Abstract
Breast cancer is the most common carcinoma in women. Comprehensive therapy on breast cancer including surgical operation, chemotherapy, radiotherapy, endocrinotherapy, etc. could help, but still has serious side effect and resistance against anticancer drugs. Complementary and alternative medicine (CAM) may avoid these problems, in which traditional Chinese medicine (TCM) has been highlighted. In this section, to analyze the mechanism through which TCM act on breast cancer, we have built a virtual model consisting of the construction of database, oral bioavailability prediction, drug-likeness evaluation, target prediction, network construction. The 20 commonly employed herbs for the treatment of breast cancer were used as a database to carry out research. As a result, 150 ingredient compounds were screened out as active molecules for the herbs, with 33 target proteins predicted. Our analysis indicates that these herbs 1) takes a ‘Jun-Chen-Zuo-Shi” as rule of prescription, 2) which function mainly through perturbing three pathways involving the epidermal growth factor receptor, estrogen receptor, and inflammatory pathways, to 3) display the breast cancer-related anti-estrogen, anti-inflammatory, regulation of cell metabolism and proliferation activities. To sum it up, by providing a novel in silico strategy for investigation of the botanical drugs, this work may be of some help for understanding the action mechanisms of herbal medicines and for discovery of new drugs from plants.
Introduction
Over about one million people would have been diagnosed with cancer and 600,000 will have died of cancer during 2004 in the United States [1]. Despite of the substantial improvements in the current treatments that are available for patients diagnosed with cancer and the positive influence of these treatments on survival, the present chemotherapy or radiation therapy still cause an array of traumatic side effects, such as sleep disturbance, depression, nausea, anxiety, fatigue, and vomiting [2]. The widespread use of a variety of nutritional, psychological and natural medical approaches, collectively-termed complementary and alternative medicine (CAM), has been well documented. It is defined as methods used in the diagnosis, treatment, or prevention of disease that complement mainstream medicine, as opposed to alternative therapies, which are used as a direct substitute for mainstream medicine [3]. The United States National Cancer Institute (NCI) has a long history demonstrating its interest in the scientific evaluation of complementary [4]. It supports CAM research which includes different methods and practices and other medical systems especially herb medicine [4]. Because of their extensive use and the therapeutic effects, there is an increasing interest and need to develop new methods for understanding of herb medicine, including the identification of active ingredients and their targets in the context of molecular network.
Over the past decade, the use of CAM therapies has been rapidly increasing among patients with cancer throughout the world [5]. A recent population-based survey of San Francisco women with breast cancer showed that 72% used at least one type of alternative modality 2 to 4 months after being diagnosed with breast cancer, especially in women with advanced disease [6]. Over the decades, researchers have developed lots of ways for the treatment of breast cancer, like surgery, hormonal therapy, adjuvant therapy, monoclonal antibodies, enzyme inhibitors and natural product therapy, et al. However, despite advances in screening, surgery, adjuvant radiation, and systemic therapy, as well as novel biologically targeted therapies, limitation still exists in their benefits, especially in advanced disease. Chemotherapy, radiation, endocrine therapy and biological agents may help, but with serious side effect and resistance against anticancer drugs they always result in low survival incidence and poor life quality.
To improve the quality and length of life when patients’ tumors have metastasized, nowadays, lots of researchers have dedicated themselves to searching for proper solutions from the resources of CAM, and indeed big progress has been made. The use of CAM has become increasingly popular in some fields, in which breast cancer is just one case for which herbal supplements are heavily promoted and easily accessible. As a whole medical system of CAM, Traditional Chinese Medicine (TCM) is derived from thousands of years of clinical application that has evolved independently from or parallel to allopathic conventional medicine and has been considered as one of the main items of the CAM [7].
As a matter of fact, TCM normally contains huge numbers of active pharmacological ingredients. Besides, herbal medicines also target several diverse genes or proteins to exert therapeutic effects, which make the investigation of their pharmacological molecular mechanisms extremely difficult. Although the concept of holistic medicine has been revealed to some extent in TCM practice, scientific evidence to illustrate the mechanisms of holistic medicine is severely lacking and many key problems associated still exist. Our previous work has firstly developed an integrated model of systems pharmacology to measure the efficacy of drugs, especially the multi-target drugs, and to reveal the functional mechanism of traditional medicine theories [3]. In recent years, many studies have shown that application of systems biology provides guidance to investigate the scientific connotation of TCM. The reason for this may be that on one hand, systems biology is generally recognized as a powerful tool to study complex life system that resembles the holism concept of channel tropism in TCM [8], and on the other hand, its applications to herbal medicine may provide new possibilities for investigating the explicit targets of medicinal herbs’ active ingredients and their interactions in the context of molecular networks, and thereby highlight the molecular mechanism of the herbs functions.
In this work, we proposed a systems-pharmacological model by combining oral bioavailability prediction, multiple drug-target prediction and validation, and network pharmacology techniques, to shed new lights on the effectiveness and mechanism of breast cancer-related TCMs. Specifically, we firstly explored 20 most frequently-used anti-breast cancer herbs and their corresponding constituents with a wide-scale text mining method. And since construction of an integrated model may be of great help for explaining the functions of herbal medicines and their relationships, we identified all candidate compounds of the herbs and their corresponding potential targets, and then mapped these compounds and targets onto functional ontologies to generate several drug-target networks to help understand the holistic and synergic essence of the herbal medicine from a systematic point of view.
Materials and Methods
As herbs usually contain considerable chemical compounds, databases of each herb were built firstly. After that, a series of combinational integrated approaches were used to screen out active components from the databases, as well as to predict the potential targets. Finally, networks of compound-target and target-pathway were constructed based on the active components and predicted targets.
Herbal medicines for breast cancer
With a text mining approach, we firstly extracted all available information about those herbs related with the treatment of breast cancer via the TCMSP database (Traditional Chinese Medicines Systems Pharmacology Database and Analysis Platform, http://lsp.nwsuaf.edu.cn/tcmspsearch.php). Since the degree of research of diverse herbs is different, presently, to lessen the possible bias of research extents and further evaluate the relationships between the herbs and depression, a parameter, i.e. the ratio of the number of anti-breast-herb-related articles/the number of herb-related articles is calculated. The p-value obtained from Eq 1 is applied to measure the significance of the relevance between each herb and breast cancer as reported literature (significant when p-value ≤ 0.01):.
| (1) |
where N is the total number of articles in PubMed and CNKI, K is the number of articles related to breast cancer, n the number of articles about one single herb and k is the number of articles about the effects of corresponding herbs on breast cancer, respectively [9]. In addition, more empirically based knowledge and TCM experience for the treatment of breast cancer are employed for the selection of herbs. Finally, a total of 20 herbs were collected. All the results are summarized in Table 1.
Table 1. Statistics and association analysis between 20 herbs and breast cancer.
| English Name | Total (n) | Relevant to breast cancer (k) | p-value |
|---|---|---|---|
| Folium Artemisiae Argyi | 52351 | 1114 | p«0.01 |
| Dysosmae Verspiellis Rhixoma Et Radix | 4339 | 499 | p«0.01 |
| Atractylodes Macrocephala Koidz | 365271 | 9673 | p«0.01 |
| Mylabris | 14229 | 2419 | p«0.01 |
| Radix Salviae | 481101 | 13666 | p«0.01 |
| Curcumae Rhizoma | 73256 | 5610 | p«0.01 |
| Stephaniae Tetrandrae Radix | 61204 | 3297 | p«0.01 |
| Curcumaelongae Rhizoma | 81457 | 8266 | p«0.01 |
| Cortex Moutan | 62681 | 2185 | p«0.01 |
| Caulis Akebiae | 61689 | 1042 | p«0.01 |
| Eriobotryae Folium | 18905 | 573 | p«0.01 |
| Pseudobulbus Cremastrae Seu Pleiones | 16264 | 2294 | p«0.01 |
| Crataegi Folium | 7699 | 377 | p«0.01 |
| Cornus Officinalis Sieb. Et Zucc | 68476 | 2209 | p«0.01 |
| Asparagi Radix | 95185 | 11382 | p«0.01 |
| Semiaquilegiae Radix | 3301 | 269 | p«0.01 |
| Artemisiae Scopariae Herba | 89803 | 2121 | p«0.01 |
| Gleditsiae Spina | 20102 | 1125 | p«0.01 |
| Hedyotis diffusa | 62308 | 4719 | p«0.01 |
| Ampelopsis japonica (Thunb.)Makino | 4311 | 131 | p«0.01 |
The total number of articles (N) in PubMed and CNKI is 104544921. The number of articles related to breast cancer (K) is 791592.
Chemical structures'construction
All ingredients of these 20 herbs were collected from (1) literature, (2) Chinese Academy of Sciences Chemistry Database (http://www.organchem.csdb.cn), and (3) Chinese Herbal Drug Database. Finally, a total of 1618 chemicals were collected. And all their chemical structures were either obtained from the Chemical Book (http://www.chemicalbook.com), NCBI PubChem database (http://www.ncbi.nlm.nih.gov/pccompound), or drawn by using ISIS Draw 2.5 (MDL Information Systems, Inc.) and further optimized by Sybyl 6.9 (Tripos Associates, St. Louis, MO). Since for oral-administrated medicine, a deglycosylation of the glucosides will appear in the intestinal tract by enteric bacteria, all compounds with glycosyl are further deglycosylated according to the rule of glycosidase hydrolysis reaction, and the resulted non-overlapping products are obtained and are further optimized according to those procedures described above [10].
Oral bioavailability (OB) prediction
Oral route, the mainly drug delivery system, is commonly used in the administration of herbal medicines. So in the development of herbal drugs intended for oral use, good drug absorption and appropriate drug delivery are very important. OB, the percentage of an oral dose able to produce a pharmacological activity, is one of the most desirable attributes of a new drug. To calculate the OB, we have utilized a novel and robust in-house system OBioavail 1.1 integrating with the metabolism (P450 3A4) and transport (P-glycoprotein) information [11, 12]. Based on P450 3A4 docking score, the set of drug compounds were grouped into four subsets. Then the descriptors of the subsets were calculated by DRAGON professional (version 5.6; Talete SRL: Milano, Italian, 2006). High predictability was achieved by using Support Vector Machine (SVM), with the regression coefficients of internal training and external test data Rtraining = 0.89 and Rtest = 0.85 and the standard errors of estimate SEEtraining = 0.35, SEEtest = 0.42, respectively. We can discard the compounds with poor OB, thus may discover the bioactive compounds or lead compounds in herbal medicine for further development by this software.
