Abstract
In the rat, a species widely used to study the neural mechanisms of sleep and motor control, lingual electromyographic activity (EMG) is minimal during non-rapid eye movement (non-REM) sleep and then phasic twitches gradually increase after the onset of REM sleep. To better characterize the central neural processes underlying this pattern, we quantified EMG of muscles innervated by distinct subpopulations of hypoglossal motoneurons and nuchal (N) EMG during transitions from non-REM sleep to REM sleep. In 8 chronically instrumented rats, we recorded cortical EEG, EMG at sites near the base of the tongue where genioglossal and intrinsic muscle fibers predominate (GG-I), EMG of the geniohyoid (GH) muscle, and N EMG. Sleep-wake states were identified and EMGs quantified relative to their mean levels in wakefulness in successive 10 s epochs. During non-REM sleep, the average EMG levels differed among the three muscles, with the order being N > GH > GG-I. During REM sleep, due to different magnitudes of phasic twitches, the order was reversed to GG-I > GH > N. GG-I and GH exhibited a gradual increase of twitching that peaked at 70–120 s after the onset of REM sleep and then declined if the REM sleep episode lasted longer. We propose that a common phasic excitatory generator impinges on motoneuron pools that innervate different muscles, but twitching magnitudes are different due to different levels of tonic motoneuronal hyperpolarization. We also propose that REM sleep episodes of average durations are terminated by intense activity of the central generator of phasic events, whereas long REM sleep episodes end as a result of a gradual waning of the tonic disfacilitatory and inhibitory processes.
Keywords: Atonia, Genioglossus, Geniohyoid, Obstructive sleep apnea, Phasic twitches, Tongue
Introduction
Rodents are extensively used to study the regulation of sleep and motor activity. Regulation of the position and movements of the tongue attracts much attention because this multifunctional organ is important for alimentary functions, vocalization and grooming (Lowe, 1981; Travers and Jackson, 1992). Additionally, in pathologic conditions such as obstructive sleep apnea, activity of lingual muscles is a major determinant of upper airway patency (Sauerland and Harper, 1976; Remmers et al., 1978; Kubin and Davies, 2002). The muscles of the tongue are innervated by motoneurons of the hypoglossal (XII) motor nucleus and are divided into intrinsic (I) fibers and fibers of the genioglossus (GG) (McClung and Goldberg, 2000). I fibers are fully contained within the tongue and not attached to any bony structures. GG fibers have one attachment point to the anterior inner surface of the lower jaw (mandibular symphysis), whereas their other ends fan out into the body of the tongue. As a result of this arrangement, contractions of GG fibers move the tongue in the anterior direction, whereas a combined action of I and GG fibers determines the shape, stiffness and dorso-ventral position of the organ (Sokoloff, 2004). The XII nucleus also innervates other muscles that line up the upper respiratory and alimentary tracts. Among those, the geniohyoid (GH) is located below the floor of the mouth, ventral to the GG, and has its fibers attached to the inner surface of the lower jaw on the one end and to the hyoid apparatus on the other end. Since the hyoid is relatively rigidly attached to the tracheal rings in most mammals other than humans, both ends of GH muscle fibers have well defined and rigid attachment points as is typical of most skeletal muscles. Thus, one would expect the neural control of the GH to be similar to that of other skeletal muscles that are joined to bony structures on both ends and different from that of the muscles that attach to rigid structures on their one end only (GG) or that have no fixed attachment points (I).
In addition to having different biomechanical arrangements, GG-I and GH muscles also have different origins of their motor innervation in the XII nucleus (Krammer et al., 1979; Uemura-Sumi et al., 1988; Altschuler et al., 1994; Aldes, 1995; Dobbins and Feldman, 1995; Fay and Norgren, 1997). While GH and GG motoneurons are all located in the ventral portion of the caudal half of the XII nucleus and have their efferent axons in the medial branch of the XII nerve, GH motoneurons form the most lateral group, GG motoneurons that innervate muscle fibers in the GG-I portion of the tongue are located more medial, and those innervating the mandibular portion of the GG are located in the medialmost part of the nucleus (Yasuda et al., 2002; McClung and Goldberg, 2002). These and other retrograde tracing studies also reveal that motoneurons innervating the GH are the largest in the XII nucleus. In addition, differences in the orientation and morphology of motoneuronal dendritic trees among XII motoneurons innervating different muscle groups suggest that different pools of XII motoneurons have distinct central sources of afferent inputs. In support of this, in ketamine-anesthetized, Wistar rats, muscle fibers located in the mandibular portion of the GG exhibited both activity related to the inspiratory phase of the respiratory cycle and to swallowing, whereas the fibers located in the GG-I portion of the tongue had activity primarily related to swallowing (Yasuda et al., 2002).
We previously reported that, in rats, lingual electromyographic activity (EMG) is minimal or absent during non-rapid eye movement (REM) sleep and then gradually increases during REM sleep due to the appearance of large phasic bursts. On the other hand, the dorsal neck muscles which have major postural (antigravity) function and are essential for orienting behaviors have moderate tonic activity during non-REM sleep that gradually declines to atonia at the onset of REM sleep and then exhibit minimal twitching activity during REM sleep (Lu et al., 2005). We also determined that lingual EMG has quantitatively similar patterns when recorded near the base of the tongue where GG and I muscle fibers are intermixed and in distal regions of the tongue where I muscles predominate (Lu and Kubin, 2009). In consideration of these neuroanatomical and functional differences, our goal was to quantify involuntary activity present in the GH and GG-I muscles during sleep. We hypothesized that GH activity during sleep and during transitions from non-REM to REM sleep is more similar to the activity of postural muscles, such as dorsal neck muscles, than to that of GG-I muscles.
