SUMMARY
Natural killer (NK) cells are important in host defense against pathogens, and they can subsequently differentiate into memory NK cells. The Ly49 and KIR gene families in rodents and humans encode both inhibitory and activating receptors for MHC class I. The physiological role of activating KIR or Ly49 receptors that recognize self-MHC class I during immune response to viral infections is unknown. Here, we address how the activating Ly49D receptor impacts the NK cell response to mouse cytomegalo-virus (MCMV) infection by comparing the activation and differentiation of Ly49D-bearing NK cells in mice lacking or expressing H-2Dd, the cognate MHC class I ligand of Ly49D. After MCMV infection, Ly49D augmented IFN-γ production by MCMV-specific Ly49H+ NK cells and preferentially promoted the generation of memory Ly49H+ NK cells. Thus, activating receptors for self-MHC class I modulate the differentiation of MCMV-specific NK cells and are beneficial for host defense against MCMV infection.
In Brief
The physiological relevance of activating receptors on NK cells that recognize polymorphic self-MHC I antigens is unclear. Nabekura and Lanier show that NK cells with activating self-MHC receptors are hypo-responsive at steady-state, and during viral infection, these receptors augment cytokine production and promote the survival of long-lived memory NK cells.
INTRODUCTION
Natural killer (NK) cells recognize virus-infected cells and transformed cells by using a repertoire of NK cell receptors that regulate their activation and effector functions (Lanier, 2005). The Ly49 gene family in rodents and the KIR gene family in primates encode both inhibitory and activating receptors that recognize polymorphic MHC class I ligands (Parham and Moffett, 2013; Schenkel et al., 2013). The inhibitory receptors for MHC class I dampen or prevent NK cell responses against host cells expressing MHC class I ligands. Additionally, during development, NK cells acquire functional competency through “licensing” by interactions between inhibitory Ly49 or KIR and self-MHC class I (Anfossi et al., 2006; Kim et al., 2005).
Whereas the inhibitory KIR and Ly49 receptors for MHC class I have been implicated in licensing NK cells and preventing attack of healthy tissues, the activating receptors for MHC class I ligands have been shown to enable NK cells to reject allogeneic bone marrow transplants and tumors. For example, NK cells in C57BL/6 (H-2Db) mice that express the activating Ly49D receptors specific for H-2Dd (George et al., 1999a) are able to recognize and reject allogeneic bone marrow grafts from H-2Dd mice (George et al., 1999b). In humans, patients with acute myeloid leukemia (AML) who express a HLA-C group 2 genotype (HLA-C alleles with N at amino acid 77 and lysine at amino acid 80) had a lower rate of relapse when they were transplanted with hematopoietic stem cells from donors expressing KIR2DS1 genes (Venstrom et al., 2012), presumably because the KIR2DS1+ NK cells arising from the donor hematopoietic stem cells are capable of killing residual allogeneic HLA-C2-bearing leukemia cells in the patients (Chewning et al., 2007; Colonna et al., 1993). KIR2DS1+ NK cells in maternal decidual tissues have been proposed to enhance placentation of semi-allogeneic fetuses expressing HLA-C2 ligands by producing GM-CSF, which facilitates trophoblast invasion (Xiong et al., 2013).
Although a role for activating KIR and Ly49 in alloantigen-induced responses has been documented, the physiological relevance of these activating receptors when they interact with self-MHC class I ligands is unknown. In general, NK cells possessing activating KIR or Ly49 receptors that can react with self-MHC class I ligands in the individual are hyporesponsive. For example, KIR2DS1+ NK cells are hyporesponsive in individuals who have a HLA-C2 genotype (Fauriat et al., 2010; Pittari et al., 2013). Similarly, there is no evidence of activation or autoimmune disease mediated by Ly49D+ NK cells in mice expressing H-2Dd (George et al., 1999b), and these Ly49D+ NK cells in H-2Dd mice are hypo-responsive when assayed ex vivo. Thus, although NK cells bearing activating KIR or Ly49 receptors in healthy donors possessing self-MHC class I ligands for these receptors are hypo-responsive or tolerant at steady state, we hypothesized that this tolerance might be broken during inflammation or infection, permitting these activating receptors to signal and enhance the response of NK cells.
Although NK cells were previously considered unable to differentiate into a long-lived memory subset, accumulating evidence demonstrates that NK cells have adaptive immune features, which include antigen-specific expansion and differentiation into self-renewing memory NK cells (Cooper et al., 2009; Min-Oo et al., 2013; Nabekura and Lanier, 2014; O’Leary et al., 2006; Paust et al., 2010; Sun et al., 2009, 2010). In some mouse models, NK cells are activated after exposure to pathogens, antigens, alloantigens, and cytokines and subsequently differentiate into memory or memory-like NK cells with augmented effector functions in response to a variety of secondary stimuli, as compared with naive NK cells (Cooper et al., 2009; Nabekura and Lanier, 2014; O’Leary et al., 2006; Sun et al., 2009). The existence of memory NK cells in humans is demonstrated by the specific expansion and persistence of NKG2ChiCD57+ NK cells after human cytomegalovirus (HCMV) infection, and by the increased frequency of the population in response to reactivation of HCMV (Foley et al., 2012a; Foley et al., 2012b; Gumá et al., 2004; Lopez-Vergès et al., 2011; Min-Oo et al., 2013). We have demonstrated previously that mouse NK cells bearing the activating Ly49H receptor, which specifically recognizes the m157 mouse cytomegalovirus (MCMV) glycoprotein on the infected cells (Arase et al., 2002; Smith et al., 2002), clonally expand and differentiate into memory NK cells, and persist for several months after MCMV infection (Sun et al., 2009, 2010). These MCMV-specific memory NK cells are capable of mounting a recall response and provide more protective host defense against re-challenge with MCMV than naive NK cells (Sun et al., 2009). The immunoreceptor tyrosine-based activation motif (ITAM)-containing DAP12 adaptor-mediated signaling through Ly49H and costimulatory signaling through the DNAM-1 receptor are essential not only for optimal expansion of effector Ly49H+ NK cells, but also for the generation of memory Ly49H+ NK cells following MCMV infection (Nabekura et al., 2014; Sun et al., 2009).