The criteria used here are based upon careful consideration of the following: (1) information from the studied herbs can be extracted as much as possible using the least number of chemical ingredients; (2) the obtained model can be reasonably explained by the reported pharmacological data. In this work, compounds with OB ≥ 30% were selected as the candidate bioactive molecules.
Drug-likeness (DL) evaluation
DL is a widely used concept in drug discovery for how “drug-like” a substance is with respect to factors which ultimately influences the absorption, distribution, metabolism, and excretion (ADME) of the substance in human body by affecting its pharmacodynamics and pharmacokinetics [13]. DL parameter between the compound structure and the drug molecular structure obtained from Drug Bank was evaluated by Tanimoto coefficient defined as:
Here, A represents the new compound, and B represents the average drug-likeness index of all 6511 molecules in DrugBank database (access time: June 1st, 2011, http://www.drugbank.ca/). In this work, compounds with DL ≥ 0.18 were selected as the candidate bioactive molecules, due to that the average value of DL for all 6511 molecules in Drug Bank is 0.18. Only those ingredients who meet both the OB and DL screening criteria are finally treated as candidate compounds.
Target prediction and validation
Previously in our work, we have predicted the potential targets of human and viral origins based on a systematic model [14], which efficiently integrated the chemical, genomic and pharmacological information for drug targeting and discovery on a large scale based on two powerful methods of Random Forest (RF) and Support Vector Machine (SVM). The sensitivity (SE) of the present models describes the ratio of correctly predicted interactions to the total number of the drug-target interactions, whereas the specificity (SP) refers to the ratio of correctly predicted non-interactions to the total number of the drug-target non-interactions. The integrated parameter concordance (the ratio of correctly predicted compound-protein pairs to the total number of tested compound-protein pairs, CO) gives an overall model performance value. This model shows an impressive performance of prediction for drug-target interaction, with a concordance of 86%, a sensitivity of 80% and a specificity of 93%, respectively. According to the possibilities of compounds interacting with candidate targets in the RF and SVM models, respectively, The proteins with the value predicted by both methods of RF and SVM greater than 0.8 and 0.7 respectively were chosen as potential targets (As displayed in the S1 Table).
Network construction
The analysis of networks has gained extensive attentions in biological and even TCM fields, due to the recent explosion of publicly available high-throughput biological data. Such analysis also provides a unifying language to describe the relations within complex systems and to understand the physiological functions. To characterize the multicomponent therapeutic mechanisms of herbal medicine in the treatment of breast cancer from a network target perspective, we constructed two kinds of visualized networks: compound-target network (C-T network) and target-pathway network (T-P network), to get insight into new possibilities for understanding of the holistic, complementary and synergic essence of herbal medicine.
C-T network
All active substances in both the herbs and their targets were used to generate a bipartite graph of drug-protein interactions. In this graph, a drug and a protein were connected to each other when the protein was a target of the drug, and thus a C-T network was built. Not all compounds have relatively potential target. Since presently 20 compounds have no potential target, they were removed from the nets accordingly.
T-P network
The information about selected pathways was initially collected by the KEGG (Kyoto Encyclopedia of Genes and Genomes), and then the T-P network was built by connecting the targets and their related signaling pathways. Targets and their related signaling pathways were represented as nodes and their interactions as links, respectively, and all visualized networks were generated by Cytoscape 2.8.3, a standard tool for biological network visualization and data integration [15]. Finally, the quantitative properties of these networks were analyzed by two plugins including Network Analyzer.
Results and Discussion
During the past few years, the pharmaceutical industry has seen a shift from the “1 disease, 1 target, 1 drug” and “1drug fits all” approaches to the pursuit of combination therapies that include more than one active ingredient [16]. It is believed that multiple components could hit multiple targets and exert synergistic therapeutic efficacies. In TCM, as one of the most important theories, “multiple herbal drugs for one disease” describes how different drugs treat the same disease, which implies that the drugs probably share common targets. The complexity of the chemical components makes it extremely difficult to understand the mechanisms of action for herbal medicines from a molecular or systems level. In the present study, novel systems pharmacology approach and network biology were applied to uncover the mechanism of the TCM rule from a molecular systematic level, in hope that it may also provide useful information for finding novel anti-breast-cancer leading compounds.
Physicochemical property analysis
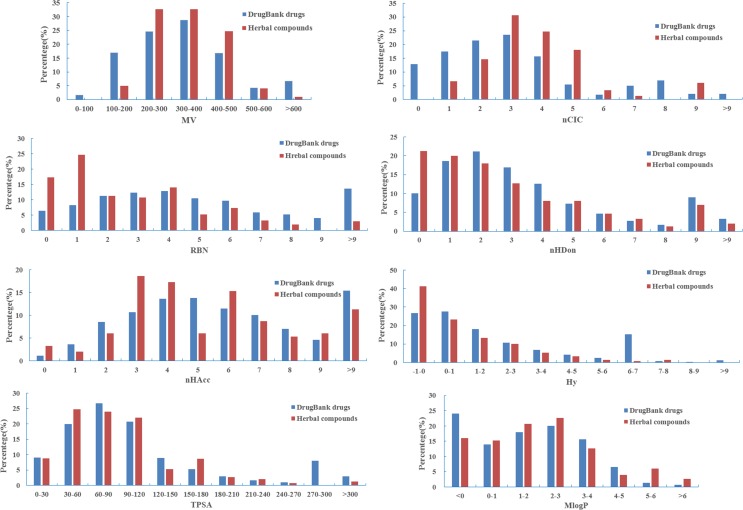
In order to analyze statistically the drug-like physicochemical properties of active compounds, herbal ingredient comparisons based on chemical properties are performed by considering 8 common molecular descriptors, which includes MW (molecular weight), nCIC (number of rings), nHDon (number of hydrogen-bond donor), nHAcc (number of hydrogen-bond acceptor), RBN (number of rotatable bonds), Hy (hydrophilic factor), TPSA (topological polar surface area) and MlogP (Moriguchi octanol-water partition coefficient), since these parameters reflect the basic characteristics of molecules including especially their pharmacokinetic properties [17]. In addition, these 8 molecular descriptors of all compounds from DrugBank are also calculated for a comparison to analyze the similarities or differences with our herbal ingredients in the property spaces. All molecular descriptors are calculated by DRAGON software in the present study.
Fig 1 depicts the distributions of selected properties among the herbal compounds and DrugBank drugs. The average values of these properties (mean and Standard Deviation) for these drugs in DrugBank as well as herbal compounds are displayed in Table 2. As shown in Fig 1, the molecular weight distribution for DrugBank drugs follows a Gaussian distribution, and the distribution characteristics are similar to those in the herbal compounds. In addition, herbal compounds exhibit an obvious narrower distribution than DrugBank from MW, nCIC and Hy descriptors, indicating that herbal compounds are less diverse than those of DrugBank drugs [18]. The calculated average values for molecular weight (see Table 2 and S2 Table) reflect the observations above. Because of the wider distribution in DrugBank drugs, the mean of molecular weight is higher for these compounds. In addition, the MlogP of herbal compounds (1.87±2.08) are larger than the ones of those DrugBank drugs (1.33±2.50), indicating that the molecules in herbal are more soluble in neutral solvents and much more hydrophobic than the DrugBank drugs. The nCIC, as a measurement for the rigidity of molecules, the average nCIC per molecule in herbal compounds (3.52±1.37) is larger than the DrugBank drugs (2.46±1.72). However, the average RBN per molecule of the herbal compounds (2.86±2.67) is lower than those of Drug-Bank ones (5.58±5.88). Thus, compounds in herbals probably have less thermodynamic advantages to achieve favorable binding properties than those in the DrugBank. Moreover, the nHDon and nHAcc of herbal compounds (2.57±2.47 and 5.63±3.50) has the lower donor/acceptor atoms for H-bonds than those (3.17±3.50 and 6.46±5.59) of DrugBank ones. Furthermore, the herbals compounds (90.15±57.95) have the lower average TPSA values than that of DrugBank compounds (99.93±90.43). All statistical analysis indicates two out of all eight descriptors between DrugBank drugs and herbal compounds are significantly different with p <0.01, including nCIC (2.46±1.72 versus 3.52±1.37) and RBN (5.58±5.88 versus 2.86±2.67).
Fig 1. The profile distributions of eight important molecular properties for DrugBank drugs and herbal compounds.
Table 2. Comparison of molecular properties between herbal compounds and DrugBank drugs.
| Index | MW (±SD) | nCIC (±SD) | RBN (±SD) | nHDon (±SD) | NHAcc (±SD) | Hy (±SD) | TPSA(Tot) (±SD | MlogP (±SD) |
|---|---|---|---|---|---|---|---|---|
| DrugBank | 346.57 (208.89) | 2.46 (1.72) | 5.58 (5.88) | 3.17 (3.50) | 6.46 (5.59) | 1.47 (2.76) | 99.93 (90.43) | 1.33 (2.50) |
| Herbal | 344.15 (113.89) | 3.52 (1.37)** | 2.86 (2.67)** | 2.57 (2.47)* | 5.63 (3.50) | 0.83 (1.80) | 90.15 (57.95) | 1.87 (2.08)* |
*p < 0.05
**p < 0.01.
OB prediction and DL calculation
For multi-compound medicinal herbs, it is usually quite difficult to distinguish those active ingredients from the compound pool of the TCMs due to the difficulties in current experimental methods, when virtual screening approaches are always of great help. In present work, a robust in-house system OBioavail 1.1 we developed previously was integrated with the metabolism information to predict a compound’s human oral bioavailability [19]. In addition, the drug-likeness property based on the Tanimoto coefficient was also applied to screen out those compounds which are deemed active in an herb. The model was built based on a set of 805 structurally diverse drug and drug-like molecules which have been critically evaluated for their human oral bioavailability. The multiple linear regression, partial least square and SVM methods were employed to build the models, resulting in an optimal model with R2 = 0.80, SEE = 0.31 for the training set, Q2 = 0.72, SEP = 0.22, respectively. At last, 150 candidate compounds were screened from the 1618 chemicals which possess not only satisfactory drug-likeness (DL>0.18), but also OB (>30%). As a result, a total of 150 bioactive ingredients of the 20 herbs are obtained (shown in Table 3). Here, in order to illuminate clearly, three representative herbal medicines are specified in detail to interpret these filtering principles.
