Abstract
The BRCA1 C-terminal (BRCT) domain has recently been implicated as a phospho-protein binding domain. We demonstrate here that a CTBP-interacting protein CtIP interacts with BRCA1 BRCT domains in a phosphorylation-dependent manner. The CtIP/BRCA1 complex only exists in G2 phase and is required for DNA damage-induced Chk1 phosphorylation and the G2/M transition checkpoint. However, the CtIP/BRCA1 complex is not required for the damage-induced G2 accumulation checkpoint, which is controlled by a separate BRCA1/BACH1 complex. Taken together, these data not only implicate CtIP as a critical player in cell cycle checkpoint control but also provide molecular mechanisms by which BRCA1 controls multiple cell cycle transitions after DNA damage.
DNA damage occurs frequently in the cell. It can be generated during normal DNA replication, UV light and X-ray exposure, oxidative base damages, or after various chemical treatments. To maintain genomic integrity, cells have developed elaborate DNA repair systems and various cell cycle checkpoints that ensure the repair of DNA lesions before cell cycle progression resumes. Two large protein kinases, ATM and ATR, are the critical upstream kinases that control the DNA damage-induced cell cycle checkpoints (1, 20). Many nuclear proteins, including BRCA1, are phosphorylated by ATM and ATR and participate in these cell cycle checkpoint pathways (29).
The breast cancer tumor suppressor gene BRCA1 encodes a 1,863-amino-acid nuclear protein with C-terminal tandem BRCA1 C-terminal (BRCT) motifs. The BRCT domains are essential for the tumor suppressor function of BRCA1. The majority of clinically relevant BRCA1 mutations lead to truncated BRCA1 gene products that lack one or both BRCT domains. Missense mutations that disrupt the secondary structure of BRCA1 BRCT domains were also identified in early-onset breast and ovarian cancer patients. Genetically removing BRCA1 BRCT domains in mice results in increased tumor incidence (17). The molecular mechanism underlying the tumor suppression function of BRCA1 BRCT domains may be linked with DNA damage-induced cell cycle checkpoint controls (10, 22, 26, 38). Recent biochemical studies demonstrated that the BRCA1 BRCT domains are a phospho-protein binding motif (18, 19, 38). These observations were recently confirmed by the structural analyses of BRCA1 BRCT domains in complexes with phospho-peptides (2, 6, 23, 30). However, since the BRCA1 BRCT domains participate in multiple cell cycle checkpoints (10, 22, 33), it is still puzzling how BRCA1 controls these distinct cellular activities.
CtIP was a protein originally identified as a binding partner of transcriptional suppressor CtBP (21). Subsequently, CtIP was also shown to interact with BRCA1 BRCT domains by two-hybrid screening (14, 31, 39). Although CtIP is phosphorylated after DNA damage, it is controversial whether or not DNA damage regulates the physical interaction between BRCA1 and CtIP (32). Moreover, the role of CtIP in BRCA1-dependent cell cycle checkpoint control has not been studied. Here we show that CtIP is a phosphorylation-dependent binding partner of the BRCA1 BRCT domains. The cell cycle-regulated BRCA1/CtIP complex is required for the G2/M transition and Chk1 activation after DNA damage.
MATERIALS AND METHODS
Cell culture and antibodies.
All cell lines were maintained in RPMI 1640 medium with 10% fetal calf serum at 37°C in 5% (vol/vol) CO2. To arrest cells at the G1/S boundary, cells were treated with 2 μM thymidine for 19 h and then released for 10 h, followed by a second thymidine (2 μM) block for 18 h. Arrested cells were released into cell cycle by removal of drug and the addition of fresh cell culture medium. Cells were harvested at the indicated time points.
Rabbit anti-BRCA1 (C23) antibody was a generous gift from David Livingston. Polyclonal anti-CtIP antibody was generated by immunizing a rabbit with glutathione S-transferase (GST)-tagged recombinant CtIP (residues 1 to 350). Monoclonal mouse anti-CtIP antibody was a gift from Richard Baer. Mouse anti-Flag (M2) antibody was purchased from Sigma. Anti-pS327 polyclonal antibody was raised against phospho-peptide TRVSS*PVFGATC and affinity purified. Mouse anti-phospho-Chk1 was from Cell Signaling Technology. Mouse anti-cyclin B and rabbit anti-phospho-histone H3 antibodies were purchased from Upstate Biotechnology, Inc.
Generation of CtIP mutants, cell transfection, immunoprecipitation, and immunoblotting.