In the present study, we addressed whether the activating Ly49D receptor that recognizes self-MHC class I molecules influences the activation of MCMV-specific Ly49H+ NK cells and the differentiation of memory NK cells generated in response to MCMV infection. Furthermore, we examined how co-expression of the inhibitory Ly49A receptor that recognizes the same self-H-2 ligand as Ly49D affects responsiveness of these NK cells to MCMV. We found that Ly49D augmented interferon (IFN)-γ production by Ly49H+ NK cells and preferentially promoted the differentiation of memory Ly49H+ NK cells by providing a survival advantage.
RESULTS
Ly49D+Ly49A−Ly49H+ NK Cells Undergo Less Homeostatic Turnover and Have Diminished Effector Functions in Naive B10.D2 Mice
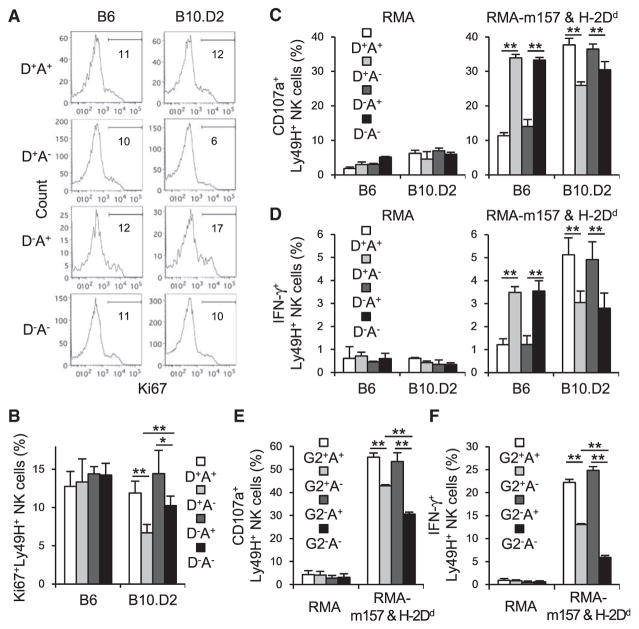
We examined the influence of the activating Ly49D receptor and the inhibitory Ly49A receptor, both of which recognize H-2Dd, on the activation and differentiation of the MCMV-specific Ly49H+ NK cells in B10.D2 mice expressing H-2Dd or B6 mice lacking H-2Dd. In resting uninfected mice, equivalent frequencies of immature (CD11b−CD27+), intermediate (CD11b+CD27+), and mature (CD11b+CD27−) NK cells were present within Ly49H+ NK cell subsets co-expressing Ly49D and/or Ly49A in the spleen and blood in B10.D2 mice, indicating that Ly49A and Ly49D do not grossly affect maturation of the Ly49H+ NK cell subsets (data not shown). Similarly, within these Ly49H+ NK cell subsets, frequencies of NK cells expressing Ly6C, an activation and differentiation marker of NK cells (Nabekura et al., 2014), and annexin V, an indicator of cellular apoptosis, were similar (data not shown). However, a statistically significant lower percentage of recently proliferated Ki67+Ly49H+ NK cells were present in the subset co-expressing the activating Ly49D receptor but lacking the inhibitory Ly49A receptor in B10.D2 mice, but not in B6 mice lacking the H-2Dd ligand (Figures 1A and 1B). To investigate effector functions of naive Ly49H+ NK cells co-expressing Ly49D, NK cells were co-cultured with RMA (H-2b) transfectants expressing m157 and H-2Dd (Figures 1C and 1D). Although Ly49A suppressed degranulation and IFN-γ production of B6 Ly49H+ NK cells against RMA transfectants expressing m157 and H-2Dd, a higher frequency of B10.D2 naive Ly49A+Ly49H+ NK cells degranulated and produced IFN-γ than naive Ly49A− Ly49H+ NK cells after co-culture with target cells (Figures 1C and 1D), consistent with prior studies analyzing NK cell licensing (Bessoles et al., 2013; Chalifour et al., 2009). The inhibitory Ly49G2 receptor also recognizes H-2Dd, although Ly49A has a higher affinity for H-2Dd (Hanke et al., 1999). However, unlike Ly49A, which is stably expressed on mature NK cells and not affected by activation of NK cells, most Ly49H+ NK cells lacking Ly49G2 acquire this receptor after MCMV infection independently of the MHC haplotype, suggesting that Ly49G2 is essentially an activation marker (Barao et al., 2011; Hanke et al., 1999; and data not shown). When B10.D2 naive Ly49H+ NK cells expressing Ly49G2 and/or Ly49A were co-cultured with target cells expressing m157 and H-2Dd, a higher frequency of B10.D2 naive Ly49H+ NK cell subsets expressing Ly49A degranulated and produced IFN-γ than naive Ly49H+ NK cell subsets not expressing Ly49A, regardless of expression of Ly49G2 (Figures 1E and 1F). Ly49H+ NK cells co-expressing Ly49G2 and Ly49A did not show a more potent licensing effect than Ly49H+ NK cells expressing Ly49A without Ly49G2 (Figures 1E and 1F). These results demonstrate that Ly49A has more potent licensing activity than Ly49G2 in B10.D2 mice. Thus, the expression of Ly49D on unlicensed (Ly49A−) NK cells in B10.D2 mice suppresses homeostatic turnover of this subset, and licensing of NK cells through Ly49A in B10.D2 mice results in enhanced effector functions of naive Ly49H+ NK cells independently of Ly49D.
Figure 1. B10.D2 Ly49D+Ly49A−Ly49H+ NK Cells Undergo Less Homeostatic Turnover and Have Diminished Effector Functions.
(A and B) Ki67 expression by naive Ly49H+ NK cell subsets expressing Ly49D and/or Ly49A in the spleen of B6 and B10.D2 mice. Data are representative of three experiments (n = 1–3 in each experiment).
(B) Percentages of Ki67+Ly49H+ NK cells were quantified. Data were pooled from three experiments (n = 7 mice in each group).
