Abstract
Rationale
Studies of hypoglossal (XII) motoneurons that innervate the genioglossus muscle, an upper airway dilator, suggested that the suppression of upper airway motor tone during REM sleep is caused by withdrawal of excitation mediated by norepinephrine and serotonin.
Objectives
Our objectives were to determine whether antagonism of aminergic receptors located in the XII nucleus region can abolish the REM sleep–like atonia of XII motoneurons, and whether both serotonergic and noradrenergic antagonists are required to achieve this effect.
Methods
REM sleep–like episodes were elicited in anesthetized rats by pontine carbachol injections before and at various times after microinjection of prazosin and methysergide combined, or of only one of the drugs, into the XII nucleus.
Measurements and Main Results
Spontaneous XII nerve activity was significantly reduced, by 35 to 81%, by each antagonist alone and in combination, indicating that XII motoneurons were under both noradrenergic and serotonergic endogenous excitatory drives. During the 32 to 81 min after microinjections of both antagonists, pontine carbachol caused no depression of XII nerve activity, whereas other characteristic effects (activation of the hippocampal and cortical EEG, and slowing of the respiratory rate) remained intact. A partial recovery of the depressant effect of carbachol then occurred parallel to the recovery of spontaneous XII nerve activity from the depressant effect of the antagonists. Microinjections of either antagonist alone did not eliminate the depressant effect of carbachol.
Conclusions
The REM sleep–like depression of XII motoneuronal activity induced by pontine carbachol can be fully accounted for by the combined withdrawal of noradrenergic and serotonergic effects on XII motoneurons.
Keywords: hypoglossal motoneurons, norepinephrine, obstructive sleep apnea, pons, serotonin
Sleep-related airway obstructions occur in patients with anatomically compromised upper airway during periods when the tone in airway-dilating muscles becomes insufficient to oppose the negative inspiratory pressure (1). The severity of obstructions varies with the anatomic conditions and the magnitude and duration of the decrements in upper airway muscle tone. Obstructive episodes are often most severe during rapid eye movement (REM) sleep, when upper airway tone is suppressed parallel to the characteristic atonia of postural muscles. For this reason, extensive efforts have been devoted toward elucidating the mechanisms of REM sleep–related upper airway hypotonia (2–5).
Four distinct neurochemical mechanisms were proposed to cause motor depression during REM sleep: two disfacilitatory, resulting from an REM sleep–related withdrawal of motoneuronal excitation mediated by serotonin (5-HT) and norepinephrine (2, 6, 7); and two active, resulting from state-specific inhibition mediated by glycine and/or GABAA receptors (8, 9). On the basis of those findings, attempts were made to determine whether any one mechanism can explain the REM sleep–related depression of activity in hypoglossal (XII) motoneurons, the source of motor innervation of the genioglossus, a major upper airway dilator. However, infusion of 5-HT into the XII nucleus only partially blunted the REM sleep–like suppression of XII nerve activity elicited in decerebrate cats by pontine carbachol injections (10), and perfusion of the XII nucleus with 5-HT in chronically instrumented rats did not prevent the depression of genioglossal activity during REM sleep (11). Microinjections into the XII nucleus of either strychnine, a glycinergic receptor antagonist, or bicuculline, a GABAA receptor antagonist, had little effect on the suppression of XII nerve activity elicited by pontine carbachol in decerebrate cats (12); a similar observation was made in chronically instrumented, naturally sleeping rats (13). These results suggested that REM sleep atonia is caused by concurrent actions mediated by more than one neurotransmitter.
We tested whether the simultaneous antagonism of the same four receptor systems can eliminate the REM sleep–like atonia of XII motoneurons (14). For this, we used urethane-anesthetized rats in which REM sleep–like episodes (comprising cortical activation, hippocampal theta rhythm, silencing of pontine nor-adrenergic neurons, and suppression of XII nerve activity) were elicited by carbachol microinjections into the dorsal pontine tegmentum (15, 16). The combined injections of serotonergic, noradrenergic, GABAA, and glycinergic receptor antagonists into the XII nucleus eliminated the depressant effect of pontine carbachol on XII motoneuronal activity (14). We then determined that strychnine was not needed for this effect (17). The goal of this study was to test whether antagonizing the excitation mediated by just norepinephrine and 5-HT is sufficient to eliminate the depressant effect of pontine carbachol on XII motoneurons, and whether antagonism of both is necessary. A preliminary report has been published (18).
METHODS
Animal Preparation and Monitoring
The Institutional Animal Care and Use Committee of the University of Pennsylvania (Philadelphia, PA) approved all procedures.
Eighteen Sprague-Dawley rats were anesthetized with urethane (1 g · kg−1) and intubated, and one femoral artery and vein were cannulated for blood pressure monitoring and fluid injections, respectively. Both vagi were cut, and openings were made in the parietal bone for inserting a carbachol-containing pipette and hippocampal recording electrode. The caudal medulla was exposed to insert a microinjection pipette into the XII nucleus. The right XII nerve was prepared for recording (19), and the cortical EEG and hippocampal activity were monitored. The animals were paralyzed with pancuronium (2 mg · kg−1, intravenous) and artificially ventilated with 30–60% O2 in air. The end-expiratory CO2 was kept constant (mean, 5.6 ± 0.1% [SE]) and rectal temperature was maintained at 36–37°C.
Drug Solutions and Microinjections
The solutions injected into the XII nucleus contained either 0.2 mM prazosin or 1.0 mM methysergide, or both drugs, in 0.9% NaCl. For injections, a glass pipette filled with the antagonist(s) was inserted into the right XII nucleus 0.3 mm lateral to the midline and 1.15 mm below the dorsal medullary surface at three locations: 0.5 mm caudal, 0.15 mm rostral, and 0.8 mm rostral to the obex. Three successive 40-nl injections were made over 5.8 ± 0.1 min (SE). The injections initially filled a 0.7-mm-diameter sphere, approximately the coronal diameter of the rat XII nucleus, and delivered the drug(s) along its entire rostrocaudal extent (17, 20). Pontine injections of carbachol (10 nl) were performed with pipettes filled with 10 mM carbachol and 2% Pontamine sky blue dye in 0.9% NaCl, inserted into the predetermined dorsomedial pontine site (16, 21).
Experimental Protocol and Data Analysis
Once the control REM sleep–like response to carbachol was established, the antagonist-filled pipette was successively inserted at the three locations in the XII nucleus and the injections made. During the subsequent 3 h (approximate time), to observe over time the effects of the antagonists on the XII nerve response to pontine carbachol, carbachol was repeatedly injected at greater than 30-min intervals. Changes in XII nerve activity were measured from the moving average of the signal as the difference between the peak during central inspiration and the expiratory level when no activity was present. The central respiratory rate was derived from XII nerve activity. Latencies and durations of the responses were measured from the onset of the carbachol injection to the start and end of changes in hippocampal activity. In each experiment, the level of XII nerve activity was normalized relative to the level before the antagonist injections.
The pontine injection sites were mapped onto standard cross-sections (22). For statistical analysis, we used analysis of variance (ANOVA) with the Bonferroni correction, and paired or unpaired Student’s t test (SigmaStat; SPSS, Chicago, IL). The variability of the means is characterized by the standard error (SE), and p values refer to paired t tests unless noted otherwise.
