Abstract
Doubly uniparental inheritance (DUI) of mitochondrial DNA, found only in some bivalve families and characterized by the existence of gender-associated mtDNA lineages that are inherited through males (M-type) or females (F-type), is one of the very few exceptions to the general rule of strict maternal mtDNA inheritance in animals. M-type sequences are often undetected and hence still underrepresented in the GenBank, which hinders the progress of the understanding of the DUI phenomenon. We have sequenced and analyzed the complete M and F mitogenomes of a freshwater mussel, Potamilus alatus. The M-type was 493 bp longer (M = 16 560, F = 16 067 bp). Gene contents, order and the distribution of genes between L and H strands were typical for unionid mussels. Candidates for the two ORFan genes (forf and morf) were found in respective mitogenomes. Both mitogenomes had a very similar A+T bias: F = 61% and M = 62.2%. The M mitogenome-specific cox2 extension (144 bp) is much shorter than in other sequenced unionid mitogenomes (531–576 bp), which might be characteristic for the Potamilus genus. The overall topology of the phylogenetic tree is in very good agreement with the currently accepted phylogenetic relationships within the Unionidae: both studied sequences were placed within the Ambleminae subfamily clusters in the corresponding M and F clades.
Introduction
Doubly uniparental inheritance (DUI), widespread through the class Bivalvia, is one of the most striking exceptions to the general rule of strict maternal inheritance of mitochondrial DNA (mtDNA) in animals [1]. DUI is an ancient system, likely around 500 million years old [2], characterized by the existence of gender-associated mtDNA lineages that are inherited through males (M type) or females (F type). Females are homoplasmic: they have only the F type mtDNA, inherited from their mothers, which they pass on to both female and male offspring. Males are heteroplasmic: they possess both M and F type; the former is received from the father and transmitted only to the male offspring, whereas the F type is inherited from the mother and not transmitted to the next generation. The M type is found predominantly in male gonads, while the F type is more abundant in somatic tissues. The two mitochondrial genomes are of similar size, comparable to mammalian mtDNA. They evolve faster than mtDNA in most other animals, and M type evolves faster than the F type (reviewed in [3–6]). Whereas marine bivalves under the DUI undergo occasional masculinisation events (a loss of the M mitogenome and its replacement with the F mitogenome) [7], the two mitogenomes in Unionidae family have been evolving independently for about 200 million years. This is believed to be a consequence of the M-specific extension of the cox2 gene, which prevents masculinisation events [8–10].
Compared to marine bivalves (particularly Mytilidae), freshwater species are less thoroughly studied with respect to DUI [4]. Bivalves of the order Unionoida are the largest group of freshwater bivalves, comprising six families and over 800 species distributed across six continents [11]. DUI has been described in over 60 of these species [4]. With 442 Unionidae species currently included in the IUCN Red List of Threatened Species [12], freshwater mussels are probably the most endangered group of freshwater animals today [13]. As the importance of their role in the maintenance of healthy freshwater ecosystems could not be overstated [14], there exists a pressing need for a better understanding of the ecology and biology (including genetics) of this understudied group of animals.
DUI is not only an excellent model system for studying the evolutionary history and phylogeographic structure of freshwater mussels, but also for studying the evolution of mtDNA genomes, and particularly the evolutionary forces that maintain the strictly maternal mtDNA inheritance in most other animals [3,15,16]. More than 50 unionid mitogenomes have been sequenced to date, e.g. [3,17–20], but none belonging to Potamilus genus. Furthermore, as researchers often use only somatic tissues as the source of DNA, and thus obtain only the F type mtDNA, this results in the underdetection of M-type mitogenomes and DUI [4]. Consequently, M type sequences are underrepresented in the GenBank, with only ten currently available. As this hinders the progress of the understanding of the DUI phenomenon, we have identified, sequenced, characterized, compared and phylogenetically analysed both F and M mitogenomes of a freshwater mussel, the pink heelsplitter Potamilus alatus (Unionidae: Ambleminae). This aquatic bivalve mollusk, native to North America, was introduced to China in 2012 as a viable candidate for the production of atropurpureus freshwater pearls [21,22], so apart from contributing to a better understanding of the DUI, the publication of the complete mtDNA sequences shall be useful for the development of the breeding technology for this species.
Materials and Methods
Ethics statement
All necessary permits were obtained from the Taihu Lake Fishery Administration, Jiangsu Province. The handling of mussels was conducted in accordance with the guidelines for the care and use of animals for scientific purposes set by the Institutional Animal Care and Use Committee of the Nanjing Agricultural University, Jiangsu, China.
Samples and DNA extraction
P. alatus samples, originating from the stock introduced to China in 2012 from the Virginia Institute of Technology [21], were obtained at the Nanquan experimental base of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (120°16’ E, 31°25’ N). Determining the presence and characteristics of DUI (mainly the M haplotype) in freshwater bivalves requires the isolation of DNA from male gonads and somatic tissues of both sexes [4]. Hence, specimens were first sexed based on the appearance of the gill marsupium during the reproduction period, when gravid females fill and expand the outer gills with glochidia, whereas in males they remain flat [23]. Afterwards, gonads of the preliminary sexed specimens were dissected and examined under a microscope to further verify the sex of the specimens. Five tissues (foot, gonads, mantle, gills and adductor muscle) of three females and three males were selected for the DNA extraction. After the presence and the ratio of the two types of mtDNA in tissues were determined by a preliminary amplification and sequencing of the cox1 gene [16], the complete mitogenomes were amplified from the adductor muscle sample of a selected female specimen and from the gonads of a selected male specimen. Both of these specimens are stored at the Freshwater Fisheries Research Center, Department of Aquaculture, Wuxi, under accession numbers N120F (F) and N466M (M). DNA was extracted using the Aidlab DNA extraction kit (Aidlab Biotechnologies, Beijing).