Full-length CtIP cDNA with three N-terminal Flag epitope tags was obtained from Richard Baer. All mutants of CtIP were generated by using a QuikChange site-directed mutagenesis kit (Stratagene, Inc.). Cell transfection, preparation of cell lysates, protein dephosphorylation, immunoprecipitation, and immunoblotting were performed as described previously (38). Polyclonal anti-CtIP antibody was used for immunoprecipitation, and monoclonal anti-CtIP antibody was used for immunoblotting.
siRNA transfection and lentivirus infection.
The small interfering RNAs (siRNAs) specific for CtIP, BACH1, and BRCA1 were chemically synthesized (Dharmacon). The sequence of CtIP siRNA1 was GCUAAAACAGGAACGAATCdTdT; the sequence of CtIP siRNA2 was GGACCUUUGGACAAAACUAdTdT. The sequences of BRCA1 and BACH1 siRNAs were previously reported (12, 38). The siRNA transfection was performed as described previously (38).
siRNA-resistant wild-type CtIP and S327A constructs were generated by changing three nucleotides in the CtIP siRNA1 targeting region (C133T, A135G, and A138G substitutions). Lentivirus packaging and infection were performed as described previously (38).
Cell cycle checkpoint assays.
For the transient G2/M transition checkpoint assay, asynchronous cells were exposed to 2 Gy of gamma irradiation and then incubated at 37°C in 5% (vol/vol) CO2 for 1 h. Cells were fixed with 70% ethanol and stained with rabbit anti-phospho-histone H3 antibody (pH 3), followed by incubation with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G secondary antibody. The stained cells were treated with RNase, incubated with propidium iodide, and then analyzed by flow cytometry or examined by immunofluorescence microscopy.
The prolonged G2 accumulation assay was performed as described previously (38) with several modifications. Cells were incubated with 10 μM bromodeoxyuridine (BrdU) for 30 min, after exposure to 8 Gy of gamma irradiation. Cells were allowed to recover for 4 h and then treated with nocodazole (1 μg/ml) for 15 h. Cells were collected and fixed with 3% paraformaldehyde. Fixed cells were stained with fluorescein isothiocyanate-conjugated mouse anti-BrdU antibody (BD Biosciences) and rabbit anti-phospho-histone H3 antibody, followed by incubation with rhodamine-conjugated goat anti-rabbit immunoglobulin G secondary antibody. Then, 2,000 BrdU-positive-staining cells were examined by immunofluorescence microscopy.
RESULTS
The formation of BRCA1/CtIP complex depends on the phosphorylation of CtIP.
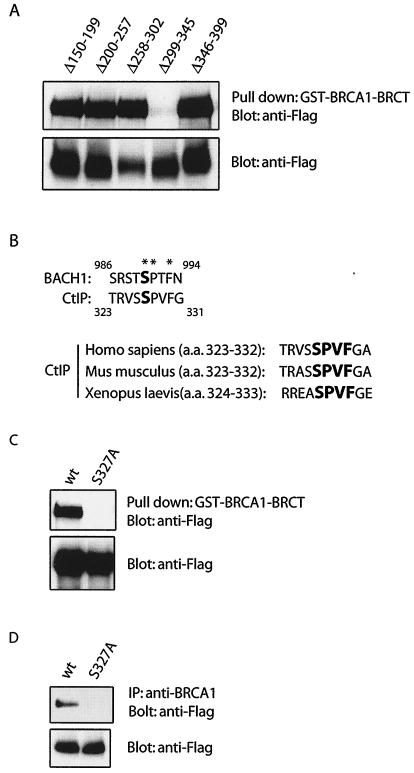
To elucidate whether CtIP is a phosphorylation-dependent binding partner of BRCA1, we first determined the BRCA1-interacting region on CtIP. Previous studies suggest that residues 133 to 369 of CtIP are required for CtIP to interact with BRCA1 BRCT domains (37). Using a series of CtIP deletion mutants, we showed that the region from residues 299 to 345 mediates the interaction between CtIP and BRCA1 BRCT domains (Fig. 1A). Since the BRCA1 BRCT domains recognize only the phosphorylated form of BACH1, a putative DEAH family DNA helicase (4), we postulated that the BRCA1 BRCT domains might also bind specifically to phosphorylated CtIP. Within the BRCA1-binding region of CtIP, the sequence surrounding Ser327 resembles the phosphorylation motif on BACH1 (Fig. 1B), which binds directly to BRCA1 BRCT domains (38). Both motifs contain Pro at the +1 position and Phe at the +3 position, two residues that are critical for the association of BACH1 and BRCA1 in vitro and in vivo (18, 19, 38). Moreover, the residues surrounding Ser327 of human CtIP is conserved in other species, suggesting this motif may be crucial for CtIP function (Fig. 1B). To test whether Ser327 of CtIP is essential for its interaction with BRCA1, we generated a Ser327-to-alanine (S327A) mutant of CtIP. Unlike wild-type CtIP, the S327A mutant did not bind to BRCA1 BRCT domains in vitro (Fig. 1C) and failed to associate with BRCA1 in vivo (Fig. 1D), suggesting that residue Ser327 of CtIP is critical for the CtIP-BRCA1 interaction.