RESULTS
REM Sleep–like Effects of Pontine Carbachol on XII Nerve Activity under Control Conditions
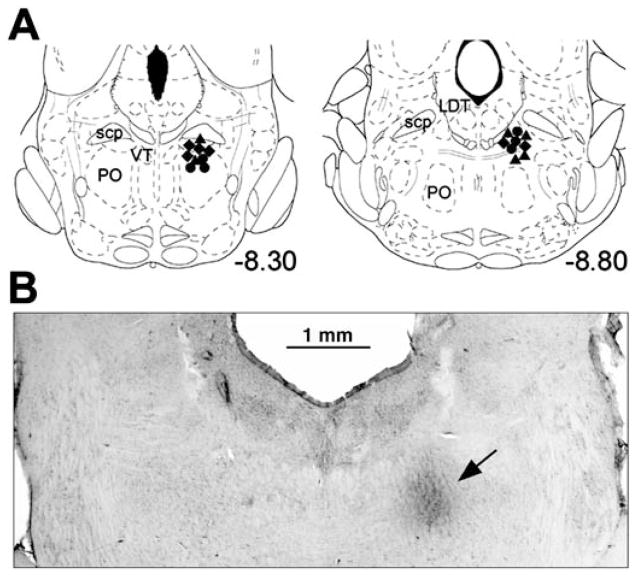
Figure 1A shows the pontine carbachol injection sites for all 18 animals superimposed on the closest standard brain sections. All injections (e.g., Figure 1B) were placed at anteroposterior levels from −8.1 to −9.0 mm caudal to the bregma (mean anteroposterior level, −8.56 ± 0.08 mm; n = 18) according to the brain atlas (22). As described previously, carbachol injections placed at these sites triggered REM sleep–like episodes characterized by activation of the cortical EEG, the appearance of hippocampal theta rhythm (3–5 Hz), suppression of XII nerve activity, and a decreased central respiratory rate (15–17, 21). Responses having similar pattern and timing could be repeatedly produced from the same site by carbachol injections made at 30-min or longer intervals. Figure 2 shows an example of two responses to pontine carbachol, one elicited before methysergide microinjections into the XII nucleus and one about 1.5 h after the antagonist had been injected. The responses were highly reproducible in terms of the respiratory rate changes, the time course and pattern of the hippocampal theta-like rhythm, and the increased power of the cortical EEG in the 6- to 12-Hz range. We also determined previously that pontine noradrenergic cells are silenced during these responses, just as they are during natural REM sleep (16, 23).
Figure 1.
Localization of pontine carbachol injection sites. Various symbols in A show the centers of carbachol injections placed on the corresponding closest standard sections of the pons (22). Circles, carbachol injection sites in the six rats with combined injections of prazosin and methysergide into the XII nucleus; triangles, sites in the six rats with only prazosin injected into the XII nucleus; diamonds, sites in the six rats with only methysergide injections. All carbachol injections were placed within a discrete region of the dorsal pontine tegmentum. (B) Carbachol injection site in one of the rats used in the present study, as seen in a coronal section of the pons. The darkened area in the dorsomedial pontine tegmentum (arrow) represents deposition of the blue dye injected with carbachol. LDT = laterodorsal tegmental nucleus; PO = nucleus pontis oralis; scp = superior cerebellar peduncle; VT = ventral tegmental nucleus.
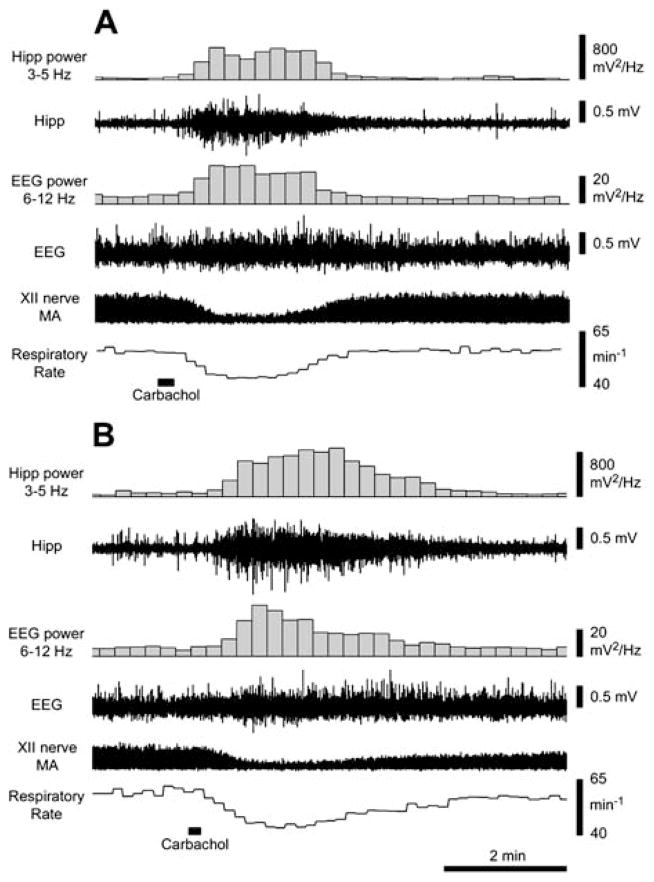
Figure 2.
Two responses to pontine carbachol elicited about 150 min apart in one experiment demonstrate long-term stability of hippocampal and cortical activations and respiratory rate slowing in response to pontine carbachol. In both records, 10 nl of carbachol was injected at the marker. Traces from the top are as follows: power of the hippocampal (Hipp) signal in the 3- to 5-Hz range, raw hippocampal recording, power of the cortical EEG in the 6- to 12-Hz range, raw cortical EEG, moving average (MA) of XII nerve activity (at this compressed time scale, the amplitude of the record represents the peak inspiratory activity in successive respiratory cycles), and the instantaneous central respiratory rate during successive 10-s intervals. The response in A was elicited before methysergide injections and the response in B was recorded about 150 min later and after methysergide injections into the XII nucleus. Note the similarity between the two responses except that the starting magnitude of XII nerve activity is different because of the methysergide actions. Two additional responses to pontine carbachol were elicited in this experiment between the two shown in A and B.
