Abstract
Bilingual language control may involve cognitive control, including inhibition and switching. These types of control have been previously associated with neural activity in the inferior frontal gyrus (IFG) and the anterior cingulate cortex (ACC). In previous studies, the DRD2 gene, related to dopamine availability in the striatum, has been found to play a role in neural activity during cognitive control tasks, with carriers of the gene’s A1 allele showing different patterns of activity in inferior frontal regions during cognitive control tasks than non-carriers. The current study sought to extend these findings to the domain of bilingual language control. Forty-nine Spanish-English bilinguals participated in this study by providing DNA samples through saliva, completing background questionnaires, and performing a language production task (picture-naming), a non-verbal inhibition task (Simon task), and a non-verbal switching task (shape-color task) in the fMRI scanner. The fMRI data were analyzed to determine whether variation in the genetic background or bilingual language background predicts neural activity in the IFG and ACC during these three tasks. Results indicate that genetic and language background variables predicted neural activity in the IFG during English picture naming. Variation in only the genetic background predicted neural activity in the ACC during the shape-color switching task; variation in only the language background predicted neural activity in the ACC and IFG during the Simon task. These results suggest that variation in the DRD2 gene should not be ignored when drawing conclusions about bilingual verbal and non-verbal cognitive control.
Keywords: DRD2, Bilingualism, Cognitive Control, Language Control
Graphical Abstract
1.1 Spanish-English bilinguals and the DRD2 gene
While the history of psychological research involves studying and measuring observable behaviors, investigations in neuroscience are key to uncovering the mechanisms connecting experiences and genes, nature and nurture, to these behaviors. However, researchers studying bilingualism have focused their attention only on bilingual experiences, ignoring the individual differences in neural activity that may result from genetic background. The current study takes an interdisciplinary approach to examine the role of the genetic background and bilingual experience in predicting fMRI activity during language control and cognitive control tasks. This study follows up on preliminary evidence from Hernandez, Greene, Vaughn, Francis, and Grigorenko (2015), which suggests that there may be important genetic variation between groups of Spanish-English bilinguals and English monolinguals.
Hernandez and colleagues (2015) pointed out that Spanish-English bilinguals may differ from their monolingual peers in the frequency distribution of the genotypes of the DRD2/ANKK1 Taq1A polymorphism. The Spanish-English bilingual group in Hernandez and colleagues’ sample contained twice as many carriers of the A1 allele than the English monolingual sample. This polymorphism is associated with variation in dopamine reuptake in the striatum: individuals with the A1 allele of this gene have fewer dopamine receptors in the striatum (Ritchie & Noble, 2003). In an experimental study in which participants were given either the striatal dopamine agonist bromocriptine or a placebo, those who were given bromocriptine showed activity in the left inferior frontal junction (IFJ) to a greater extent during a cognitive control measure of task switching than those given the placebo (Stelzel, Fiebach, Cools, Tafazoli, & D’Esposito, 2013). These results indicate that increased dopamine presence in the striatum can result in increased frontal activity during cognitive control tasks. Stelzel, Basten, Montag, Reuter, and Fiebach (2010) also found that variation in the DRD2/ANKK1 Taq1A polymorphism is associated with activity in the inferior frontal junction (IFJ, MNI: x = −54, y = 8, z = 38) during the same task. Specifically, activity for switching tasks in comparison to repeating the same task is greater in the IFJ for individuals who do not carry the A1 allele than individuals who carry the A1 allele. When interpreting these seemingly contradictory results, it is important to understand that the administration of bromocriptine is a short-term effect, whereas DRD2 genotype is a lifelong factor impacting the brain. The authors of these studies suggest that over time, the fronto-striatal dopamine system may decrease dopamine production if DRD2 receptors are reduced, as is seen in the A1 carriers. Therefore, although in the short term, having fewer dopamine receptors may lead to a greater presence of dopamine in the striatum, in the long-run, the system may adapt so that fewer receptors may be related to decreased dopamine presence.
The above studies focused on non-verbal control, but to date, no studies have examined how the variation at the Taq1A restriction site of the DRD2 gene might be associated with bilingual language control. Studies have suggested that the bilingual language experience can shape the brain to better handle domain-general cognitive control (e.g., Abutalebi et al., 2012). If there are genetic differences that also underlie neural activity during cognitive control tasks, and the distribution of genotypes differs for bilinguals, the question then becomes what role does genetic variation play in predicting neural activity during a variety of cognitive control tasks? Disentangling the effects of genetic variation and bilingual language background in language control and domain-general cognitive control is the focus of the current study.
1.2 Language control and domain-general cognitive control
A direct comparison of brain activity relating language control and domain-general control comes from Weissberger, Gollan, Bondi, Clark, and Wierenga (2015), who compared bilingual language switching to a task involving naming the shape or color of an image presented. Similar brain areas were active when participants performed both tasks. Specifically, the switching aspect of both tasks led to nearly identical patterns of widespread neural activity, including frontal regions, sensory and motor regions, parietal regions, temporal and occipital regions, cingulate gyrus, insula, and subcortical regions (see supplementary material from Wiessberger et al., 2015 for a more detailed list). The staying aspect (staying in one language or staying with either the shape or color rule) in comparison with the switching aspect involved less activity for the language task, but not for the shape-color task. In other words, these bilinguals demonstrated less neural activity to stay in one language than to stay with one rule, or to switch between languages or rules. This study suggests that while the bilingual brain can adapt to language control, the adaptation may not necessarily transfer to non-verbal cognitive control.
Another study relating language and non-verbal control in terms of neural activity comes from Abutalebi and colleagues (2012), who compared monolingual and bilingual verbal switching and conflict monitoring. Verbal switching involved producing names of pictures in a cued language for bilinguals and producing nouns or verbs as cued for monolinguals. Conflict monitoring for both groups was measured using the computerized flanker task in which participants must determine the direction of a central arrow while ignoring the direction of arrows on either side of the target. Across bilinguals and monolinguals, both verbal switching and conflict monitoring involved the anterior cingulate cortex (ACC). The authors note that the monolinguals show increased activation in this region during conflict monitoring to a greater extent than bilinguals, suggesting that bilingual language experience conditions the ACC to perform the conflict monitoring task more efficiently.
Other studies have compared monolinguals and bilinguals in an attempt to determine how bilingual experience might change the neural processing of non-verbal cognitive control. Garbin and colleagues (2010) compared neural activity in bilinguals and monolinguals during a shape-color task similar to that used by Weissberger et al. (2015). For the switching condition, neural activity for the bilinguals was observed in the left inferior frontal gyrus (IFG), while for the monolinguals, activity in the right IFG, the ACC, and the left inferior parietal lobule was observed, suggesting that bilingualism may change the neural systems used for cognitive control. A similar study by Rodriguez-Pujadas and colleagues (2013) found that bilinguals showed more activity than monolinguals during the switching condition in the left IFG, left ACC, and bilateral caudate. It is important to note that these two studies involve group comparisons rather than within-subjects comparisons, which may ignore individual differences within the bilingual group such as language proficiency or age of second language acquisition (AOA). In other words, contradictory findings in comparisons of bilinguals and monolinguals may be better understood when the bilingual group is treated as heterogeneous, and differences in AOA and proficiency are taken into consideration.
If bilingual language experience changes the neural processing of non-verbal cognitive control, then bilinguals with earlier AOAs or higher proficiency in both languages should be more efficient in processing this information. To date, the only studies considering these language background differences in fMRI activity have examined bilingual language control. Abutalebi et al. (2013) found that bilinguals recruit the ACC and pre-supplemental motor area during language switching, but switching from a more proficient language to a less proficient language involved greater recruitment of the left caudate. Perani et al. (2003) demonstrated that bilinguals with lower proficiency in a second language show more activity in the left IFG while producing that language, and that the second language is related to increased activity in the left IFG relative to the first language. Studies to date have failed to examine how differences within the bilingual population relate to neural activity during non-verbal cognitive control. If language switching is related to non-verbal cognitive control, the same effects on language, with greater activity for the less proficient or late bilinguals, should be seen during non-verbal task switching. It is expected that learning the second language early in life and achieving a high proficiency in both the first and second language is related to less activity, or greater efficiency, in the IFG and ACC during language control and cognitive control tasks.