FIG. 1.
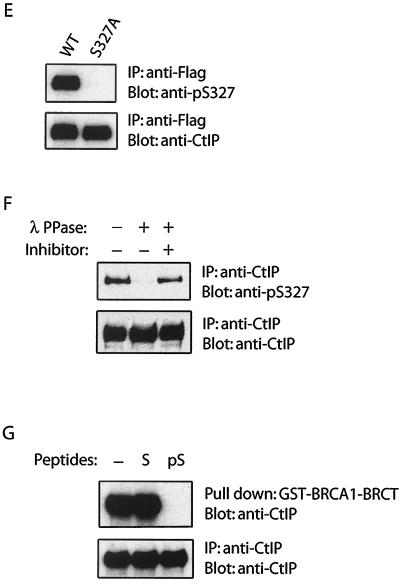
The BRCA1 BRCT domain interacts specifically with phosphorylated CtIP. (A) Flag-tagged CtIP deletion mutants were expressed in 293T cells. Cell lysates were incubated with GST-BRCA1-BRCT protein immobilized on beads. Associated CtIP proteins were eluted and detected with anti-Flag antibody (upper panels). Whole-cell lysates were also blotted with anti-Flag antibody to ensure similar expression levels of various CtIP mutants (lower panels). (B) Potential phospho-motif on CtIP. (C to E) Wild-type or S327A mutant of CtIP was expressed in 293T cells. Cell lysates were incubated with beads containing GST-BRCA1-BRCT for pull-down assay (C) or incubated with the indicated antibodies for immunoprecipitation (D and E). Associated or immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with indicated antibodies. (F) Endogenous CtIP from 293T cell lysate was immunoprecipitated with anti-CtIP antibody, followed by treatment with or without λ phosphatase (PPase) or phosphatase inhibitor. Immunoprecipitated CtIP was then immunoblotted with anti-phospho-S327 antibody (upper panels). An identical blot was also blotted with anti-CtIP antibody to ensure similar amounts of CtIP in these experiments. (G) Phosphorylated CtIP peptide competes with endogenous CtIP for BRCA1 binding. 293T cell lysates were incubated with beads containing GST-BRCA1-BRCT proteins in the presence of 10 μM phosphorylated peptide (pS, TRVSpSPVFGAT) or unphosphorylated control peptide (S, TRVSSPVFGAT). Endogenous CtIP associated with BRCA1 BRCT domains was eluted and detected by using anti-CtIP antibody.
To confirm that Ser327 of CtIP is phosphorylated in vivo, we raised an antibody to the Ser327 phosphorylated CtIP. This antibody only recognized wild-type CtIP, but not the S327A mutant (Fig. 1E). It also failed to recognize CtIP pretreated with phosphatase (Fig. 1F), suggesting that Ser327 of CtIP is indeed phosphorylated in vivo. Furthermore, only the phosphorylated CtIP peptide but not the unphosphorylated control peptide competed with endogenous CtIP for binding to the BRCA1 BRCT domains (Fig. 1G), further demonstrating that BRCA1 interacts selectively with Ser327 phosphorylated CtIP.
Cell cycle regulation of BRCA1/CtIP complex.
To examine whether phosphorylation of CtIP is cell cycle regulated, HeLa cells were first arrested in M phase by nocodazole (1 μg/ml) and then released into the cell cycle. To our surprise, Ser327 of CtIP was only phosphorylated transiently in G2 phase (Fig. 2A). This is very different from that of BACH1, which is phosphorylated from S to M phase (38). We repeated these experiments by using a thymidine block (at the G1/S boundary) and release protocol and confirmed that CtIP and BACH1 are differentially regulated during cell cycle. First, CtIP was not phosphorylated in M phase (Fig. 2A), but BACH1 was hyperphosphorylated when cells were arrested in M phase (38). Second, whereas Ser990 of BACH1 was phosphorylated in S phase, phosphorylation of CtIP at Ser327 was not observed until cells started to enter G2 phase (Fig. 2B). In addition, in agreement with a previous report (37), the protein level of CtIP is also tightly regulated through the cell cycle. Although phosphorylation of BACH1 and the BRCA1/BACH1 complex were detected from S to M phase, phosphorylation of Ser327 of CtIP and formation of BRCA1/CtIP complex were only observed in the G2 phase (Fig. 2B). These data demonstrate that phosphorylation of CtIP at Ser327 and BRCA1/CtIP complex formation start and end in different phases of the cell cycle than the BACH1 phosphorylation and BRCA1/BACH1 complex formation.