Effects of Aminergic Antagonists on Spontaneous XII Nerve Activity
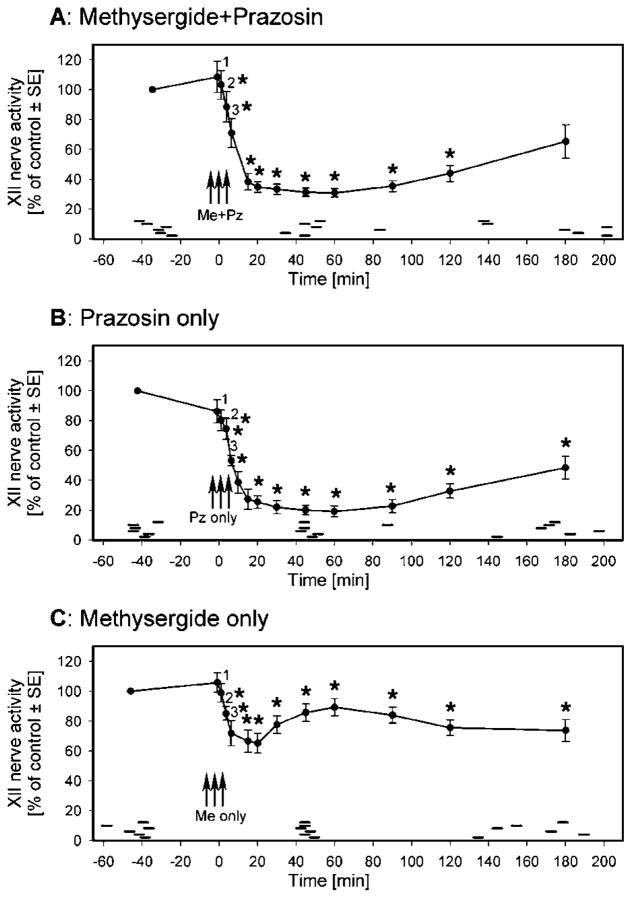
Six rats received combined injections of prazosin and methysergide at the three sites in the XII nucleus, as described in METHODS. At each level, the injections were aimed at the center of the nucleus (see Figure 1B in Fenik and coworkers [14] and Figure 1B in Fenik and coworkers [17]). The injections elicited a gradual decrease in XII nerve activity that developed over about 20 min and was not accompanied by changes in respiratory rate or arterial blood pressure. Figure 3A shows the average time course of the effect of combined microinjections of prazosin and methysergide on spontaneous XII nerve activity. A depression greater than 90% of maximum was present 15 to 90 min after the antagonists. The maximum occurred at about 60 min, at which time activity was depressed to 30.8 ± 2.7% of the control level. XII nerve activity was depressed by the antagonists to a level similar to that attained during the preantagonist responses to pontine carbachol (24.9 ± 3.6% of the control level, p = 0.14; n = 6). Subsequently, the activity gradually recovered and, 180 min after antagonist administration, reached 65.3 ± 11% of the preantagonist level (not significantly different from control, p = 0.06).
Figure 3.
Average time course of the changes in spontaneous XII nerve activity after microinjections of prazosin and methysergide (A), prazosin only (B), and methysergide only (C) into the XII nucleus. (A) Combined antagonists were injected at three sites in the XII nucleus between time 0 and the point numbered 3; points 1–3 show the average activity levels measured 1 min after each of the three injections. XII nerve activity reached a minimum of 30.8 ± 2.7% of the control activity level about 60 min after the injections. A recovery to 65.3 ± 11% of the preantagonist level occurred over the next 2 h. (B) Prazosin only was injected at three sites in the XII nucleus (same format as in A). XII nerve activity declined to a minimum level corresponding to 19.1 ± 3.6% of the preantagonist level and then gradually recovered to 48.4 ± 7.7% over the next 2 h. (C) Methysergide only was injected at three sites in the XII nucleus (same format as in A). After these injections, XII nerve activity declined to 65.1 ± 6.6% of the control level during the first 20 min. It then transiently increased over the next 40 min to 89.2 ± 5.7% of the control level, suggesting that the drug diffused into a region where its action resulted in enhancement of XII nerve activity. Three hours after methysergide administration, XII nerve activity was still significantly depressed to 73.7 ± 7.4% of the control level. * Points at which XII nerve activity was significantly depressed (p < 0.05 relative to the level of activity at the beginning of these experiments); each curve is based on data from six animals. Pontine carbachol injections were made at greater than 30-min intervals during these experiments, both before and after antagonist injections, and each elicited characteristic responses, such as those illustrated in Figures 2 and 4, which lasted 3 to 6 min. The short horizontal bars mark the periods when carbachol responses described in text were elicited in individual animals.
In another six rats, three injections of prazosin only were made into the XII nucleus. These injections also caused a gradual decrease in spontaneous XII nerve activity (Figure 3B). A depression greater than 90% of the maximum was present 20 to 90 min after the injections, with the maximum also at about 60 min, at which time the activity was depressed to 19.1 ± 3.6% of the control level. At the time of maximal depression, the level of XII nerve activity tended to be lower than during the preprazosin response to pontine carbachol in this group of animals (26.1 ± 4.9%; p = 0.17). The depression caused by prazosin alone was also greater than that following the combined injections of prazosin and methysergide (19.1 ± 3.6 versus 30.8 ± 2.7%; p < 0.05, unpaired t test). Subsequently, the activity gradually increased and, 180 min after administration of the antagonists, reached 48.4 ± 7.7% of the control level, still significantly below the preantagonist level (p < 0.01).
In a third group of six rats, three injections of methysergide only were made into the XII nucleus. These injections caused a biphasic change in spontaneous XII nerve activity: first a fast decrease and then an increase at a rate faster than that during the recovery from either combined methysergide and prazosin or prazosin-only injections (Figure 3C). During the initial phase, XII nerve activity was reduced to a minimum of 65.1 ± 6.6% of control (p < 0.01) within 20 min of the injections. This level of activity was significantly higher than the lowest points after either combined antagonist or prazosin-only injections (p < 0.001 for both, unpaired t tests). During the subsequent 40 min, an increase to 89.2 ± 5.7% of control occurred, which was still significantly below the premethysergide level (p < 0.01). After this time, XII nerve activity declined slightly and remained steady over the next 2 h. At 180 min after methysergide administration, XII nerve activity was at 73.7 ± 7.4% of its premethysergide level (p < 0.01).
Effect of Combined Injections of Prazosin and Methysergide on Carbachol-induced Depression of XII Nerve Activity
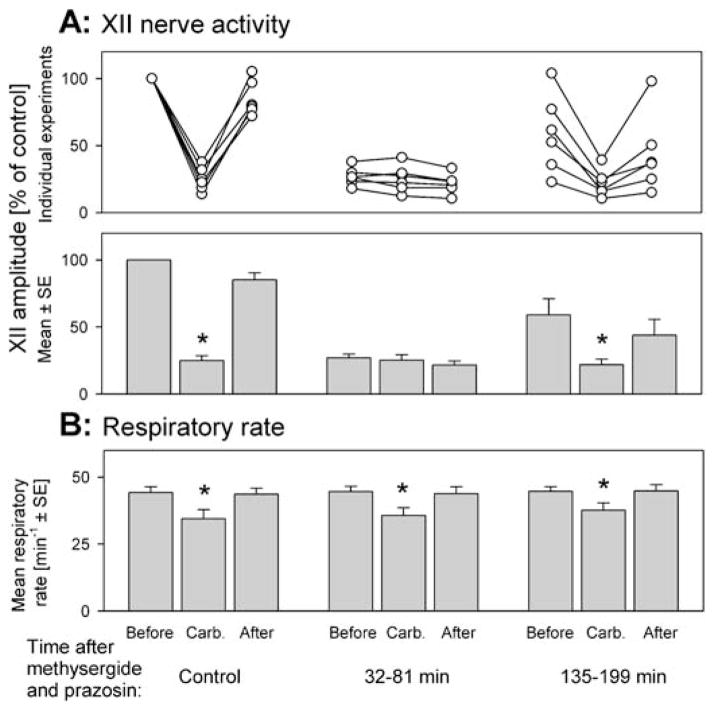
In the group of six rats receiving combined microinjections of prazosin and methysergide, the preantagonist responses to carbachol had a mean latency and duration of 62.5 ± 26 and 185 ± 13 seconds, respectively. During the responses, XII nerve activity was depressed to 24.9 ± 3.6% of the precarbachol value (p < 0.001), and the respiratory rate decreased from 44.2 ± 2.1 to 34.5 ± 3.5 min−1 (p < 0.01). Figure 4 shows examples of responses to pontine carbachol elicited before, and at two different times after, antagonist administration. Individual and average decreases in XII nerve activity and respiratory rate after carbachol administration, both before and at different times after the combined injections of prazosin and methysergide, are shown in Figure 5.