Methods
Experiments were conducted on 8 adult male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA). After instrumentation, the animals were housed individually under a 12 h light (7:00–19:00)/12 h dark cycle and had standard rodent chow and water available ad lib. All surgical and animal handling procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Animal instrumentation and habituation
Instrumentation was conducted after at least four days of adaptation to housing in our facility. Under ketamine (60 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.) anesthesia followed by isoflurane administered through a nose mask (0.5–0.8%), the animals were instrumented for recording of the cortical EEG and lingual (GG-I), GH, nuchal (N) and sternal diaphragmatic electromyographic activity (EMG). One recording wire (10-stranded, stainless steel, Teflon-coated, with tips rounded and wires exposed over ~0.1 mm; catalog number AS361; Cooner Wire, Chatsworth, CA) was inserted into the tongue using the approach described previously (Lu et al., 2005) aiming at a site near the base of the tongue where GG and I muscle fibers are intermixed; these sites are thereafter referred to as GG-I sites. Another wire of the same type was inserted into the GH muscle using the ventral approach. The GH and GG-I muscles were selected for this study because they both receive motor innervation from the ventral part of the caudal XII motor nucleus but GH fibers are innervated by ventrolateral XII motoneurons, whereas GG-I muscles by ventromedial XII motoneurons (see Introduction). The GG-I and GH wires were used for monopolar recordings against a reference point on the skull (Lu and Kubin, 2009). A pair of teflon-coated, stainless steel wires (64 strands, with tips exposed over 10–15 mm; AS636, Cooner Wire) was sutured to the surface of the dorsal neck muscles, and another pair of wires of the same type was implanted into the sternal diaphragm for bipolar recordings of diaphragmatic activity. All leads were tunneled subcutaneously and attached to a mini-socket (220-9 pin ABS Plug, Ginder Scientific, Ottawa, Canada) that was then attached to the skull with dental acrylic and all skin openings were tightly sutured. At the conclusion of the instrumentation surgery, the animals were given gentamicin (5 mg/kg, i.m), yohimbine (5 mg/kg, i.m.) and an analgesic (Metacam, 2 mg/kg, s.c.), and then their recovery was periodically observed over the subsequent 3–5 days.
On day 6 and several subsequent days after instrumentation, the home cage housing each animal was individually placed in a ventilated, dimly illuminated and sound-attenuated recording chamber for 1–4 h at least three times to habituate the animal to the environment and the brief immobilization needed to attach it to the recording apparatus. During these procedures, we also established optimal signal amplification parameters.
Recording procedures, signal processing, scoring of sleep-wake states and data analysis
The cortical EEG and the EMGs were amplified with a Grass Instruments polygraph (Model 8–10B) using bandwidths of 0.3–100 Hz for the EEG and 30–1000 Hz for the EMGs. All signals were continuously monitored and digitally stored using sampling rates of 100 Hz and 1000 Hz, respectively (Power-1401 A/D converter and Spike-2 v.7 data acquisition hardware and software; Cambridge Electronic Design, Inc., Cambridge, England). Gains were set to obtain maximal amplification without saturation of the amplifiers or converters during periods of maximal activity. During data collection, the animal was left undisturbed in its own home cage with free access to food and water.
Because GG-I and GH EMGs were recorded between one wire implanted into the target muscle and a reference electrode on the frontal bone, the signals were high-pass filtered at 125 Hz to eliminate any contribution from the EEG to the EMG signals. For consistency, the same filtering was also applied to N and diaphragmatic EMGs. In combination with the sampling rate of 1000 Hz, this set the final bandwidth of the EMG recordings at 125–500 Hz. The EEG was band-pass filtered at 1.25–40 Hz. Behavioral states were scored based on the characteristic changes in the filtered signals observed in 60 s increments on the computer screen using commercial sleep-scoring software (Somnologica; Medcare, Buffalo, NY). Three behavioral states, wakefulness (W), non-REM sleep and REM sleep, were distinguished in successive 10 s epochs based on the appearance of the cortical EEG, nuchal EMG and the shape of the EEG power spectrum which was simultaneously displayed during scoring for each scored segment. To reduce overestimation of EMG levels during sleep introduced by large bursts of activity associated with awakenings, the epochs in which awakenings occurred were scored as non-REM or REM sleep only when these states occupied at least 75% of the duration of the epoch; all other epochs were scored according to the state that occupied more than 50% of the epoch duration. After the initial scoring, root mean square (RMS) values of the EMG signals were calculated for successive scoring intervals and sequentially exported to a spreadsheet together with concurrently calculated cortical EEG powers in selected bands (delta-2: 1.25–2.0 Hz; delta-1: 2.0–4.5 Hz; theta: 5.0–8.0 Hz; beta-2: 20–25 Hz). Two procedures were then used to verify the accuracy of scoring. First, a hypnogram was plotted for the entire recording session together with sequential plots of EEG delta-1 and theta powers, the beta2/delta-2 power ratio and the RMS values for the N EMG because these outputs assume characteristic levels in each of the three distinguished behavioral states (cf. Fig. 2 in Lu et al., 2005). Then, scatter plots of RMS values for N and GG-I EMGs vs. the beta-2/delta-2 band EEG power ratio were generated for all scoring intervals because such plots exhibit distinct clustering in relation to behavioral states (Lu et al., 2005; Lu and Kubin, 2009). The diaphragmatic EMG was not subjected to quantitative analysis, but it was used as an additional aid during scoring of behavioral states because central respiratory rate is characteristically regular during non-REM sleep and characteristically irregular during REM sleep.