Table 3. 150 bioactive compounds of the 20 herbs with their predicted OB and DL values.
| ID | Compound | OB | DL | Medicine |
| AY01 | B-sitosterol | 36.91 | 0.75 | Folium Artemisiae Argyi |
| AY02 | Quercetin | 3.15 | 0.28 | Folium Artemisiae Argyi |
| AY03 | Luteolin | 13.36 | 0.25 | Folium Artemisiae Argyi |
| AY04 | Apigenin | 46.23 | 0.34 | Folium Artemisiae Argyi |
| AY05 | Jaceosidin | 17.26 | 0.38 | Folium Artemisiae Argyi |
| AY06 | Eupatilin | 18.44 | 0.45 | Folium Artemisiae Argyi |
| BJ01 | Kaempferol | 41.88 | 0.24 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ02 | Quercitrin | 4.04 | 0.74 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ03 | Beta-sitosterol | 36.91 | 0.75 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ04 | 4'-demethylpodophyllotoxin | 27.05 | 0.82 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ05 | Deoxypodophyllotoxin | 37.75 | 0.83 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ06 | Picropodophyllin | 51.77 | 0.86 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ07 | Podophyllotoxin | 59.94 | 0.86 | Dysosmae Verspiellis Rhixoma Et Radix |
| BJ08 | Podophyllotoxone | 49.61 | 0.86 | Dysosmae Verspiellis Rhixoma Et Radix |
| BZ01 | Atractylenolide I | 37.37 | 0.15 | Atractylodes Macrocephala Koidz |
| BZ02 | AtractylenolideII | 47.5 | 0.15 | Atractylodes Macrocephala Koidz |
| BZ03 | Atractylenolide III | 68.11 | 0.18 | Atractylodes Macrocephala Koidz |
| BZ04 | 8β-ethoxy atractylenolide III | 35.95 | 0.21 | Atractylodes Macrocephala Koidz |
| BZ05 | 3β-acetoxyatractylone | 54.07 | 0.22 | Atractylodes Macrocephala Koidz |
| BM01 | Norcantharidin | 97.71 | 0.07 | Mylabris |
| BM02 | Cantharidin | 51.23 | 0.1 | Mylabris |
| DS01 | Digallate | 61.85 | 0.26 | Radix Salviae |
| DS02 | Dehydrotanshinone II A | 43.76 | 0.4 | Radix Salviae |
| DS03 | 2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxypropyl)-7-methoxy-3-benzofurancarboxaldehyde | 62.78 | 0.4 | Radix Salviae |
| DS04 | Formyltanshinone | 73.44 | 0.42 | Radix Salviae |
| DS05 | Methylene tanshinquinone | 37.07 | 0.36 | Radix Salviae |
| DS06 | Przewalskin B | 110.32 | 0.44 | Radix Salviae |
| DS07 | Przewaquinone B | 62.24 | 0.41 | Radix Salviae |
| DS08 | Przewaquinone C | 55.74 | 0.4 | Radix Salviae |
| DS09 | Tanshinaldehyde | 52.47 | 0.45 | Radix Salviae |
| DS10 | Danshenol B | 57.95 | 0.56 | Radix Salviae |
| DS11 | Danshenol A | 56.97 | 0.52 | Radix Salviae |
| ID | Compound | OB | DL | Medicine |
| DS12 | Cryptotanshinone | 52.34 | 0.4 | Radix Salviae |
| DS13 | Danshenspiroketallactone | 50.43 | 0.31 | Radix Salviae |
| DS14 | Deoxyneocryptotanshinone | 49.4 | 0.29 | Radix Salviae |
| DS15 | Dihydroisotanshinone I | 20.91 | 0.36 | Radix Salviae |
| DS16 | Dihydrotanshinone I | 45.04 | 0.36 | Radix Salviae |
| DS17 | Epidanshenspiroketallactone | 68.27 | 0.31 | Radix Salviae |
| DS18 | Isocryptotanshi-none | 54.98 | 0.39 | Radix Salviae |
| DS19 | Isotanshinone IIB | 21.07 | 0.45 | Radix Salviae |
| DS20 | Isotanshinone II | 49.92 | 0.4 | Radix Salviae |
| DS21 | Isotanshinone I | 29.72 | 0.36 | Radix Salviae |
| DS22 | Miltionone II | 71.03 | 0.44 | Radix Salviae |
| DS23 | Neocryptotanshinone II | 39.46 | 0.23 | Radix Salviae |
| DS24 | Neocryptotanshinone | 52.49 | 0.32 | Radix Salviae |
| DS25 | Prolithospermic acid | 64.37 | 0.31 | Radix Salviae |
| DS26 | (2R)-3-(3,4-dihydroxyphenyl)-2-[(Z)-3-(3,4-dihydroxyphenyl)acryloyl]oxy-propionic acid | 109.38 | 0.35 | Radix Salviae |
| DS27 | (Z)-3-[2-[(E)-2-(3,4-dihydroxyphenyl)vinyl]-3,4-dihydroxy-phenyl]acrylic acid | 88.54 | 0.26 | Radix Salviae |
| DS28 | (6S)-6-hydroxy-1-methyl-6-methylol-8,9-dihydro-7H-naphtho[8,7-g]benzofuran-10,11-quinone | 75.39 | 0.46 | Radix Salviae |
| DS29 | Tanshinone IIA | 49.89 | 0.4 | Radix Salviae |
| DS30 | (6S)-6-(hydroxymethyl)-1,6-dimethyl-8,9-dihydro-7H-naphtho[8,7-g]benzofuran-10,11-dione | 65.26 | 0.45 | Radix Salviae |
| DS31 | Tanshinone VI | 45.64 | 0.3 | Radix Salviae |
| DS32 | Tanshinone I | 29.27 | 0.36 | Radix Salviae |
| WZ01 | γ-elemene | 23.79 | 0.06 | Curcumae Rhizoma |
| WZ02 | Beta-elemene | 25.63 | 0.06 | Curcumae Rhizoma |
| WZ03 | δ-elemene | 21.47 | 0.06 | Curcumae Rhizoma |
| WZ04 | (1R,10R)-epoxy-1,10-dihydrocurdione | 36.73 | 0.12 | Curcumae Rhizoma |
| WZ05 | Isocurcumenol | 97.67 | 0.13 | Curcumae Rhizoma |
| WZ06 | Bisdemethoxycurcumin | 77.38 | 0.26 | Curcumae Rhizoma |
| WZ07 | Curcumenol | 96.72 | 0.08 | Curcumae Rhizoma |
| WZ08 | Curdione | 35.05 | 0.11 | Curcumae Rhizoma |
| No. | Compound | OB | DL | Medicine |
| WZ09 | Curzerenone | 52.68 | 0.13 | Curcumae Rhizoma |
| WZ10 | Isocurcumol | 101.1 | 0.08 | Curcumae Rhizoma |
| FJ01 | Tetrandrine | 26.64 | 0.1 | Stephaniae Tetrandrae Radix |
| FJ02 | Hesperetin | 70.31 | 0.27 | Stephaniae Tetrandrae Radix |
| JH01 | (+)-alpha-curcumene | 26.56 | 0.06 | Curcumae longae Rhizoma |
| JH02 | Alpha-curcumene | 4.68 | 0.06 | Curcumae longae Rhizoma |
| JH03 | Procurcumadiol | 69.82 | 0.13 | Curcumae longae Rhizoma |
| JH04 | Curcumol | 103.55 | 0.13 | Curcumae longae Rhizoma |
| JH05 | Bisdemethoxycurcumin | 3.55 | 0.26 | Curcumae longae Rhizoma |
| JH06 | Demethoxycurcumin | 4.37 | 0.33 | Curcumae longae Rhizoma |
| JH07 | Dihydrocurcumin | 5.91 | 0.41 | Curcumae longae Rhizoma |
| JH08 | Curcumin | 4.37 | 0.04 | Curcumae longae Rhizoma |
| MD01 | Gallate acid | 25.01 | 0.79 | Cortex Moutan |
| MD02 | Paeoniflorin | 19.92 | 0.78 | Cortex Moutan |
| MD03 | Mairin | 55.38 | 0.78 | Cortex Moutan |
| MD04 | (+)-catechin | 54.83 | 0.24 | Cortex Moutan |
| MD05 | Paeonol | 28.79 | 0.04 | Cortex Moutan |
| MD06 | Paeoniflorin_qt | 68.18 | 0.4 | Cortex Moutan |
| MD07 | Oxypaeoniflorin_qt | 19.4 | 0.44 | Cortex Moutan |
| MD08 | Benzoyl paeoniflorin | 31.14 | 0.54 | Cortex Moutan |
| MD09 | Oxypaeoniflorin | 12.98 | 0.78 | Cortex Moutan |
| MD10 | 4-O-methylpaeoniflorin_qt | 67.24 | 0.43 | Cortex Moutan |
| MD11 | Paeonidanin_qt | 65.31 | 0.35 | Cortex Moutan |
| MT01 | Ariskanina | 109.51 | 0.4 | Caulis Akebiae |
| MT02 | Aristolochic acid | 62.71 | 0.55 | Caulis Akebiae |
| MT03 | Hederagenin | 36.91 | 0.75 | Caulis Akebiae |
| MT04 | Oleanolic acid | 15.32 | 0.74 | Caulis Akebiae |
| SZ01 | Quercetin | 46.43 | 0.28 | Crataegi Folium |
| SZ02 | Rutin | 3.2 | 0.68 | Crataegi Folium |
| SZ03 | Vitexin | 3.05 | 0.71 | Crataegi Folium |
| SZ04 | Hyperin | 6.94 | 0.77 | Crataegi Folium |
| SZ05 | Vitexin-2-o-rhamnoside | 6.98 | 0.8 | Crataegi Folium |
| PP01 | Corosolic acid | 14.97 | 0.76 | Eriobotryae Folium |
| PP02 | Oleanolic acid | 29.02 | 0.76 | Eriobotryae Folium |
| PP03 | Ursolic acid | 16.77 | 0.75 | Eriobotryae Folium |
| PP04 | Maslinic acid | 15.54 | 0.74 | Eriobotryae Folium |
| No. | Compound | OB | DL | Medicine |
| PP05 | (2R,3R,10S)-2,10-bis(3,4-dihydroxyphenyl)-3,5-dihydroxy-3,4,9,10-tetrahydro-2H-pyrano[6,5-h]chromen-8-one | 65.26 | 0.93 | Eriobotryae Folium |
| PP06 | (4R,8R,9R)-4,8-bis(3,4-dihydroxyphenyl)-5,9-dihydroxy-4,8,9,10-tetrahydro-3H-pyrano[6,5-h]chromen-2-one | 58.16 | 0.93 | Eriobotryae Folium |
| PP07 | (2R,3R,4S)-2-(3,4-dihydroxyphenyl)-4-(2,4,6-trihydroxyphenyl)chroman-3,5,7-triol | 72.41 | 0.64 | Eriobotryae Folium |
| SC01 | Cirrhopetalanthrin | 24.68 | 0.93 | Pseudobulbus Cremastrae Seu Pleiones |
| SC02 | Shanciol | 5.44 | 0.92 | Pseudobulbus Cremastrae Seu Pleiones |
| SC03 | Shanciol E | 15.62 | 0.92 | Pseudobulbus Cremastrae Seu Pleiones |
| SC04 | Shanciol F | 17.49 | 0.11 | Pseudobulbus Cremastrae Seu Pleiones |
| SC05 | Cremastrine | 17.3 | 0.59 | Pseudobulbus Cremastrae Seu Pleiones |
| SC06 | Sanjidin A | 3.82 | 0.59 | Pseudobulbus Cremastrae Seu Pleiones |
| SC07 | Sanjidin B | 3.82 | 0.53 | Pseudobulbus Cremastrae Seu Pleiones |
| SC08 | Pleionin A | 35.68 | 0.65 | Pseudobulbus Cremastrae Seu Pleiones |
| SC09 | Pleionol | 41.98 | 0.04 | Pseudobulbus Cremastrae Seu Pleiones |
| SY01 | Gallic acid | 25.01 | 0.44 | Cornus Officinalis Sieb. Et Zucc |
| SY02 | Loganin | 16.8 | 0.5 | Cornus Officinalis Sieb. Et Zucc |
| SY03 | Morroniside | 13.86 | 0.04 | Cornus Officinalis Sieb. Et Zucc |
| SY04 | Protocatechuic acid | 25.47 | 0.09 | Cornus Officinalis Sieb. Et Zucc |