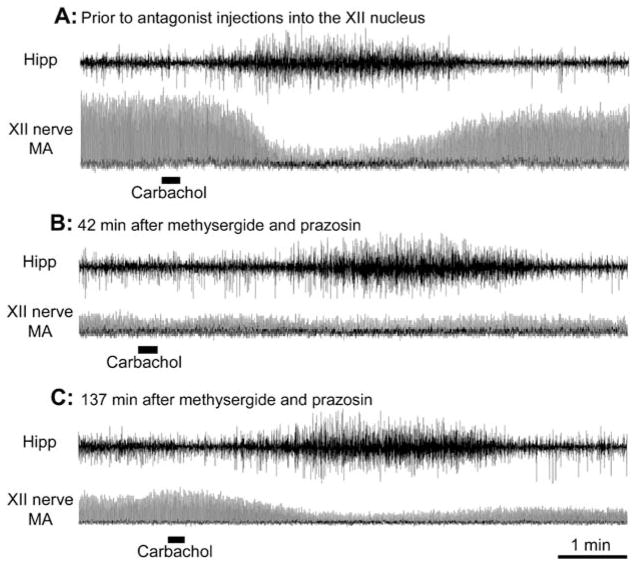
Figure 4.
Combined microinjections of prazosin and methysergide into the XII nucleus eliminate the REM sleep–like depression of XII nerve activity. In each panel, the top trace shows hippocampal activity (Hipp) and the bottom trace shows the moving average (MA) of XII nerve activity, both recorded at the same gains in all three panels. Each peak in the XII nerve MA represents one compressed inspiratory burst. In each record, 10-nl carbachol injections were made at the markers. (A) The preantagonist response to pontine carbachol, during which XII nerve activity is profoundly depressed and the respiratory rate is reduced from 52.5 to 47.5 min−1. (B) A response to carbachol elicited 42 min after antagonists were injected into the XII nucleus; at this time, the precarbachol level of XII nerve activity was reduced to a level similar to that observed at the time of maximal response to carbachol before antagonist administration (A), and no further depression occurred after carbachol injection. The characteristic hippocampal activation and slowing of the respiratory rate from 52.5 to 46.7 min−1 indicate that carbachol was effective but did not depress XII nerve activity. (C) By 137 minutes after administration of the antagonists, the precarbachol level of XII nerve activity increased compared with that in B, and XII nerve activity was visibly depressed after pontine carbachol injection, both indicating a partial recovery from the effect of the antagonists.
Figure 5.
Individual and average effects of combined prazosin and methysergide microinjections into the XII nucleus on the carbachol-induced depression of XII nerve activity (A) and slowing of the respiratory rate (B). (A) Individual (top graph) and average (bottom graph) responses of the XII nerve to pontine carbachol. Each data triplet shows the level of XII nerve activity just before, at the peak of, and just after the response to carbachol. XII nerve activity is normalized relative to the activity level at the beginning of each experiment. The three data sets illustrate the effects of carbachol before and during two periods after antagonist administration, as indicated at the bottom. XII nerve activity was significantly depressed by pontine carbachol under the control conditions and when tested 135 to 199 min after the antagonists. However, during the tests with carbachol conducted 32 to 81 min after antagonist administration, XII nerve activity was on average not altered, and only minimally increased or decreased in individual animals (middle data set, top graph). Thus, during this period, the combined antagonism of adrenergic and serotonergic receptors reversibly eliminated the depressant effect of pontine carbachol on XII nerve activity. (B) Average changes in respiratory rate elicited by carbachol at successive stages of these experiments, shown using the same format as in the bottom graph in A. The baseline respiratory rate and its carbachol-induced decreases were well maintained at all times after antagonist administration. * Significantly different from the precarbachol level at p < 0.05.
The first postantagonist tests with pontine carbachol were conducted 5–10 min after antagonist injections. As in our previous study with microinjections of three or more distinct antagonists into the XII nucleus (14, 17), XII nerve activity was significantly suppressed by pontine carbachol at this time. For simplicity, the results of these tests are not reported.
During the carbachol injections made 32–81 min (mean, 49.2 ± 6.9 min) after the combined prazosin and methysergide injections, carbachol did not depress XII nerve activity. These responses had a mean latency of 58.7 ± 17 s and a duration of 211 ± 17 s (not different from the control values in this group: p = 0.88 and p = 0.06, respectively). The respiratory rate decreased from 44.6 ± 2.0 to 35.7 ± 2.9 min−1 (p < 0.01) (Figure 5B), also not different from the responses elicited before antagonist administration (F1,1,5 ± 1.83, p = 0.23, two-way repeated measures ANOVA). During these responses, characteristic hippocampal and cortical activations occurred (cf. Figure 2). However, XII nerve activity, reduced at this time by the antagonists (Figure 3A), was not altered in association with the response to carbachol. Figure 4B shows one example of such a response. The mean XII nerve activity was 27.0 ± 2.7% of control before and 25.2 ±4.1% at the peak of the responses to pontine carbachol (p = 0.4) (Figure 5A). This absence of depression of XII nerve activity was significantly different from the depressant effect of carbachol before antagonist administration (F1,1,5 =56, p < 0.001, two-way repeated measures ANOVA).
Another series of tests with carbachol was performed 135–199 min (mean, 172 ± 12 min) after antagonist administration. At this time, the depressant effect of pontine carbachol on XII nerve activity partially recovered (Figures 4C and 5A). The activity was suppressed from 51.8 ± 8.0 to 20.8 ± 3.3% of the preantagonist control (p < 0.01). In contrast to the previous set of responses to carbachol, the depression was significant (F1,1,5 = 23, p < 0.01, two-way repeated measures ANOVA), but also significantly weaker than before antagonist administration (F1,1,5 = 43, p < 0.01, two-way repeated measures ANOVA). The latencies and durations of these responses were not different from those before antagonist administration, 87.5 ± 45 s (p = 0.27) and 195 ± 24 s (p = 0.57), respectively, and the respiratory rate decreased from 44.6 ± 1.8 to 37.6 ± 2.8 min−1 (p < 0.01) (Figure 5B). The latter was slightly less than during previous carbachol responses (F1,1,5 = 6.7, p < 0.05, two-way repeated measures ANOVA), but not different from the control responses before antagonist administration (F1,1,5 = 4.9, p = 0.08, two-way repeated measures ANOVA).