The data for this report were obtained from the middle 2 hours of one 4.0 h recording session conducted with each of the 8 animals between noon and 4 pm on day 18 after instrumentation (except one animal in which only a 1.5 h recording was available for analysis). The first 1 h of recording was always excluded to allow the animal to settle down after it was connected to the recording system. Thus, the data sets comprised 540–720 sequential measurements of RMS values for EMGs and selected measures of EEG power derived from all 10 s scoring epochs that covered the analyzed portion of each recordings. For quantification and comparison of GG-I, GH and N EMGs among the animals, we measured the RMS of each EMG signal during the 10 s interval when no activity was present (electrical noise only), which we defined as the lowest value among all 10 s intervals in each recording session (Lu and Kubin, 2009). This lowest value was then subtracted from all RMS values in each data set. Subsequently, the data sets were sorted by behavioral state, the mean RMS levels of GG-I, GH and N EMGs were calculated for all epochs scored as wakefulness (W), and this mean activity level was used to normalize EMG levels in all epochs in the record.
Paired or unpaired Student’s t-tests were used to compare EMG levels among behavioral states and muscles. The variability of the means is characterized by the standard error (SE).
Verification of the location of recording sites in the tongue and GH muscle
The animals were deeply anesthetized (Nembutal; 100 mg/kg, i.p.) 22–55 days after the recording session that yielded the data for this report (mean: 38 days). They were decapitated, the head was fixed in 10% formalin, and the GG-I and GH recording sites were verified by localizing the tips of the recording wires under microscopic observation. The locations of the recording sites were then plotted onto a parasgittal cross-section of the tongue generated in-house and stained with Neutral red.
Results
Distribution of sleep-wake states and recording sites within the GG-I and GH muscles
The average percentages of recording time that the eight rats spent in different sleep-wake states were: 16% ± 2 (SE) for W, 64% ± 1 for non-REM sleep, and 20% ± 1 for REM sleep, with the corresponding ranges being 8.3–22.8%, 59.4–69.7%, and 15.5–26.8%, respectively.
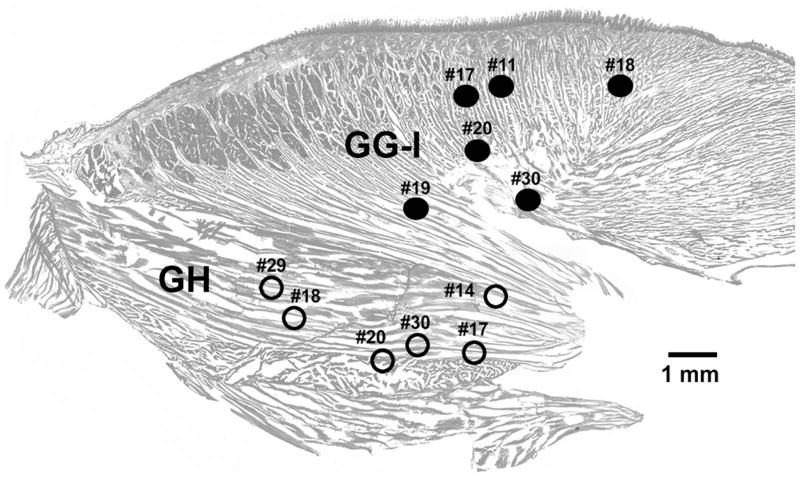
Among the eight rats included in this study, in four we simultaneously recorded EMG activity from both the GG-I region of the tongue and the GH muscle, in two rats only GG-I activity was recorded, and in the remaining two we recorded GH EMG only. The locations of these 12 recording sites are shown in Fig. 1. The numbers correspond to animal numbers, thus indicating the animals in which recordings were obtained from GG-I only, GH only, or both muscles. Different recording sites within the GG-I region (filled circles) contain variable proportions of muscle fibers of the GG and I muscles. Recordings from all GG-I sites are grouped together as one category because we previously determined that EMG activities recorded across sleep-wake states anywhere in the GG-I region shown in Fig. 1 and also at more anterior sites within the tongue were quantitatively indistinguishable (Lu and Kubin, 2009).
Fig. 1.
Location of the recording sites in the proximal region of the tongue where genioglossus and intrinsic muscle fibers are intermixed (GG-I) and in the geniohyoid (GH) muscle. The sites were localized post-mortem and superimposed on a parasagittal cross-section of the tongue and GH muscle (only the proximal part of the tongue near its base is shown). GG-I EMG was recorded from 6 rats (filled circles) and GH activity also from 6 rats (open circles). Simultaneous recordings from both locations were obtained from 4 of the 8 rats included in this study. The numbers denote different animals.