(C and D) Degranulation (C) and IFN-γ production (D) of B6 and B10.D2 naive Ly49H+ NK cell subsets expressing Ly49D and or Ly49A after co-culture with RMA or RMA cells expressing m157 and H-2Dd. Data are representative of three experiments (n = 5 or 6 in each group).
(E and F) Degranulation (E) and IFN-γ production (F) of B10.D2 naive Ly49H+ NK cell subsets expressing Ly49G2 and/or Ly49A after co-culture with RMA or RMA cells expressing m157 and H-2Dd. Data are pooled from two experiments (n = 8 in each group).
*p < 0.05, **p < 0.005. Error bars show SEM.
Ly49D and Ly49A Enhance Activation and IFN-γ Production of B10.D2 Ly49H+ NK Cells in the Early Phase of MCMV Infection
We addressed how the activating Ly49D and inhibitory Ly49A receptors, both recognizing H-2Dd, impact the activation and IFN-γ production of Ly49H+ NK cells in B6 and B10.D2 mice in the early course of MCMV infection. On day 1.5 post-infection (pi), B6 Ly49H+ NK cell subsets expressing Ly49D and/or Ly49A produced equivalent amounts of IFN-γ (Figures 2A and 2B). In contrast to B6 mice, in B10.D2 mice, Ly49D+Ly49H+ NK cells and Ly49A+Ly49H+ NK cells produced more IFN-γ after MCMV infection than the Ly49D−Ly49A−Ly49H+ NK cell subset (Figures 2A and 2B). Furthermore, B10.D2 Ly49D+Ly49A+ Ly49H+ NK cells produced the largest amount of IFN-γ within these Ly49H+ NK cell subsets (Figures 2A and 2B). Thus, although the activating Ly49D receptor induced hypo-responsiveness at steady state, it augmented the activation and IFN-γ production of Ly49H+ NK cells in B10.D2 mice in the early course of MCMV infection.
Figure 2. Ly49D and Ly49A Enhance Activation and IFN-γ Production of B10.D2 Ly49H+ NK Cells in the Early Course of MCMV Infection.
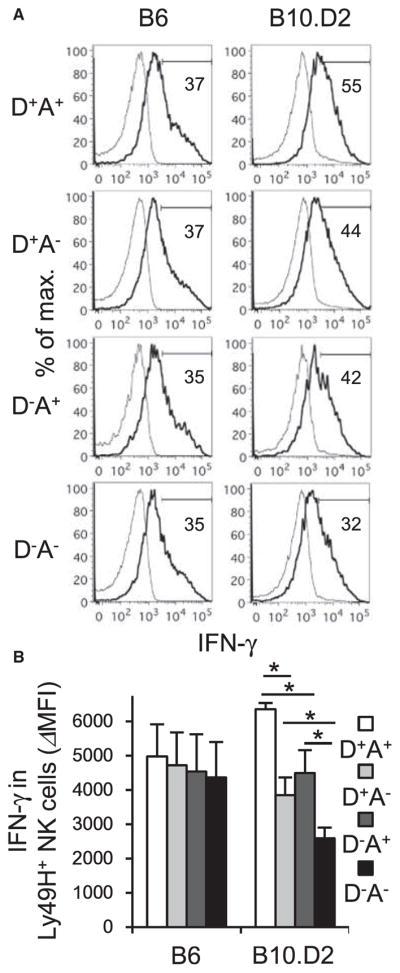
B6 mice were infected with 1 × 104 pfu MCMV. B10.D2 mice were depleted of CD8+T cells onthe day before infection and theninfected with 5×104 pfu MCMV.
(A) IFN-γ production of Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on day 1.5 pi. Data are representative of two experiments (n = 3–4 in each experiment).
(B) Delta mean fluorescent intensity (ΔMFI) of IFN-γ in Ly49H+ NK cells was quantified. Data were pooled from two experiments (n = 7 mice in each group). Bold and thin lines represent Ly49H+ NK cells in infected mice and Ly49H+ NK cells in uninfected mice, respectively. *p < 0.05. Error bars show SEM.
Ly49D Suppresses Activation and Cell-Cycle Progression of B10.D2 Ly49H+ NK Cells at the Peak of NK Cell Response after MCMV Infection
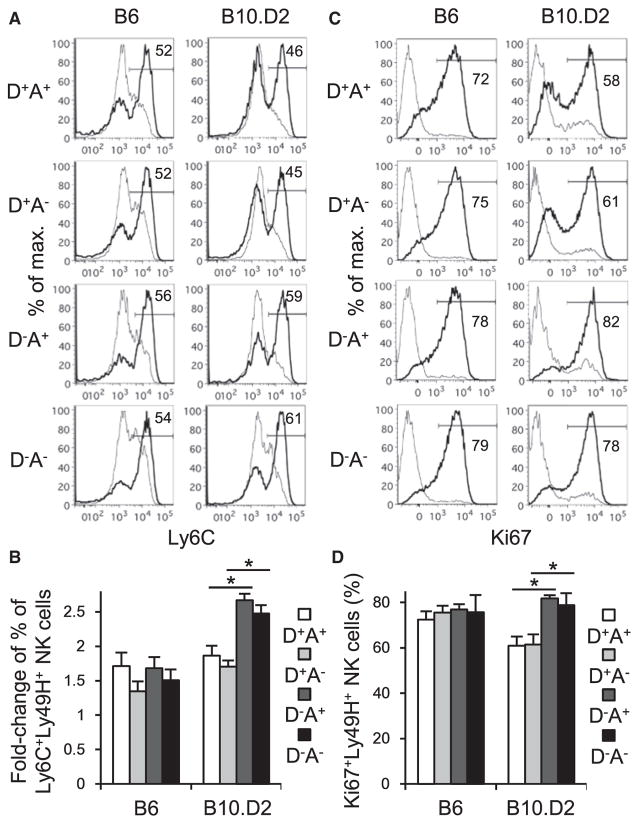
B6 MCMV-specific Ly49H+ NK cells preferentially proliferate, peaking at 7 days after MCMV infection (Sun et al., 2009). We investigated the influence of Ly49D and Ly49A, both recognizing H-2Dd, on the activation and proliferation of Ly49H+ NK cells at the peak of the NK cell response after MCMV infection. B6 Ly49H+ NK cell subsets expressing Ly49D and/or Ly49A equivalently upregulated Ly6C and KLRG1 on day 7 pi (Figures 3A and 3B and data not shown). Unlike activation of NK cells by inflammatory cytokines in the early phase of MCMV infection (Figures 2A and 2B) (Krug et al., 2004; Sun et al., 2011), B10.D2 Ly49D+Ly49H+ NK cells showed less upregulation of Ly6C and KLRG1 than Ly49D−Ly49H+ NK cells on day 7 pi (Figures 3A and 3B and data not shown). These B10.D2 Ly49D+Ly49H+ NK cells were less proliferative, as demonstrated by the smaller percentages of Ki67+ NK cells, than Ly49D−Ly49H+ NK cells on day 7 pi (Figures 3C and 3D). Although Ly49A did not affect the proliferation of these B10.D2 Ly49D+Ly49H+ NK cells during peak expansion after MCMV infection, in naive B10.D2 mice, Ly49D+Ly49H+ NK cell subsets expressing Ly49A exhibited more homeostatic turnover than LY49D+Ly49H+ NK cells lacking L49A in uninfected mice (Figures 1A and 1B and 3C and 3D). Neither upregulation of Ly6C nor increased frequencies of Ki67+ cells were observed in Ly49H− NK cells in B6 and B10.D2 mice (data not shown). These results demonstrate that in B10.D2 mice, Ly49D suppresses activation and subsequent cell-cycle progression of MCMV-specific Ly49H+ NK cells at the peak of the NK cell response after MCMV infection.
