
- 22.Savulescu C, Jiménez-Jorge S, Delgado-Sanz C, de Mateo S, Pozo F, Casas I, et al. Higher vaccine effectiveness in seasons with predominant circulation of seasonal influenza A(H1N1) than in A(H3N2) seasons: test-negative case-control studies using surveillance data, Spain, 2003–2011. Vaccine. 2014;32(35):4404–11. 10.1016/j.vaccine.2014.06.063 [DOI] [PubMed] [Google Scholar]
- 23.Rimmelzwaan GF, de Jong JC, Bartelds AI, Wilbrink B, Fouchier RA, Osterhaus ADME. The 2003/2004 influenza season in the Netherlands with a limited epidemic of the virus variant A/Fujian, and the vaccine composition for the 2004/2005 season (Article in Dutch). Ned Tijdschr Geneeskd. 2004;148(40):1984–8. [PubMed] [Google Scholar]
- 24.de Jong JC, Rimmelzwaan GF, Bartelds AI, Meijer A, Fouchier RA, Osterhaus ADME. The influenza season 2004/’05 in the Netherlands with the largest epidemic of the last 5 years caused by the virus variant A/California and the composition of the vaccine for the season 2005/’06 (Article in Dutch). Ned Tijdschr Geneeskd. 2005;149(42):2355–61. [PubMed] [Google Scholar]
- 25.Rimmelzwaan GF, de Jong J.C, Donker GA, Meijer A, Fouchier RA, Osterhaus ADME. Het influenzaseizoen 2005/’06 in Nederland en de vaccinsamenstelling voor het seizoen 2006/’07 (Article in Dutch). Ned Tijdschr Geneeskd. 2006 7;150(40): 2209–14. [PubMed] [Google Scholar]
- 26.de Jong JC, Rimmelzwaan GF, Donker GA, Meijer A, Fouchier RA, Osterhaus ADME. The 2006/’07 influenza season in the Netherlands and the vaccine composition for the 2007/’08 season (Article in Dutch). Ned Tijdschr Geneeskd. 2007;151(39):2158–65. [PubMed] [Google Scholar]
- 27.Rimmelzwaan GF, de Jong JC, Donker GA, Meijer A, Fouchier RA, Osterhaus ADME. Influenza season 2007/’08 in the Netherlands: antigenic variation, oseltamivir resistance and vaccine composition for the 2008/’09 season (Article in Dutch). Ned Tijdschr Geneeskd. 2008;152(39):2138–44. [PubMed] [Google Scholar]
- 28.de Jong JC, Rimmelzwaan GF, Donker GA, Meijer A, Fouchier AM, Osterhaus ADME. Het influenza winterseizoen 2008/’09 in Nederland en de vaccinsamenstelling voor het seizoen 2009/’10 (Article in Dutch). Available at: http://www.erasmusmc.nl/viro/influenza-news-letter/2008-20091/Raport_epidemie_2008_-_2009.pdf
- 29.de Jong JC, Rimmelzwaan GF, Donker GA, Meijer A, van der Hoek W, Osterhaus ADME. De Mexicaanse grieppandemie van 2009: een overzicht met focus op Nederland(Article in Dutch). Ned Tijdschr Med Microbiol 2011;19(3): 6–12. [Google Scholar]
- 30.de Jong JC, Donker GA, Meijer A, van der Hoek W, Rimmelzwaan GF, Osterhaus ADME. Het influenzaseizoen 2010/2011 in Nederland: het nieuwe A(H1N1)-virus van 2009 blijft actief (Article in Dutch). Ned Tijdschr Med Microbiol 2011;19(4): 21–27. [Google Scholar]
- 31.de Jong JC, Meijer A, Donker GA, van der Hoek W, Rimmelzwaan GF, Osterhaus ADME. Het influenzaseizoen 2011/12 in Nederland Een kleine epidemie gedomineerd door het A(H3N2)-virus (Article in Dutch). Ned Tijdschr Med Microbiol 2012;20(4): 142–148. [Google Scholar]
- 32.de Jong JC, Donker GA, Meijer A, Hoek W. van der, Lange M.M.A. de, Rimmelzwaan G.F, et al. Het influenzaseizoen 2012/2013 in Nederland: een milde maar langdurige epidemie (Article in Dutch). Ned Tijdschr Med Microbiol 2013;21(4): 135–142. [Google Scholar]
- 33.de Jong JC, Meijer A, Donker GA, Hoek W. van der, Lange M.M.A. de, Rimmelzwaan G.F, et al. Het influenzaseizoen 2013/2014 in Nederland: lage influenza-activiteit (Article in Dutch). Ned Tijdschr Med Microbiol 2014;22(4): 153–161. [Google Scholar]
- 34.Janjua NZ, Skowronski DM, De Serres G, Dickinson J, Crowcroft NS, Taylor M, et al. Estimates of influenza vaccine effectiveness for 2007–2008 from Canada's sentinel surveillance system: cross-protection against major and minor variants. J Infect Dis. 2012;205(12):1858–68. 10.1093/infdis/jis283 [DOI] [PubMed] [Google Scholar]
- 35.Valenciano M, Kissling E, I-MOVE case–control study team. Early estimates of seasonal influenza vaccine effectiveness in Europe: results from the I-MOVE study, 2012/13. Euro Surveill. 2013;18(7):3 [PubMed] [Google Scholar]
- 36.Turbelin C, Souty C, Pelat C, Hanslik T, Sarazin M, Blanchon T, et al. Age distribution of influenza like illness cases during post-pandemic A(H3N2): comparison with the twelve previous seasons, in France. PloS One. 2013;8(6):e65919 10.1371/journal.pone.0065919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belongia EA, Kieke BA, Donahue JG, Coleman LA, Irving SA, Meece JK, et al. Influenza vaccine effectiveness in Wisconsin during the 2007–08 season: comparison of interim and final results. Vaccine. 2011;29(38):6558–63. 10.1016/j.vaccine.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 38.Beran J, Vesikari T, Wertzova V, Beran J, Vesikari T, Wertzova V, Karvonen A, Honegr K, Lindblad N, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis. 2009;200(12):1861–9. 10.1086/648406 [DOI] [PubMed] [Google Scholar]
- 39.Xie H, Wan XF, Ye Z, Plant EP, Zhao Y, Xu Y, et al. H3N2 Mismatch of 2014–15 Northern Hemisphere Influenza Vaccines and Head-to-head Comparison between Human and Ferret Antisera derived Antigenic Maps. Sci Rep. 2015;5:15279 10.1038/srep15279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523(7559):217–20. 10.1038/nature14460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of placebo-controlled clinical trials. Euro Surveill. 2013;18(37). [DOI] [PubMed] [Google Scholar]
- 42.Kelly HA, Sullivan SG, Grant KA, Fielding JE. Moderate influenza vaccine effectiveness with variable effectiveness by match between circulating and vaccine strains in Australian adults aged 20–64 years, 2007–2011. Influenza Other Respir Viruses. 2013;7(5):729–37. 10.1111/irv.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(DOCX)
Data Availability Statement
Related data will be made available to interested researchers upon request under a Data Transfer Agreement restricting onward transfer of the data to third parties without prior agreement with the National Institute of Public Health and the Environment (RIVM), Netherlands. A request should initially be submitted to Dr. Adam Meijer (adam.meijer@rivm.nl).