Differences between the mean GG-I, GH and N EMG levels during non-REM and REM sleep
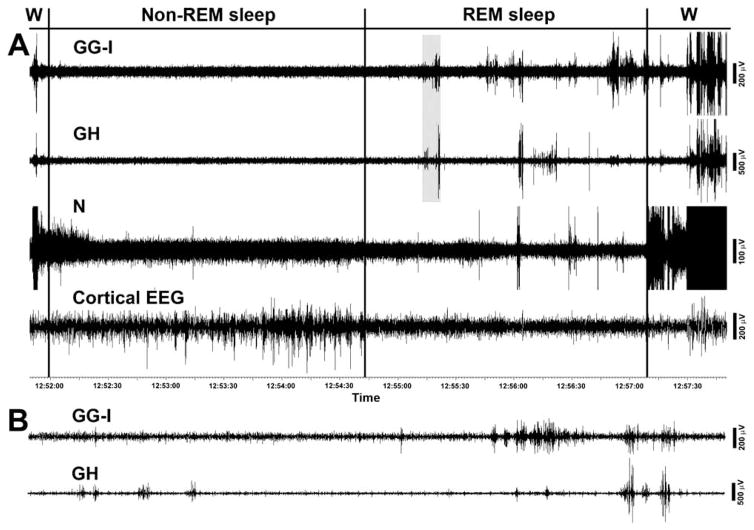
Fig. 2A shows a typical example of raw, filtered EMG records from a GG-I region of the tongue, GH and N muscles and cortical EEG during a typical wake-sleep cycle that starts with an awakening and then successively progresses through non-REM sleep, REM sleep and another awakening. Of note here is that N EMG gradually declines during non-REM sleep and then exhibits a distinct further decline at the onset of REM sleep, whereas GG-I and GH EMGs are atonic, or nearly atonic, already during non-REM sleep (there is no distinct decline of GG-I and GH signals at the onset of REM sleep). After the onset of REM sleep, all three muscles exhibit some degree of phasic twitching that starts with a delay after entry into REM sleep and is most intense in the GG-I EMG. The awakening is first signaled by an intense burst of activity in N EMG, while GG-I and GH EMGs are activated later. This sequence of N activation preceding GG-I and GH activation at awakening is typical of these muscles. The signals are displayed as saturated at this time to achieve a better expansion of the magnitude and pattern of twitches occurring during REM sleep. During the second half of the period of REM sleep, when intense twitching occurs in GG-I and GH EMGs, twitches also occur in N EMG, but their times of occurrence show only a loose association with twitches in GG-I and GH EMGs. Individual twitches in GG-I and GH appear to be better synchronized, but many bursts in one muscle do not have their counterparts in the other muscle, or their magnitudes differ. The two bottom traces in Fig. 2B show, on an expanded time scale, the portion of the GG-I and GH records highlighted in Fig. 2A. The records show that twitches in the two muscles occur with distinct timing.
Fig. 2.
Example of GG-I, GH and N activities during the transition from wakefulness to non-REM sleep and then REM sleep that ends with an awakening. In panel A, note that, during non-REM sleep, the nuchal (N) EMG gradually declines and then exhibits a distinct further decline at the onset of REM sleep, whereas GG-I and GH EMGs are atonic, or nearly atonic and show no distinct decline at the onset of REM sleep. After the onset of REM sleep, all three muscles exhibit some degree of phasic twitching that starts with a delay after the entry into REM sleep and is most intense in the GG-I EMG. The awakening is characteristically first signaled by an intense burst of activity in N EMG, while GG-I and GH EMGs are activated later. Panel B shows the shaded in panel A portion of GG-I and GH records (9 s). The expanded records show that twitches in the two muscles occur with distinct timing. The GG-I and GH recordings are from the sites designated in Fig. 1 as #18.
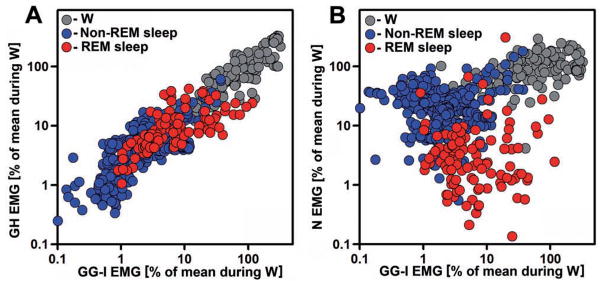
Figure 3 shows the relationship between the levels of GG-I, GH and N EMGs across the entire recording session in one animal, with the EMGs quantified as their RMS values in successive 10 s intervals used for scoring of behavioral states. GG-I and GH activity levels are more closely correlated with each other (Fig. 3A) than GG-I activity is with N activity across all behavioral states. This is consistent with GG-I and GH activities originating in the same motor nucleus and N activity in a different nucleus, and with supporting the orienting reaction at awakening being the main function of N muscles. However, the close correlation between GG-I and GH activities is not as narrow as the one we previously observed between different regions within the tongue (Fig. 5A in Lu and Kubin, 2009). In particular, the scatter plot in Fig. 3A contains many points with relatively lower GH than GG-I EMG levels for the scoring epochs that correspond to the periods with intense twitching during REM sleep (the upper 50% range for the red symbols). The relationship between GG-I and N EMGs shown in Fig. 3B indicates a much weaker relationship on the epoch-by-epoch basis than in Fig. 3A. Characteristic for the GG-I vs. N scatter plots is the upward shift of N EMG levels relative to GG-I EMG levels for many epochs during non-REM sleep (blue symbols) and a major downward shift of N EMG levels relative to GG-I EMG levels during REM sleep (red symbols). Since the measurements shown in Fig. 3 are normalized by the mean level of each muscle’s activity during W, the REM sleep data points in these plots point to a relatively lower magnitude of twitching in the GH than GG-I EMGs and an even lower level of twitching in the N EMG. They also indicate that N EMG is higher than GG-I EMG during non-REM sleep.