Figure 3. Ly49D Suppresses Activation and Cell-Cycle Progression of B10.D2 Ly49H+ NK Cells at the Peak of NK Cell Response after MCMV Infection.
B6 mice were infected with 1 × 104 pfu MCMV. B10.D2 mice were depleted of CD8+ T cells on the day before infection and then infected with 5 × 104 pfu MCMV.
(A) An activation and differentiation marker Ly6C on Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on day 7 pi. Data are representative of four experiments (n = 3–4 in each experiment).
(B) Fold change of percentages of Ly6C+Ly49H+ NK cells was quantified. Data were pooled from two experiments (n = 7 mice in each group).
(C) Ki67 expression by Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on day 7 pi. Data are representative of two experiments (n = 3 or 4 in each experiment).
(D) Percentages of Ki67+Ly49H+ NK cells were quantified. Data were pooled from two experiments (n = 7 mice in each group). Bold and thin lines represent Ly49H+ NK cells in infected mice and Ly49H+ NK cells in uninfected mice, respectively.
*p < 0.005. Error bars show SEM.
Ly49D on B10.D2 Ly49H+ NK Cells Provides a Survival Advantage
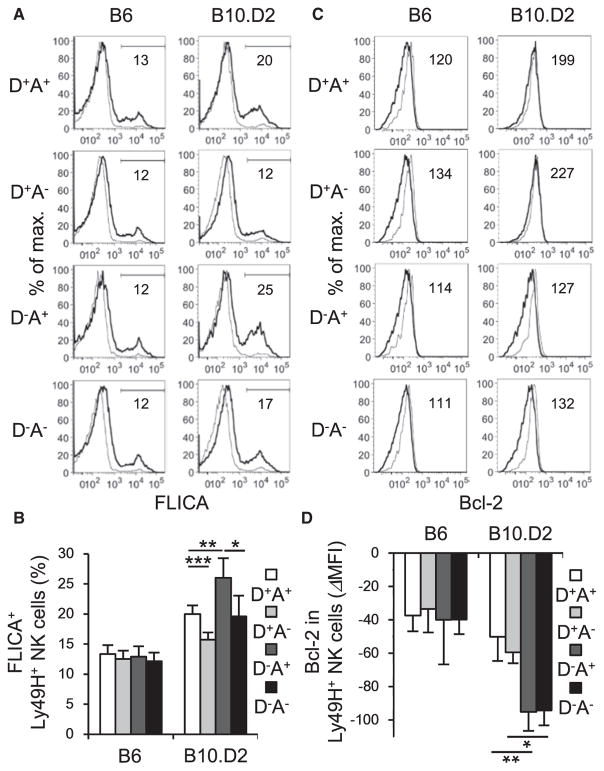
B6 Ly49H+ NK cells clonally expand and subsequently undergo a contraction after the peak of the NK cell response after MCMV infection (Sun et al., 2009). In B10.D2 mice, but not in B6 mice, the licensed Ly49A+Ly49H+ NK cell subset demonstrated a higher proportion of apoptotic cells than unlicensed Ly49A−Ly49H+ NK cells at the peak of the NK cell response during MCMV infection, as determined by staining with a fluorescent-labeled inhibitor of caspases (FLICA), a membrane-permeable probe retained in cells containing active caspases (Figures 4A and 4B). Within these Ly49H+ NK cell subsets, in uninfected mice, frequencies of apoptotic NK cells were equivalent. Interestingly, B10.D2 Ly49D+Ly49H+ NK cells maintained expression of higher amounts of Bcl-2 than Ly49D−Ly49H+ NK cells did in the contraction phase (Figures 4C and 4D). Unlike B10.D2 Ly49H+ NK cells, B6 Ly49H+ NK cell subsets equivalently expressed Bcl-2 during the contraction phase (Figures 4C and 4D). These results indicate that licensing through Ly49A renders B10.D2 Ly49H+ NK cells sensitive to cell death after MCMV infection, whereas, although Ly49D renders NK cells in B10.D2 mice hypo-responsive in steady state, it contributes to survival of B10.D2 Ly49H+ NK cells during MCMV infection.
Figure 4. B10.D2 Ly49A+Ly49H+ NK Cells are More Sensitive to Cell Death and B10.D2 Ly49D+Ly49H+ NK Cells Maintain Expression of Bcl-2 during MCMV Infection.