Relevant to the DRD2/ANKK1-TaqIa polymorphism of interest in this study, Stocco and Prat (2014) have found that bilinguals outperformed monolinguals on a novel rule-switching task, and that this enhanced performance was associated with increased activity in the basal ganglia. This study highlights the potential role of the basal ganglia, including the striatum, in bilingual non-verbal cognitive control, which will be further investigated in the current study through this DRD2 polymorphism related to striatal dopamine.
A few gaps arise from the previous research comparing language control to non-verbal control. First, studies should be conducted comparing neural activity of bilingual language control and non-verbal control with methodology that includes a wider variety of cognitive control tasks. The current study addresses this issue by including both a measure of inhibitory cognitive control and a measure of switching abilities and working memory, in order to measure non-verbal cognitive control. To assess language control, the current study uses a picture-naming task, in which participants name pictures in either English or Spanish, or switch between the two languages. This task is similar to that used by previous researchers (Abutalebi et al., 2012; Hernandez, Dapretto, Mazziotta, & Bookheimer, 2001; Weissberger et al., 2015). In addition, previous research has not examined how individual differences within the bilingual population could be related to neural activity during non-verbal cognitive control, which is a goal of the current study.
Relevant to the goals of this study, it is important to also consider that linguistic factor and non-linguistic factors may have unique influences on the structure and function of the brain during domain-general cognitive control. In a review of the structural brain changes that occur as a result of linguistic and non-linguistic training, Li, Legault, and Litcofsky (2014) describe grey matter density increases in the frontal lobe found to be induced by linguistic training, and increases in the parietal lobe found to be induced by non-linguistic training. It is unclear whether these results will align with functional differences in the current study, but this review does suggest that linguistic and non-linguistic factors may be related to distinct brain regions in the frontal-parietal network predicted to be involved in cognitive control tasks.
1.3 Racial/Ethnic Confounds
Variations in genetic makeup are known to differ based on race or ethnicity (Ossorio & Duster, 2005). Therefore, when studying the role of this genetic variant in neural activity, it is important to control for race and ethnicity. The previous study by Hernandez and colleagues (2015) compared a group of Hispanic bilinguals to a group of Caucasian, non-Hispanic monolinguals. The ethnic difference between these two groups is a potential explanation for the differing distributions of genotypes in the sample (i.e., more A1 carriers than non-carriers in the bilingual sample and the reverse pattern in the monolingual sample). The current study will focus exclusively on the Hispanic bilingual group in order to determine the role of both genetic variation and language background without confounding these effects with racial or ethnic differences (i.e., not comparing Hispanic bilinguals to Caucasian monolinguals). If genetic variation within this sample of bilinguals is predictive of neural activity during language and non-verbal cognitive control tasks, future studies should consider racial and ethnic differences between their bilingual and monolingual samples in order to avoid potential genetic differences, on top of any other cultural or social differences that may exist between different racial and ethnic groups.
1.4 Current study
The current study examines both language control and non-verbal control in a group of Spanish-English bilinguals. These bilinguals completed a picture-naming task in English, Spanish, and a mixed condition as a measure of language control. They also completed a shape-color switching task (similar to Rodriguez-Pujadas et al., 2013) and the Simon task (Simon & Rudell, 1967). The focus of this study is to determine whether the genetic variation (or, rather, the variation in a single candidate gene at a single polymorphism) or in language background predict neural activity in the IFG and ACC during these tasks. These regions of interest (ROIs) were selected based on their recurrence throughout the previously conducted studies with bilinguals and the DRD2/ANKK1 Taq1A polymorphism. Specifically, inferior frontal regions (gyrus and junction) have been implicated both in studies investigating the role of the DRD2 gene (Stelzel et al., 2010; Stelzel et al., 2013) and studies focused on bilingualism (Garbin et al., 2010; Perani et al., 2003; Rodriguez-Pujadas et al., 2013). The ACC has been one of the major areas associated with bilingual language control and cognitive control (Abutalebi et al., 2012; Abutalebi et al., 2013; Garbin et al., 2010; Rodriguez-Pujadas et al., 2013), and has been investigated in relation to the DRD2 genotype in one study by Fossella, Green, and Fan (2006), who found that A1 carriers showed less activity than non-carriers in the ACC during the attentional networks task, a non-verbal measure of cognitive control. Therefore, it is important to understand whether genetic differences are associated with differences in the neural activity of the ACC as well. The purpose of this study was to help determine whether experience with two languages shapes the brain for language and non-verbal control, or whether the genetic variation within the Hispanic, Spanish-English bilingual population equips the bilingual brain to handle language and non-verbal control. If language experience is essential for the development of language and non-verbal control, the results should indicate that, controlling for variation in the DRD2/ANKK1 Taq1A polymorphism, age of second language acquisition and proficiency in each language predict neural activity during language and non-verbal control. If genetic variation alone can explain differences in neural activity during these tasks that have previously been attributed to bilingualism, the results should indicate that, regardless of age of acquisition and proficiency in each language, activity during language and non-verbal switching in the bilingual brain can be predicted by whether an individual is a carrier or non-carrier of the A1/T allele of the DRD2/ANKK1 Taq1A polymorphism. It is also possible that bilingualism and genetic background both play a role in verbal and non-verbal cognitive control, and that considering the effects of both is an important next step in understanding how the brain handles cognitive control tasks. The current study tested these hypotheses.
2. Method
2.1 Participants
All 49 participants were right-handed Spanish-English bilinguals (34 females) recruited from the University of Houston (mean age = 23.27, SD = 4.4). All indicated that they and their parents were of Latino or Hispanic ethnicity. Information about genetic ancestry was not collected from participants, but information about birthplace indicated that 35 participants were born in the USA, ten were born in Mexico, three were born in Venezuela, and one was born in Colombia. This sample is representative of the University of Houston student population in terms of country of origin; the most popular Hispanic country of origin is Mexico, followed by Venezuela. Compensation included gift cards or class credit. Age of English acquisition ranged from 0 to 17 (mean age = 7.02 years, SD = 3.5), and was assessed with an in-house questionnaire. Participants reported socioeconomic status based on parental education (mother and father) on a scale from 1–6, where 1 = Some elementary school or less, 2 = Some high school or less, 3 = High school graduate, 4 = Some college, 5 = College graduate, 6 = Advanced degree. The average socioeconomic status of our participants was 3.01 (high school graduate) with a standard deviation of 1.48. Participants were also screened for exclusionary criteria preventing them from participating in the fMRI study (e.g., claustrophobia), for handedness, socioeconomic status, and language history. All participants reported no history of psychiatric or neurological disorders and had normal or corrected-to-normal vision.
Six participants were excluded due to unusable DNA samples. Of the 43 participants who provided usable DNA samples, one participant was removed from the analyses of all three tasks due to problems with preprocessing the fMRI images. In addition, one participant was excluded from the picture naming, three participants were excluded from the shape-color task, and three participants were excluded from the Simon task for the same reason. The results presented, therefore, include n=41 for the picture-naming task and n=39 each for the shape-color task and the Simon task. Table 1 displays the proportion of A1 carriers, as well as the demographic information for the participants included in the analyses of each task. A1 carriers were defined as those individuals who had at least one A1/T allele.
Table 1.
Descriptive statistics for participants included in the analyses for each task.
| Picture Naming | Shape-Color | Simon | |
|---|---|---|---|
| Sample Size | 41 | 39 | 39 |
| Number of A1 Carriers | 26 | 24 | 24 |
| Mean AOA | 7.17 (3.69) | 7.08 (3.8) | 7.05 (3.78) |
| Mean English Proficiency * | 75.89 (7.08) | 76.06 (6.90) | 76.27 (6.74) |
| Mean Spanish Proficiency * | 76.10 (10.51) | 75.56 (10.66) | 76.11 (10.31) |
Mean proficiency in each language determined by the sum of the raw scores from the picture vocabulary and passage comprehension subtests of the Woodcock-Muñoz Language Survey - Revised
On average, proficiency in English and Spanish was equal for this group of bilinguals; however, there was much more variability in Spanish proficiency than English proficiency (see Figure 4). See Figure 5 for distribution of Spanish and English proficiency scores on the Woodcock-Muñoz Language Survey-Revised based on age of English acquisition.