Effect of Prazosin Only on Carbachol-induced Depression of XII Nerve Activity
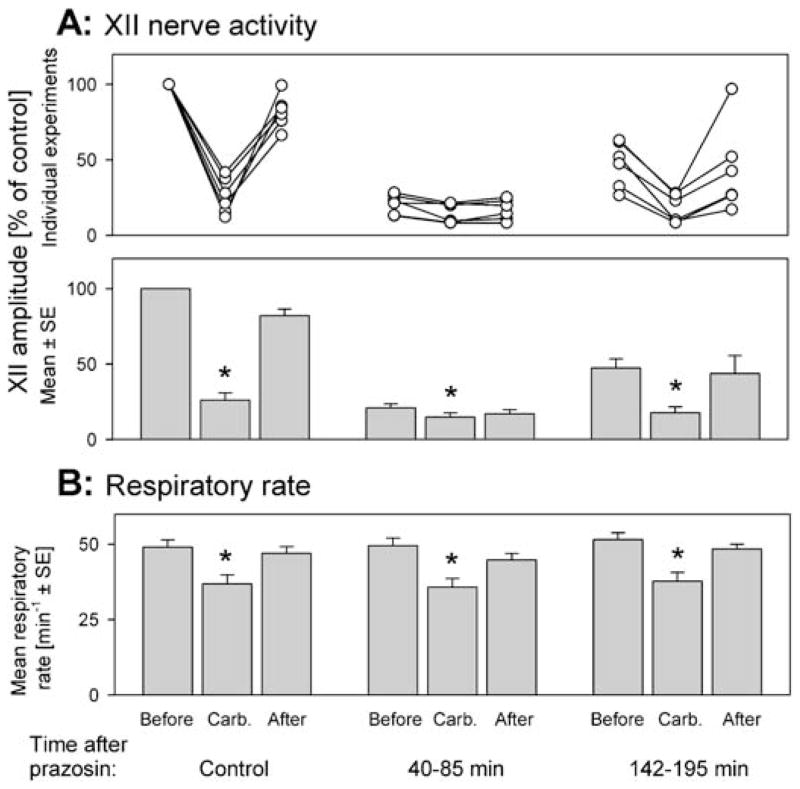
In the six rats with injections only of prazosin into the XII nucleus, the preprazosin responses to carbachol had a mean latency and duration of 56.3 ± 7.5 and 203 ± 14 s, respectively; XII nerve activity was depressed to 26.1 ± 4.9% of the control (p < 0.001); and the respiratory rate decreased from 49.1 ± 2.3 to 36.9 ± 3.0 min−1 (p < 0.01). The individual and average XII nerve and respiratory rate responses to carbachol before, and at two different times after, prazosin injections are shown in Figure 6.
Figure 6.
Microinjections into the XII nucleus of prazosin only did not eliminate the depressant effect of pontine carbachol on XII nerve activity. (A) The effect of pontine carbachol on XII nerve activity before, and at two times after, prazosin microinjections, shown in the same format as in Figure 5A. Each data set shows XII nerve activity just before, at the peak of the response to pontine carbachol, and just after the response. The three data sets illustrate the effects of carbachol, as tested under control conditions and then during two periods after prazosin administration (indicated at the bottom). Note that pontine carbachol injections made 40–85 minutes after prazosin had a significant depressant effect on XII nerve activity; during the same period, combined microinjections of prazosin and methysergide eliminated the carbachol-induced depression (cf. Figure 5A). XII nerve activity is normalized relative to its level at the beginning of the experiments. (B) The baseline respiratory rate and its carbachol-induced changes were well maintained at all times after prazosin administration. * Significantly different from the precarbachol level at p < 0.05.
Carbachol injected 40–85 min (mean, 50.7 ± 7.0 min) after prazosin elicited responses with a mean latency of 76.0 ± 25 s and a duration of 216 ± 7.7 s (not different from the control values in this group: p = 0.34 and p = 0.37, respectively). Responses included the characteristic hippocampal and cortical activations, and the respiratory rate decreased from 49.5 ± 2.5 to 35.8 ± 2.8 min−1 (p < 0.01) (Figure 6B); not different from the responses elicited before prazosin (F1,1,5 = 1.46, p = 0.28, two-way repeated measures ANOVA). Despite the fact that the XII nerve activity was reduced by prazosin more than by the combined injections of prazosin and methysergide, XII nerve activity was still significantly reduced after pontine carbachol administration, from 20.8 ± 2.6 to 14.8 ± 2.7% (p < 0.05) of the preantagonist control (Figure 6A). The average level of XII nerve activity during these responses to carbachol was significantly lower than that during the preprazosin responses to carbachol (14.8 ± 2.7 versus 26.1 ± 4.9%, p < 0.01).
Subsequent to carbachol injections performed 142–195 min (mean, 171 ± 7.2 min) after prazosin administration, XII nerve activity was suppressed from 47.3 ± 6.2 to 17.7 ± 3.8% of the control (p < 0.001) (Figure 6A). This suppression was significantly stronger than that during the preceding tests (F1,1,5 = 75, p < 0.001, two-way repeated measures ANOVA), but also significantly weaker than the effect of carbachol before prazosin (F1,1,5 = 43, p < 0.01, two-way repeated measures ANOVA). The latencies and durations of these responses were not different from those before prazosin, 77.0 ± 23 s (p = 0.28) and 247 ± 32 s (p = 0.26), respectively. The respiratory rate decreased from 51.5 ± 2.2 to 37.7 ± 2.9 min−1 (p < 0.05) (Figure 6B); not different from that during the preprazosin responses to carbachol (F1,1,5 = 0.47, p = 0.52, two-way repeated measures ANOVA).
Effect of Methysergide Only on Carbachol-induced Depression of XII Nerve Activity
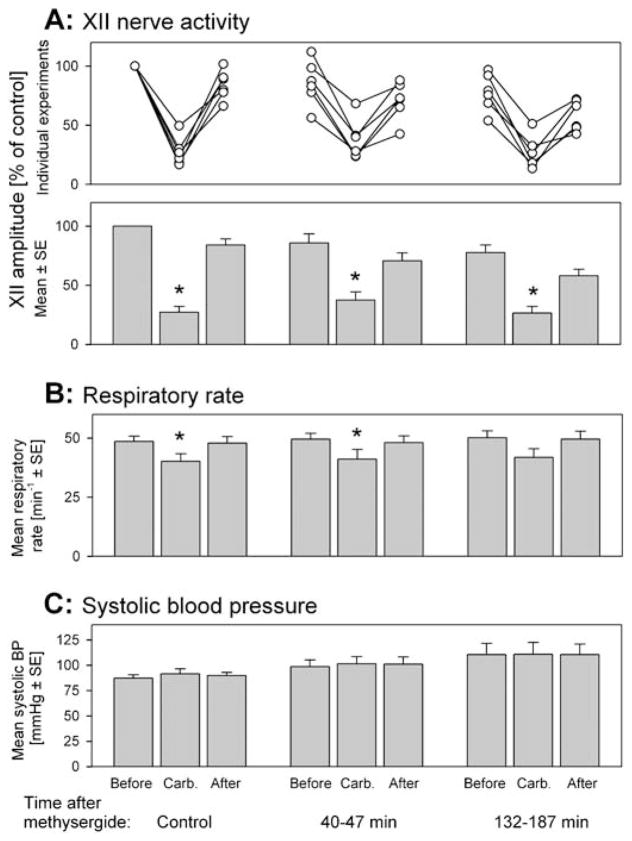
In these six rats, the control response to carbachol had a mean latency and duration of 75.0 ± 23 and 185 ± 15 s, respectively. XII nerve activity was depressed to 27.2 ± 4.9% of control (p < 0.001), and the respiratory rate decreased from 48.6 ± 2.3 to 40.2 ± 3.2 min−1 (p < 0.01). These control carbachol responses were not different from those of the two other animal groups (F2,5 = 0.21, p = 0.81 and F2,5 = 0.056, p = 0.58 for latency and duration, respectively [one-way ANOVA]; F1,2,5 = 0.07, p = 0.94 and F1,2,5 = 0.77, p = 0.48 for the depression of XII activity and respiratory rate, respectively [two-way repeated measures ANOVA]). The average XII nerve and respiratory rate responses to carbachol before, and at different times after, methysergide injections are shown in Figures 7A and 7B.