B6 mice were infected with 1 × 104 pfu MCMV. B10.D2 mice were depleted of CD8+ T cells on the day before infection and infected with 5 × 104 pfu MCMV.
(A) FLICA in Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on day 7 pi. Data are representative of two experiments (n = 2–4 in each experiment).
(B) Percentages of FLICA+Ly49H+ NK cells were quantified. Data were pooled from two experiments (n = 6 mice in each group).
(C) Expression of Bcl-2 in Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on day 10 pi. Data are representative of two experiments (n = 2 or 3 in each experiment).
(D) ΔMFI of Bcl-2 in Ly49H+ NK cells was quantified and calculated by subtracting the MFI values for Bcl-2 staining in the indicated NK cells subsets in uninfected mice from the MFI values in infected mice. Data were pooled from two experiments (n = 5 mice in each group). Bold and thin lines represent Ly49H+ NK cells in infected mice and Ly49H+ NK cells in uninfected mice, respectively.
*p < 0.05, **p < 0.01, ***p < 0.005. Error bars show SEM.
B10.D2 Ly49D+Ly49A−Ly49H+ NK Cells Preferentially Differentiation into Memory NK Cells
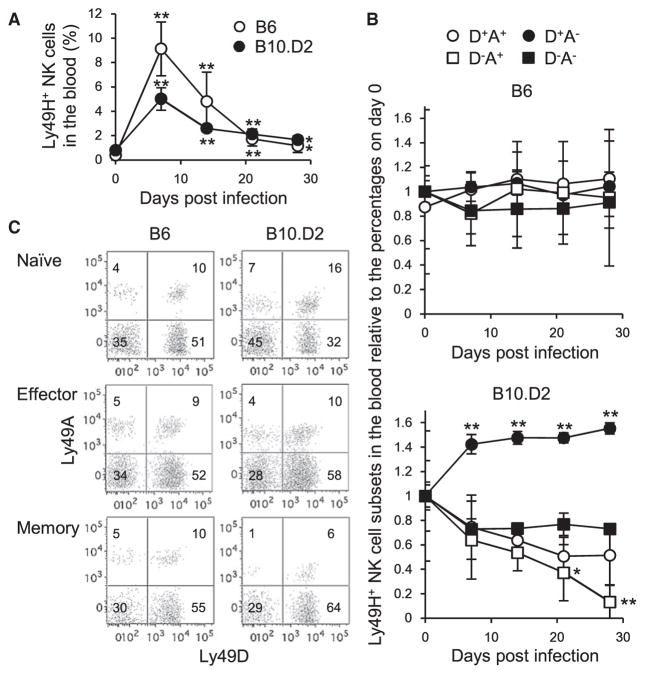
To determine how the activating Ly49D and inhibitory Ly49A, both recognizing H-2Dd, modulate expansion of effector Ly49H+ NK cells, as well as differentiation into memory Ly49H+ NK cells, B6 and B10.D2 Ly49H+ NK cells were adoptively transferred into syngeneic Ly49H-deficient recipient mice and infected with MCMV. Like B6 donor Ly49H+ NK cells, B10.D2 donor Ly49H+ NK cells preferentially expanded and differentiated into long-lived memory NK cells in the blood and spleen (Figures 5A and 5C). The frequencies of B6 donor Ly49H+ NK cells co-expressing Ly49D and/or Ly49A, which lack self-MHC class I ligands, remained constant during MCMV infection (Figures 5B and 5C). On the contrary, B10.D2 donor Ly49D+ Ly49A−Ly49H+ NK cells predominated in the effector phase (Figures 5B and 5C). These B10.D2 Ly49D+Ly49A−Ly49H+ NK cells preferentially differentiated into memory NK cells (characterized by the memory phenotype KLRG1+, Ly6C+, DNAM-1−, CD11b+, and CD27−, not shown), whereas Ly49D−Ly49A+Ly49H+ NK cells generated fewer memory NK cells detected in the blood and spleen 1 month after the infection (Figures 5B and 5C). Donor Ly49H− NK cells did not differentiate into Ly49H+ NK cells after MCMV infection (data not shown). These findings demonstrate that the activating Ly49D receptor modulates the expansion and differentiation of MCMV-specific Ly49H+ NK cells in the course of MCMV infection.
Figure 5. B10.D2 Ly49D+Ly49A−Ly49H+ NK Cells Predominate and Preferentially Differentiate into Memory NK Cells during MCMV Infection.
100,000 B6 Ly49H+ NK cells were transferred into syngeneic Ly49H-deficient B6 mice on the day before infection and then infected with 2.5–5 × 103 pfu MCMV. 100,000 B10.D2 Ly49H+ NK cells were transferred into syngeneic Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with 1–2.5 × 104 pfu MCMV.
(A) The percentages of donor Ly49H+ NK cells in the blood over the course of infection are shown.
(B) The percentages of donor Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the blood over the course of infection were represented as the ratio relative to the percentages of donor Ly49H+ NK cell subsets in the blood on day 0 (pre-infection). Data are pooled from two experiments (n = 6 or 7 in each group).
(C) Ly49H+ NK cell subsets expressing Ly49D and or Ly49A in the spleen on days 0, 10, and 29 pi. Data are representative of four experiments (n = 2–4 in each experiment).
*p < 0.05, **p < 0.005 versus day 0. Error bars show SEM.
B10.D2 Memory Ly49H+ NK Cells Exert Augmented Effector Functions
To determine whether B10.D2 memory Ly49H+ NK cells exhibit enhanced effector functions as compared to those of naive Ly49H+ NK cells, B10.D2 naive and memory Ly49H+ NK cells were co-cultured with RMA transfectants expressing m157 and H-2Dd (Figures 6A and 6B). Each memory Ly49H+ NK cell subset expressing Ly49D and/or Ly49A degranulated more efficiently and produced more IFN-γ than naive Ly49H+ NK cell subsets after co-culture with target cells expressing m157 and H-2Dd (Figures 6A and 6B). Augmented effector functions of licensed Ly49A+ NK cell subsets, compared with unlicensed Ly49A− NK cell subsets, were preserved in memory Ly49H+ NK cells (Figures 6A and 6B).