| SY05 | Swertiamarin_qt | 2.58 | 0.09 | Cornus Officinalis Sieb. Et Zucc |
| SY06 | Swertiamarin | 21.9 | 0.42 | Cornus Officinalis Sieb. Et Zucc |
| SY07 | Malkangunin | 57.71 | 0.63 | Cornus Officinalis Sieb. Et Zucc |
| SY08 | Telocinobufagin | 69.99 | 0.79 | Cornus Officinalis Sieb. Et Zucc |
| SY09 | Cornuside | 2.61 | 0.71 | Cornus Officinalis Sieb. Et Zucc |
| SY10 | Cornuside_qt | 2.37 | 0.39 | Cornus Officinalis Sieb. Et Zucc |
| SY11 | Gemin D | 68.83 | 0.56 | Cornus Officinalis Sieb. Et Zucc |
| TD01 | Asparaside A | 9.48 | 0.02 | Asparagi Radix |
| TD02 | Aspafilioside A_qt | 17.66 | 0.81 | Asparagi Radix |
| TD03 | Diosgenin | 80.88 | 0.81 | Asparagi Radix |
| TD04 | Asparaside a_qt | 30.6 | 0.86 | Asparagi Radix |
| TK01 | Griffonilide | 43.15 | 0.05 | Semiaquilegiae Radix |
| TK02 | Berberrubine | 35.74 | 0.73 | Semiaquilegiae Radix |
| TK03 | Thalifendine | 44.41 | 0.73 | Semiaquilegiae Radix |
| No. | Compound | OB | DL | Medicine |
| YC01 | Capillarin | 87.01 | 0.08 | Artemisiae Scopariae Herba |
| YC02 | Artepillin A | 68.32 | 0.24 | Artemisiae Scopariae Herba |
| YC03 | Chlorogenic acid | 13.99 | 0.41 | Artemisiae Scopariae Herba |
| YC04 | Areapillin | 48.96 | 0.41 | Artemisiae Scopariae Herba |
| YC05 | Isoarcapillin | 57.4 | 0.41 | Artemisiae Scopariae Herba |
| YC06 | Capillarisin | 57.56 | 0.31 | Artemisiae Scopariae Herba |
| YC07 | 4'-methylcapillarisin | 72.18 | 0.35 | Artemisiae Scopariae Herba |
| YC08 | Demethoxycapillarisin | 52.33 | 0.25 | Artemisiae Scopariae Herba |
| ZJ01 | Quercetin | 46.43 | 0.28 | Gleditsiae Spina |
| ZJ02 | (-)-taxifolin | 60.51 | 0.27 | Gleditsiae Spina |
| ZJ03 | Fisetin | 52.6 | 0.24 | Gleditsiae Spina |
| ZJ04 | Fustin | 50.91 | 0.24 | Gleditsiae Spina |
| ZJ05 | 3β-acetoxyolean-12-en-28-oic acid | 40.21 | 0.4 | Gleditsiae Spina |
| BH01 | Deacetyl asperulosidic acid | 3.24 | 0.45 | Hedyotis Diffusa |
| BH02 | Deacetyl asperulosidic acid _qt | 30.29 | 0.1 | Hedyotis Diffusa |
| BH03 | Deacetyl asperulosidic acid methyl ester | 4.29 | 0.48 | Hedyotis Diffusa |
| BH04 | Deacetyl asperuloside acid_qt | 62.46 | 0.11 | Hedyotis Diffusa |
| BL01 | Cis-resveratrol | 41.13 | 0.11 | Ampelopsis Japonica (Thunb.) Makino |
| BL02 | Oleanolic acid | 29.02 | 0.76 | Ampelopsis Japonica (Thunb.) Makino |
| BL03 | (2R,3R,4S)-4-(4-hydroxy-3-methoxy-phenyl)-7-methoxy-2,3-dimethylol-tetralin-6-ol | 66.51 | 0.39 | Ampelopsis Japonica (Thunb.) Makino |
| BL04 | (+)-catechin | 54.83 | 0.24 | Ampelopsis Japonica (Thunb.) Makino |
| BL05 | Digallate | 61.85 | 0.26 | Ampelopsis Japonica (Thunb.) Makino |
| BL06 | (-)-catechin gallate | 53.57 | 0.75 | Ampelopsis Japonica (Thunb.) Makino |
Radix salvia
Radix Salviae, derived from the dried root or rhizome of Salvia miltiorrhiza Bge (Chinese Pharmacopoeia Committee, 2005), is one of the oldest and most frequently used TCMs. It was recorded in The China Pharmacopoeia with the Chinese name Danshen and has been widely used for the treatment of various kinds of diseases [20]. Various Fufang Danshen prescriptions are commercially available, such as Fufang Danshen tablet, Guanxin Danshen tablet, Compound Danshen dripping pill and Danqi tablet [21]. Pharmacological research has revealed that these preparations have the effects of activating blood circulation, dilating coronary artery and antagonizing myocardial ischemia, and potent therapeutic effects have been demonstrated for treating coronary heart disease, cardiac angina and atherosclerosis in the clinic [22]. It also has been widely used for the treatment of menstrual disorders, menorrhealgia, insomnia, menostasis, blood circulation diseases, and other cardiovascular diseases [22].
The major bioactive constituents of Radix Salviae can be classified into hydrophilic depsides derived from caffeic acid such as salvianolic acids and lipophilic components including diterpenoids tanshinones [19]. In this work, 174 compounds of various types have been identified in Radix Salviae, out of which 28 molecules demonstrate good OB and DL. It is interesting that among all the 28 compounds, some were already reported to be pharmacologically effective for the treatment of various symptoms. For instance, tanshinone IIA has been confirmed to have anti-inflammatory, anti-ischemic, antioxidant, and antitumor activities [23]. Tanshinone IIA not only has a powerful inhibitory effect on the proliferation of ER-positive human breast cells in vitro, but also significantly inhibits the growth of human ER-negative breast in vivo, without any untoward toxicity. The anticancer activity of tanshinone IIA could be attributed in part to its inhibition of proliferation and apoptosis induction of cancer cells through cell proliferation, apoptosis, signal transduction, transcriptional regulation, angiogenesis, invasive potential and metastatic potential of cancer cells [24].
Besides the above 28 molecules, 4 compounds, though with low OB or DL values, are also deemed as active components due to their reported therapeutical effects, like tanshinone I that actually significantly induced the apoptosis in MCF-7 and MDA-MB-231 human breast cancer cells. Otherwise, the activation of caspase 3, anti-apoptotic protein Bcl-2, pro-apoptotic protein Bax have been reported to mediate the induction of apoptotic cell death [25]. Owing to the profound pharmacological effects, these compounds are also selected for further research as well. In brief, 32 ingredients from Radix Salviae are assumed playing major roles in the treatment of breast cancer, and thus chosen as active compounds for further study.
Atractylodes macrocephala koidz
Atractylodes Macrocephala Koidz (Bai-Zhu), as a valuable traditional Chinese medicine, is an important component of several Chinese herbal prescriptions and has been used for treating various diseases for thousands of years all over the world duo to its special pharmacological activities [23]. Atractylodes Macrocephala Koidz, a member of the Compositae can invigorate the spleen, and cure patients with splenic asthenia, anorexia, oedema, excessive perspiration and abnormal fetal movement. The rhizomes of Atractylodes Macrocephala Koidz have also shown a variety of other pharmacological activities such anti-inflammatory, antioxidant and as antitumor [26].
The chemical investigations on the rhizomes of Atractylodes Macrocephala Koidz have demonstrated the anti-tumor and anti-inflammatory activities of the main active constituents of the herb [27]. In these ingredients, eudesmane-type sesquiterpenoids, such as atractylenolides I, II, III, etc. are the major ones which exhibit the activity of gastro protective, anti-inflammatory and anti-tumor properties [28, 29]. Notably, atractylenolides I could reduce the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 [30]. In addition, at a concentration of 10 μM, atractylenolide I, atractylenolide II and atractylenolide III also had inhibition ratios of 94.56 ± 0.70%, 90.93 ± 1.41% and 86.31 ± 8.46%, respectively, which indicates that atractylenolide and its derivatives may serve as potential aromatase inhibitors for the treatment of breast cancer [27].
Hedyotis diffusa
Hedyotis diffusa, belonging to the Rubiaceae family, is a medicinal herb widely distributed in Northeast Asia. As a well-known traditional Chinese folk-medicine, Hedyotis diffusa has long been used as a major component in many TCM formulas for the clinical treatment of hepatitis, tonsillitis, sore throat, appendicitis, urethral infection, and malignant tumors of the liver, lung, and stomach [31]. It is reported to possess various pharmacological activities such as anti-cancerous, anti-oxidative, anti-inflammatory, hepato-protective, and neuroprotective activities [32]. Hedyotis diffusa has also been used for treating many types of cancer and tumor in Taiwan and China for a long time [32]. Hedyotis diffusa has been demonstrated significant anti-proliferative effects and apoptotic responses by inducing p53 gene expression in ERα-positive MCF-7 breast cancer cells. Furthermore, Hedyotis diffusa inhibits anchorage-dependent and-independent cell growth in ERα-positive breast cancer cells in a dose-related manner [33].