PCR amplification and mitogenome sequencing
Degenerate primers (Table 1) were designed to match the generally conserved regions of mtDNA genes and used to amplify and sequence short fragments of 12s, cox1, cox3, nad4, cytb, nad1 and nad2 genes. These sequences were then used to design the specific primers for the amplification and sequencing of the remaining mitogenomic sequences in several PCR steps. Primers were designed to amplify products with overlaps of about 100 bp. Sequences were assembled in a stepwise manner, ensuring that the overlaps are identical. All obtained fragments were BLASTed [24] to confirm that the amplicon is the actual target sequence. Reaction volume of 50 μL contained 5 U/μL of TaKaRa LA Taq polymerase (TaKaRa, Japan), 10×LATaq Buffer II (Mg2+Plus), 2.5 μM of dNTP mixture, 0.2–1.0 μM of each primer, 60 ng of DNA template, and PCR-grade H2O. PCR conditions were optimized for each reaction, with the annealing temperature adjusted to suit the specific primer pair, extension time set to 1 min per Kb of the expected product size, and cycles (35 on average) adjusted depending on the amplification efficiency of the primers. PCR products were sequenced directly, using Sanger method, on an ABI 3730 automatic sequencer. When that was not possible, the products were cloned into a pMD18-T vector (TaKaRa, Japan) and then sequenced. DNAstar v7.1 (Dnastar Inc., USA) package was used to assemble the sequences and locate the putative ORFs for protein-coding genes. Then we used BLAST and Blastx to compare the putative ORFs with the published nucleotide and amino acid sequences of related species, and manually determine the actual initiation and termination codon positions. Annotation of tRNAs was performed using tRNAscan [25] and ARWEN [26] tools and the results checked manually. GenBank accession numbers for the mitogenomes are KU559011 (F) and KU559010 (M). Genomes were visualized using CLC Sequence Viewer 7.7.1 (QIAGEN Bioinformatics).
Table 1. Primers, degenerate and specific, used for the PCR amplification of Potamilus alatus male (M) and female (F) type mitochondrial genomes.
| Fragment No. | Gene or region | Primer name | Sequence (5’-3’) | Length (bp) |
|---|---|---|---|---|
| F1 | cox3 | HBCOX3F | ACRTCNACAAARTGYCARTAYCA | 342 |
| HBCOX3R | GCTGTDTTDYTDGGBTCHGGDGT | |||
| M1 | cox1 | HBCOX1F | CTAARCAHTTYAAYCCDAGYAGG | 938 |
| HBCOX1R | ACYAATCRTAARGAYATTDGGAC | |||
| F2/M2 | nad2 | HBND2F | AAYTGRYTAAYHACHTGRATWGG | 308 |
| HBND2R | GGDGCRATYTTTTGYCADGT | |||
| F3/M3 | 12S | HB12SF | TGGTGCCAGCAKTCGCGGT | 600 |
| HB12SR | ACYCCYACTDTGTTACGACTT | |||
| F4/M4 | cytb | HBCYTBF | GGBTAYGTNCTHCCNTGRGGNCA | 434 |
| HBCYTBR | AARTAYCAYTCDGGYTGAATRTG | |||
| F5 | nad5 | HBND5F | AAHAADGCYTTAAANANHGCRTG | 314 |
| HBND5R | GCDGCNATRGCHGCYCCWACNCC | |||
| M5 | nad1 | HBND1F | GCNCAAARAAYTTDATACGARGT | 407 |
| HBND1R | ATDAGNARATCRTAGBGRTAACRCGG | |||
| M6/F6 | nad4 | HBND4F | GGNGCYTCNRCATGVGCYTTNGG | 251 |
| HBND4R | GGNTGRGGNTAYCADCCDGARCG | |||
| F7 | cox3-nad2 | 2HBF1 | TACACCTAAGAACCTCACC | 4741 |
| 2HBR1 | TGTTGGGTGAAGAGAGGAG | |||
| F8 | cox3-nad2 | 2HBF1 | TACACCTAAGAACCTCACC | 4741 |
| 2HBR1 | TGTTGGGTGAAGAGAGGAG | |||