Figure 6. B10.D2 Memory Ly49H+ NK Cells Exert Augmented Effector Functions and Provide Protective Host Defense against Re-challenge with MCMV.
B10.D2 Ly49H+ NK cells were transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with 1 × 104 pfu MCMV. Memory Ly49H+ NK cells were enriched from spleens of recipient mice 25–29 days pi.
(A and B) Degranulation (A) and IFN-γ production (B) of B10.D2 naive and memory Ly49H+ NK cell subsets expressing Ly49D and or Ly49A after co-culture with RMA or RMA cells expressing m157 and H-2Dd. Data are pooled from three experiments (n = 9–11 in each group). *p < 0.05, **p < 0.005 versus naive NK cell subsets.
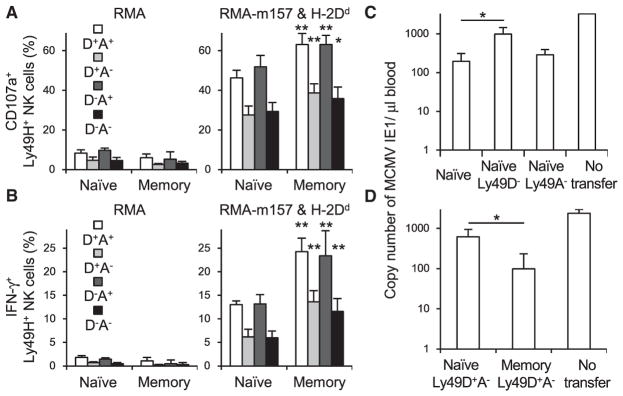
(C) 50,000 B10.D2 naive Ly49H+ NK cells, Ly49D−Ly49H+ NK cells, and Ly49A−Ly49H+ NK cells were purified from spleens of uninfected mice and transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with MCMV. The copy number of MCMV IE1 gene in the blood on day 7 pi was analyzed by qPCR.
(D) B10.D2 naive Ly49D+Ly49A−Ly49H+ NK cells were purified from spleens of uninfected mice. B10.D2 memory Ly49D+Ly49A−Ly49H+ NK cells were purified from spleens of recipient mice 25 days pi. 20,000 B10.D2 naive and memory Ly49D+Ly49A−Ly49H+ NK cells were transferred into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and then infected with MCMV. The copy number of MCMV IE1 gene in the blood on day 7 pi was analyzed by qPCR. Data are pooled from two experiments (n = 5–8 in each group).
*p < 0.05. Error bars show SEM.
We investigated the contribution of Ly49D+Ly49H+ NK cells and Ly49A+Ly49H+ NK cells in host defense of B10.D2 mice against MCMV infection. When an equivalent number of naive B10.D2 Ly49H+ NK cells, naive Ly49D−Ly49H+ NK cells, or naive B10.D2 Ly49A−Ly49H+ NK cells were transferred separately into syngeneic Ly49H-deficient mice, followed by infection with MCMV, recipient mice that received Ly49D−Ly49H+ NK cells showed a higher viral burden in the blood on day 7 pi than did mice that received total naive Ly49H+ NK cells or Ly49A−Ly49H+ NK cells (Figure 6C). To confirm whether B10.D2 memory Ly49D+Ly49A−Ly49H+ NK cells, which preferentially differentiate into memory NK cells during MCMV infection, provide effective host protection against a secondary MCMV infection, donor B10.D2 Ly49D+Ly49A−Ly49H+ NK cells were purified from recipient mice 1 month after infection and transferred into naive Ly49H-deficient recipient mice, followed by re-challenge with MCMV. Recipient mice transferred with B10.D2 memory Ly49D+Ly49A−Ly49H+ NK cells exhibited improved viral control, as compared with mice that received B10.D2 naive Ly49D+Ly49A−Ly49H+ NK cells (Figure 6D). These findings demonstrate that B10.D2 memory Ly49H+ NK cells exert augmented effector functions and mount an effective anti-viral response against re-challenge with MCMV.
DISCUSSION
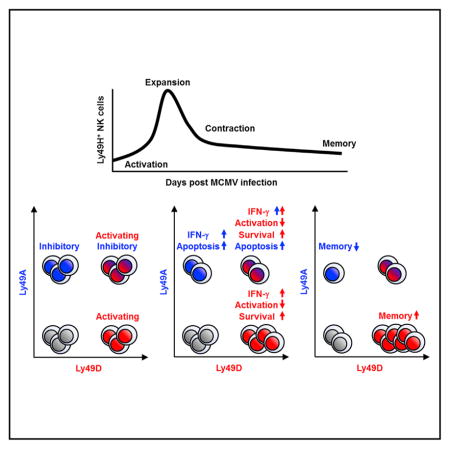
Here, we examined whether activating Ly49 receptors for self-MHC class I influence the activation, effector functions, and adaptive immune features, including antigen-specific expansion and generation of Ly49H+ memory NK cells, during MCMV infection. Furthermore, we investigated how MCMV-specific Ly49H+ NK cells co-expressing both inhibitory and activating Ly49 receptors recognizing the same self-MHC class I ligand respond and differentiate in response to MCMV. Prior studies have established that, in uninfected mice, NK cells expressing an inhibitory receptor for self-MHC class I are licensed and respond more vigorously when stimulated ex vivo (Anfossi et al., 2006; Kim et al., 2005). However, it has previously been reported that NK cells in β2-microglobulin-deficient B6 mice, although unlicensed and hypo-responsive at steady-state, mount an efficient response to MCMV infection, indicating that this anergic state can be broken during infection (Sun and Lanier, 2008; Tay et al., 1995). Similarly, unlicensed NK cells in B6 mice, hypo-responsive at steady-state, have been shown to dominate the response to MCMV infection (Orr et al., 2010). O’Leary and colleagues have reported that adoptively transferred liver NK cells from hapten-sensitized mice expressing an inhibitory receptor for MHC class I are preferentially capable of mounting a memory response upon recall (O’Leary et al., 2006); however, because the receptor responsible for hapten recognition is not yet identified, it is unclear whether the NK cells lacking an inhibitory receptor for self-MHC class I lack the hapten-specific activating receptor. The role of activating Ly49 receptors in mice or activating KIR in humans that recognize self-MHC class I have not previously been investigated during an NK cell response to infection. Our findings reveal that the activating Ly49D receptor for self-MHC class I augments IFN-γ production by Ly49H+ NK cells in an acute phase of MCMV infection and thereafter provides a survival advantage by reducing activation-induced apoptosis during the course of MCMV infection, which contributes to the preferential differentiation of memory Ly49H+ NK cells expressing Ly49D and enhances host defense against challenge with MCMV. Each NK cell subset expressing the activating Ly49D and/or inhibitory Ly49A receptors has a distinct role in the control of MCMV at different phases of the response. Furthermore, our findings imply that the activating receptors for self-MHC class I have an importance for host protection against viral infection, especially in a situation where tolerance induced through the activating receptors at steady state is broken by an acute viral infection.