In this study, 4 components from Hedyotis diffusa which include deacetyl asperulosidic acid and its derivatives demonstrate good bioavailability, and thus were chosen as candidate components. It is reported that asperulosidic acid has numerous pharmacological activities including anti-inflammatory, anticancer and hepatoprotective effects [34]. They have an inhibitory effect on tumor cell proliferation through cell cycle arrest [35]. The mechanism of anti-tumor activity is that they have effects on the inhibition of cell proliferation and stimulation of cell apoptosis in human cancer cells [34]. In addition, deacetyl asperulosidic acid methyl ester and deacetyl asperulosidic acid have the activity of analgesia which reduces the risk of cancer recurrence [36, 37].
To sum it up, 150 ingredients of all these 20 herbs are finally screened and regarded as candidate compound.
Drug targeting and validating for the therapy of breast cancer
Identification of the protein targets of a bioactive small molecule is the most important step in new drug development because small molecules typically exert their bioactive effects through interactions with proteins. The interactions between the small molecule and its target protein are the key to understanding the small molecule’s cellular mechanism. Traditionally, expressed-sequence tags (ESTs), serial analysis of gene expression (SAGE), homology cloning and relevant approaches are adopted to confirm the therapeutic targets of active chemicals [38]. However, given the time-consuming, expensive, challenging feature and a narrow application scope of this process, computational methods always become the first choice. In this section, in order to probe the binding of Chinese herbs to their targets in breast cancer, we developed a simple, robust, unbiased and universally applicable target identification approach on the basis of the RF and SVM techniques. It combines the chemical, genomic and pharmacological information for drug targeting and discovery on a large scale.
As mentioned above, 150 compounds were screened out. However, the way how they interact with their specific acting targets still remains unknown. Based on the application of RF and SVM, a total of 83 proteins were identified as targets to interact with those candidate compounds of the 20 herbs. Among these, finally 33 targets are chosen which are highly associated with breast cancer, such as hormonal receptors, inflammatory mediators. Further analysis about the connections between the drugs and these targets are referred in network construction and analysis. The detailed information of the 33 target proteins is described in Table 4.
Table 4. The information of breast cancer targets.
| Protein Name | Gene Name | UniProt ID |
|---|---|---|
| Muscarinic acetylcholine receptor M4 | CHRM4 | P08173 |
| Muscarinic acetylcholine receptor M5 | CHRM5 | P08912 |
| Alpha-1A adrenergic receptor | ADRA1A | P35348 |
| Alpha-1B adrenergic receptor | ADRA1B | P35368 |
| Calcium-activated potassium channel subunit alpha-1 | KCNMA1 | Q12791 |
| Mu-type opioid receptor | OPRM1 | P35372 |
| Progesterone receptor | PGR | P06401 |
| Muscarinic acetylcholine receptor M3 | CHRM3 | P20309 |
| Muscarinic acetylcholine receptor M2 | CHRM2 | P08172 |
| Muscarinic acetylcholine receptor M1 | CHRM1 | P11229 |
| Retinoic acid receptor RXR-alpha | RXRA | P19793 |
| DNA topoisomerase 2-alpha | TOP2A | P11388 |
| Carbonic anhydrase 2 | CA2 | P00918 |
| Prostaglandin G/H synthase 1 | PTGS1 | P23219 |
| Prostaglandin G/H synthase 2 | PTGS2 | P35354 |
| Androgen receptor | AR | P10275 |
| Nitric oxide synthase, inducible | NOS2 | P35228 |
| Nitric oxide synthase, endothelial | NOS3 | P29474 |
| Estrogen receptor | ESR1 | P03372 |
| Estrogen receptor beta | ESR2 | Q92731 |
| Serine/threonine-protein kinase Chk1 | CHEK1 | O14757 |
| Serine/threonine-protein kinase pim-1 | PIM1 | P11309 |
| Alpha-1D adrenergic receptor | ADRA1D | P25100 |
| Alpha-2A adrenergic receptor | ADRA2A | P08913 |
| Alpha-2B adrenergic receptor | ADRA2B | P18089 |
| Alpha-2C adrenergic receptor | ADRA2C | P18825 |
| Delta-type opioid receptor | OPRD1 | P41143 |
| Coagulation factor X | F10 | P00742 |
| Glucocorticoid receptor | NR3C1 | P04150 |
| Vascular endothelial growth factor receptor 2 | KDR | P35968 |
| Sodium-dependent dopamine transporter | SLC6A3 | Q01959 |
| Sodium-dependent noradrenaline transporter | SLC6A2 | P23975 |
| Sodium-dependent serotonin transporter | SLC6A4 | P31645 |
GOBP enrichment analysis for targets
For further exploring the functional annotation of our 33 potential targets of active compounds, Gene Ontology (GO) enrichment analysis was performed by linking the targets to DAVID (The Database for Annotation, Visualization and Integrated Discovery, http://david.abcc.ncifcrf.gov) bioinformatics resources to systematically analyzing their biological process. One of the three broad GO categories (the other two being “Molecular Function” and “Cellular Component”) Biological Process” (GOBP) was utilized to symbol gene function. Only GO terms with p-value ≤ 0.05 were selected.
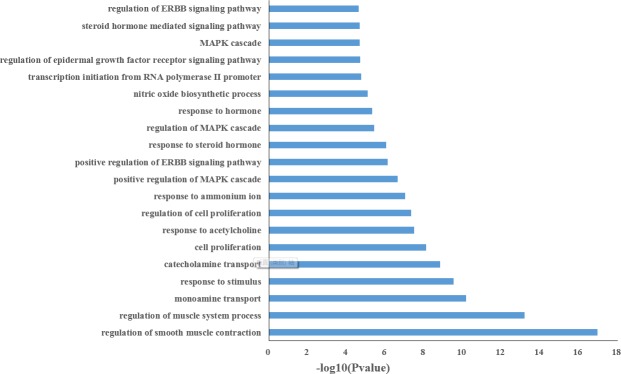
Fig 2 and S3 Table list the top 20 significantly enriched GO terms, the results show that the majority of 33 targets are strongly associated with various biological processes by cluster analysis. As shown in Fig 2, it is interesting to note that a larger number of targets involve in inflammatory and hormones associated with a variety of biological processes such as regulation of MAPK cascade, response to steroid hormone, steroid hormone mediated signaling pathway, cell proliferation, which are closely related to the pathogenesis of breast cancer. For example, a hormone receptor estrogen is widely recognized for its role in breast cancer and the presence of estrogen receptor is of outstanding importance for treating breast cancer [39]. In addition, androgen receptor, another hormone receptor, it is a predictive factor for response to endocrine treatment in patients with breast cancer [40]. These results suggest that the active compounds of herbal medicines might exert the therapeutic effects on breast cancer through regulating the biological processes of their protein targets.
Fig 2. Gene Ontology (GO) analysis of therapy target genes.
The y-axis shows significantly enriched ‘Biological Process ‘ (BP) categories in GO relative to the target genes, and the x-axis depicts the enrichment scores of these terms (p-value≤0.05).
Network construction and analysis
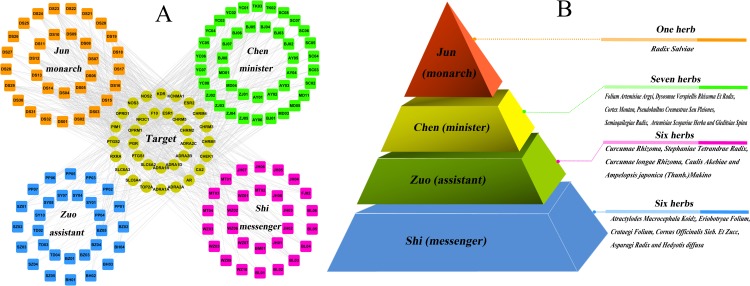
With the growing understanding of the breast cancer, the focus of analyzing its treatment has shifted from the well-accepted ‘one target, one drug’ model design toward a new ‘multi-target, multi-drug’ model aimed at systemically modulating multiple targets in body. In the following part, the network pharmacology approaches are applied in the investigations. Based on these outcomes and to uncover the synergistic effects, our aim is to assess the mechanism and relationship between breast cancer and these targets by using compound-target (C-T) network analysis. As mentioned above, 33 predicted targets are screened out, which are targeted by 150 candidate components. After deleting all those compounds (altogether 20) with no targets, the resultant 130 candidate compounds and all their candidate targets are used to generate a graph of C-T interactions. As illustrated in Fig 3, the C-T network is constructed by all the active ingredients of 20 herbs. The squares and circles represent the potential compounds and targets, respectively.
Fig 3. The global view of C-T network for the 20 breast cancer-related herbs.
(A) 32 bioactive compounds (orange squares) from Radix Salviae applied as monarch herbal medicine play principal roles in therapeutic effect. 41 bioactive compounds (green squares) from 7 herbs represent those minister herbal medicine which increase the effects of Radix Salviae. 57 bioactive compounds (magenta and blue squares) from 12 herbs serve as assistant and messenger drugs, respectively. The yellow circles represent the target proteins of the active compounds. (B) The combination principle of Jun-Chen-Zuo-Shi.
The reasonability of the combination rule of Jun-Chen-Zuo-Shi
Traditional Chinese prescriptions organically combine various herbal drugs based on a certain composition principle. According to the traditional Chinese medicine theory, a TCM formula contains multiple interactive herbs. It is customary to rank the compositions into four categories, namely, Jun (monarch), Chen (minister), Zuo (assistant) and Shi (guide) with proper herbs to synergize the desirable effects and to minimize side effects integrally when analyzing the roles they play in the TCM formula. The monarch drug is an essential ingredient in a formula, which plays leading pharmacological effects aiming at the cause or the main syndrome of a disease. The minister drug helps strengthen the curative effect of the monarch drug, or, is used for treatment of accompanying symptoms [41]. The assistant drug is used to promote the treatment efficacy of the minister drug on chief syndromes/disease, or to aim at minor symptoms [42]. The Guide drug is to lead the ‘herbs’ impact to the disease location and to modulate the interaction among the herbs [43]. Various drugs in a prescription have their respective importance of effect in the order of ‘monarch, minister, assistant and messenger. The principles of “monarch”, “minister”, “assistant” and “messenger” signify the fact that each herbal ingredient of the recipe has a specific function within the composition, and is organized and arranged integrally under this principle to generate its specific effect [43].