Figure 4.
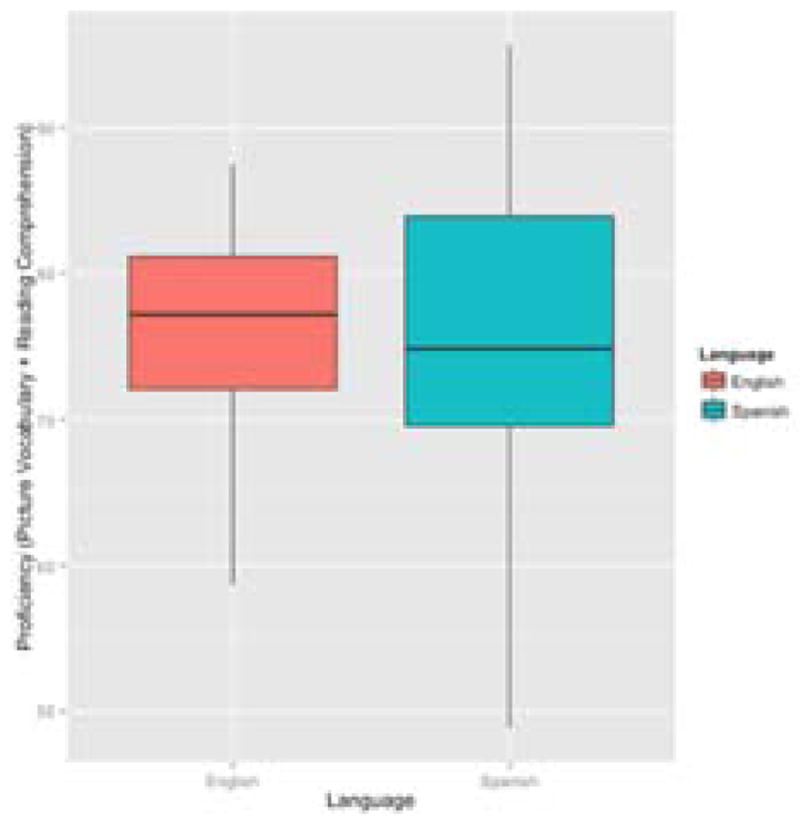
Distribution of English and Spanish Proficiency Scores
Figure 5. Relationship Between Age of English Acquisition and English and Spanish Proficiency.
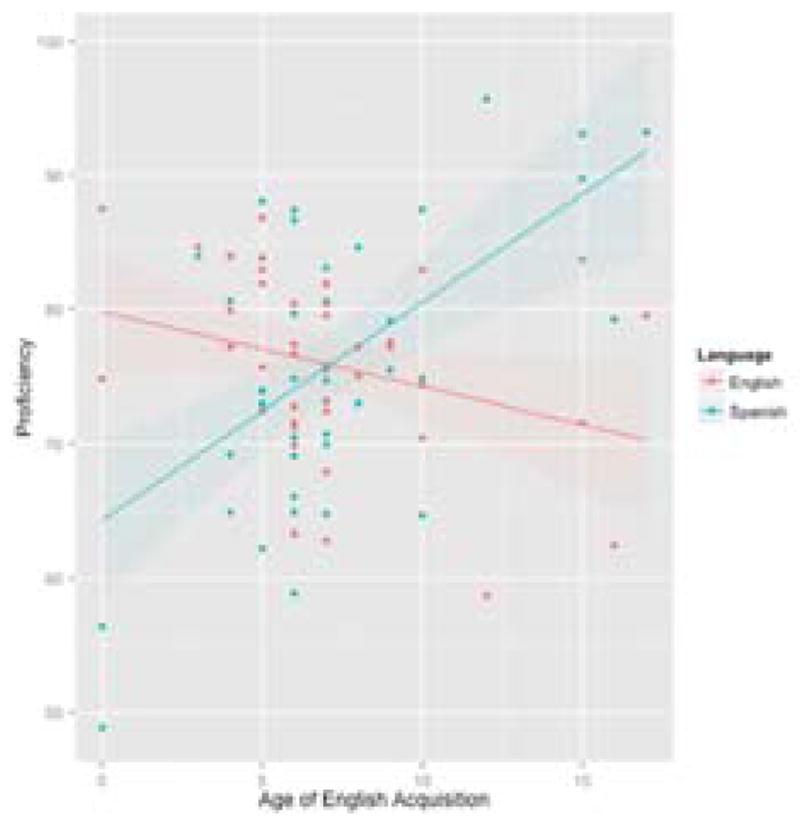
This graph shows the relationship between the age at which these Spanish-English bilinguals began learning English and their achieved proficiency in both English and Spanish. Bilinguals who began learning English earlier in life achieved a proficiency in English that exceeded their Spanish proficiency. After about the age of seven, learning English no longer appears to be detrimental to learning Spanish. Bilinguals maintained a higher Spanish than English proficiency when English was introduced later in life.
2.2 Materials
2.2.1 Language History Questionnaire
Bilingual participants were screened with a language history questionnaire in order to ensure they qualified as Spanish-English bilinguals for the current experiment. The questionnaire included questions about daily language usage, age of acquisition of the second language, self-rated proficiency, and knowledge of additional languages.
2.2.2 Woodcock-Muñoz Language Survey-Revised
Participants’ language proficiency was measured using the picture vocabulary and passage comprehension subtests of the Woodcock-Muñoz Language Survey-Revised test (Woodcock, Muñoz-Sandova, Ruef, & Alvarado, 2005). For the vocabulary subscale, participants were required to name a series of up to 59 pictures. For the passage comprehension subscale, participants read up to 33 sentences that were missing a word and were asked to fill in the missing word with one that made sense in the context of the sentence. Both subscales were completed in English and Spanish and increased in difficulty as they progressed. Researchers ended the testing if participants missed 4 or more items in a row. Summing the scores on both subscales in English and Spanish led to an English proficiency score and a Spanish proficiency score, each out of 92 possible points.
2.2.3 Picture Naming Task
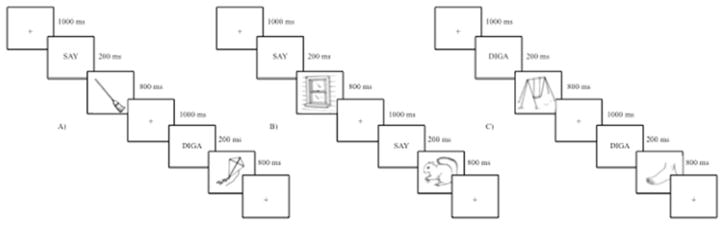
The current experiment used a picture-naming paradigm with 80 black and white line drawings from the UCSD Center for Research in Language International Picture Naming Project normalized database (Bates et al., 2003). The pictures were all concrete objects selected and matched across conditions based on high frequency, word length, and imageability. The task was divided into three conditions: mixed (naming switched from Spanish to English and vice versa), Spanish (naming in Spanish only) and English (naming in English only). The 80 pictures were all included in the mixed condition, but were divided randomly into a set of 40 pictures for each the English condition and the Spanish condition. The picture-naming task required participants to name a picture in Spanish or English. A given picture-naming trial began with a fixation cross presented on the screen for 1000 ms; then a 200 ms-cue appeared indicating which language should be used to respond (“diga” for Spanish and “say” for English). After the cue disappeared, a picture appeared and stayed on the screen for 800 ms at which point participants were required to name the picture as quickly and accurately as possible. This task was created with a block design, so that each naming condition was a single block, and the entire task consisted of 2 runs (see Figure 1).
Figure 1. Picture Naming Task Design.
A) Mixed block. Participants name pictures in English or Spanish based on the cues “diga” or “say.” B) English block. Participants name pictures in English following the cue “say.” C) Spanish block. Participants name pictures in Spanish following the cue “diga.”