Our prior studies demonstrated that unlicensed Ly49H+ NK cells in B6 mice, lacking the inhibitory Ly49C and/or Ly49I receptors that recognize self-MHC class I H-2Kb, preferentially expand during MCMV infection (Orr et al., 2010). In humans, HCMV-specific memory CD94-NKG2C+ NK cells express distinct patterns of activating and inhibitory KIR repertoires (Schlums et al., 2015). Notably, some HCMV-seropositive subjects who possess a homozygous KLRC2 (NKG2C)-null allele display expanded NK cell subpopulations exclusively expressing an activating KIR but not an inhibitory KIR that recognizes the same HLA class I molecule as the activating KIR (Béziat et al., 2013). Further, whereas expression of self HLA-Bw4 ligands for the inhibitory KIR3DL1 does not result in the preferential expansion or survival of KIR3DL1+CD94-NKG2C+ NK cells (Lopez-Vergès et al., 2011), a preferential response of KIR2DL+CD94-NKG2C+ NK cells is observed during HCMV infection in individuals bearing self-HLA-C ligands (Béziat et al., 2013; Foley et al., 2012a), suggesting that different inhibitory receptors could have different impacts on memory formation. Our findings in mice provide insights into the involvement of activating and inhibitory KIRs in the differentiation of HCMV-responsive NK cells in humans.
Our prior studies have reported that the antigen specificity of memory Ly49H+ NK cells is defined by the expression of activating receptors, and memory NK cells show enhanced responses when stimulated ex vivo through various activating receptors, including Ly49D (Nabekura and Lanier, 2014; Sun et al., 2009). Although these findings were obtained under conditions without NK cell tolerance mediated by an activating Ly49 recognizing a self-H-2 ligand, they have important implications for allogeneic transplantation in humans. Patients who receive allogeneic hematopoietic stem cell transplantation for the treatment of AML have a reduced risk of the relapse when the donor and/or recipient are HCMV seropositive before the transplantation (Behrendt et al., 2009; Elmaagacli et al., 2011). These observations raise the intriguing possibility that HCMV-induced memory NK cells bearing an activating KIR capable of recognizing allogeneic HLA class I on AML cells might contribute to the eradication of minimal residual disease. AML-affected patients who undergo allogeneic hematopoietic stem cell grafts with the activating KIR2DS1, which recognizes group 2 HLA-C ligands of KIR2DS1 but not group 1 HLA-C ligands not recognized by KIR2DS1, have a lower rate of relapse (Venstrom et al., 2012). KIR2DS1-positive allografts from donors who were homozygous or heterozygous for group 1 HLA-C could mediate this anti-leukemic effect, whereas those from donors who were homozygous for group 2 HLA-C, in which donor NK cells are tolerized, did not provide any advantage (Venstrom et al., 2012). Taken together, these results suggest that the choice of donors who have memory NK cells with activating KIRs that recognize the recipient’s HLA class I ligands might have the most beneficial effect on the prevention of relapse of hematological malignancies. Further studies of NK cell tolerance, licensing, and their interplay with adaptive immune features of NK cells will provide opportunities for developing effective and safe NK-cell-based therapies against infectious diseases and cancers.
EXPERIMENTAL PROCEDURES
Mice and MCMV
C57BL/6 (B6) mice were purchased from the National Cancer Institute. B10.D2 (B10.D2-Hc1H2dH2-T18c/nSnJ) mice were purchased from the Jackson Laboratory. Wild-type (WT) and Ly49H-deficient (Klra8−/−) (Fodil-Cornu et al., 2008) (generously provided by Dr. S. Vidal, McGill University) mice on B6 and B10.D2 backgrounds were maintained at the University of California, San Francisco in accordance with the guidelines of the institutional animal care and use committee. Smith-strain MCMV was prepared by homogenizing salivary glands harvested from BALB/c mice as described previously (Brune et al., 2001). B6 mice were infected by intraperitoneal injection of 2.5–10 × 103 plaque-forming units (pfu) MCMV. B10.D2 mice were depleted of CD8+ T cells on the day before infection by intraperitoneal injection of 200 μg anti-CD8 (clone 2.43) monoclonal antibody (mAb) and infected by intraperitoneal injection of 1–5 × 104 pfu MCMV. We observed that B10.D2 mice mount a more robust response against MCMV than B6 mice do; therefore, CD8+ T cells were depleted in the B10.D2 mice prior to infection to achieve a similar NK cell response early after MCMV infection (data not shown). Depletion of CD8+ T cells did not affect the differentiation of Ly49H+ NK cells expressing Ly49D and/or Ly49A (data not shown).
NK Cell Enrichment and Adoptive Transfer
NK cells were enriched by incubating splenocytes with purified rat mAbs against mouse CD4, CD5, CD8, CD19, Gr-1, and Ter119, followed by anti-rat IgG antibodies conjugated to magnetic beads (QIAGEN), as described previously (Nabekura et al., 2014). In some experiments, enriched NK cells were stained with antibodies against TCRβ and B220, and NK cells were purified by sorting non-T cell and non-B cell lymphocytes with a FACSAria III (BD Biosciences). In some experiments, enriched NK cells were stained with anti-Ly49D (clone 4E5) mAb and anti-Ly49A (clone YE1/48.10.6) mAb, and Ly49D− NK cells, Ly49A− NK cells, and Ly49D+Ly49A− NK cells were purified with a FACSAria III (BD Biosciences). 100,000 Ly49H+ NK cells were injected intravenously into syngeneic Ly49H-deficient mice on the day before MCMV infection.