Figure 7.
Microinjections into the XII nucleus of methysergide only did not eliminate the depressant effect of pontine carbachol on XII nerve activity. (A) The effect of pontine carbachol on XII nerve activity before, and at two times after, methysergide administration, shown in the same format as in Figure 5. Each data set shows XII nerve activity levels just before, at the peak of the response to pontine carbachol, and just after the response. The three data sets illustrate the effects of carbachol under control conditions and then during two periods after methysergide administration (indicated at the bottom). XII nerve activity is normalized relative to the activity level at the beginning of the experiments. Note that pontine carbachol had a significant depressant effect on XII nerve activity when tested 40 to 47 min after methysergide administration, whereas the combined microinjections of prazosin and methysergide eliminated the depression (cf. Figure 5A). (B) The baseline respiratory rate and its carbachol-induced changes were well maintained throughout these experiments. * Significantly different from the precarbachol level at p < 0.05. (C) Mean systolic arterial blood pressure measured at the same times as XII nerve activity and respiratory rate shown in A and B. Neither the small arterial pressure increases after carbachol injections nor the gradual increasing trend across the entire duration of these experiments were significant. See the last section of RESULTS for more details on blood pressure across all conditions.
Carbachol injected 40–47 min (mean, 43.0 ± 1.0 min) after the methysergide injections elicited responses with a mean latency of 98.0 s ± 35 and a duration of 230 s ± 33 (not different from the control values in this group: p = 0.14 for both). The responses included the characteristic hippocampal and cortical activations, and XII nerve activity was significantly depressed from 85.8% ± 7.7 of the control to 37.6% ± 6.9 (p < 0.001) (Figure 7A). The minimum level of XII nerve activity after carbachol administration was significantly higher than during the control responses (37.6 ± 6.9 versus 27.2 ± 4.9%, p < 0.05). The respiratory rate decreased from 49.5 ± 2.5 to 41.2 ± 4.1 min−1 (p < 0.05) (Figure 7B), not different from the responses elicited before methysergide (F1,1,5 = 0.0008, p = 0.98, two-way repeated measures ANOVA).
In the tests performed 132–187 min (mean, 160 ± 8.7 min) after methysergide administration, XII nerve activity was suppressed by carbachol from 77.7 ± 6.4% of the control level to 26.5 ± 5.7% (p < 0.001) (Figure 7A). The latencies and durations of these responses were not different from those before methysergide administration, 108 ± 46 s (p = 0.25) and 257 ± 51 s (p = 0.15), respectively. The respiratory rate decreased in five animals and did not change in one animal, with the average decrease from 50.2 ± 2.9 to 41.9 ± 3.6 min−1 (n = 6, p = 0.07) (Figure 7B).
Effects of Antagonists and Carbachol on Arterial Blood Pressure
Neither the antagonists nor the carbachol injections, nor the time elapsed between the start and end of each experiment, was associated with major changes in arterial blood pressure. Overall, blood pressure tended to increase with time, and most responses to carbachol were associated with small blood pressure increases when measured during the response maximum. Figure 7C shows a typical data set for systolic blood pressure in the series of animals receiving methysergide injections.
For the combined data from the three series of studies (n = 18 rats), the time-dependent increase in systolic blood pressure measured before carbachol injections was from 80 ± 3 mm Hg at the beginning of the study to 82 ± 4 mm Hg 32–85 min after administration of the antagonists, and to 90 ± 6 mm Hg 132–199 min after administration of the antagonists, with the difference between the beginning and the end of the study approaching statistical significance (p = 0.06, paired t test, n = 18). This trend was likely to be related to a decreasing level of anesthesia despite additional doses of urethane administered between the tests with carbachol to suppress any signs of spontaneous cortical or hippocampal activation (see the online supplement).
The carbachol-induced blood pressure increases were significant in four of the nine data sets (in the control and first postantagonist carbachol test in animals with prazosin injections, and in the two postantagonist tests in rats with combined methysergide and prazosin injections). For all three antagonist conditions combined, blood pressure increases that followed each carbachol injection were 5.8 ± 1.2mm Hg for the responses before antagonist administration, 5.9 ± 1.5 mm Hg for the responses elicited 32–85 minutes after antagonist administration, and 3.0 ± 1.2 mm Hg for those elicited 132–199 min after antagonist administration (n = 18 for each). These increases were statistically significant (p < 0.05 each, paired t tests) and not significantly different among the responses to carbachol elicited at the successive stages of the experiments.
DISCUSSION
Our main finding is that combined microinjections into the XII nucleus of antagonists of α-adrenergic and serotonergic receptors are necessary and sufficient to eliminate the REM sleep–like depression of XII motoneuronal activity elicited by pontine carbachol injections. The effect was reversible and selective in that the carbachol-induced depression of XII nerve activity was abolished, whereas other REM sleep–like changes, such as the cortical and hippocampal activations and slowing of the respiratory rate, remained intact. The most parsimonious explanation of this result is that the adrenergic and serotonergic receptor antagonists injected into the XII nucleus removed aminergic excitation from XII motoneurons. By doing so, they preempted the ability of pontine carbachol to exert its effects because they acted through the same disfacilitatory mechanism that would otherwise occur during the REM sleep–like depression of XII motoneuronal activity. Accordingly, we propose that, at least in the carbachol model used, a combined withdrawal from motoneurons of adrenergic and serotonergic effects is the main cause of the REM sleep–like suppression of their activity.
Urethane-anesthetized Rat as a Model of Neural Phenomena of REM Sleep
Pontine carbachol injections have been extensively used to study REM sleep (15, 24). As this and other studies show, 5- to 20-nl carbachol injections into a discrete site within the dorsal pontine tegmentum of urethane-anesthetized rats can repeatedly activate neural events similar to those characterizing natural REM sleep (15, 16, 21). The effects of carbachol last 3 to 4 min and include activation of the cortical EEG, appearance of rhythmic theta-like activity in the hippocampus, silencing of pontine noradrenergic neurons, and a profound suppression of XII nerve activity (15, 16). The site from which these changes are elicited most readily is analogous to that identified in cats as most effective for triggering an REM sleep–like state (25, 26). Thus, the model used in this study has important features similar to the neural events during natural REM sleep. Although this is a reduced model, and additional mechanisms may influence the activity of XII motoneurons during REM sleep in behaving animals, it is noteworthy that the mechanisms of upper airway motor control during REM sleep delineated with carbachol models (2, 10, 12, 27) were subsequently found to function in a similar way during natural REM sleep in behaving animals (11, 13).