2.2.4 Shape-Color Task
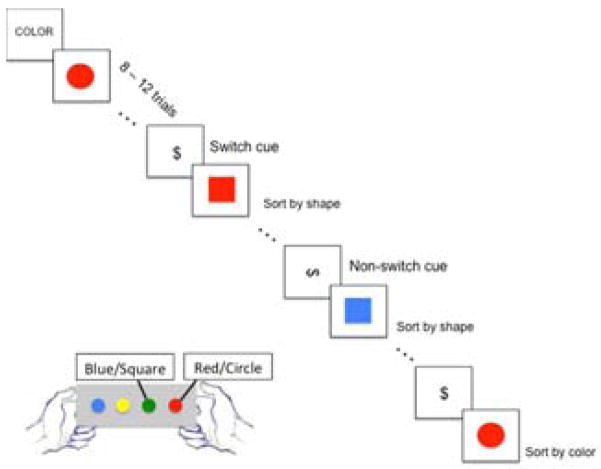
As a measure of the switching aspect of cognitive control, participants completed a shape-color task with stimuli that consisted of circles or squares that were either red or blue, presented one at a time in the center of the screen. The goal of the task was to distinguish stimuli based on either their color or their shape. The rule of response determined which stimulus characteristic (color or shape) participants responded to. In the color response, participants responded to whether the stimuli were red or blue, and in the shape response, participants responded to whether the stimuli were circles or squares. Participants were asked to press one of two buttons when a stimulus was presented. One button would be pressed for a red or circle stimulus, and another would be pressed for a blue or square stimulus. The specific buttons used (left or right) were counter-balanced across participants. The task was divided into switch and repeat conditions. Repeat trials immediately followed a cue (horizontal dollar sign), indicating to participants that they should continue responding to the stimulus according to the rule they were using before (color or shape). Switch trials immediately followed a cue (vertical dollar sign) signaling participants to switch their responses from one aspect of the stimulus (e.g., color) to the other (e.g., shape). In order to calculate behavioral switch costs, response times for each trial immediately following a vertical dollar sign (switch) were averaged, and the same was done for response times for each trial immediately following a horizontal dollar sign (repeat). The difference in response times for these two conditions was considered the “switch cost.” The design of this task was event-related, with the cues occurring after 8–12 trials (see Figure 2). Each participant completed 5 runs of this task. The order of the runs was counterbalanced across participants. Each run included 7 switch cues and 7 repeat cues, leading to a total of 35 repeat cues and 35 switch cues, which were entered into fMRI analyses.
Figure 2. Shape-Color Task Design.
Participants respond to either the shape (circle/square) or color (red/blue) of the image by pressing a left or right button. When the dollar sign cues appear, participants switch sorting rules (vertical dollar sign), or repeat the same sorting rule (horizontal dollar sign). This task was completed for 5 runs, with 14 cues presented per run.
2.2.5 Simon Task
In order to assess the inhibitory aspect of cognitive control, researchers administered the Simon task (Simon & Rudell, 1967). Stimuli in this task were circles that were either red or green, presented one at a time on the left, center, or right side of the screen. The goal of the task was to distinguish between red and green circles while ignoring their location. Responses were recorded via button presses on the left or right side of a button box. Responses were therefore divided into three conditions: congruent (same stimuli location as correct button press), incongruent (opposite stimuli location as correct button press), and neutral (stimuli located in the center) conditions (see Figure 3). The task included approximately 60 trials for each condition. This task involved an event-related design, with a varied interstimulus interval (ISI) of 2000, 4000, 6000, or 8000 ms.
Figure 3. Simon Task Design.
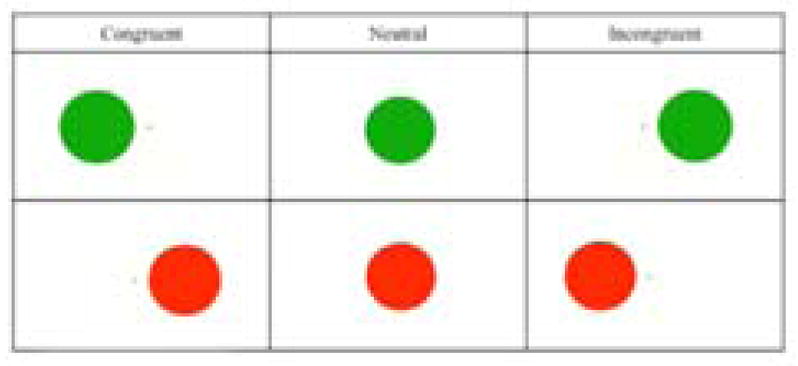
Participants press a right button for red circles and a left button for green circles. Congruent trials: red circles appearing on the right and left circles appearing on the left. Incongruent trials: red circles appearing on the left and green circles appearing on the right. Neutral trials: circles appearing in the center of the screen. Color-button associations were counterbalanced across participants.
2.2.6 FMRI Parameters
Structural and functional MRI data was collected at the Core for Advanced MR Imaging (CAMRI) at Baylor College of Medicine using a 3.0 Tesla Siemens Magnetom Trio scanner. The researchers collected a localizer scan from each participant to determine their head position. The MRI scanning took place across two sessions. One session included an anatomical scan and eight epi sequences to collect functional images during each the shape-color task (five runs) and the Simon task (three runs). The parameters for the shape-color fMRI images were: 34 slices with a voxel size of 3.4×3.4×4.0, TR = 2500ms, TE = 30ms, flip angle = 90°, and a 64×64 matrix. The Simon task fMRI parameters were: 34 slices with a voxel size of 3.4×3.4×4.0, TR = 2000ms, TE = 30ms, flip angle = 90°, and a 64×64 matrix. The second session included an anatomical scan and two echo-planar imaging sequence (epi) of functional images collected during the block-designed picture-naming task. These functional images contained 34 slices and had a voxel size of 3.4×3.4×4.0, TR = 2000ms, TE = 30ms, flip angle = 90°, and a 64×64 matrix. The anatomical images were high-resolution T1-weighted images acquired using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with the following parameters: voxel size 0.48 × 0.48 ×1.00 mm, TR = 1200ms, TE = 2.66ms reconstructed into 192 slices.
2.2.7 DNA Samples
Participants provided 2mL saliva samples into Oragene (OG-500) kits from DNA Genotek. This kit consists of a tube that participants were asked to fill with saliva to a “fill line.” Then, participants closed the lid on the top of the tube, unscrewed the top, and replaced it with a small cap. They then shook the tube for five seconds and returned it to the researcher. This is a simple, non-invasive way to collect DNA samples.
2.3 Procedure
The University of Houston’s Institutional Review Board approved the procedure for this study. Participation in this study involved three sessions: one screening session at the University of Houston and two MRI scanning sessions at Baylor College of Medicine’s CAMRI. During the screening session, participants gave informed consent and completed the paper questionnaires, screenings, and proficiency assessments. Then, participants for whom it was safe to complete the MRI scanning returned for the two MRI sessions separated by approximately a week. The first MRI session involved both non-verbal tasks, the shape-color task and the Simon task, and during the second MRI session, participants completed the picture-naming task. A localizer scan allowed researchers to check the participant’s head alignment in the scanner. Then researchers collected a four-and-a-half minute anatomical scan, during which the participants were encouraged to relax and were shown a short movie clip. The shape-color and Simon tasks were counterbalanced across participants during this session. The shape-color task involved 5 runs, and each run lasted 4 minutes and 20 seconds. Between runs, the researcher ensured that the participants were still comfortable in the scanner and did not have any trouble with the task. The Simon task consisted of 3 runs, which lasted less than 9 minutes each. Because the Simon task involved a variable ISI, the exact length of each run varied. The picture-naming task, administered during the second MRI session, consisted of two 10-minute runs each containing four one-minute and 20 second naming blocks including one Spanish, one English, and two mixed, and five one-minute rest periods between blocks in which participants saw the following message: “Rest. Keep your eyes open”. Each condition block lasted one minute and 20 seconds and the rest periods lasted one minute. The order of block presentation during the picture-naming task was counterbalanced in order to avoid order effects. The Simon task, shape-color task, and picture-naming task were each presented on a Windows computer using E-Prime 2 (Psychology Software Tools, Inc., 2012), which was projected onto a screen in the back of the MRI scanner. Participants could view the screen through a mirror positioned on top of the head coil. Upon completion of the MRI scans, participants received compensation and a CD of their anatomical images. They also provided saliva samples for DNA analyses.