FIG. 2.
Phosphorylation-dependent formation of BRCA1/CtIP complex is cell cycle regulated. (A and B) HeLa cells were arrested in M phase by treatment with nocodazole (1 μg/ml) for 24 h (A) or at G1/S boundary by double thymidine block (B). Cells were then released and harvested at the indicated time points. Immunoprecipitations and immunoblotting were performed as indicated. Since Chk2 protein level is not cell cycle regulated, an anti-Chk2 immunoblot was included as a protein loading control. Both anti-cyclin B and anti-phospho-histone H3 blots were used as controls for cell cycle progress. Cell cycle distributions were analyzed by fluorescence-activated cell sorting and summarized here.
The BRCA1/CtIP complex is required for DNA damage-induced G2/M transition checkpoint.
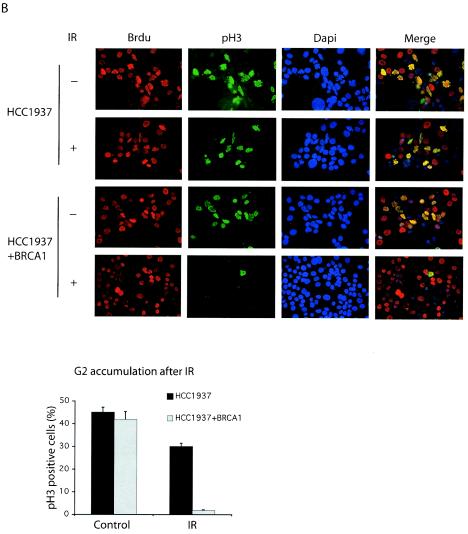
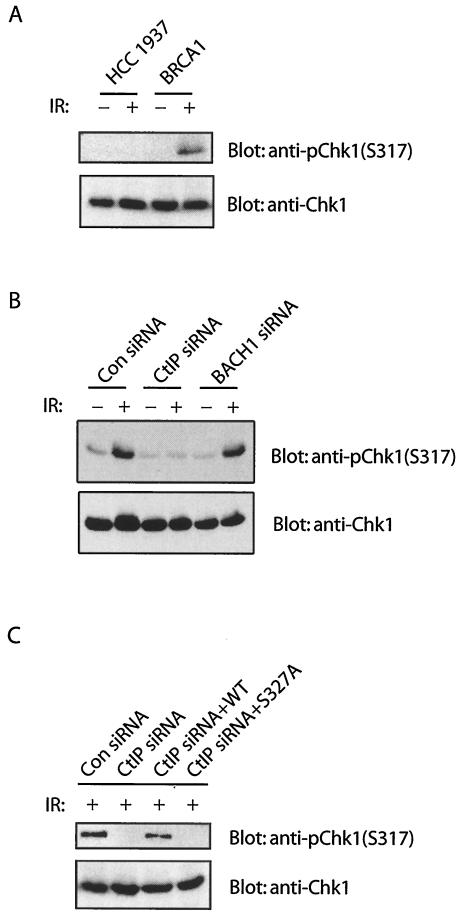
The difference in cell-cycle-dependent regulation of BRCA1-containing complexes implies the possible distinct functions of these complexes. After exposure to ionizing radiation, normal cells are arrested in the G2 phase of the cell cycle and will not enter mitosis until DNA repair is completed. However, murine BRCA1−/− fibroblasts have a G2/M checkpoint defect after DNA damage (35). HCC1937 cells, derived from human breast cancer, which express only a truncated form of BRCA1 also show G2/M checkpoint defects in response to DNA double-strand breaks (33, 36, 38). Recent evidence indicates that there are two distinct G2/M checkpoints (34), which involve the ATM and ATR pathways, respectively (3). The activation of ATM-dependent G2/M checkpoint transiently delays the cell cycle transition from G2 to M phase, whereas the ATR-dependent G2 accumulation arrests cells in G2 phase for a prolonged period (3, 7). Since BRCA1 is a substrate of both ATM and ATR in vivo (5, 8, 25), we examined whether both of these checkpoints would be defective in BRCA1-deficient HCC1937 cells. To investigate whether BRCA1 controls the transient G2/M checkpoint, HCC1937 cells were irradiated, and cells undergoing G2/M transition were monitored by using a mitotic marker (phosphorylated histone H3) 1 h after gamma irradiation. In agreement with previous publications (33, 35), BRCA1-deficient HCC1937 cells failed to arrest in G2 and entered into mitosis despite the presence of DNA damage (Fig. 3A). As a control, reconstitution with wild-type BRCA1 restored this G2/M checkpoint (Fig. 3A), suggesting that BRCA1 is indeed essential for the transient G2/M checkpoint after DNA damage. To test DNA damage-dependent G2 accumulation, S phase population of HCC1937 cells was labeled with BrdU. BrdU-labeled HCC1937 cells were irradiated and incubated for 4 h to allow cells to pass through the transient G2/M checkpoint before the addition of nocodazole. The integrity of G2 accumulation checkpoint was assessed 15 h later. As previously reported (36, 38), BRCA1 is also required for this G2 accumulation after DNA damage (Fig. 3B).