Effect of Noradrenergic and Serotonergic Antagonists on Spontaneous XII Nerve Activity
Injections of either prazosin or methysergide into the XII nucleus reduced XII nerve activity. Prazosin is a relatively specific antagonist of the excitatory adrenergic α1 receptors (28). Therefore, the reduction of XII nerve activity after its microinjection into the XII nucleus demonstrates that there is an endogenous adrenergic excitatory action on XII motoneurons in urethane-anesthetized rats. This was not previously reported in vivo, but was suggested by studies showing that brainstem noradrenergic neurons become silent during REM sleep and the carbachol-induced REM sleep–like state (15, 16, 23, 29, 30), and that the primary effect of norepinephrine and phenylephrine on XII motoneurons is excitation (31, 32). Accordingly, microinjections of prazosin into the XII nucleus may be interpreted as mimicking the withdrawal of noradrenergic excitation to XII motoneurons that occurs during REM sleep.
In contrast to the paucity of studies with adrenergic antagonists, the effects of serotonergic antagonists on spontaneous activity of XII motoneurons have been investigated relatively extensively. In decerebrate cats (10, 27), behaving dogs and rats (11, 33, 34), and urethane-anesthetized rats (35), methysergide and various 5-HT2 receptor antagonists reduced XII nerve activity and that of XII nerve–innervated muscles. Consistent with earlier reports, our microinjections initially depressed XII nerve activity by about 35%. As with noradrenergic neurons, brainstem 5-HT–containing neurons are silenced during the atonia of REM sleep (36–38). Therefore, methysergide microinjections into the XII nucleus may mimic the effects of the withdrawal of serotonergic excitation from XII motoneurons that is likely to occur during REM sleep.
Rationale for Composition of the Antagonist Mix
Our study design was based on evidence that aminergic excitation of XII motoneurons is mediated mainly by α1-adrenergic and 5-HT2 receptors (32, 35, 39–42). These receptors are effectively blocked in vivo by prazosin and methysergide, respectively. Although the main aminergic receptor subtypes mediating excitation of XII motoneurons have been further identified as α1B adrenergic and 5-HT2A (39–42), the in vivo actions of drugs specific for these subtypes are less well established than for the classical, broad-spectrum α-adrenergic and serotonergic receptor antagonists, such as prazosin and methysergide. For this reason, and for consistency with our earlier studies (14, 17), we used these two antagonists.
We injected the antagonists directly into the XII nucleus, rather than systemically, in a manner that ensured their relatively uniform spread throughout the entire rostrocaudal extent of the nucleus. With this mode of drug administration, the initial depression of XII nerve activity caused by methysergide was followed by a period during which the depression relatively rapidly diminished, suggesting that the drug diffused into an area from which it exerted effects on XII motoneurons opposite to those produced within the XII nucleus. The model of diffusion with which we designed and interpreted our study predicts that 20 min after the injection, that is, when this secondary effect of methysergide appeared, the drug could spread about 1 mm from the center of the XII nucleus (17, 20). Thus, it could act on receptors in the vicinity of the XII nucleus whose antagonism results in an increase in XII motoneuronal activity. Such a hypothetical excitatory action of methysergide may explain why the maximal suppression of spontaneous XII nerve activity produced by combined microinjections of methysergide and prazosin tended to be smaller than that with prazosin only (Figure 3). We also cannot rule out that this secondary effect of methysergide played a role in the abolition of the carbachol-induced suppression of XII nerve activity. However, because the depressant effect of pontine carbachol on XII nerve activity was not abolished by either antagonist alone, this secondary effect of methysergide is unlikely to have played a decisive role. It is also noteworthy that the full abolition of the effect of carbachol did not occur under the conditions when XII nerve activity was most suppressed (by prazosin), but when both serotonergic and adrenergic receptors were simultaneously antagonized. This argues against the possibility that the elimination of the depressant effect of pontine carbachol on XII nerve activity was a nonspecific consequence of the reduced level of XII nerve activity after administration of the antagonists.
Previous Attempts to Define Mechanisms of REM Sleep–related Depression of Motoneuronal Activity
In addition to the withdrawal of aminergic excitation and active inhibition as mechanisms by which XII motoneuronal activity can be reduced during REM sleep, one can also consider disfacilitatory effects mediated by excitatory peptides such as thyrotropin-releasing hormone or substance P, as both are present in medullary 5-HT–containing neurons (43, 44). Although the role of these peptides in the mechanisms of REM sleep has not been studied and our data show that they do not play a role in the atonia produced by carbachol in the model used, considerable attention was given to the effects caused by postsynaptic inhibition present in XII and other motoneurons during the atonia of REM sleep (45–48).
To test the role of either disfacilitatory or inhibitory mechanisms, serotonergic agonists or antagonists were injected into the XII nucleus (10, 11, 27) and glycinergic or GABAA receptor–mediated inhibitions were antagonized (12, 13, 49). All studies indicated that the REM sleep–like motoneuronal depression was not mediated by any single receptor system. In contrast, we found, using the same experimental design as in the present study, that combined antagonism of aminergic excitation and amino acid–mediated inhibition by prazosin, methysergide, and bicuculline, with or without strychnine, eliminates the depressant effect of pontine carbachol on XII motoneurons (14, 17). These results provided, for the first time, proof of the concept that the REM sleep–like depression of XII motoneuronal activity can be fully accounted for by the combined actions mediated by at most four major types of neurotransmitter receptor. However, we did not test whether all antagonists used were necessary. In the present study, we demonstrate that, at least in the carbachol model, the disfacilitatory actions resulting from the combined withdrawal of noradrenergic and serotonergic influences are both necessary and sufficient.
Time Course of the Abolition of REM Sleep–like Depression of XII Nerve Activity by Antagonists
The volumes were adequate to deliver the antagonists throughout the entire XII nucleus, but, as in our previous studies (14, 17), the depression of XII nerve activity was still significant during tests performed within the first 5 to 10 min after the injections. As discussed elsewhere, when the depressant effect of pontine carbachol was eliminated 30 to 80 min after the antagonists, the drugs had spread 0.9–1.4 mm from the center of the XII nucleus (17). The time course of the effects of methysergide on spontaneous XII nerve activity (discussed previously) also suggests that diffusion of drugs beyond the XII nucleus occurred. This is not surprising; some diffusion beyond the XII nucleus was expected and desired to ensure that the antagonists spread far enough to reach all dendrites of XII motoneurons, some of which extend outside the XII nucleus and have 5-HT2A and adrenergic receptors (40, 50, 51). However, the diffusion must have been relatively limited because the antagonists did not cause changes in the respiratory rate, the magnitude of the decrease in respiratory rate after pontine carbachol administration, or arterial blood pressure. It is also noteworthy that the depressant effect of carbachol was eliminated when tested 30 to 80 min after antagonist administration, but was present both 5 to 10 min afterward, and 2 to 3 h afterward. This is compatible with a genuine antagonistic action of the drugs during their gradual diffusion and washout.