| F9 | nad2-12S | 2HBF2 | CAGACCCCAACCTATGGTC | 545 |
| 2HBR2 | TCCCATGGGACAGCTTTCC | |||
| F10 | 12S-cytb | 2HBF3 | CTAAAGCGAAATCTAGTAC | 1573 |
| 2HBR3.5 | AGAATCCTCCTCACAGCC | |||
| F11 | cytb-nad5 | 2HBF3.1 | ACACTAACCTCCATTTGCC | 1582 |
| HBR3 | AACTCTTAGGCAGCTTGGTC | |||
| F12 | nad5-nad4 | HBF4 | TCACAAAATCTCTGGACTCTC | 2226 |
| 2HBR5 | TGGTGATGAGGAAACGTAAG | |||
| F12 | nad4-cox3 | 2HBF6 | ACAAAACACTACACTAATCG | 2097 |
| 2HBR6 | TTAGATATCTTGTGTTGGTG | |||
| M7 | cox3 | HBCOX3F | ACRTCNACAAARTGYCARTAYCA | 342 |
| HBCOX3R | GCTGTDTTDYTDGGBTCHGGDGT | |||
| M8 | cox3-cox1 | 1HBF8 | CTCAAAAACTTGTAATACGG | 1327 |
| HBR7 | CTTAGAATTGGGGTGATAGG | |||
| M9 | cox1-nd2 | HBF6 | AACTGGTCATCACACATCAC | 1543 |
| 1HBR6-1 | AGTGTATGTGGGTTTGTG | |||
| M10 | nad2-12S | 1HBF1 | ATCCAGACCACTAACAAG | 1361 |
| 1HBR1 | GTTTGGGTGTAAACTTAAGG | |||
| M11 | 12S-cytb | 1HBF2-0 | AAGTAAGACCACAACACT | 2258 |
| 1HBR2 | AGAAGTCTTGTTCCAGAC | |||
| M12 | cytb-nad1 | 2HBF4 | ACGAAACATACACATGGC | 3975 |
| 13HB-B | GGTTAGAATTATAGGAATTTCG | |||
| M13 | nad1-nad4 | 13HBF4 | GATTCAACGTAGAATACTCAAGAG | 1686 |
| 1HBR4 | TGGAGTTCTTTGGAGTGAG | |||
| M14 | nad4-cox3 | 1HBF5 | ATAGCTTCATAACACAAACC | 2650 |
| 1HBR7 | TGTTAGTGTGTTTGTGACG |
Phylogenetic analyses
To provide additional evidence that our samples were correctly identified, and that sequencing and annotation were conducted with high precision and reliability, we have selected a subset of unionid mitogenomes available from the GenBank and conducted phylogenetic analyses. To maximise the phylogenetic resolution for the two studied sequences, we have included all of the available sequences belonging to the Ambleminae subfamily; two M mitogenomes: Venustaconcha ellipsiformis and Quadrula quadrula [3]; and five F mitogenomes: Toxolasma parvus [27], Leptodea leptodon [28], Lampsilis ornata [17], Venustaconcha ellipsiformis and Quadrula quadrula [3]. Additional twelve unionid sequences, two Margaritiferidae (Bivalvia: Unionoida), and a Mytilus edulis (Bivalvia: Mytilioda) sequence as outgroup were also retrieved from the GenBank. Analyses were performed on concatenated twelve mitochondrial protein-coding genes and two rRNA genes. Atp8 was not used in the analysis because it is not present in all unionid mitogenomes [3]. MitoTool (https://github.com/dongzhang0725/MitoTool) package was used to retrieve the sequences from the GenBank. Each sequence was aligned separately (in batches) by MAFFT [29] integrated into BioSuite program (https://github.com/dongzhang0725/BioSuite): rRNAs were aligned directly, whereas PCGs were translated into amino acid sequences (by MitoTool), aligned (MAFFT), and then back-translated into the corresponding codon-based DNA alignments using pal2nal.pl [30]. Alignments were visually inspected and ambiguously aligned parts manually deleted. Finally, BioSuite was used to concatenate the alignments (S1 File) and produce input files for the two programs used to conduct the phylogenetic analysis: maximum-likelihood (with 1000 bootstrap replications) using raxmlGUI [31,32], and Bayesian inference using MrBayes 3.2.6 [33], with 4×106 generations. GTR+G+I evolution model, selected using Modelgenerator [34], was used in both analyses.