FIG. 3.
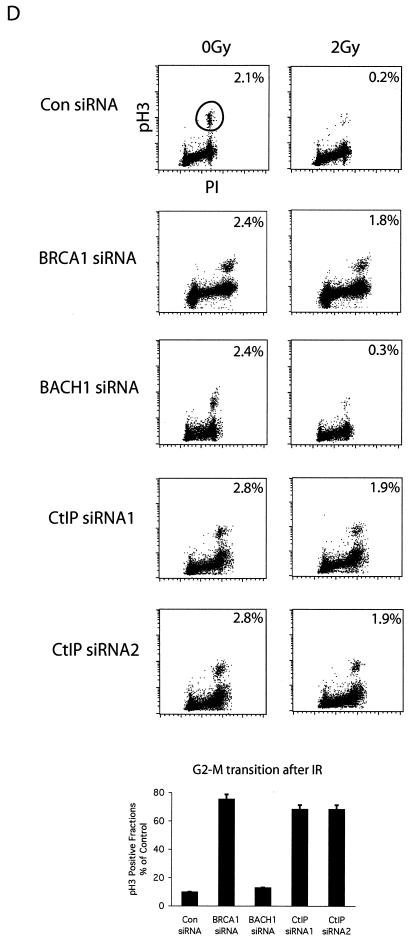
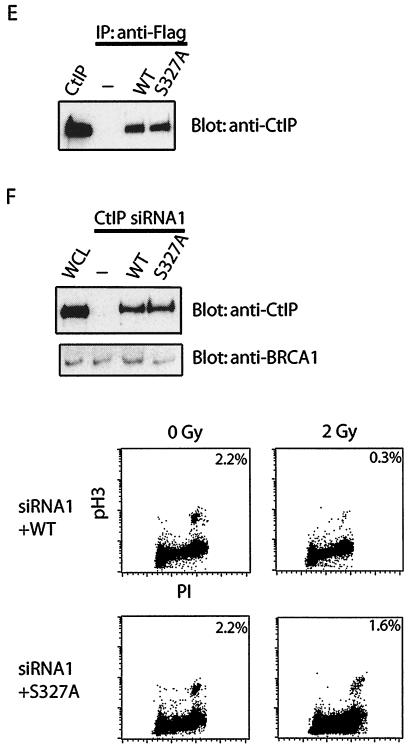
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
HCC1937 cells express a truncated mutant of BRCA1 that lacks one of the C-terminal tandem BRCT motifs. Because HCC1937 cells are defective in both the transient G2/M and the G2 accumulation checkpoints, it is likely that the BRCA1 BRCT domain and its binding partners are critical for these checkpoint controls. Since the BRCA1/BACH1 and BRCA1/CtIP complexes form at different cell cycle phases, we next tested the possibility that these two complexes may be involved in two distinct checkpoint pathways. We have shown previously that BRCA1/BACH1 complex participates in the prolonged G2 accumulation checkpoint (38). Here, we examined whether CtIP is involved in the same checkpoint pathway. Two different CtIP siRNAs were used to downregulate CtIP expression. As shown in Fig. 3C, BACH1, but not CtIP, participates in this G2 accumulation checkpoint. We next examined the transient G2/M transition checkpoint control in cells treated with BACH1 or CtIP siRNA. Interestingly, only CtIP and not BACH1 is required for this G2/M transition checkpoint control (Fig. 3D). To exclude the side effect of siRNA transfection, we used control and BRCA1 siRNAs as negative and positive controls, respectively (Fig. 3C and D). To further confirm that the role of CtIP in checkpoint control depends on its interaction with BRCA1, we generated siRNA-resistant wild-type CtIP and the S327A mutant that cannot bind BRCA1 (Fig. 3E and F). Expression of siRNA-resistant wild-type CtIP, but not the S327A mutant, restored the transient G2/M transition checkpoint (Fig. 3F), suggesting that the phosphorylation-dependent BRCA1/CtIP complex participates in the transient G2/M checkpoint control.
The BRCA1/CtIP complex is important for Chk1 activation.