Fig. 3.
Distribution of GG-I, GH and N EMG levels relative to each other during different behavioral states. GG-I, GH and N EMG levels were normalized by their mean levels during wakefulness (W), color-coded by behavioral state, and their root mean square (RMS) values plotted for all 720 successive 10 s intervals of a 2 h recording of undisturbed behavior. GG-I and GH activity levels are more closely correlated with each other across all behavioral states (A) than GG-I activity is with N activity (B). Characteristic for the GG-I vs. N scatter plot is the upward shift of N EMG levels relative to GG-I EMG levels for many epochs during non-REM sleep (blue symbols) and a major downward shift of N EMG levels relative to GG-I EMG levels during REM sleep (red symbols). The GG-I and GH recording sites for this example correspond to animal #18 in Fig. 1.
To conduct a quantitative comparison of EMGs among different muscles across multiple animals, we normalized RMS values of EMG activities in successive 10 s scoring epochs (measured in mV·s) within each recording session by the mean RMS value calculated for all intervals scored as W in each recording. After subtraction of electrical noise levels and adjustment for different amplifier gains used in different recordings, the absolute mean EMG levels during W (the normalizing factors for subsequent calculations) across all animals were: 330 ± 80 mV·s for GG-I, 275 ± 45 mV·s for GH, and 260 ± 50 mV·s for N. These values did not statistically differ from each other. It is also worth noting that the trend of GG-I > GH > N for these absolute mean EMG levels during W is the same as the order of maximal normalized EMG activities for the three muscles during REM sleep illustrated in the subsequent Figs. 4–6. Thus, the mean levels of activity during W used as the normalizing factors for quantification of EMGs in the other two behavioral states were not the cause of the significant differences among the muscles that our analysis revealed during REM sleep.
Fig. 4.
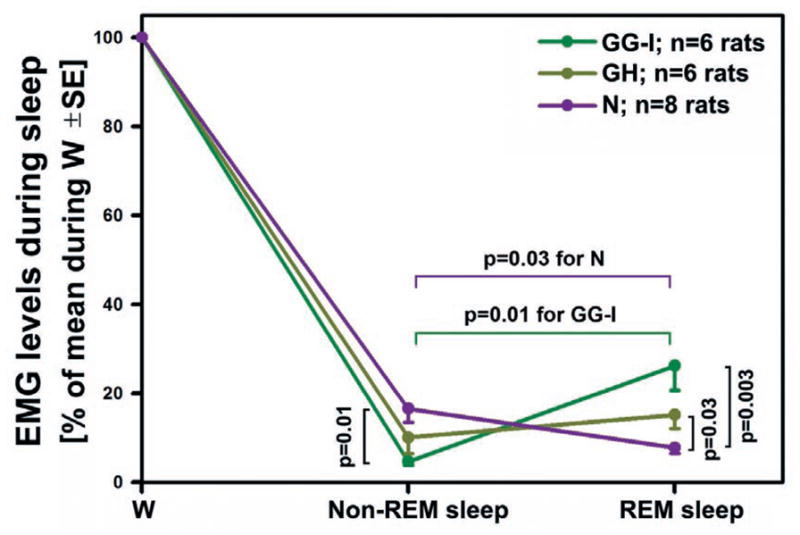
Mean GG-I, GH and N EMG levels during non-REM sleep and REM sleep normalized by the mean activity during W. The levels differ significantly among the three muscles during both stages of sleep. During non-REM sleep, GG-I EMG is minimal (atonic) and significantly lower than N EMG. The mean GH EMG level is intermediate between the GG-I and N EMG levels. During REM sleep, N EMG is minimal, whereas GG-I EMG is largest and GH EMG retains an intermediate position. N EMG declines significantly between non-REM sleep and REM sleep, which is an opposite change to that for the mean GG-I. GH EMG shows only a small trend towards increase during REM sleep compared to its mean level during non-REM sleep because it is slightly elevated above the level of atonia during non-REM sleep and when this tonic activity disappears during REM sleep it is replaced by a moderate level of twitching. During REM sleep, both the mean GG-I and GH EMG levels are significantly higher than the N EMG due to the low level of phasic activity in the latter.