Results and Discussion
Genome size and organization
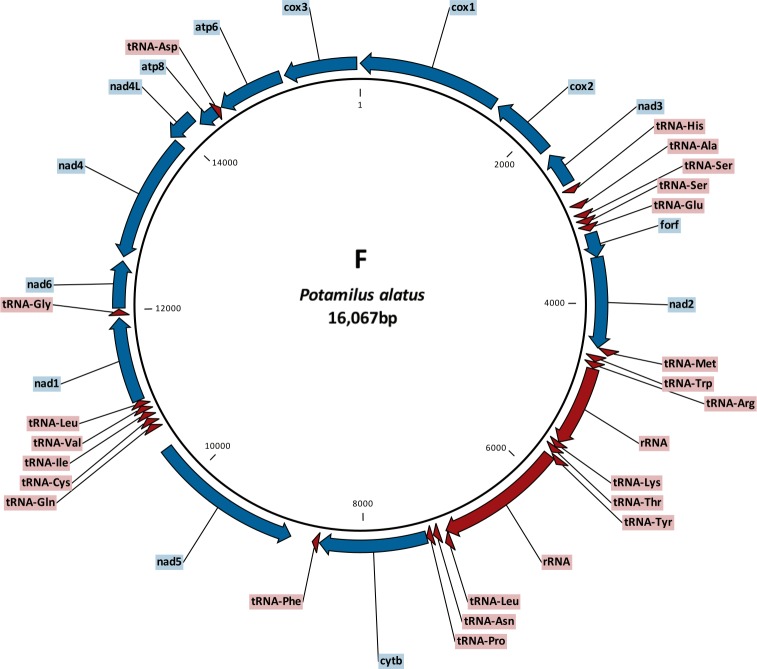
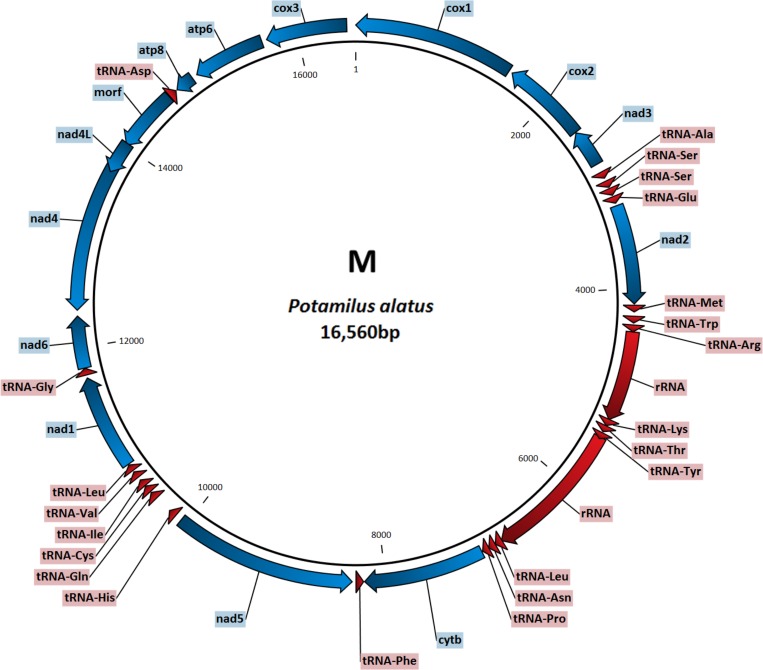
In unionid bivalves, the M mitogenome is usually about 550 bp longer than the F type, mostly as a result of the M-specific extension of cox2 gene [4,8]. In P. alatus, the M genome (16 560 bp) is also longer than the F genome (16 067 bp), but the difference is only 493 bp. The F genome is somewhat larger than the usual unionid F mitogenomes, whereas the M genome is shorter than most other unionid counterparts [18,20,35–39]. Both genomes contained all 37 genes commonly found in animal mtDNAs [40]: 13 protein-coding genes, 22 tRNAs (including double serine and leucine tRNAs) and two (12S and 16S) rRNAs (Figs 1 and 2, S1 Table and S2 Table). Control region was not found, but candidates for two ORFan [3,41] genes (forf and morf) were found in respective genomes. The gene order in both genomes, as well as the distribution of genes between L and H strands, was typical for unionid mussels [3]. Non-coding regions ranged from 2 to 233 bp in length in the F mitogenome (26 in total) and from 1 to 170 bp in the M mitogenome (31 in total). These numbers are within the standard range (22–33) of unassigned regions usually found in unionoid mitogenomes [3,17,36,37,42]. Two gene overlaps were found in the F genome: nad2 / tRNAMet (1 bp) and nad4 / nad4L (8 bp). There is a possibility that nad2 uses the unfinished T—top codon instead of TAA, in which case it does not overlap with the tRNAMet. Overlap between nad4L and nad4 is common in unionoid mitogenomes [3,4]. Two gene overlaps were found in the M genome as well: 3 bp between 12S rRNA and tRNALys and 17 bp between nad4L and morf. Gene overlaps in mitogenomes usually involve tRNA genes, because their sequences are under lesser evolutionary constraints [43]. However, the large (17 bp) overlap between nad4L and morf indicates the possibility of an annotation mistake. We have compared the translated morf amino acid product with the remaining four morf sequences available in the GenBank, and the results indicate the existence of four extra bases at the end of the P. alatus morf polypeptide. However, this would require GGA to be used as the stop codon, which is not likely. It is also possible that such a large overlap was made possible by the low functional constraints on morf evolution, as evidenced by very low level of sequence similarity between the available orthologs [3]. This problem further highlights the need for a larger number of fully annotated unionid M type genomes to be available in public databases. Four different start codons were found in the F genome: AUG (6 genes), AUU (5), GUG (2), AUA (1); and two stop codons: UAG (8) and UAA (6). Three start codons were found in the M genome: AUG (9 genes), AUU (3), AUA (2); and two (complete) stop codons: UAA (8), UAG (5). We did not count U—stop codon separately, as we presumed that it would be completed (UAA) by the addition of 3' A residues to the mRNA [17,44–46]. All these codons are typical for the invertebrate mitochondrial DNA [40].
Fig 1. Gene map of the F type mitochondrial genome of Potamilus alatus.
Fig 2. Gene map of the M type mitochondrial genome of Potamilus alatus.
Base composition
A+T bias has been observed in many unionid mitogenomes [4], and the two P. alatus genomes are no exception: F = 61% (38% A, 23% T, 11% G, 28% C) and M = 62.2% (39.2% A, 23.0% T, 10.6% G, 27.2% C). Almost identical A and G ratios were observed in the F mitogenome of U. pictorum [35]. There was very little variation in base composition among different functional parts of the genome: cox2 had the lowest A+T bias in both genomes (56.2% in F, 58.9% in M), whereas nad3 (63.0%) and nad6 (63.9%) had the highest bias in F and M genomes, respectively.