2.4 Analysis
DNA kits from participants of this study were analyzed with a larger sample of participants for whom MRI data was not available. Genomic DNA for the larger sample was isolated from 190 Oragene Saliva Collection Kits and assessed for quality and 181 of the DNAs for these participants met the minimum requirement for further analysis. Genotyping was performed on a total of 188 DNAs, 7 of which were randomly chosen as technical replicates to verify protocol efficiency, and analyzed using a custom GoldenGate® Genotyping Assay Panel (Illumina Inc.) consisting of 96 single-nucleotide polymorphism (SNP) markers across 31 genes located on 15 chromosomes (see Supplementary Table 1). Raw data were generated at the Yale Center for Genomic Analysis using Illumina’s standard GoldenGate genotyping protocols and scanned on an Illumina iScan. Raw data were then clustered using Illumina’s GenomeStudio software generating allelic differentiation for each marker. From the panel generated, the genetic marker for the DRD2/ANKK1-Taq1A polymorphism was selected a priori for further analyses.
All fMRI data was analyzed using Analysis of Functional Neuroimages (AFNI) software (Cox, 1996). The first two scans during each scanning sequence (i.e., run/block) were dropped from analyses. The remaining scans were processed using AFNI’s proc.py procedure, which involved slice timing alignment, volume registration, blurring to 4mm fwhm, aligning the anatomical and functional scans, and warping to the standard Talairach space. Stimuli onset times for each task were used to create contrasts for the cognitive control components of each task. For the Simon task, the key cognitive control component was the difference between the incongruent and congruent conditions. For the shape-color task, the key cognitive control component was the difference between seeing a switch cue and seeing a repeat cue. For the picture-naming task, all three conditions (English, Spanish, and Mixed) may require some level of cognitive control, so each was considered separately. Previous research has demonstrated that bilingual production of a single language may require inhibition of the other language (e.g., Guo, Liu, Misra, & Kroll, 2011). Research has also demonstrated that mixed production of a bilingual’s two languages may involve a switching component of cognitive control (e.g., Hernandez, Dapretto, Mazziotta, & Bookheimer, 2001). Therefore, the mixed conditions and the two single language conditions were each considered separately. The specific comparisons made were: Mixed vs. Spanish & English, English vs. Spanish & Mixed, and Spanish vs. English & Mixed.
Once the contrasts were created for each task for each participant, the images were corrected using a false detection rate (FDR) method, and anatomical ROI masks for the left and right ACC and IFG were created from the Destrieux, Fischl, Dale, and Halgren (2010) atlas. The ACC was defined as the “G_and_S_cingul-Ant” region from this atlas, and the IFG was defined as both the “G_front_inf-Triangul” and the “G_front_inf-Opercular.” The t-statistic for the contrasts within each of these ROIs for each participant was extracted from AFNI. Each of these measures of neural activity was entered into a regression model using R statistics software (R Core Team, 2015) to determine the unique contributions of the A1 allele and the language background variables (English proficiency, Spanish proficiency, and AOA). Regression models took the form of: “A1 carrier status + AOA + English proficiency + Spanish proficiency = Contrast Activity in left/right ACC/IFG”. Separate regression models were calculated for each contrast and each ROI (left ACC, right ACC, left IFG, right IFG). These analyses in R were followed up with multiple regressions calculated in AFNI in order to determine the specific clusters or voxels within each ROI that were responsible for significant findings from the regression models calculated for each contrast.
3. Results
3.1 Picture naming results
Detailed results for each regression model are presented in Supplementary Table 2. Additional details about the significant clusters for each regression are presented in Supplementary Table 3. Effect sizes for each significant predictor are presented in Supplementary Table 4. For the picture-naming task, A1 carrier status and AOA significantly predict activity in the left IFG during naming in English, and A1 carrier status alone predicts activity in the right IFG during this condition. Each of these predictors has a positive relationship with activity, meaning that carriers of the A1 allele present with activity in left and right IFG to a greater extent than non-carriers, and individuals who learned English later in life show more activity in the left IFG than those who learned English earlier (see Figure 6). There were no relationships between the genetic or language variables and activity in the left or right ACC during English naming. Neither A1 carrier status nor language variables predicted activity in the ACC and IFG during picture naming in Spanish, nor when switching between Spanish and English.
Figure 6. IFG activity relationships during English Picture Naming.
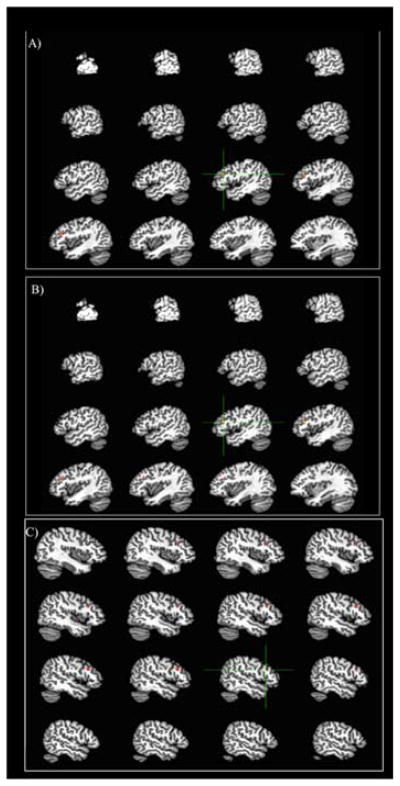
Red/yellow = positive relationship. Blue = negative relationship A) Left IFG relationship with A1 carrier status (carriers > non-carriers) controlling for AOA, English proficiency, and Spanish proficiency B) Left IFG relationship with AOA controlling for A1 carrier status, English proficiency, and Spanish proficiency C) Right IFG relationship with A1 carrier status (carriers > non-carriers) controlling for AOA, English proficiency, and Spanish proficiency
Attempts were made to collect voice recordings in response to the picture naming task in order to include behavioral results. Unfortunately, the voice recordings could not be analyzed because the microphone that was used in the scanner did not have noise cancellation properties; therefore the noise of the scanner washed out the voices of the participants in the recordings.
3.2 Shape-color results
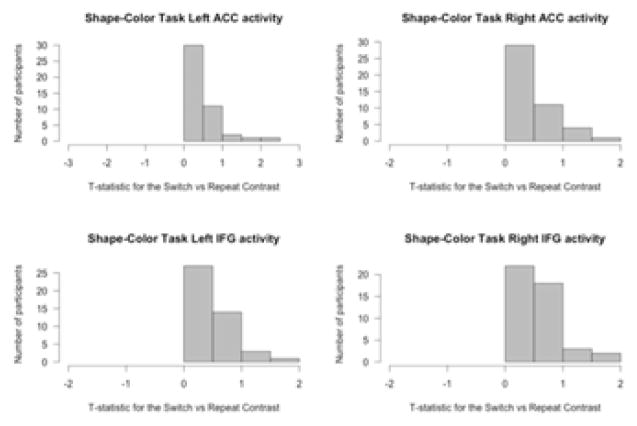
The shape-color task was examined in terms of the activity for the switch cues compared to activity for the repeat cues. All participants showed more activity for the switch cues than the non-switch cues in the ACC and IFG (see Figure 7). Activity in the left and right ACC was significantly predicted by A1 carrier status (see Figure 8). No other variables were significant. A1 carriers showed smaller differences in activity in the left and right ACC for the switch condition compared with the non-switch condition. Activity in the left and right IFG was not predicted by any of the variables in the regression model.
Figure 7. Histogram of t-statistics for differences in neural activity in the IFG and ACC for the contrast “switch cues – repeat cues” during the shape-color task.
All participants showed more activity for switch cues compared to repeat cues in the regions of interest.
Figure 8. ACC activity relationship during shape-color task.