The present work shows that the Jun herb possesses two extraordinary characteristics: many active molecules and powerful pharmacological effects. As shown in Fig 3, 32 ingredients from Radix Salviae which contain the major bioactive compounds in the 20 herbs are assumed playing major roles in the treatment of breast cancer. In order to quantify the polypharmacological effect, we count the number of targets for each drug, that is, the degree for each drug node in the drug-target network. As a result, Radix Salviae has the largest number of degree in the 20 herbs. Network analysis also shows that the average number of targets per compound is 12. All these 32 compounds are linked with more than two targets, proving the polypharmacology of this herb. For example, Radix Salviae has long time ago been used for treating atherosclerosis, cardiovascular diseases, stroke, inflammatory diseases and cancers. Its major bioactive constituents are multi-target drugs, whose molecular targets include transcription factors, scavenger receptors, ion channels, kinases, pro- and anti-apoptotic proteins, growth factors, inflammatory mediators, microRNA, and others [44]. This good hit rate indicates the rationality and reliability to find active compounds by using network-based analytical methods. Thus, it is assumed that Radix Salviae as monarch herbal medicine plays a principal role in therapeutic effect.
In this work, by compound structural comparisons and target analysis, to certain extent, 7 herbs include 41 ingredients contain about half of whole bioactive compounds. In addition, these herbs have the middle number of degree in the 20 herbs; the average number of potential targets per candidate compound is 7.24, and these compounds have many overlapping targets. This indicates that these 7 herbs and Radix Salviae could produce enhancing pharmacological synergism, due to that these herbs direct at a similar receptor target or physiological system. Thus, 7 herbs are assumed as minister herbal medicine increasing the effect of Radix Salviae.
The rest herbs (Fig 3) serve as assistant and messenger drugs which have the lowest number of active ingredients and also target comparatively less proteins displayed. The average number of potential targets per candidate compound is only 6.74. However, despite the small number of the active compounds and low degree, these herbs may have broad pharmacological actions with Jun/Chen herb, which was used to improve the pharmacological activity of monarch drug and ministerial drug. For example, Curcumae Rhizoma is an important TCM with ‘‘Huoxuehuayu” (promoting blood circulation and removing blood stasis) activity, and its essential oils contribute to several of its pharmacological effects [45]. It exerts synergistic antitumor activity with other herbs and thus Curcumae Rhizoma can increase the function of the herb combination [46]. Beta-elemene is the main components of essential oils in Curcumae Rhizoma. Beta-elemene -induced alteration of cell membrane permeability, which potentially results in the enhanced cellular uptake of taxanes, may contribute to the synergistic interactions of the combination treatment [47]. Beta-elemene increases anti-cancer in by combination with etoposide, which is mediated by the cleavage of poly (ADP-ribose) polymerase [47]. Therefore, Curcumae Rhizoma demonstrates the potentially beneficial clinical combination with other drugs. Curcumae longae Rhizomaextracts show a high anti-inflammatory effect after parenteral application in standard animal models of inflammation [48]. Its main activity component Curcumin has been shown to be synergistic with chemotherapy such as 5-fluorouracil, oxaliplatin, and gemcitabine in inhibiting tumor cell growth [49]. This synergistic effect appears to be partly due to the inhibition of NF-κB and growth factor receptors, which indicates that Curcumae longae Rhizoma might affect the pharmacological effects of other herbs due to the drug-drug interactions.
Taken together, these 20 herbs work together harmoniously to achieve an ideal therapeutic effect. All these results also explain why this formula takes the ‘‘Jun-Chen-Zuo-Shi” as the rule of prescription.
Illustrating the mechanism of action on treating breast cancer based on the C-T interactions
Hormones play a critical role in breast carcinogenesis [50]. The sex hormones control the activation of responsive genes by first binding to specific receptors and forming complexes that can in turn bind to sequences in the promoters of downstream, hormone-responsive receptors, and thus steroid hormone receptors are candidates for breast cancer susceptibility receptors [51]. These hormonal receptors, namely, androgen receptor, glucocorticoid receptor, estrogen receptor, and progesterone receptor, have been widely targeted for developing treatments for breast cancer. As shown in Fig 4, several targets belong to the estrogen receptor-like family that displays the highest degree, such as ESR1 (estrogen receptor-α, degree = 119), ESR2 (estrogen receptor-β, degree = 76), AR (androgen receptor, degree = 119), NR3C1 (glucocorticoid receptor, degree = 26) and PGR (progesterone receptor, degree = 7), which are major therapeutic targets of breast cancer due to their dominant positions in this net.
Fig 4. Distribution of the target proteins versus the drug node degree in the drug-target network.
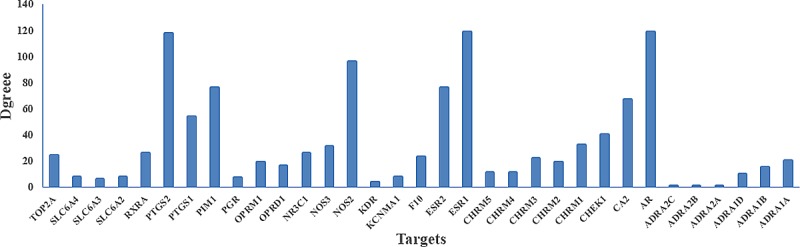
For instance, (1) estrogen is widely recognized for its role in breast cancer; elevated urinary levels of estrogens is associated with an increased risk of breast cancer [52]. Knowledge of the central role of estrogen receptor in breast cancer has already led to the development of new preventive and therapeutic interventions that block receptor function or drastically reduce the levels of endogenous estrogen through the inhibition of its synthesis [52]. Additionally, endocrine therapies that target the ER are the cornerstone of breast cancer treatment for the majority of patients [39]. (2) AR, a member of the steroid receptor subfamily with well-known biological and therapeutic importance has been implicated in breast cancer pathogenesis [40]. AR may be both a prognostic factor for survival and a predictive factor for response to endocrine treatment in patients with breast cancer [53]. In addition, AR is expressed in normal breast epithelial cells and in approximately 70% to 90% of invasive breast carcinomas [40]. (3) PGR is ligand-activated transcription factor member of the steroid hormone family of nuclear receptor [54]. Progesterone is also critically involved in the development of the mammary gland and breast cancer, and its effects are mostly mediated via the progesterone receptor [55]. Progestational agents are widely used for oral contraception, menopausal hormone replacement therapy, and for treating breast cancer [54]. As a result, PGR is an important therapeutic target in breast cancer. (4) GR is a classic nuclear hormone receptor that dimerizes after ligand binding, translocates to the nucleus, and modulates the expression of many target genes [56]. NR3C1 activation has been demonstrated an inhibition capacity to apoptosis in breast epithelial cells, which play an important role in breast epithelial and breast cancer cell biology [57]. NR3C1 directly mediates the gene expression pathways associated with the aggressive behavior and early relapse in the breast cancer subtype. NR3C1-induced MAPK phosphatase-1 (MPK-1) expression inhibits the paclitaxel-associated MAPK activation and contributes to breast cancer cell survival [57]. Thus, NR3C1 expression may represent a novel therapeutic target for breast cancer. These results confirm the reliability of our network and these high-degreed targets are major therapeutic targets in the treatment of breast cancer.
Chronic inflammation is a key contributor to cancer development and progression [58]. Epidemiological studies have shown that chronic inflammation predisposes individuals to various types of cancer [59]. Cancer survivors with chronic inflammation may have an elevated risk of recurrence as a result of the effects of inflammatory processes on cell growth or the presence of cancer cells that induce inflammation [58]. Chronic inflammation is believed to contribute to the development and progression of breast cancer. Indeed, further observation of the C-T network shows that most of the high-degreed targets are associated with inflammatory mediators, such as COX-1 (PTGS1), COX-2 (PTGS2) and VEGF2 (KDR). There is a growing body of evidence that two primary cyclooxygenase (COX) isoforms (COX-1 and COX-2) play an important role in carcinogenesis and angiogenesis of human tumors [60]. COX inhibitors have potent activity in a model of highly aggressive breast cancer [61]. Epidemiologic and laboratory investigations suggest that non-steroidal anti-inflammatory drugs have chemopreventive effects against breast cancer due to their activity against COX-2, the rate-limiting enzyme of the prostaglandin cascade [62]. Epidemiological studies as well as early clinical trials also suggest that administration of either dual COX-1/COX-2 or selective COX-2 inhibitors may reduce the risk of cancer development [61]. Inhibition of COX-2 (and COX-1) results from the use of non-steroidal anti-inflammatory drugs and a case-controlled study has reported a reduction in the incidence of breast cancer in patients reporting a regular intake of these drugs [60]. VEGF2 is also a viable target for pharmacological intervention in cancer, and the aberrant expression of VEGF2 is a hallmark of malignant tumor development required for the colonization of endothelial cells that allow tumor nutrition [63]. Therefore, these high-degreed targets should be treated as the crucial ones of these herbs.
Furthermore, mu-type opioid receptor and delta-type opioid receptor are also targeted by candidate components from 20 herbs, which have the effect of sedation. Opioids, most common drugs used for treatment of cancer pain, are immunosuppressive, and therefore, they might directly and/or indirectly influence long-term cancer recurrence. For this reason, OPRM1 (mu-type opioid receptor) genotype may play a key role in both the short-term postmastectomy outcome and the long-term follow-up, becoming a new biomarker for breast cancer recurrence in patients suffering from chronic postmastectomy pain managed by opioid therapy [64]. In addition, OPRD1 (delta-type opioid receptor) is also reported to have sedative effect [65].
T-P network and systems analysis
Drug action is not only related to its targets, but also affects various metabolic enzymes, transporter proteins, as well as the downstream effects of drug action and pathways related to the specific disease. Most drugs act by binding to specific proteins, thereby changing their biochemical and/or biophysical activities, with multiple consequences on various functions. Multiple compounds can jointly perturb the same disease-related signal pathways [66]. For this reason, to understand the therapeutic mechanisms of a drug, it is also critical to identify the signal pathways its targets participate in. Thus presently, network-based analyses among compounds, targets and pathways are carried out to study the pharmacological mechanism of the herb in a systematical level as well as to unfold the main targeting proteins and signaling pathways in the process of treatment. As a matter of fact, for better elaborating the major pathways involved in herbal medicines for breast cancer therapy, we extract three canonical pathways that are highly associated with breast cancer from KEGG database (http://www.genome.jp/kegg/) to construct the target-pathway (T-P) network (Fig 5) with corresponding targets of herbal compounds.
Fig 5. The T-P network.
A link is created between a target and a pathway if the pathway is lighted at the target, where blue, green and red nodes represent compounds, targets and pathways, respectively. The information of pathways is obtained by mapping the target proteins to the KEGG pathway database.