ORFan genes
All bivalve mollusks under DUI appear to possess lineage-specific mitochondrial ORFs (open reading frames). Their origin in bivalves may be extremely ancient, it is believed that forf has been maintained for at least 200 million years in unionids [1,4,41]. Even though both genes appear to be rapidly evolving [3], their long-term conservation in bivalves, as well as their transcriptional levels, suggest that they are functional: they could be directly involved in sex determination, reproduction, embryo development, spermatogenesis, or just the maintenance of sperm mitochondria during the embryo development [1,5,27]. We have found a 264 bp-long candidate for the forf gene between nad2 and nad3 genes in the studied F mitogenome, and a 612 bp-long candidate for the morf gene between nad4l and tRNAAsp in the M genome. This is standard for ORFan genes: their position generally differs between F and M genomes and their length varies from 66 to 92 codons (198 to 276 bp) in F genomes and from 101 to 234 codons (303 to 702 bp) in M genomes (reviewed in [5,47]).
Gene sizes
Assuming that there were no major annotation oversights, a somewhat larger number of longer genes was found in the M genome, but the differences in size for most (26) of the genes were in a single-digit range. Hence, the extra 493 bases in the male genome can be attributed mostly to the differences in sizes between cox1 (28 bp), cox2 (144 bp), nad5 (84 bp), and morf/forf (348 bp) genes. Only one gene, atp8, was much larger in the F genome: 27 bp.
cox2 extension
Unionid M genomes are characterised by the presence of a unique 3’-end coding extension of the cytochrome c oxidase subunit II (cox2) gene [8]. It is functional, rapidly evolving and its absence from the F genome suggests that it plays an important role in the mechanism of DUI [3,47]. It usually spans 531 to 576 bases [4,9,19], but in P. alatus, the extension is much shorter, only 144 bases: F cox2 is 681 bp-long, whereas the M cox2 is 825 bp-long. As an identical (144 bp) extension was also found in the closely related Potamilus purpuratus [9], this significantly shortened extension might be characteristic for this genus.
Similarity and phylogeny
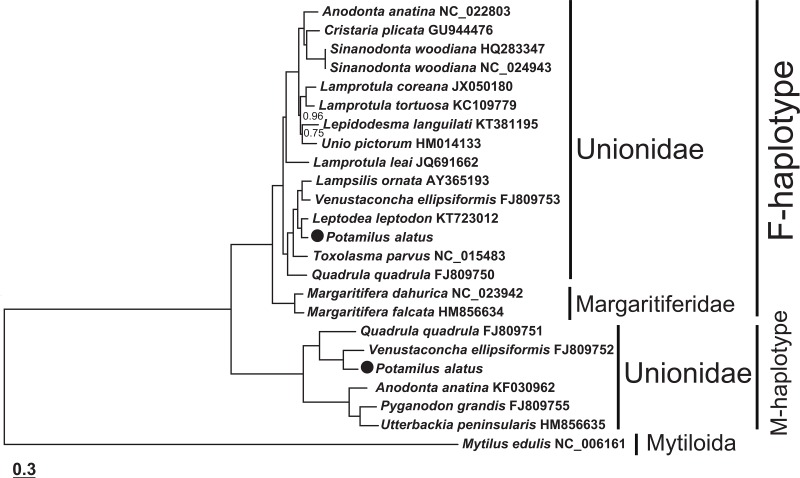
Unionoid bivalves are characterized by extreme intraspecific sequence divergences between gender-associated mtDNAs [10]. Both synonymous and non-synonymous substitutions accumulate faster in the M genome. As expression studies indicate that the M genome is active only at spermatogenesis, these observations suggest that the M genome is under a more relaxed selective constraint than the F genome [3,47,48]. Whereas several masculinisation events are believed to have occurred during the evolutionary history of Mytillidae, the two mitogenomes have been evolving independently in Unionidae for at least 200 million years [4,7–9,47]. As the divergence between male and female genomes starts de novo with each masculinisation event, the genetic divergence between F and M genomes in the marine genus Mytilus is only 21–26% [49,50], whereas in Unionidae it is usually in the range of 40 to 50% [10,19]. The genetic divergence between the two P. alatus genomes (40%) is at the lower end of this range. As a result of the high diversity and the absence of recent masculinisation of mtDNA in freshwater bivalves, separate F and M clades are formed in phylogenetic analyses and can be independently used for phylogeny reconstruction [9,47]. The two approaches (Maximum Likelihood and Bayesian inference) used in this research to estimate the phylogenetic position of P. alatus produced identical dendrogram topologies (consensus tree is shown in Fig 3) with very high statistical support. The overall topology of the phylogenetic tree is in very good agreement with the results of the recent review of the phylogenetic relationships within the Unionidae based on cox1 and 28S genes [51]: both studied sequences were placed within the Ambleminae subfamily clusters in the corresponding M and F clades. Furthermore, the topology of both clades corroborates the close relationships of Potamilus with other Lampsilini tribe genera included in the analysis: Leptodea, Lampsilis, Venustaconcha and Toxolasma [51,52].
Fig 3. Phylogenetic positions of the two studied P. alatus mitogenomes.