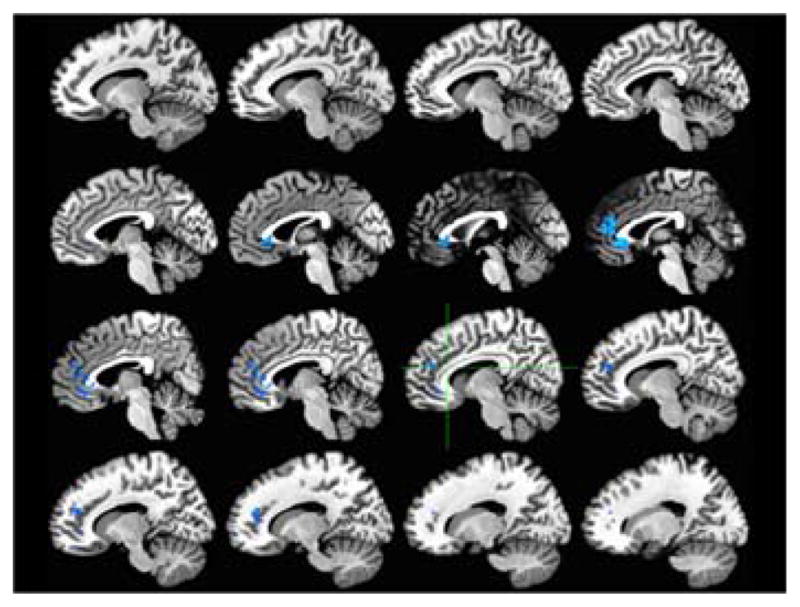
Relationship to A1 carrier status (carriers > non-carriers) for Switch - Repeat conditions Red/yellow = positive relationship. Blue = negative relationship
A paired-sample t-test for the behavioral results revealed significantly slower response times (RTs) for the first trials after the switch cue compared to the first trials after the repeat cue (average difference in RT = 33.70, t = 5.26, p < 0.0001). After the first trial, the response times for both conditions (switch and repeat) returned to baseline and no longer differed significantly (average difference in RT = −8.80, t = −1.65, p > 0.05). A multiple regression was conducted to predict the switch cost in the first trial after the cue from the variables: A1 carrier status, AOA, English proficiency, and Spanish proficiency. None of these variables significantly predicted the first trial switch cost during this task.
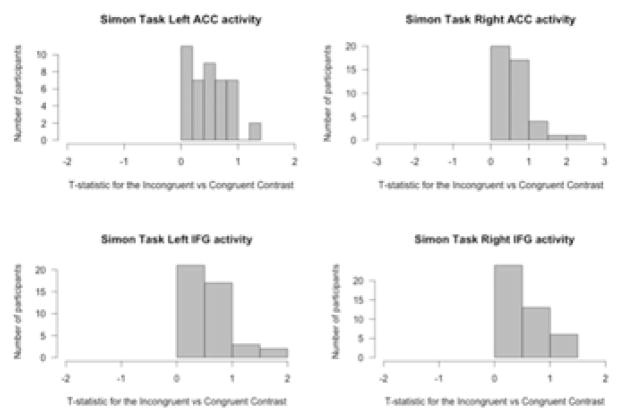
3.3 Simon results
The Simon task was examined in terms of the activity for the incongruent trials compared to activity for the congruent trials. All participants showed more activity for the incongruent trials than the congruent trials in the ACC and IFG (see Figure 9). For the Simon task, A1 carrier status was not a significant predictor of activity in any of the ROIs for the incongruent versus congruent condition. AOA, English proficiency, and Spanish proficiency all significantly predict activity in the left ACC, and AOA significantly predicts activity in the left IFG (see Figure 10). However, the significant clusters in the IFG are only 2 voxels each, and the effect size of this relationship is 0.05, so these results are difficult to interpret in any meaningful way. In the left ACC, earlier age of English acquisition, lower English proficiency, and higher Spanish proficiency are associated with more activity for the incongruent trials compared to the congruent trials. In the left IFG, earlier AOA is associated with more activity for the same comparison.
Figure 9. Histogram of t-statistics for differences in neural activity in the IFG and ACC for the contrast “incongruent trials – congruent trials” during the Simon task.
All participants showed more activity for incongruent trials compared to congruent trials in the regions of interest.
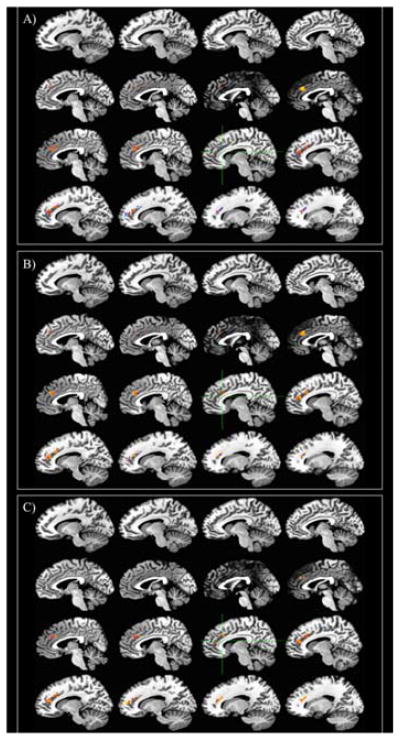
Figure 10. ACC activity relationships during Simon task (Incongruent - Congruent conditions).
Red/yellow = positive relationship. Blue = negative relationship A) Relationship with AOA controlling for A1 carrier status, English proficiency, and Spanish proficiency B) Relationship with English proficiency controlling for A1 carrier status, AOA, and Spanish proficiency C) Relationship with Spanish proficiency controlling for A1 carrier status, AOA, and English proficiency
Behavioral results based on a paired-samples t-test for the Simon task revealed the expected Simon effect (incongruent RT > congruent RT; average RT difference = 29.77 ms, t = 8.18, p < 0.0001). A multiple regression was conducted to examine whether the Simon effect could be predicted by A1 carrier status, AOA, English proficiency, and Spanish proficiency (Simon effect = A1 carrier status + AOA + English proficiency + Spanish proficiency). The only variable that significantly predicted the Simon effect was Spanish proficiency (p < 0.05). This relationship was negative, with lower Spanish proficiency predicting a larger Simon effect.
4. Discussion
4.1 Picture Naming
Language background and DRD2 genotype did not significantly predict neural activity during Spanish picture naming or mixed picture naming (switching between English and Spanish); however, age of English acquisition and DRD2 genotype significantly predicted neural activity during English picture naming. Participants who learned English at a later age, controlling for DRD2 genotype, English proficiency, and Spanish proficiency, demonstrated increased activity in the left IFG. Participants who carry the A1 allele of the DRD2 gene showed increased activity in the left and right IFG during English picture naming, while controlling for age of English acquisition, English proficiency, and Spanish proficiency.
Previous studies employing bilingual picture naming have found that the mixed condition is more cognitively-demanding than blocks of picture naming in either language (Guo, Liu, Misra, & Kroll, 2011), and that naming in the second language is more demanding than naming in the first language (Guo et al., 2011). However, in a study involving a Spanish-English bilingual sample from the same population as the current study, Hernandez (2009) observed activity in the superior parietal lobule, supplementary motor area, and dorsolateral prefrontal cortex in response to the mixed vs. blocked conditions, and activity in the pre-supplementary motor area, superior temporal gyrus, somatosensory cortex, and importantly, IFG for the blocked vs. mixed condition. It is possible that the IFG specifically is therefore more relevant for blocked than mixed conditions, and that selecting other regions of interest may change the results. Many fMRI studies to date have been underpowered, with small sample sizes for comparisons of activity across every voxel imaged (Yarkoni, 2009). The current study used a region-of-interest approach in order to avoid issues with power, yet this approach is hypothesis-driven, and cannot account for all differences between conditions that may exist in this sample.