Several studies imply that Chk1 is required for the transient G2/M checkpoint, probably through its ability to regulate Cdc25A/C (16, 24, 28, 40). In addition, BRCA1 is known to be required for Chk1 activation after DNA damage (9, 13, 36). To explore the role of BRCA1-containing complexes in DNA damage signal transduction, we examined damage-induced Chk1 phosphorylation in cells treated with CtIP or BACH1 siRNA. Only downregulation of CtIP inhibits Chk1 phosphorylation (Fig. 4B). In addition, expression of siRNA-resistant wild-type CtIP but not the S327A mutant restored Chk1 activation after DNA damage (Fig. 4C). Taken together, these results suggest that the BRCA1/CtIP complex regulate Chk1 activation and thus control the transient G2/M checkpoint after DNA damage.
FIG. 4.
CtIP is required for Chk1 activation after DNA damage. (A) HCC1937 cells or HCC1937-BRCA1 cells were treated with or without irradiation (IR; 8 Gy). Cell lysates were collected and immunoblotted with anti-phospho-Chk1 (anti-pS317) or anti-Chk1 antibodies. (B) HeLa cells were transfected with control, CtIP or BACH1 siRNAs. Cells were treated with or without irradiation (IR; 8 Gy). Cell lysates were collected and immunoblotted with indicated antibodies. (C) HeLa cells were transfected with CtIP siRNA after infection with lentivirus expressing wild type (WT) or the S327A mutant of CtIP. Cells were treated with irradiation (IR; 8 Gy) at 48 h after CtIP siRNA transfection. Cell lysates were immunoblotted with anti-phospho-Chk1 (anti-pS317) or anti-Chk1 antibodies.
DISCUSSION
CtIP was previously identified as a BRCA1-interacting protein (14, 31, 39). The role of CtIP in DNA damage checkpoint control has not been studied. Here, we provide evidence demonstrating that CtIP is involved in BRCA1-dependent G2/M transition checkpoint after DNA damage. It appears that CtIP is only phosphorylated and associated with BRCA1 in G2 cells. The G2-phase-specific BRCA1/CtIP complex is required for Chk1 phosphorylation and the G2/M transition after DNA damage, suggesting that CtIP cooperates with BRCA1 in cell cycle checkpoint control.
The BRCA1 BRCT domain recognizes phospho-peptides (18, 19, 38). Here, we mapped the phosphorylation site of CtIP that is critical for its interaction with BRCA1. The CtIP phosphorylation site matches perfectly with the consensus BRCA1-binding motif based on in vitro studies on the BRCA1 BRCT domain (18, 19, 38). This confirms that the BRCA1 BRCT domain recognizes a conserved phospho-motif with valine or threonine at position +2 and phenylalanine at position +3. We also measured the affinity between the recombinant BRCT domain and the phospho-CtIP peptide. The Kd is fivefold higher than that between BRCT and BACH1, suggesting that BRCA1/CtIP complex is less stable (unpublished data). This probably explains that BRCA1/CtIP complex is only transiently formed in G2 cells when CtIP protein level is also accumulated. On the contrary, BRCA1/BACH1 complex is much more stable and exists in S-to-M-phase cells.
The Ser327 site of CtIP is phosphorylated mainly in G2 phase, which is distinct from the phosphorylation of BACH1 at Ser990. The phosphorylation of Ser327 is cell cycle regulated and also appears to correlate with the expression level of CtIP. Since the Ser327 site is a Ser-Pro site, a preferred phosphorylation site by cyclin-dependent kinases, it is possible that CtIP is phosphorylated by one or more of the cyclin-dependent kinases in G2 phase. Although the Ser327 phosphorylation of CtIP is required for the G2/M transition checkpoint, this phosphorylation event is not induced by DNA damage (data not shown). One hypothesis is that the phosphorylation of CtIP at Ser327 site may be required for its further phosphorylation after DNA damage. It is likely that the Ser327 phosphorylation of CtIP and its association with BRCA1 may facilitate the ATM/ATR-dependent phosphorylation of CtIP (15). In support of this hypothesis, recent studies suggest that the damage-induced phosphorylation of CtIP requires BRCA1 (11).
Distinct BRCA1 complexes existing in different cell cycle stages reflect the diverse functions conducted by the BRCA1 BRCT domain and its binding partners. In the present study, we characterize two different BRCA1 BRCT domain-dependent G2 checkpoints. Although BRCA1/BACH1 is important for prolonged G2 accumulation after DNA damage, BRCA1/CtIP only exists transiently in G2 and is required for the transient G2/M checkpoint. These two different G2 checkpoints may be initiated by ATM and ATR, respectively (3). Both ATM and ATR regulate BRCA1 after DNA damage (29). Our data provide the molecular mechanism for how ATM/ATR and BRCA1 control two distinct checkpoints. We postulate that the G2/M transition checkpoint controls the entering of late G2 cells to mitosis after DNA damage, whereas the G2 accumulation checkpoint reflects how S and early G2 cells response to DNA damage. We demonstrate here that BRCA1, acting together with CtIP or BACH1, controls these two distinct checkpoints after DNA damage.