Phylogenetic dendrogram showing the evolutionary relationships among 24 almost complete bivalve mtDNA sequences: 21 Unionidae (including P. alatus), two Margaritiferidae and Mytilus edulis (outgroup). Scale bar corresponds to the estimated number of substitutions per site. Statistical support values were all 100 (not shown), except where noted otherwise: Bayesian posterior probability values (Bayesian inference analysis) are bolded and larger, bootstrap support values (Maximum Likelihood) are italicized and smaller. Potamilus alatus sequences are highlighted by a black dot. GenBank accession numbers are indicated in the figure.
Conclusions
Both M and F Potamilus alatus mitogenomes are relatively similar to other described unionid mitogenomes, and phylogenomic analysis results are in good agreement with the phylogenetic relationships inferred using other molecular markers. The publication of this sequence shall be valuable for the future studies of the DUI phenomenon in bivalves. Apart from focusing on further identification and sequencing of (still) underrepresented M genomes in freshwater mussels, future studies should also further explore the phenomenon of the shortened cox2 extension in the M genome of the Potamilus genus.
Supporting Information
Sequences include concatenated twelve mitochondrial protein-coding genes (Atp8 was not used in the analysis) and two rRNA genes.
(FAS)
Gene lengths are in bp, St = strand, and NCR = non-coding region, where a negative value indicates an overlap between two genes.
(DOCX)
Gene lengths are in bp, St = strand, and NCR = non-coding region, where a negative value indicates an overlap between two genes.
(DOCX)
Acknowledgments
This study was supported by the Special Scientific Research Funds for Central Non-profit Institutes of Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Grant number: 2015JBFM02), and Fisheries Sanxin Engineering Funds of Ocean and Fishery Bureau of Jiangsu Province (Grant number: Y2014-38). We would like to express our gratitude to the two reviewers, whose detailed comments have helped us to improve our manuscript.
Data Availability
Apart from the two genomic sequences, all relevant data are within the paper and its Supporting Information files. GenBank accession numbers for the two genomic sequences are KU559011 (F) and KU559010 (M). The two specimens used for the DNA extraction are stored at the Freshwater Fisheries Research Center, Department of Aquaculture, Wuxi, China, under accession numbers N120F (F) and N466M (M).
Funding Statement
This study was supported by the Special Scientific Research Funds for Central Non-profit Institutes of Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Grant number: 2015JBFM02), and Fisheries Sanxin Engineering Funds of Ocean and Fishery Bureau of Jiangsu Province (Grant number: Y2014-38). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Breton S, Milani L, Ghiselli F, Guerra D, Stewart DT, Passamonti M. A resourceful genome: Updating the functional repertoire and evolutionary role of animal mitochondrial DNAs. Trends Genet. 2014;30: 555–564. 10.1016/j.tig.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Boyle EE, Etter RJ. Heteroplasmy in a deep-sea protobranch bivalve suggests an ancient origin of doubly uniparental inheritance of mitochondria in Bivalvia. Mar Biol. Springer-Verlag; 2013;160: 413–422. [Google Scholar]
- 3.Breton S, Beaupré HD, Stewart DT, Piontkivska H, Karmakar M, Bogan AE, et al. Comparative mitochondrial genomics of freshwater mussels (Bivalvia: Unionoida) with doubly uniparental inheritance of mtDNA: gender-specific open reading frames and putative origins of replication. Genetics. Genetics; 2009;183: 1575–89. 10.1534/genetics.109.110700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soroka M. Characteristics of mitochondrial DNA of unionid bivalves (Mollusca: Bivalvia: Unionidae). I. Detection and characteristics of doubly uniparental inheritance (DUI) of unionid mitochondrial DNA. Folia Malacol. 2010;18: 147–188. [Google Scholar]
- 5.Milani L, Ghiselli F, Maurizii MG, Passamonti M. Doubly uniparental inheritance of mitochondria as a model system for studying germ line formation. PLoS One. Public Library of Science; 2011;6: e28194 10.1371/journal.pone.0028194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart DT, Hoeh WR, Bauer G, Breton S. Mitochondrial genes, sex determination and hermaphroditism in freshwater mussels (Bivalvia: Unionoida). Evolutionary Biology: Exobiology and Evolutionary Mechanisms. 2013. pp. 245–255. [Google Scholar]
- 7.Hoeh WR, Stewart DT, Saavedra C, Sutherland BW, Zouros E. Phylogenetic evidence for role-reversals of gender-associated mitochondrial DNA in Mytilus (Bivalvia: Mytilidae). Mol Biol Evol. 1997;14: 959–967. [DOI] [PubMed] [Google Scholar]
- 8.Curole JP, Kocher TD. Ancient sex-specific extension of the cytochrome c oxidase II gene in bivalves and the fidelity of doubly-uniparental inheritance. Mol Biol Evol. Oxford University Press; 2002;19: 1323–8. [DOI] [PubMed] [Google Scholar]
- 9.Curole JP, Kocher TD. Evolution of a unique mitotype-specific protein-coding extension of the cytochrome c oxidase 5 gene in freshwater mussels (Bivalvia: Unionoida). J Mol Evol. 2005;61: 381–389. 10.1007/s00239-004-0192-7 [DOI] [PubMed] [Google Scholar]
- 10.Doucet-Beaupré H, Breton S, Chapman EG, Blier PU, Bogan AE, Stewart DT, et al. Mitochondrial phylogenomics of the Bivalvia (Mollusca): searching for the origin and mitogenomic correlates of doubly uniparental inheritance of mtDNA. BMC Evol Biol. 2010;10: 50 10.1186/1471-2148-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogan AE, Roe K. Freshwater bivalve (Unioniformes) diversity, systematics, and evolution: status and future directions. J North Am Benthol Soc. 2008;27: 349–369. [Google Scholar]
- 12.IUCN. IUCN Red List of Threatened Species. In: Version 2016.2. 2016 p. www.iucnredlist.org.