The T-P network consists of 153 nodes and 865 edges, with blue, green and red nodes representing compounds, targets and pathways, respectively. As can be observed, most of the target proteins (27/33) appear in multiple pathways, indicating that these targets may intercede on the interactions and cross-talk between different pathways. Similarly, major pathways are also modulated through multiple target proteins, and many of them have been reported as suitable target pathways for breast cancer therapies, including the EGFR signaling pathway, Estrogen signaling pathway, and Wnt signaling pathway. The three pathways are interdependent with each other through the compounds, indicating that the herbal medicines may exert synergistic influences through multiple different pathways. Additionally, visually we find that most of compounds target more than one proteins involved in the same pathway or different pathways, which implies again the “multiple compounds, multiple targets” features of the TCMS.
Of all 33 candidate targets, 24 participate in the EGFR signaling pathway, indicating that the EGFR signaling pathway plays an important role in the treatment of breast cancer. Actually, aberrant epidermal growth factor receptor (EGF/EGFR) signaling is considered as a major characteristic of many human malignancies including breast cancer [67]. The signals mediated by EGFR can be deregulated by an increase in the amount of the receptor on the cell surface or by structural alterations of the protein that constitutively activates the signal pathway independent of its ligand [68]. The EGFR pathway is composed of several key transduction cascades, namely, those ones that are involved in the PI3K/Akt/mTOR, Ras/Raf/MAPK and JAK/Stat. For example, one of the pathways implicated in cell growth and metabolic regulation is the IGF1R/PI3K/Akt/mTOR pathway, which has been largely looked into the past decade as a target to treat breast cancer. The IGF1R activation by IGF1 leads to the phosphorylation of tyrosine residues in the intracellular domain of the receptor and phosphorylation of tyrosine and serine residues of the insulin receptor substrate (IRS) and Src which will activate the MAPK and PI3K/Akt/mTOR pathways. IGF pathway is regulated in many critical points, from ligand availability to negative feedback mechanisms exerted by mTOR [68]. As for its role in human cancer, preclinical models show that the IGF1R is frequently overexpressed by tumors, and mediates proliferation and apoptosis protection [69]. Thus, the EGFR pathway participates in cell metabolism regulation, in growth and survival, and changes in this pathway may contribute to various subtypes of breast cancer [68].
The second pathway, Estrogen, also plays an important role in the growth and differentiation of the normal mammary gland [70]. However, there is considerable evidence that estrogens are also mammary carcinogens in the breast, involving the metabolism of estrogen to genotoxic, mutagenic metabolites and the stimulation of tissue growth [50]. For instance, ERα, an estrogen-inducible transcription factor, is member of the nuclear receptor super family, the dysfunction of which accounts for 70% breast tumors [71]. Indeed, clinical and experimental studies have suggested an important role for Estrogen signaling pathway in the treatment of breast cancer and the reduction for estrogen deprivation of mammary tumors [70]. In breast cell, studies from several laboratories strongly support that estrogen also triggers signaling cascades typically linked to membrane receptors that possess tyrosine kinase activity or couple to heterotrimeric G proteins, such as MAPK, PI3K and PKB/Akt [72]. Therefore, given the role of this Estrogen signaling pathway in regulation of cell proliferation, mammary gland growth and development, estrogen is a major oncogenic factor and an attractive therapeutic target.
The third pathway, Wnt signaling pathway plays an important role in modulating the inflammatory response and the regulation of many cellular processes, including cell fate decisions and cell proliferation, and aberrant Wnt signaling also is associated with tumorigenesis [73, 74]. Presently, 12 compounds like WZ07 (Curcumenol), JH03 (Procurcumadiol), JH07 (Dihydrocurcumin), curcumin and its derivative, may be able to down regulate the Wnt signaling pathway through regulating β-catenin itself or downstream components, and then provide synergistic therapeutic effects to benefit patients [75, 76]. Many Wnt proteins act via a signaling pathway which always results in stabilization of β-catenin and consequent transcriptional activation of specific target genes. Mutations in β-catenin or other Wnt pathway components, which result in β-catenin accumulation, are found in a wide range of human cancers. Moreover, studies in animal model systems clearly demonstrate that activated Wnt signaling leads to mammary tumorigenesis [74]. Thus, Wnt signaling contributes to the genesis of cancers in a wide range of human tissues. These findings reveal the importance of Wnt signaling pathway in inducing breast cancer.
To sum it up, essential pathways for breast cancer mediate many of the characteristics of the malignant phenotype, such as increased cell proliferation, decreased apoptosis and metastasis. Since the three pathways are closely associated with epidermal growth factor receptor, estrogen receptor, and inflammatory, we speculate that the 20 herbs probably perturb the pathways, and thereby display anti-estrogen, anti-inflammatory, regulation of cell metabolism and proliferation activities. The three pathways are interdependent with each other through compounds, which further indicate that 20 herbs can exert synergistic influences on different pathways (Fig 5). In addition, a compound may target different proteins involved in the same pathway or different pathways, which also illustrates the mechanism of multiple targets for a TCM.
Conclusion
Breast cancer is the most common malignancy in women. Traditional Chinese medicine has been practiced for three thousand years. This discipline is practiced worldwide that TCM may serve as a useful model for scientific inquiry since there is a standardized system of diagnostics and therapies. However, unlike conventional pharmacological medications used in western medicine, bioactive compounds and mechanisms of action of herbal medications have not been specified and measured precisely. Thus, in the present study, we have constructed a systems pharmacology approach including the OB screening, DL evaluation, target identification, and network pharmacology analysis, which combines the use of computational modeling and wide-scale text-mining methods, to elucidate the mechanisms of action of the most widely studied medicinal herbs for the treatment of breast cancer. The main findings are as follows:
Through OB and DL screening, 150 compounds out of 1618 components in 20 traditional Chinese medicines are identified as candidate compounds. 33 targets related to breast cancer are predicted to interact with the candidate compounds which generate the compound-target network. Based on the deep investigation of the function and the synergistic effect of each herb in the molecular/systems level, the combination principle of TCM formula can be explained as follows: Radix Salviae as monarch herbal medicine plays a principal role in therapeutic effect. 7 herbs are minister herbal medicine increasing the effect of Radix Salviae. The rest herbs serve as assistant and messenger drugs. All these results also explain why these herbs takes the ‘‘Jun-Chen-Zuo-Shi” as the rule of prescription.
Three pathways are closely associated with epidermal growth factor receptor, estrogen receptor, and inflammatory. Therefore, we speculate that the 20 herbs display anti-estrogen, anti-inflammatory, regulation of cell metabolism and proliferation activities by probably perturbing these pathways. The T-P network of these herbs constructed presently demonstrates that the herbal medicines may simultaneously target several pathways like EGFR, Estrogen and Wnt signaling pathways, thereby exhibiting synergistic benefits in breast cancer treatment.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful for the Key Program of National Natural Science Foundation of China (Grant No. 81530100) and the Oasis Scholar Fund of Shihezi University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Key Program of National Natural Science Foundation of China (Grant No. 81530100) and the Oasis Scholar Fund of Shihezi University.
References
- 1.Yates JS, Mustian KM, Morrow GR, Gillies LJ, Padmanaban D, Atkins JN, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005; 13(10):806–11. 10.1007/s00520-004-0770-7 [DOI] [PubMed] [Google Scholar]
- 2.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000; 14(4):275–90. [PubMed] [Google Scholar]
- 3.Lengacher CA, Bennett M, Kip KE, Keller R, LaVance MS, Smith LS, et al. Frequency of use of complementary and alternative medicine in women with breast cancer. Oncol Nurs Forum. 2002; 29(10):1445–52. 10.1188/02.ONF.1445-1452 [DOI] [PubMed] [Google Scholar]
- 4.Jia L. Cancer complementary and alternative medicine research at the US National Cancer Institute. Chin J Integr Med. 2012; 18(5):325–32. 10.1007/s11655-011-0950-5 [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Shu X-O, Gao Y, Wen W, Ruan Z-X, Jin F, et al. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res Tr. 2004; 85(3):263–70. [DOI] [PubMed] [Google Scholar]
- 6.Cohen I, Tagliaferri M, Tripathy D, editors. Traditional Chinese medicine in the treatment of breast cancer Semin Oncol; 2002: Elsevier. [DOI] [PubMed] [Google Scholar]
- 7.Cheung F. TCM: made in China. Nature. 2011; 480(7378):S82–S3. 10.1038/480S82a [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Wang Y. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery. J Ethnopharmacol. 2014; 151(1):66–77. 10.1016/j.jep.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Erhardt RA, Schneider R, Blaschke C. Status of text-mining techniques applied to biomedical text. Drug Discov Today. 2006; 11(7):315–25. [DOI] [PubMed] [Google Scholar]
- 10.Nmeth K, Plumb GW, Berrin J-G, Juge N, Jacob R, Naim HY, et al. Deglycosylation by small intestinal epithelial cell-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003; 1(42). [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Zhang W, Huang C, Li Y, Yu H, Wang Y, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012; 13(6):6964–82. 10.3390/ijms13066964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Xu X, Wang J, Yu H, Wang X, Yang H, et al. A system-level investigation into the mechanisms of Chinese Traditional Medicine: Compound Danshen Formula for cardiovascular disease treatment. PLoS One. 2012; 7(9):e43918 10.1371/journal.pone.0043918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Chen J, Xu X, Li Y, Zhao H, Fang Y, et al. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PloS one. 2012; 7(5):e37608 10.1371/journal.pone.0037608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao W, Xu X, Wang X, Li B, Wang Y, Li Y, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013; 145(1):1–10. 10.1016/j.jep.2012.09.051 [DOI] [PubMed] [Google Scholar]