Future Studies and Implications for Pharmacotherapy for Obstructive Sleep Apnea Syndrome
Our finding that antagonism of just two aminergic receptor systems is sufficient to abolish REM sleep–like depressant effects in XII motoneurons narrows the range of neurotransmitter receptors that also may be important for mediating upper airway hypotonia during REM sleep. However, caution is needed because our results were obtained in a reduced model of REM sleep. In addition, even in this model, by varying the doses and placement of the antagonists and assessing the timing of their effect on the REM sleep–like atonia, one can further delineate the region that needs to be affected. Also, by using antagonists specific for the receptor subtypes likely to be most important in mediating the endogenous aminergic excitatory drive in XII motoneurons (35, 39–42, 52), one can further refine the underlying pharmacology.
An improved understanding of the neurochemical mechanisms of REM sleep–related hypotonia of upper airway muscles may offer new opportunities for pharmacologic treatments of the obstructive sleep apnea syndrome. However, a transition from local manipulations with aminergic transmission in an experimental setting to an effective treatment by systemic drug administration will pose problems because the same receptors that are involved in motoneuronal control across the sleep–wake states are also involved in many other functions, including sleep. For example, systemic administration of aminergic agonists or reuptake blockers to enhance upper airway motor tone is likely to suppress sleep (53, 54). This and other negative side effects will limit the doses of drugs that otherwise could be effective, and may be one reason for the unsatisfactory outcomes of attempts to treat obstructive sleep apnea with drugs targeting aminergic transmission (55, 56). An improved understanding of the receptor subtypes involved in state-dependent control of upper airway motoneurons may help establish more effective treatments. Eventually, a combination therapy with low doses of drugs that elevate central norepinephrine and 5-HT levels, stimulate or sensitize appropriate receptors, and improve sleep continuity may prove useful.
Supplementary Material
Footnotes
Conflict of Interest Statement: V.B.F. does not have a financial relationship with a commercial entity that has an interest in the subject matter of this manuscript. R.O.D. does not have a financial relationship with a commercial entity that has an interest in the subject matter of this manuscript. L.K. acted as a one-time consultant for the following commercial entities interested in pharmacologic treatments for the obstructive sleep apnea syndrome for which he received honoraria not exceeding $2,500 per any one instance: Hypnion, Sepracor, and Cypress Bioscience.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
References
- 1.Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 2.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 3.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis, and treatment. New York: Marcel Dekker; 2002. pp. 99–154. [Google Scholar]
- 4.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnoea. Respir Res. 2001;2:286–294. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 6.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during the carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 7.Lai YY, Kodama T, Siegel J. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chase MH, Morales F. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 9.Mori S. Contribution of postural muscle tone to full expression of posture and locomotor movements: multi-faceted analyses of its setting brainstem–spinal cord mechanisms in the cat. Jpn J Physiol. 1989;39:785–809. [PubMed] [Google Scholar]
- 10.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep–like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- 11.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep–wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 13.Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep–like depression of hypoglossal motoneuronal activity. Arch Ital Biol. 2004;142:237–249. [PubMed] [Google Scholar]
- 15.Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 16.Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep–like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- 17.Fenik V, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep–like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005;14 doi: 10.1111/j.1365-2869.2005.00461.x. (In press) [DOI] [PubMed] [Google Scholar]
- 18.Fenik V, Davies RO, Kubin L. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. [accessed September 2005]. Microinjections of serotonergic and noradrenergic antagonists into the XII nucleus abolish the carbachol-induced, REM sleep–like atonia of hypoglossal motoneurons. Program No. 769.9. Available from: http://sfn.scholarone.com/itin2003/ [Google Scholar]
- 19.Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Methods. 2001;116:147–150. doi: 10.1016/s0165-0270(01)00340-5. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson C. Diffusion from an injected volume of a substance in the brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 21.Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. San Diego, CA: Academic Press; 1991. [Google Scholar]
- 23.Fenik V, Ogawa H, Davies RO, Kubin L. The activity of locus coeruleus neurons is reduced in urethane-anesthetized rats during carbachol-induced episodes of REM sleep–like suppression of upper airway motor tone. Soc Neurosci Abstr. 1999;25:2144. [Google Scholar]
- 24.Baghdoyan HA. Cholinergic mechanisms regulating REM sleep. In: Schwartz WJ, editor. Sleep science: integrating basic research and clinical practice. Basel: Karger; 1997. pp. 88–116. [Google Scholar]
- 25.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- 26.Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: locus of the sensitive region. Neuroscience. 1990;39:279–293. doi: 10.1016/0306-4522(90)90267-8. [DOI] [PubMed] [Google Scholar]
- 27.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 28.Bylund DB. Subtypes of α1- and α2-adrenergic receptors. FASEB J. 1992;6:832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- 29.Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 30.Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- 31.Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- 32.Fenik V, Davies RO, Kubin L. Adrenergic receptor subtypes mediating excitatory effects in hypoglossal motoneurons. Sleep. 1999;22:S37. [Google Scholar]
- 33.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- 34.Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with L-trytophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160:1659–1667. doi: 10.1164/ajrccm.160.5.9812007. [DOI] [PubMed] [Google Scholar]
- 35.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 36.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 37.Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- 38.Woch G, Davies RO, Pack AI, Kubin L. Behavior of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol. 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons: a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- 40.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- 41.Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 42.Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 43.Johansson O, Hokfelt T, Pernow B, Jeffcoate SL, White N, Steinbusch HW, Verhofstad AA, Emson PC, Spindel E. Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience. 1981;6:1857–1881. doi: 10.1016/0306-4522(81)90028-2. [DOI] [PubMed] [Google Scholar]
- 44.Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1998;391:491–505. [PubMed] [Google Scholar]
- 45.Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–1129. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- 46.Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- 47.Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res. 2000;885:262–272. doi: 10.1016/s0006-8993(00)02955-3. [DOI] [PubMed] [Google Scholar]
- 48.Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soja PJ, Finch DM, Chase MH. Effect of inhibitory amino acid antagonists on masseteric reflex suppression during active sleep. Exp Neurol. 1987;96:178–193. doi: 10.1016/0014-4886(87)90179-8. [DOI] [PubMed] [Google Scholar]
- 50.Aldes LD, Shaw B, Chronister RB, Haycock JW. Catecholamine-containing axon terminals in the hypoglossal nucleus of the rat: an immunoelectron microscopic study. Exp Brain Res. 1990;81:167–178. doi: 10.1007/BF00230113. [DOI] [PubMed] [Google Scholar]
- 51.Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- 52.Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 53.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;1:57–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 54.Qureshi A, Lee-Chiong T., Jr Medications and their effects on sleep. Med Clin N Am. 2004;88:751–766. doi: 10.1016/j.mcna.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Veasey SC. Pharmacotherapies for obstructive sleep apnea: how close are we? Curr Opin Pulm Med. 2001;7:399–403. doi: 10.1097/00063198-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Smith IE, Quinnell TG. Pharmacotherapies for obstructive sleep apnoea: where are we now? Drugs. 2004;64:1385–1399. doi: 10.2165/00003495-200464130-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.