Flow Cytometry
Fc receptors (CD16 and CD32) were blocked with 2.4G2 mAb before surface or intracellular staining with the indicated fluorochrome-conjugated mAbs or isotype-matched control antibodies (BD Biosciences, eBioscience, BioLegend, or TONBO Biosciences). For measuring apoptosis, we stained cells by using the FAM FLICA poly caspase assay kit (ImmunoChemistry Technologies) according to the manufacturer’s protocol and with antibodies against cell surface molecules. Samples were acquired on an LSRII or a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Measurement of MCMV Load
20,000 or 50,000 B10.D2 naive or memory Ly49H+ NK cells were transferred separately into Ly49H-deficient B10.D2 mice that had been depleted of CD8+ T cells on the day before infection and infected with 1 × 104 pfu MCMV. The copy number of MCMV IE1 gene in DNA prepared from peripheral blood on day 7 pi was determined by qPCR analysis with a SYBR green master mix reagent (Roche), as described previously (Kamimura and Lanier, 2014; Nabekura et al., 2014).
Ex Vivo Stimulation of NK Cells
100,000 NK cells were co-cultured with 1 ×105 RMA or RMA transfectants expressing m157 and H-2Dd for 5 hr at 37°C in the presence of PE-conjugated anti-CD107a mAb and GolgiStop (BD Biosciences), followed by staining for surface molecules and intracellular IFN-γ as described previously (Nabekura and Lanier, 2014).
Statistical Methods
Student’s t test was used to compare results. The Mann-Whitney U test was used to compare MCMV viral titers. p < 0.05 was considered statistically significant.
Highlights.
Ly49D-recognizing self-MHC class I augments IFN-γ production from Ly49H+ NK cells
Ly49D contributes to survival of Ly49H+ NK cells responding to MCMV
Ly49A impedes the formation of memory Ly49H+ NK cells responding to MCMV
Acknowledgments
We thank the L.L.L. laboratory for comments and discussions. The work was supported by NIH grant AI068129. L.L.L. is an American Cancer Society Professor, and the Friends of Leukemia Research Fund and the Nakajima Foundation support T.N.
Footnotes
AUTHOR CONTRIBUTIONS
T.N. contributed to project planning, experimental work, data analysis, and writing the manuscript. L.L.L. contributed to project planning, data analysis, and writing the manuscript.
References
- Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Barao I, Alvarez M, Ames E, Orr MT, Stefanski HE, Blazar BR, Lanier LL, Anderson SK, Redelman D, Murphy WJ. Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independently of MHC. Blood. 2011;117:7032–7041. doi: 10.1182/blood-2010-11-316653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15:54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessoles S, Angelov GS, Back J, Leclercq G, Vivier E, Held W. Education of murine NK cells requires both cis and trans recognition of MHC class I molecules. J Immunol. 2013;191:5044–5051. doi: 10.4049/jimmunol.1301971. [DOI] [PubMed] [Google Scholar]
- Béziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Björklund AT, Retière C, Sverremark-Ekström E, Traherne J, Ljungman P, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune W, Hengel H, Koszinowski UH. Current Protocols in Immunology. John Wiley and Sons; 2001. A mouse model for cytomegalovirus infection. http://dx.doi.org/10.1002/0471142735.im1907s43. [DOI] [PubMed] [Google Scholar]
- Chalifour A, Scarpellino L, Back J, Brodin P, Devèvre E, Gros F, Lévy F, Leclercq G, Höglund P, Beermann F, Held W. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179:854–868. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- Colonna M, Brooks EG, Falco M, Ferrara GB, Strominger JL. Generation of allospecific natural killer cells by stimulation across a polymorphism of HLA-C. Science. 1993;260:1121–1124. doi: 10.1126/science.8493555. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- Fodil-Cornu N, Lee SH, Belanger S, Makrigiannis AP, Biron CA, Buller RM, Vidal SM. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012a;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012b;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TC, Mason LH, Ortaldo JR, Kumar V, Bennett M. Positive recognition of MHC class I molecules by the Ly49D receptor of murine NK cells. J Immunol. 1999a;162:2035–2043. [PubMed] [Google Scholar]
- George TC, Ortaldo JR, Lemieux S, Kumar V, Bennett M. Tolerance and alloreactivity of the Ly49D subset of murine NK cells. J Immunol. 1999b;163:1859–1867. [PubMed] [Google Scholar]
- Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Hanke T, Takizawa H, McMahon CW, Busch DH, Pamer EG, Miller JD, Altman JD, Liu Y, Cado D, Lemonnier FA, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- Kamimura Y, Lanier LL. Rapid and sequential quantitation of salivary gland-associated mouse cytomegalovirus in oral lavage. J Virol Methods. 2014;205C:53–56. doi: 10.1016/j.jviromet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Lanier LL. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med. 2014;211:2455–2465. doi: 10.1084/jem.20140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier LL. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittari G, Liu XR, Selvakumar A, Zhao Z, Merino E, Huse M, Chewning JH, Hsu KC, Dupont B. NK cell tolerance of self-specific activating receptor KIR2DS1 in individuals with cognate HLA-C2 ligand. J Immunol. 2013;190:4650–4660. doi: 10.4049/jimmunol.1202120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel AR, Kingry LC, Slayden RA. The ly49 gene family. A brief guide to the nomenclature, genetics, and role in intracellular infection. Front Immunol. 2013;4:90. doi: 10.3389/fimmu.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J Immunol. 2008;181:7453–7457. doi: 10.4049/jimmunol.181.11.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CH, Welsh RM, Brutkiewicz RR. NK cell response to viral infections in beta 2-microglobulin-deficient mice. J Immunol. 1995;154:780–789. [PubMed] [Google Scholar]
- Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]