- 13.Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark S a., et al. The Global Decline of Nonmarine Mollusks. Bioscience. 2004;54: 321. [Google Scholar]
- 14.Vaughn CC, Hakenkamp CC. The functional role of burrowing bivalves in freshwater ecosystems. Freshw Biol. Blackwell Science Ltd; 2001;46: 1431–1446. [Google Scholar]
- 15.Doucet-Beaupré H, Blier PU, Chapman EG, Piontkivska H, Dufresne F, Sietman BE, et al. Pyganodon (Bivalvia: Unionoida: Unionidae) phylogenetics: a male- and female-transmitted mitochondrial DNA perspective. Mol Phylogenet Evol. 2012;63: 430–44. 10.1016/j.ympev.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 16.Walker JM, Curole JP, Wade DE, Chapman EG, Bogan AE, Watters GT, et al. Taxonomic Distribution and Phylogenetic Utility of Gender- Associated Mitochondrial Genomes in the Unionoida (Bivalvia). Malacologia. 2006;48: 265–282. [Google Scholar]
- 17.Serb JM, Lydeard C. Complete mtDNA sequence of the North American freshwater mussel, Lampsilis ornata (Unionidae): an examination of the evolution and phylogenetic utility of mitochondrial genome organization in Bivalvia (Mollusca). Mol Biol Evol. 2003;20: 1854–66. 10.1093/molbev/msg218 [DOI] [PubMed] [Google Scholar]
- 18.Soroka M, Burzyński A. Complete female mitochondrial genome of Anodonta anatina (Mollusca: Unionidae): confirmation of a novel protein-coding gene (F ORF). Mitochondrial DNA. 2013;1736: 1–3. [DOI] [PubMed] [Google Scholar]
- 19.Huang XC, Rong J, Liu Y, Zhang MH, Wan Y, Ouyang S, et al. The complete maternally and paternally inherited mitochondrial genomes of the endangered freshwater mussel Solenaia carinatus (Bivalvia: Unionidae) and implications for unionidae taxonomy. PLoS One. 2013;8: e84352 10.1371/journal.pone.0084352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soroka M, Burzyński A. Complete male mitochondrial genome of Anodonta anatina (Mollusca: Unionidae). Mitochondrial DNA. 2016;27: 1679–1680. 10.3109/19401736.2014.958725 [DOI] [PubMed] [Google Scholar]
- 21.Wen HB, Hua D, Ma XY, Jin W, Zhuang YB, Gu RB, et al. Byssogenesis in the juvenile pink heelsplitter mussel, Potamilus alatus (Bivalvia: Unionidae). J Morphol. 2015;276: 1273–1282. 10.1002/jmor.20406 [DOI] [PubMed] [Google Scholar]
- 22.Hua D, Neves RJ. Captive survival and pearl culture potential of the pink heelsplitter Potamilus alatus. N Am J Aquac. 2007;69: 147–158. [Google Scholar]
- 23.Hua D. Biology, captive propagation, and feasibility of pearl culture in the pink heelsplitter (Potamilus alatus) (Say, 1817) (Bivalvia: Unionidae). MSc thesis. Virginia Polytechnic Institute and State University, Blacksburg, USA. 2005.