- 15.Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011; 27(3):431–2. PubMed Central PMCID: PMC3031041. 10.1093/bioinformatics/btq675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang A, Sun H, Yuan Y, Sun W, Jiao G, Wang X. An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents. Fitoterapia. 2011; 82(8):1160–8. 10.1016/j.fitote.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 17.Grabowski K, Schneider G. Properties and architecture of drugs and natural products revisited. Current Chemical Biology 2007; 1: 115–27. [Google Scholar]
- 18.Li P, Chen J, Wang J, Zhou W, Wang X, Li B, et al. Systems pharmacology strategies for drug discovery and combination with applications to cardiovascular diseases. J Ethnopharmacol. 2014; 151(1); 93–107. 10.1016/j.jep.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Wang J, Zhou W, Wang Y, Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol. 2013; 146(3):773–93. 10.1016/j.jep.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Wei YJ, Qi LW, Li P, Qian ZM, Luo HW, et al. Determination of fifteen bioactive components in Radix et Rhizoma Salviae Miltiorrhizae by high‐performance liquid chromatography with ultraviolet and mass spectrometric detection. Biomed Chromatogr. 2008; 22(2):164–72. 10.1002/bmc.911 [DOI] [PubMed] [Google Scholar]
- 21.Wei YJ, Li P, Shu B, Li Hj, Peng Yr, Song Y, et al. Analysis of chemical and metabolic components in traditional Chinese medicinal combined prescription containing Radix Salvia miltiorrhiza and Radix Panax notoginseng by LC‐ESI‐MS methods. Biomed Chromatogr. 2007; 21(8):797–809. 10.1002/bmc.775 [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Lu L. Pharmacology study and clinical application of Fufang danshen tablet. HeilongJiang Medical Journal. 2005; 18:59. [Google Scholar]
- 23.Wang J, Chen J, Pan K. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut R. 2013; 20(3):1441–9. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang J, et al. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J cancer. 2005; 116(5):799–807. 10.1002/ijc.20880 [DOI] [PubMed] [Google Scholar]
- 25.Nizamutdinova IT, Lee GW, Son KH, Jeon SJ, Kang SS, Kim YS, et al. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int J oncol. 2008; 33(3):485–91. [PubMed] [Google Scholar]
- 26.Peng W, Han T, Xin W-B, Zhang X-G, Zhang Q-Y, Jia M, et al. Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res. 2011; 20(2):146–51. [Google Scholar]
- 27.Jiang H, Shi J, Li Y. Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules. 2011; 16(4):3146–51. 10.3390/molecules16043146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang KT, Chen LG, Wu CH, Chang CC, Wang CC. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol‐induced gastric ulcer in vitro and in vivo. J Pharm Pharmacol. 2010; 62(3):381–8. 10.1211/jpp.62.03.0014 [DOI] [PubMed] [Google Scholar]
- 29.Dong H, He L, Huang M, Dong Y. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat Pro Res. 2008; 22(16):1418–27. [DOI] [PubMed] [Google Scholar]
- 30.Wang A, Xiao Z, Zhou L, Zhang J, Li X, He Q. The protective effect of atractylenolide I on systemic inflammation in the mouse model of sepsis created by cecal ligation and puncture. Pharm Biol. 2016; 54(1):146–50. 10.3109/13880209.2015.1024330 [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Wei L, Shen A, Cai Q, Xu W, Li H, et al. Hedyotis diffusa Willd extract suppresses Sonic hedgehog signaling leading to the inhibition of colorectal cancer angiogenesis. Int J Oncol. 2013; 42(2):651–6. 10.3892/ijo.2012.1753 [DOI] [PubMed] [Google Scholar]
- 32.Lin C-C, Kuo C-L, Lee M-H, Hsu S-C, Huang A-C, Tang N-Y, et al. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T-and B-cell proliferation in leukemic mice. In Vivo. 2011; 25(4):633–40. [PubMed] [Google Scholar]
- 33.Gu G, Barone I, Gelsomino L, Giordano C, Bonofiglio D, Statti G, et al. Oldenlandia diffusa extracts exert antiproliferative and apoptotic effects on human breast cancer cells through ERα/Sp1‐mediated p53 activation. J Cell Physiol. 2012; 227(10):3363–72. 10.1002/jcp.24035 [DOI] [PubMed] [Google Scholar]
- 34.Lee H-Z, Bau D-T, Kuo C-L, Tsai R-Y, Chen Y-C, Chang Y-H. Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am J Chinese Med. 2011; 39(01):201–13. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Guo W-J, Yang Q-Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J Gastroentero. 2002; 8(3):493–5. PubMed Central PMCID: PMC4656428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y-f, Zhang J-y, Zhao M-h, Yan M, Zhao Q-c, Wu Q, et al. The analgesic activity and possible mechanisms of deacetyl asperulosidic acid methyl ester from Ji shi teng in mice. Pharmacol Biochem Be. 2012; 102(4):585–92. [DOI] [PubMed] [Google Scholar]
- 37.Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat M-O, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer?: Methodology of a multicenter randomized trial. Contemp Clin Tials. 2008; 29(4):517–26. [DOI] [PubMed] [Google Scholar]
- 38.Fryer RM, Randall J, Yoshida T, Hsiao L-L, Blumenstock J, Jensen KE, et al. Global analysis of gene expression: methods, interpretation, and pitfalls. Nephron Exp Nephrol. 2002; 10(2):64–74. [DOI] [PubMed] [Google Scholar]
- 39.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011; 20(1):119–31. 10.1016/j.ccr.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011; 17(7):1867–74. PubMed Central PMCID: PMC3076683. 10.1158/1078-0432.CCR-10-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Gai Y, Wu X, Wan H. Quantitatively analyze composition principle of Ma Huang Tang by structural equation modeling. J Ethnopharmacol. 2012; 143(3):851–8. 10.1016/j.jep.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 42.Liang Y, Zhou Y, Zhang J, Rao T, Zhou L, Xing R, et al. Pharmacokinetic compatibility of ginsenosides and Schisandra Lignans in Shengmai-san: from the perspective of p-glycoprotein. PloS One. 2014; 9(6):e98717 PubMed Central PMCID: PMC4055595. 10.1371/journal.pone.0098717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tingxiu L, Yuhang L. Discussion on the three levels of the prescription-composition principle of TCM. Deutsche Zeitschrift für Akupunktur. 2009; 52(3):27–30. [Google Scholar]
- 44.Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opinion Ther Pt. 2013; 23(2):149–53. [DOI] [PubMed] [Google Scholar]
- 45.Lu J-J, Dang Y-Y, Huang M, Xu W-S, Chen X-P, Wang Y-T. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae–A review. J Ehnopharmacol. 2012; 143(2):406–11. [DOI] [PubMed] [Google Scholar]
- 46.Gao J-L, He T-C, Li Y-B, Wang Y-T. A traditional Chinese medicine formulation consisting of Rhizoma Corydalis and Rhizoma Curcumae exerts synergistic anti-tumor activity. Oncol Rep.2009; 22(5):1077–83. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Xu L, Qu X, Zhao M, Jin B, Kang J, et al. Synergistic antitumor effect of β-elemene and etoposide is mediated via induction of cell apoptosis and cell cycle arrest in non-small cell lung carcinoma cells. Mol Med Rep. 2011; 4(6):1189–93. 10.3892/mmr.2011.537 [DOI] [PubMed] [Google Scholar]
- 48.Miquel J, Bernd A, Sempere J, Dıaz-Alperi J, Ramırez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geri. 2002; 34(1):37–46. [DOI] [PubMed] [Google Scholar]
- 49.Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutr Cancer. 2009; 61(6):842–6. 10.1080/01635580903285106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Bio. 2002; 7(1):3–15. [DOI] [PubMed] [Google Scholar]
- 51.Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidem Bioma. 1999; 8(10):843–54. [PubMed] [Google Scholar]
- 52.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. New Engl J Med. 2006; 354(3):270–82. 10.1056/NEJMra050776 [DOI] [PubMed] [Google Scholar]
- 53.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009; 69(15):6131–40. 10.1158/0008-5472.CAN-09-0452 [DOI] [PubMed] [Google Scholar]
- 54.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002; 277(7):5209–18. 10.1074/jbc.M110090200 [DOI] [PubMed] [Google Scholar]
- 55.Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003; 17(4):575–88. 10.1210/me.2002-0318 [DOI] [PubMed] [Google Scholar]
- 56.Wu W, Zou M, Brickley DR, Pew T, Conzen SD. Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol. 2006; 20(10):2304–14. 10.1210/me.2006-0131 [DOI] [PubMed] [Google Scholar]
- 57.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005; 280(6):4117–24. 10.1074/jbc.M411200200 [DOI] [PubMed] [Google Scholar]
- 58.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009; 27(21):3437–44. PubMed Central PMCID: PMC2717751. 10.1200/JCO.2008.18.9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454(7203):436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 60.Kirkpatrick K, Ogunkolade W, Elkak A, Bustin S, Jenkins P, Ghilchik M, et al. The mRNA expression of cyclo-oxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in human breast cancer. Curr Med Res Opin. 2002; 18(4):237–41. 10.1185/030079902125000633 [DOI] [PubMed] [Google Scholar]
- 61.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002; 62(8):2343–6. [PubMed] [Google Scholar]
- 62.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006; 6(1):1. PubMed Central PMCID: PMC1373658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal. 2010; 22(9):1350–62. PubMed Central PMCID: PMC2928711. 10.1016/j.cellsig.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Gregori M, Diatchenko L, Belfer I, Allegri M. OPRM1 receptor as new biomarker to help the prediction of post mastectomy pain and recurrence in breast cancer. Minerva Anestesiol. 2015; 81:894–900. [PubMed] [Google Scholar]
- 65.Chiorean M, Chiorean M. Genetics of Pain. Acta Medica Marisiensis. 2013; 59(4):231–33. [Google Scholar]
- 66.Yıldırım MA, Goh K-I, Cusick ME, Barabási A-L, Vidal M. Drug—target network. Nat Biotechnol. 2007; 25(10):1119–26. 10.1038/nbt1338 [DOI] [PubMed] [Google Scholar]
- 67.Lo H-W, Hsu S-C, Hung M-C. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Tr. 2006; 95(3):211–8. [DOI] [PubMed] [Google Scholar]
- 68.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012; 38(6):698–707. 10.1016/j.ctrv.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 69.Riedemann J, Macaulay V. IGF1R signalling and its inhibition. Endocr-Relat Cancer. 2006; 13(Supplement 1):S33–S43. [DOI] [PubMed] [Google Scholar]
- 70.Cos S, González A, Martínez-Campa C, Mediavilla MD, Alonso-González C, Sánchez-Barceló EJ. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect Prev. 2006; 30(2):118–28. 10.1016/j.cdp.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 71.Tang B, Hsu H-K, Hsu P-Y, Bonneville R, Chen S-S, Huang TH, et al. Hierarchical modularity in ERα transcriptional network is associated with distinct functions and implicates clinical outcomes. Sci Rep-UK. 2012; 2:875. PubMed Central PMCID: PMC3500769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem. 2002; 80(2):231–8. [DOI] [PubMed] [Google Scholar]
- 73.Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediat Inflamm. 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004; 3(1):36–41. [DOI] [PubMed] [Google Scholar]
- 75.Handral HK, Duggi S, Handral R, Tulsianand G, Shruthi S. TURMERIC: NATURE’S PRECIOUS MEDICINE. Asian J Pharm Clin Res. 2013; 6(3). [Google Scholar]
- 76.Park CH, Hahm ER, Park S, Kim H-K, Yang CH. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS Lett. 2005; 579(13):2965–71. 10.1016/j.febslet.2005.04.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.