Although the finding of significant predictors in the English condition alone was surprising, these results uncover an important combination of experience and genetic factors in production of the second language. Regardless of (i.e., controlling for) proficiency in either Spanish or English, bilinguals who learned English later in life showed more activity in the bilateral IFG when naming pictures in English than bilinguals who learned English earlier in life. This finding fits with the sensorimotor hypothesis put forth by Hernandez and Li (2007). This view suggests that the neural mechanisms involved in linguistic and non-linguistic tasks change as a function of the age at which the requisite skill is acquired; skills acquired earlier recruit a neural system that relies on more subcortical regions, and is related to procedural or implicit memory. On the other hand, skills acquired later in life may rely on more cortical, particularly frontal, regions of the brain, and be more explicit or controlled. A study by Fiebach and his colleagues (2003) examining the role of age of acquisition of words within one language (i.e. English words learned as a toddler vs. English words learned in adulthood) could predict neural activity during the presentation of those words. The findings in that study revealed that hearing later learned words led to increased activity in the IFG than hearing earlier learned words. The current study extends these results to a bilingual language production setting: bilinguals who acquire their second language later in life show more activity in the IFG for producing words in the second language.
The sensorimotor hypothesis can also shed some light on the connection between genetic variability in the basal ganglia and neural activity in the IFG during English picture naming. This hypothesis assumes that subcortical structures are associated with more automatic, procedural processes, and cortical structures are associated with more controlled, explicit processes. If there is ample dopamine available in the basal ganglia, as is the case for non-carriers of the A1 allele, tasks such as learning a second language may be able to become automatized; however, a lack of dopamine availability, as can be seen in carriers of the A1 allele, might lead to difficulties in automatizing the second language. Therefore, carriers of the A1 allele may need to rely on frontal control regions of the brain in order to name pictures in their second language. This would explain the results of the current study, in which carriers of the A1 allele show increased activity compared to non-carriers in the left and right IFG during the English condition.
4.2 Shape-Color Switching Task
The only significant predictor of ACC activity during the shape-color task, for the switch conditions compared to the non-switch conditions, was A1 carrier status; age of English acquisition and proficiency in English and Spanish did not predict neural activity for non-verbal task switching. None of the variables entered predicted activity in the IFG during this task. In both the left and right ACC, bilinguals who carry the A1 allele showed a smaller difference in activity for the switch compared to non-switch condition than bilinguals who were not carriers of the A1 allele. This could be interpreted as more efficient switching for the A1 carriers than for the non-carriers.
As stated in the introduction, the ACC was selected as a region of interest because of its recurrence in studies comparing monolinguals and bilinguals during non-verbal cognitive control, not because of its relationship to the DRD2-ANKK1 polymorphism. Previous research by Stelzel and her colleagues (2010, 2013) did not investigate the relationship between genotype of this polymorphism (A1 carrier or non-carrier) and activity in the ACC during non-verbal switching, but findings by Fossella, Green, and Fan (2006) support these results. In their study, participants completed the attentional networks task (ANT), which is also a non-verbal measure of cognitive control. The participants were genotyped for the DRD2 gene and fMRI data was collected during the ANT. Results indicated that carriers of the A1 allele show a smaller difference in ACC activity between the incongruent and congruent conditions of this task than non-carriers of an A1 allele. These results match the results of the current study, indicating that the relationship between the DRD2-ANKK1 polymorphism and ACC activity during cognitive control is similar in Hispanic, Spanish-English bilinguals as in the general population sampled by Fossella, Green, and Fan (2006). In other words, bilingualism does not appear to change the functioning of the connection between D2 dopamine and the ACC during cognitive control.
Supporting this idea, none of the bilingualism factors (AoA, English proficiency, or Spanish proficiency) were related to ACC or IFG activity during this non-verbal task switching. This is surprising, as researchers have previously connected bilingual language experience to non-verbal task switching using fMRI. Garbin and her colleagues (2010) found that non-verbal task switching resulted in increased activity in the left IFG for bilinguals and increased activity in the right IFG, ACC, and left inferior parietal lobule for monolinguals. In a similar comparison, Rodriguez-Pujadas and her colleagues (2013) revealed more activity for bilinguals than monolinguals in the left IFG, left ACC, and bilateral caudate during non-verbal task switching. In order to support either of these findings, suggesting that bilingualism is associated with either more or less activity during non-verbal task switching, the bilingual background variables in the current study should be predictive of activity in either the ACC or IFG. It is possible that bilingualism might be associated with differences in neural activity during the shape-color task in terms of bilingual/monolingual comparisons, but not specifically tied to age of second language acquisition or proficiency, and therefore not evident in a sample of only bilingual participants. Future studies should combine analyses of individual differences within bilinguals and monolinguals in order to determine whether differences are indeed only observable at the group level.
4.3 Simon Task
The results for the Simon task were quite different from the shape-color task. For the Simon task comparison of incongruent trials and congruent trials, activity in the left ACC was predicted by AOA, English proficiency, and Spanish proficiency, but not by DRD2 genotype. Controlling for proficiency, AOA is negatively correlated with activity, meaning that bilinguals who learned their second language earlier in life show a larger difference between the incongruent and congruent conditions in terms of left ACC activity. Controlling for AOA and Spanish proficiency, English proficiency is also negatively related to this difference, suggesting that bilinguals with a lower English (L2) proficiency show a larger difference in the ACC between the incongruent and congruent trials. Finally, controlling for AOA and English proficiency, Spanish (L1) proficiency is positively related to ACC activity for the incongruent – congruent contrast, indicating that bilinguals who attain a higher proficiency in their first language show a smaller difference in the ACC for the incongruent and congruent conditions of the Simon task. In addition, activity for this contrast in the left IFG was negatively related to age of English acquisition alone; earlier AOA indicates a larger difference in left IFG activity for incongruent and congruent trials.
The ability to disentangle the first and second language proficiency and AOA provides insight into the relationship between the bilingual experience and inhibition or attentional control, as measured by the Simon task. The relationship between AOA and neural activity can be interpreted developmentally; in other words, AOA reflects the stage of neural development impacted by bilingualism. The relationship between English proficiency and neural activity can be interpreted in this sample as reflecting education; for the Spanish-English bilingual population sampled for this study, English is the language of schooling, regardless of the age at which English was acquired. This population of bilinguals attends an American university in which they receive an education in English, and their English abilities are not highly variable. The Spanish proficiencies of these bilinguals, on the other hand, are quite variable, and therefore reflect the extent to which a person could be considered bilingual. For example, a participant who presents with a low level of Spanish proficiency would be considered less bilingual than a participant with a high level of Spanish proficiency, as they both have a fairly equal grasp of English (see Figure 4).
The issue of whether more or less neural activity during a task is better is still up for debate (Paap, Sawi, Dalibar, Darrow, & Johnson, 2014). Some researchers argue that regardless of direction, differences in neural activity related to bilingualism, whether through a comparison of monolinguals and bilinguals, or through an examination of individual differences within bilinguals, can demonstrate that bilingualism changes the brain in a way that relates to cognitive control (Kousaie & Taler, 2015; Vaughn, Greene, Ramos Nunez, & Hernandez, 2015). In the current study, neither AOA nor English proficiency was associated with response times for the Simon task, but both were associated with ACC activity during the task. Generally, earlier AOA and higher proficiency in the second language are conceptualized as better than later AOA and lower second language proficiency in terms of bilingualism (e.g., Hernandez, 2016), and in this case, both are associated with a larger difference between incongruent and congruent trial activity unrelated to behavioral performance. These results are difficult to interpret because studies to date have not considered the role of these individual differences in language background in neural activity during non-verbal cognitive control. It is unclear whether these results are unique to the bilingual population sampled as a part of this study, or whether these patterns exist across bilingual populations. Future studies should attempt to replicate these results with other populations of bilinguals in order to better understand these findings.
Spanish proficiency is the only relationship that exists both in terms of neural activity and in terms of behavioral performance. Higher proficiency is associated with a smaller difference between incongruent trials and congruent trials in the ACC, and a smaller response time difference between incongruent and congruent trials. Therefore, proficiency in this first language seems to be important for the way bilinguals handle the Simon task. This fits with the role of the ACC in conflict monitoring and error detection put forth by Abutalebi and Green (2007). For this group of bilinguals, higher Spanish proficiency could indicate more conflict between the two languages if Spanish needs to be inhibited in order to use English, as Green’s inhibitory control model would suggest (Green, 1998). This heightened level of language conflict could train the ACC to be more efficient, fitting with the findings of Abutalebi and his colleagues (2012) during the flanker task. As stated in the introduction, studies of non-verbal cognitive control have yet to consider individual differences within the bilingual population, so more studies should examine age of acquisition and first and second language proficiency separately in order to support or dispute this explanation.