- 24.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research. 1997. pp. 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33: W686–9. 10.1093/nar/gki366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laslett D, Canbäck B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 2008;24: 172–175. 10.1093/bioinformatics/btm573 [DOI] [PubMed] [Google Scholar]
- 27.Breton S, Stewart DT, Shepardson S, Trdan RJ, Bogan AE, Chapman EG, et al. Novel protein genes in animal mtDNA: a new sex determination system in freshwater mussels (Bivalvia: Unionoida)? Mol Biol Evol. 2011;28: 1645–59. 10.1093/molbev/msq345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng L, Zhang X, Zhao G-F. The complete mitochondrial genome of the scaleshell Leptodea leptodon (Bivalvia: Unionidae). Conserv Genet Resour. 2016; [Google Scholar]
- 29.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suyama M, Torrents D, Bork P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestro D, Michalak I. RaxmlGUI: A graphical front-end for RAxML. Org Divers Evol. 2012;12: 335–337. [Google Scholar]
- 32.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 34.Keane TM, Creevey CJ, Pentony MM, Naughton TJM, Mclnerney JO. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol. 2006;6: 29 10.1186/1471-2148-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soroka M, Burzyński A. Complete sequences of maternally inherited mitochondrial genomes in mussels Unio pictorum (Bivalvia, Unionidae). J Appl Genet. 2010;51: 469–76. 10.1007/BF03208876 [DOI] [PubMed] [Google Scholar]
- 36.Song X-L, Ouyang S, Zhou C-H, Wu X-P. Complete maternal mitochondrial genome of freshwater mussel Anodonta lucida (Bivalvia: Unionidae: Anodontinae). Mitochondrial DNA. 2016;27: 549–550. 10.3109/19401736.2014.905852 [DOI] [PubMed] [Google Scholar]
- 37.An C, Ouyang S, Zhou C-H, Wu X-P. The complete F-type mitochondrial genome of Chinese Anodonta arcaeformis (Bivalvia: Unionidae: Anodontinae). Mitochondrial DNA. 2016;27: 1552–3. 10.3109/19401736.2014.953133 [DOI] [PubMed] [Google Scholar]
- 38.Zhou C-H, Ouyang S, Wu X-P, Ding M-H. The complete maternal mitochondrial genome of rare Chinese freshwater mussel Lepidodesma languilati (Bivalvia: Unionidae: Unioninae). Mitochondrial DNA. 2015; [DOI] [PubMed] [Google Scholar]
- 39.Wu R-W, An C-T, Wu X-P, Zhou C-H, Ouyang S. Complete maternal mitochondrial genome of freshwater mussel Aculamprotula tientsinensis (Bivalvia: Unionidae: Unioninae). Mitochondrial DNA. 2015; [DOI] [PubMed] [Google Scholar]
- 40.Wolstenholme DR. Animal Mitochondrial DNA: Structure and Evolution. International Review of Cytology. 1992. pp. 173–216. [DOI] [PubMed] [Google Scholar]
- 41.Milani L, Ghiselli F, Guerra D, Breton S, Passamonti M. A comparative analysis of mitochondrial ORFans: new clues on their origin and role in species with doubly uniparental inheritance of mitochondria. Genome Biol Evol. 2013;5: 1408–34. 10.1093/gbe/evt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JH, Kim SK, Hwang UW. Complete mitochondrial genome of Lamprotula coreana (Unionidae, Unionoida, Bivalvia). Mitochondrial DNA. 2016;27: 1026–7. 10.3109/19401736.2014.926537 [DOI] [PubMed] [Google Scholar]
- 43.Doublet V, Ubrig E, Alioua A, Bouchon D, Marcade I, Marechal-Drouard L. Large gene overlaps and tRNA processing in the compact mitochondrial genome of the crustacean Armadillidium vulgare. RNA Biol. 2015;12: 1159–1168. 10.1080/15476286.2015.1090078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temperley RJ, Wydro M, Lightowlers RN, Chrzanowska-Lightowlers ZM. Human mitochondrial mRNAs-like members of all families, similar but different. Biochim Biophys Acta—Bioenerg. Elsevier; 2010;1797: 1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290: 470–474. [DOI] [PubMed] [Google Scholar]
- 46.Schuster G, Stern D. RNA Polyadenylation and Decay in Mitochondria and Chloroplasts. Progress in Molecular Biology and Translational Science. 2009. pp. 393–422. 10.1016/S0079-6603(08)00810-6 [DOI] [PubMed] [Google Scholar]
- 47.Zouros E. Biparental Inheritance Through Uniparental Transmission: The Doubly Uniparental Inheritance (DUI) of Mitochondrial DNA. Evol Biol. 2012;40: 1–31. [Google Scholar]
- 48.Stewart DT, Kenchington ER, Singh RK, Zonros E. Degree of selective constraint as an explanation of the different rates of evolution of gender-specific mitochondrial DNA lineages in the mussel Mytilus. Genetics. 1996;143: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zbawicka M, Burzyński A, Skibinski D, Wenne R. Scottish Mytilus trossulus mussels retain ancestral mitochondrial DNA: Complete sequences of male and female mtDNA genomes. Gene. 2010;456: 45–53. 10.1016/j.gene.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 50.Breton S, Burger G, Stewart DT, Blier PU. Comparative analysis of gender-associated complete mitochondrial genomes in marine mussels (Mytilus spp.). Genetics. 2006;172: 1107–1119. 10.1534/genetics.105.047159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes-Lima M, Froufe E, Do VT, Ghamizi M, Mock KE, Kebapçı Ü, et al. Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): Defining modern subfamilies and tribes. Mol Phylogenet Evol. 2017;106: 174–191. 10.1016/j.ympev.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 52.Zanatta DT, Murphy RW. Evolution of active host-attraction strategies in the freshwater mussel tribe Lampsilini (Bivalvia: Unionidae). Mol Phylogenet Evol. 2006;41: 195–208. 10.1016/j.ympev.2006.05.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences include concatenated twelve mitochondrial protein-coding genes (Atp8 was not used in the analysis) and two rRNA genes.
(FAS)
Gene lengths are in bp, St = strand, and NCR = non-coding region, where a negative value indicates an overlap between two genes.
(DOCX)
Gene lengths are in bp, St = strand, and NCR = non-coding region, where a negative value indicates an overlap between two genes.
(DOCX)
Data Availability Statement
Apart from the two genomic sequences, all relevant data are within the paper and its Supporting Information files. GenBank accession numbers for the two genomic sequences are KU559011 (F) and KU559010 (M). The two specimens used for the DNA extraction are stored at the Freshwater Fisheries Research Center, Department of Aquaculture, Wuxi, China, under accession numbers N120F (F) and N466M (M).