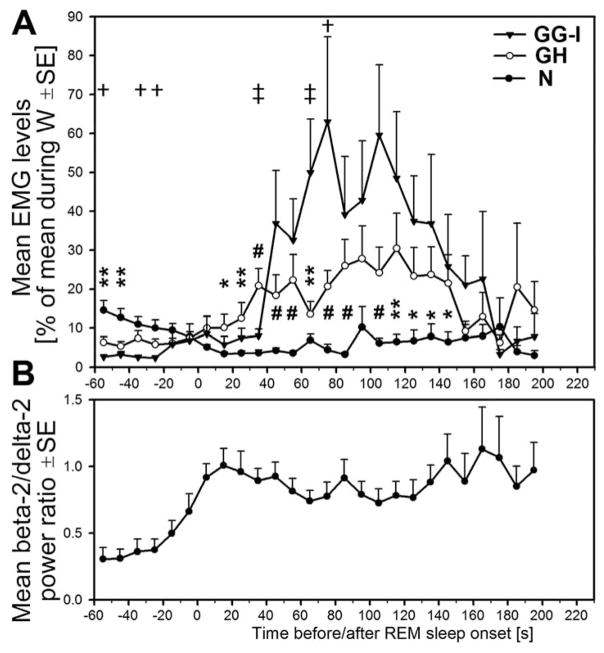
Fig. 6.
Quantitative comparison of mean GG-I, GH and N activities during transitions from non-REM sleep to REM sleep. A: the average time course of GG-I, GH and N EMG levels during the same transitions from non-REM to REM sleep as those individually illustrated in Fig. 5. B: the mean level of the ratio of cortical EEG powers in the beta-2 to delta-2 range at the corresponding time points before and after the onset of REM sleep. During the early phase of non-REM sleep, N EMG level is significantly higher than GH or GG-I EMGs and then declines. From about 20 s after the onset of REM sleep onwards, both GG-I and GH EMG levels begin to increase (twitching), whereas for the N EMG only a small contribution of twitching can be seen 60 s after the onset of REM sleep. The average GH EMG level is significantly higher than that of the N EMG at most times between 20 and 150 s after the onset of REM sleep, and GG-I EMG becomes higher than GH EMG at 60 s. Both the GG-I and GH reach a maximum at 70–120 s after REM sleep onset and then decline precipitously when REM sleep episodes last longer than 2 min. Thus, during the non-REM sleep period preceding the onset of REM sleep by about 30 s, the three muscles have different magnitudes with the order being N > GH > GG-I. In contrast, at 70–120 s into REM sleep, the order is N < GH < GG-I. *, ** and # = N EMG level significantly different from GH EMG level at p < 0.05, p < 0.02 and p < 0.005, respectively. † and ‡ = GG-I significantly different from GH EMG level at p < 0.05 and p < 0.02, respectively. For clarity, and because N and GG-I EMG levels are more separated than either N from GH or GH from GG-I EMG levels, markers for comparisons between N and GG-I EMG levels are omitted.
The mean normalized levels of EMG activities during entire periods of non-REM and REM sleep across all animals are shown in Fig. 4. The levels differ significantly among the three muscles during both sleep stages. During non-REM sleep, GG-I activity is minimal (4.7% of activity in W ± 0.8; n = 6) and significantly lower than that of N EMG (16.6% of activity in W ± 3.1; n = 8, p < 0.01 vs. GG-I). Indeed, the presence of a residual level of GG-I activity during non-REM sleep is largely due to measurements contributed by the scoring epochs with transitions from W to non-REM sleep in which the latter state occupied more than 50% but activity during the preceding W was also present. This, as well as a certain minimal level of purely electrical noise that our methodology does not allow us to exclude, result in the mean GG-I levels being larger than zero even though direct observation of the records indicates that GG-I muscles are atonic during most of non-REM sleep. In contrast, the mean GH EMG level is genuinely elevated during non-REM sleep relative to the mean GG-I level and assumes an intermediate level between the latter and the mean N EMG level.
In contrast to non-REM sleep, during REM sleep, N EMG is minimal, whereas GG-I EMG is largest and GH EMG retains an intermediate position between the other two. In this state, N EMG exhibits atonia most of the time but its average level is slightly elevated relative to the mean level of GG-I EMG during non-REM sleep due to occasional twitches in N EMG during REM sleep. Overall, N EMG declines significantly between non-REM sleep and REM sleep (to 7.8% of activity in W ± 1.2); n = 8, p < 0.03 vs. N EMG during non-REM sleep). This is an opposite change to that for the mean GG-I, which is significantly higher during REM sleep than during non-REM sleep; 26.2% of activity in W ± 5.4; n = 6, p < 0.01 vs. the GG-I EMG during non-REM sleep). The mean GH EMG shows only a small trend towards increase during REM sleep compared to its mean level during non-REM sleep. The cause for this minimal change is that the mean GH EMG is slightly elevated above the level of atonia during non-REM sleep due to the presence of a small level of tonic activity but when this tonic activity disappears during REM sleep it is replaced by a moderate level of twitching. Thus, during REM sleep both the GG-I and GH EMGs are significantly higher than the N EMG (p < 0.003 and p < 0.03, respectively) as a result of different levels of phasic activity in the three muscles.