Failures in DNA damage checkpoint controls allow cells to enter next cell cycle phase without proper DNA repair and subsequently lead to genomic instability. Because of their roles in the maintenance of genomic integrity, many cell cycle checkpoint proteins, including p53, BRCA1, ATM, and Chk2, are also tumor suppressors. It is not yet clear whether CtIP functions as a tumor suppressor. Missense mutations of CtIP have been identified in several cancer cell lines (31). Genetic studies also indicate that CtIP might be the gene targeted in colon cancer with microsatellite instability (27). A C-terminal truncation mutation of CtIP has been reported in these tumors (27). Interestingly, this truncated form of CtIP still retains the BRCA1 BRCT domain-binding site. The Ser327 site of this truncated CtIP is phosphorylated in vivo, and this mutant still interacts with BRCA1. However, it loses the ability to control G2/M transition after DNA damage (unpublished data), suggesting that this mutant may function as a dominant-negative mutant in cancer cells. All of these findings raise the question of whether CtIP is a bona fide tumor suppressor. Germ line mutations of BACH1, the other BRCA1 BRCT domain-binding partner, were identified in familial breast cancer patients, suggesting that BACH1, like BRCA1, may function as a tumor suppressor (4). Further genetic analysis of CtIP will reveal whether CtIP contributes to tumorigenesis in breast, ovarian, or other cancers.
Taken together, our data not only unveil the physiological function of CtIP but also provide molecular mechanisms by which BRCA1 controls G2/M checkpoints. More importantly, the functional specificity of various BRCT-domain containing proteins and their corresponding binding partners is likely to be essential for our understanding of other cellular processes.
Acknowledgments
We thank D. M. Livingston and R. Baer for valuable reagents. We thank Carolin Merkle and Katherine Minter-Dykhouse for proofreading the manuscript.
This study is supported in part by grants from the National Institutes of Health (CA89239 and CA92312), the Prospect Creek Foundation, and the Breast Cancer Research Foundation. J.C. received a DOD breast cancer career development award (DAMD17-02-1-0472).
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Botuyan, M. V., Y. Nomine, X. Yu, N. Juranic, S. Macura, J. Chen, and G. Mer. 2004. Structural basis of BACH1 phosphopeptide recognition by BRCA1 tandem BRCT domains. Structure 12:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, E. J., and D. Baltimore. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. Wahrer, D. C. Sgroi, W. S. Lane, D. A. Haber, and D. M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105:149-160. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. 2000. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 60:5037-5039. [PubMed] [Google Scholar]
- 6.Clapperton, J. A., I. A. Manke, D. M. Lowery, T. Ho, L. F. Haire, M. B. Yaffe, and S. J. Smerdon. 2004. Structure and mechanism of BRCA1 BRCT domain recognition of phosphorylated BACH1 with implications for cancer. Nat. Struct. Mol. Biol. 11:512-518. [DOI] [PubMed] [Google Scholar]
- 7.Cortez, D., S. Guntuku, J. Qin, and S. J. Elledge. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713-1716. [DOI] [PubMed] [Google Scholar]
- 8.Cortez, D., Y. Wang, J. Qin, and S. J. Elledge. 1999. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286:1162-1166. [DOI] [PubMed] [Google Scholar]
- 9.Deming, P. B., C. A. Cistulli, H. Zhao, P. R. Graves, H. Piwnica-Worms, R. S. Paules, C. S. Downes, and W. K. Kaufmann. 2001. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA 98:12044-12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, C. X., and S. G. Brodie. 2000. Roles of BRCA1 and its interacting proteins. Bioessays 22:728-737. [DOI] [PubMed] [Google Scholar]
- 11.Foray, N., D. Marot, A. Gabriel, V. Randrianarison, A. M. Carr, M. Perricaudet, A. Ashworth, and P. Jeggo. 2003. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 22:2860-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan, S., D. P. Silver, R. A. Greenberg, D. Avni, R. Drapkin, A. Miron, S. C. Mok, V. Randrianarison, S. Brodie, J. Salstrom, T. P. Rasmussen, A. Klimke, C. Marrese, Y. Marahrens, C. X. Deng, J. Feunteun, and D. M. Livingston. 2002. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell 111:393-405. [DOI] [PubMed] [Google Scholar]
- 13.Lee, E. Y. 2002. BRCA1 and Chk1 in G2/M checkpoint: a new order of regulation. Cell Cycle 1:178-180. [PubMed] [Google Scholar]