4.4 Limitations and Future Directions
Although the current study highlighted important individual differences in terms of bilingual experiences and genetic variation that may influence neural processing during bilingual language and cognitive control, there are limitations to these findings. This study focused only on one particular group of bilinguals (Spanish-English), so future studies should attempt to replicate these findings in other groups of bilinguals. In the current sample, language background and genetic variation were related only to production of the second language; future studies should examine whether this is universal across groups of bilinguals or whether this is a finding that is unique to American Spanish-English bilinguals who are enrolled in college, who may have different language experiences than other groups of bilinguals. The current study also only considered the relationship between neural activity and one particular polymorphic site (ANKK1-Taq1a) in the DRD2 gene. There are many different genetic factors associated with dopamine, as well as with cognitive control. The sample size of the current study limited the examination of genetic variation to one allele, but future studies should examine the role of other alleles and recruit increasingly large samples in order to better examine the various individual differences that might impact the relationship between language and cognition. Finally, because of issues with sample size and power, the current study focused exclusively on the IFG and ACC as regions of interest. It is possible that this approach could neglect other regions of the brain involved in cognitive control that are related to the DRD2 genotype or language background. Larger samples should be recruited in order to conduct whole-brain analyses of these regression models.
4.5 Conclusions
The reason for investigating the role of the A1 allele along with language background in predicting neural activity during bilingual cognitive control is to determine whether population differences in the proportion of the A1 allele are relevant to consider in future fMRI studies of cognitive control and language control. The findings that A1 carrier status predicts neural activity in the left IFG during English picture naming and the ACC during non-verbal task switching suggest that understanding genetic differences across populations is important. Hernandez and colleagues (2015) found, using pilot data, that Spanish-English bilingual university students carry the A1 allele at twice the rate of the English monolinguals studied. If the genetic variation is an important source of individual differences and affects neural activity, as suggested by the results of the current study, then it may be incorrect to assume that the difference between bilinguals and monolinguals in neural activity is caused solely by differing language experiences. Indeed, a better method to study the impact of bilingualism on the brain during language control and non-verbal cognitive control might be to measure individual differences within the bilingual population, such as proficiency in each language or age of second language acquisition. Finding that bilinguals with higher proficiencies in each language have different patterns of neural activity than bilinguals with lower proficiencies, for example, would support the idea that language itself is shaping the brain to handle cognitive control tasks, and not other variables that may differ between bilingual and monolingual groups. As stated in a recent paper by Vaughn, Greene, Ramos-Nuñez, and Hernandez (2015), the authors believe that understanding differences within the bilingual population is important for understanding the relationship between language and cognition in the brain.
Supplementary Material
Highlights.
Bilingual language control is associated with activity in the inferior frontal gyrus
Non-verbal control is associated with activity in the anterior cingulate cortex
Specific genotypes predict fMRI activity during language control and task switching
Bilingual experience predicts fMRI activity during language control and inhibition
Acknowledgments
Thank you to Maria Lee who assisted with data analysis and interpretation of the genetic aspects of the study.
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R03HD079873 (“Effects of genetic differences and bilingual status on cognitive control”; 2015–2017) and R21HD059103 (“Neural correlates of lexical processing in child L2 learners”; 2009–2013).
Support was also provided by the Alexander von Humboldt foundation.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abutalebi J, Della Rosa PA, Ding G, Weekes B, Costa A, Green DW. Language proficiency modulates the engagement of cognitive control areas in multilinguals. Cortex. 2013;49(3):905–911. doi: 10.1016/j.cortex.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FI, Klein R, Viswanathan M. Bilingualism, aging, and cognitive control: evidence from the Simon task. Psychology and Aging. 2004;19(2):290–303. doi: 10.1037/0882-7974.19.2.290. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Green DW, Gollan TH. Bilingual Minds. Psychological Science in the Public Interest. 2009;10(3):89–129. doi: 10.1177/1529100610387084. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. http://dx.doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E-Prime (Version 2.0) Sharpsburg, PA: Psychology Software Tools, Inc; 2012. Retrieved from http://www.pstnet.com/eprime.cfm. [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY, Hernandez AE. Distinct brain representations for early and late learned words. NeuroImage. 2003;19(4):1627–1637. doi: 10.1016/s1053-8119(03)00227-1. [DOI] [PubMed] [Google Scholar]
- Fossella J, Green AE, Fan J. Evaluation of a structural polymorphism in the ankyrin repeat and kinase domain containing 1(ANKK1) gene and the activation of executive attention networks. Cognitive Affection Behavivour Neuroscience. 2006;6:71–78. doi: 10.3758/cabn.6.1.71. [DOI] [PubMed] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez-Pujadas A, Belloch V, Avila C. Bridging language and attention: brain basis of the impact of bilingualism on cognitive control. Neuroimage. 2010;53(4):1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism: Language and cognition. 1998;1(02):67–81. [Google Scholar]
- Guo T, Liu H, Misra M, Kroll JF. Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. Neuroimage. 2011;56(4):2300–2309. doi: 10.1016/j.neuroimage.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: what’s next? Brain and Language. 2009;109(2–3):133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez AE. Bilingual Development and Age of Acquisition. In: Hickok G, Small S, editors. Neurobiology of Language. London, UK: Academic Press; 2016. pp. 407–418. [DOI] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: an fMRI study. Neuroimage. 2001;14(2):510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Greene MR, Vaughn KA, Francis DJ, Grigorenko EL. Beyond the bilingual advantage: The potential role of genes and environment on the development of cognitive control. Journal of Neurolinguistics. 2015;35:109–119. doi: 10.1016/j.jneuroling.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Li P. Age of acquisition: Its neural and computational mechanisms. Psychological Bulletin. 2007;133(4):638–650. doi: 10.1037/0033-2909.133.4.638. [DOI] [PubMed] [Google Scholar]
- Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex. 2014;58:301–24. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Ossorio P, Duster T. Race and genetics: Controversies in biomedical, behavioral, and forensic sciences. American Psychologist. 2005;60(1):115–128. doi: 10.1037/0003-066X.60.1.115. [DOI] [PubMed] [Google Scholar]
- Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cognitive Psychology. 2013;66(2):232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, Fazio F. The role of age of acquisition and language usage in early, high-proficient bilinguals: an fMRI study during verbal fluency. Human Brain Mapping. 2003;19(3):170–182. doi: 10.1002/hbm.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard W, Woodcock AFM-S, Ruef Mary L, Alvarado Criselda G. Woodcock-Muñoz Language Survey - Revised. Riverside Publishing; 2005. [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemical Research. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pujadas A, Sanjuan A, Ventura-Campos N, Roman P, Martin C, Barcelo F, Costa A, Avila C. Bilinguals use language-control brain areas more than monolinguals to perform non-linguistic switching tasks. PLoS One. 2013;8(9):e73028. doi: 10.1371/journal.pone.0073028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. Journal of Neuroscience. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Fiebach CJ, Cools R, Tafazoli S, D’Esposito M. Dissociable fronto-striatal effects of dopamine D2 receptor stimulation on cognitive versus motor flexibility. Cortex. 2013;49(10):2799–2811. doi: 10.1016/j.cortex.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A, Prat CS. Bilingualism trains specific brain circuits involved in flexible rule selection and application. Brain and Language. 2014;137:50–61. doi: 10.1016/j.bandl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Team, R. C. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Retrieved from http://www.R-project.org. [Google Scholar]
- Vaughn KA, Greene MR, Ramos Nunez AI, Hernandez AE. The importance of neuroscience in understanding bilingual cognitive control. Cortex. 2015 doi: 10.1016/j.cortex.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberger GH, Gollan TH, Bondi MW, Clark LR, Wierenga CE. Language and task switching in the bilingual brain: Bilinguals are staying, not switching, experts. Neuropsychologia. 2015;66:193–203. doi: 10.1016/j.neuropsychologia.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power—Commentary on Vul et al. Perspectives on Psychological Science. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.