Comparison of GG-I, GH and N EMGs during transitions from non-REM to REM sleep
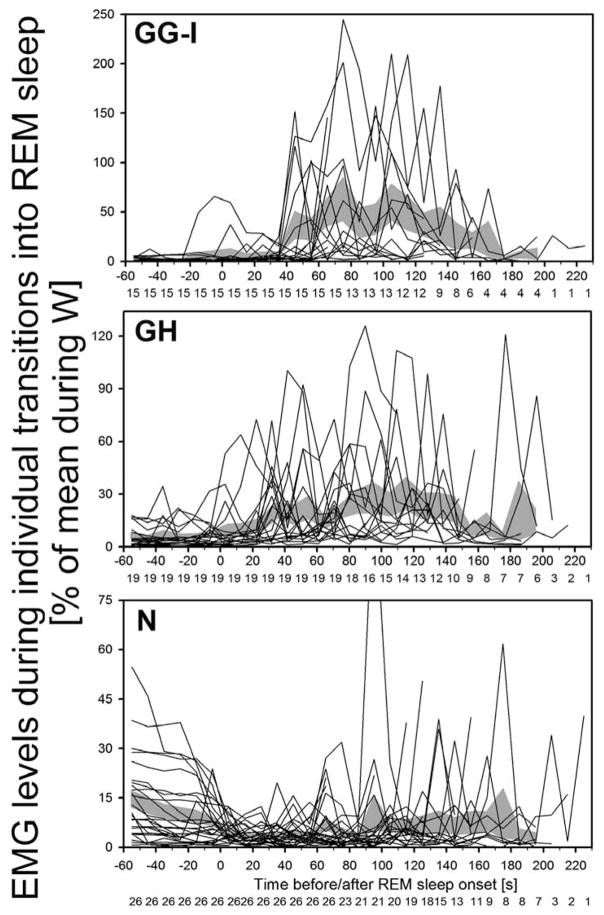
Because the levels of tonic activity during non-REM sleep and the intensity of twitching during REM sleep are not uniformly distributed across the duration of these states, we examined and compared the time course of changes in GG-I, GH and N EMGs during the transitions from non-REM to REM sleep. For this analysis, we selected from our records all those segments that contained at least 60 s of continuous non-REM sleep followed by at least 60 s of continuous REM sleep. EMG levels (RMS values) were then plotted sequentially for successive 10 s epochs for all three muscles and all transition periods that met our selection criteria. In Fig. 5, the initial 60 s of non-REM sleep and the subsequent 60 s of REM sleep contain sequential EMG levels for all the state transition periods that met our selection criteria (15 for GG-I from 6 rats, 19 for GH from 6 rats, and 26 for N from 8 rats). For REM sleep episodes lasting longer than 60 s, there is a gradual drop-out of traces due to variable duration of the episodes beyond 60 s (see Fig. 5 for specific numbers of episodes included at each time point). As in Fig. 4, EMG levels were normalized by their mean levels in W. What is apparent in these plots is that GG-I activity is nearly absent during non-REM sleep in most episodes, whereas GH and N EMGs have tonic activity that gradually declines in the case of N EMG. Then, GG-I and GH, and to a much lesser extent N, EMG show a gradually increasing magnitude of twitching after the onset of REM sleep (time zero) that reaches a maximum at 70–120 s and then declines.
Fig. 5.
Time course of GG-I, GH and N EMG levels during transitions from non-REM sleep to REM sleep in individual segments of records that had at least 60 s of stable non-REM sleep followed by at least 60 s of REM sleep. EMG levels in successive 10 s intervals are shown for all segments of records with the state transitions that contained at least 60 s of continuous non-REM sleep followed by at least 60 s of continuous REM sleep (15 segments for GG-I from 6 rats, 19 for GH from 6 rats, and 26 for N from 8 rats). There is a gradual drop-out of traces due to variable duration of REM sleep episodes beyond 60 s; the numbers of segments included at each time point are shown below the abscissae. EMG levels are normalized by their mean levels in W. GG-I activity is nearly absent during non-REM sleep in most episodes, whereas GH and N EMGs have tonic activity. Then, GG-I and GH, and to a much lesser extent N, EMG show a gradually increasing magnitude of twitching after the onset of REM sleep (time zero) that reaches a maximum at 70–120 s and then declines. Shaded areas show the corresponding mean EMG levels ± SE during successive 10 s epochs.
Fig. 6 shows, on a common amplitude scale, the average time courses of GG-I, GH and N EMG levels during the transitions from non-REM to REM sleep for the data sets individually illustrated in Fig. 5 and their temporal relationship to the mean level of the ratio of cortical EEG powers in the beta-2 to delta2 range (used as one of our the criteria for scoring of behavioral states). The average beta-2 to delta-2 power ratio was generated from the segments of records with N EMG data shown in Fig. 5. The mean power ratio begins to increase before the transition from non-REM sleep to REM sleep, reaches a maximum within the first 20 s after REM sleep onset and then remains elevated throughout the subsequent period of REM sleep (Fig. 6B). During the early phase of non-REM sleep, N EMG level is significantly higher than GH or GG-I EMGs and then declines. From about 20 s after the onset of REM sleep, both GG-I and GH EMG levels begin to increase (twitching), whereas the contribution of twitching to the slight increase of N EMG is not clear at least till 60 s after the onset of REM sleep. The average GH EMG level is significantly higher than that of the N EMG at most times between 20 and 150 s after the onset of REM sleep, and GG-I EMG is significantly higher than the GH EMG at some points within this range. The GG-I EMG reaches a maximum of 50–60% of the mean level of activity in W at 70–120 s after REM sleep onset and then declines precipitously when REM sleep episodes last longer than 2 min. The same pattern is also present in the GH EMG trace, whereas for the N EMG it is difficult to ascertain the presence of any distinct pattern due to the overall low level of activity. Thus, during the non-REM sleep period preceding the onset of REM sleep by about 30 s, the three muscles have different magnitudes with the order being N > GH > GG-I. In contrast, at 70–120 s into REM sleep, the order is N < GH < GG-I. The subsequent decline of GG-I and GH EMGs is characteristic of REM sleep episodes that last considerably longer than 90 s, which is the average duration of REM sleep episodes in rats (e.g., Trachsel et al., 1991).