- 14.Li, S., P. L. Chen, T. Subramanian, G. Chinnadurai, G. Tomlinson, C. K. Osborne, Z. D. Sharp, and W. H. Lee. 1999. Binding of CtIP to the BRCT repeats of BRCA1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J. Biol. Chem. 274:11334-11338. [DOI] [PubMed] [Google Scholar]
- 15.Li, S., N. S. Ting, L. Zheng, P. L. Chen, Y. Ziv, Y. Shiloh, E. Y. Lee, and W. H. Lee. 2000. Functional link of BRCA1 and ataxia telangiectasia gene product in DNA damage response. Nature 406:210-215. [DOI] [PubMed] [Google Scholar]
- 16.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig, T., P. Fisher, S. Ganesan, and A. Efstratiadis. 2001. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 15:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manke, I. A., D. M. Lowery, A. Nguyen, and M. B. Yaffe. 2003. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302:636-639. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez, M., X. Yu, J. Chen, and Z. Songyang. 2003. Phosphopeptide binding specificities of BRCA1 COOH-terminal (BRCT) domains. J. Biol. Chem. 278:52914-52918. [DOI] [PubMed] [Google Scholar]
- 20.Rouse, J., and S. P. Jackson. 2002. Interfaces between the detection, signaling, and repair of DNA damage. Science 297:547-551. [DOI] [PubMed] [Google Scholar]
- 21.Schaeper, U., T. Subramanian, L. Lim, J. M. Boyd, and G. Chinnadurai. 1998. Interaction between a cellular protein that binds to the C-terminal region of adenovirus E1A (CtBP) and a novel cellular protein is disrupted by E1A through a conserved PLDLS motif. J. Biol. Chem. 273:8549-8552. [DOI] [PubMed] [Google Scholar]
- 22.Scully, R., and D. M. Livingston. 2000. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 408:429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiozaki, E. N., L. Gu, N. Yan, and Y. Shi. 2004. Structure of the BRCT repeats of BRCA1 bound to a BACH1 phosphopeptide: implications for signaling. Mol. Cell 14:405-412. [DOI] [PubMed] [Google Scholar]
- 24.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, and M. Nakanishi. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 25.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 27.Vilkki, S., V. Launonen, A. Karhu, P. Sistonen, I. Vastrik, and L. A. Aaltonen. 2002. Screening for microsatellite instability target genes in colorectal cancers. J. Med. Genet. 39:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walworth, N. C. 2001. DNA damage: Chk1 and Cdc25, more than meets the eye. Curr. Opin. Genet. Dev. 11:78-82. [DOI] [PubMed] [Google Scholar]
- 29.Welcsh, P. L., and M. C. King. 2001. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 10:705-713. [DOI] [PubMed] [Google Scholar]
- 30.Williams, R. S., M. S. Lee, D. D. Hau, and J. N. Glover. 2004. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat. Struct. Mol. Biol. 11:519-525. [DOI] [PubMed] [Google Scholar]
- 31.Wong, A. K., P. A. Ormonde, R. Pero, Y. Chen, L. Lian, G. Salada, S. Berry, Q. Lawrence, P. Dayananth, P. Ha, S. V. Tavtigian, D. H. Teng, and P. L. Bartel. 1998. Characterization of a carboxy-terminal BRCA1 interacting protein. Oncogene 17:2279-2285. [DOI] [PubMed] [Google Scholar]
- 32.Wu-Baer, F., and R. Baer. 2001. Effect of DNA damage on a BRCA1 complex. Nature 414:36.. [DOI] [PubMed] [Google Scholar]
- 33.Xu, B., S. Kim, and M. B. Kastan. 2001. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21:3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu, B., S. T. Kim, D. S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 22:1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, X., Z. Weaver, S. P. Linke, C. Li, J. Gotay, X. W. Wang, C. C. Harris, T. Ried, and C. X. Deng. 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3:389-395. [DOI] [PubMed] [Google Scholar]
- 36.Yarden, R. I., S. Pardo-Reoyo, M. Sgagias, K. H. Cowan, and L. C. Brody. 2002. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 30:285-289. [DOI] [PubMed] [Google Scholar]
- 37.Yu, X., and R. Baer. 2000. Nuclear localization and cell cycle-specific expression of CtIP, a protein that associates with the BRCA1 tumor suppressor. J. Biol. Chem. 275:18541-18549. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X., C. C. Chini, M. He, G. Mer, and J. Chen. 2003. The BRCT domain is a phospho-protein binding domain. Science 302:639-642. [DOI] [PubMed] [Google Scholar]
- 39.Yu, X., L. C. Wu, A. M. Bowcock, A. Aronheim, and R. Baer. 1998. The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J. Biol. Chem. 273:25388-25392. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, H., J. L. Watkins, and H. Piwnica-Worms. 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA 99:14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]