Discussion
We confirmed that the time course of GG-I EMG changes during sleep is quantitatively different from that of the N EMG, as reported earlier (Lu et al., 2005). We also quantified the corresponding changes in another muscle innervated by XII motoneurons (GH), and we extended our observations of the time course of GG-I, GH and N EMGs during REM sleep to episodes lasting longer than 2 min, which was the upper limit in our previous study. This resulted in two new findings. First, we found that GH EMG changes during sleep with a time course that shares a common feature with N EMG (maintains tonic activity during non-REM sleep) and a common, albeit less prominently expressed, feature with GG-I EMG (twitching that gradually increases after the onset of REM sleep). Second, we determined that the high level of twitching in GG-I and GH muscles is not sustained during particularly long-lasting episodes of REM sleep. Since the same appears to be the case with flurries of eye movements (REMs) during REM sleep in humans (Arnulf, 2011 in this Special Issue), these observations show that long episodes of REM sleep include an initial quiescent period, a period when intensity of phasic muscle twitches gradually increases and then declines, and a terminal period with little phasic activity. This scheme differs from the classic division of REM sleep into its “tonic” and “phasic” phases because it emphasizes a continuous and gradual process of augmenting and then decrementing phasic activity that is superimposed on separate tonic processes that maintain the state. Furthermore, since the time of occurrence of the peak of phasic activity during REM sleep corresponds to the average duration of REM sleep episodes, our findings suggest that intense phasic activity contributes to termination of typical REM sleep episodes. If this is the case, then the mechanisms causing termination of long-lasting episodes may be different from those that terminate REM sleep episodes of the average duration.
Phasic twitches of distal limb and facial muscles have been noted as one of the cardinal signs of REM sleep (Aserinsky and Kleitman, 1953), and many subsequent studies recorded high intensity of twitching in all orofacial muscles innervated by the XII, facial, trigeminal and ambiguous motor nuclei (Megirian et al., 1978; Chokroverty, 1980; Richard and Harper, 1991; Kuna et al., 1991; Morrison et al., 2003; Brooks and Peever, 2008; Anaclet et al., 2010; Rivera-Garcia et al., 2011). In our earlier study, we quantified the magnitude of phasic activity in lingual muscles and argued that the process underlying this type of activity gradually intensifies after the onset of REM sleep (Lu et al., 2005). In the present study, we show that twitching is not sustained but declines during particularly long REM sleep episodes and that different muscles innervated by the XII nucleus exhibit different magnitudes but the same temporal pattern of twitching activity. It is less clear whether twitching in N muscles originates in the same time-dependent process as twitches in the GG-I and GH muscles. This is difficult to ascertain, in part, due to the low intensity of phasic excitatory inputs seen in the form of twitches in N muscles. It is possible that tonic hyperpolarization of N motoneurons during REM sleep is stronger than that of XII motoneurons and this prevents many phasic excitatory inputs to motoneurons that innervate N muscles from eliciting phasic motoneuronal activity. It is of note that chemical lesions of cells in the dorsomedial pontine reticular formation that reduce tonic motoneuronal hyperpolarization result in greatly increased frequency of twitches in N muscles and only slightly elevated phasic activity in the masseter muscle innervated by trigeminal motoneurons (Anaclet et al., 2010). This is consistent with a common origin of twitches in neck and orofacial motoneurons and with their different magnitudes under the natural conditions being dependent on the level of tonic motoneuronal hyperpolarization. On the other hand, cellular lesions of certain parts of the medullary parvocellular and paramedian reticular regions (PCR-PM) significantly reduced the frequency of twitches in the masseter and caused only a small decrease of phasic activity in N muscles (Anaclet et al., 2010). The small decline observed in N muscles could be due to the low baseline level of twitching in these muscles which impeded detection of further decreases. An alternative explanation offered by Ancelet et al. (2010) was that the medullary PCR-PM regions are distinct sources of phasic activity for trigeminal motoneurons. However, it remains to be determined whether the PCR-PM regions contain the network responsible for the generation of phasic orofacial muscle activity during REM sleep or whether they serve as relay sites for a generator that is located upstream.
We used a method of EMG quantification that measures the average intensity of muscle activity (both phasic and tonic) in successive 10 s epochs. This approach captures the combined activity composed of the tonic activity (if present), the magnitude of individual phasic bursts and their frequency. As such, it does not allow one to distinguish between intensity of individual twitches that usually last fractions of a second and their instantaneous frequency. In another recent study with human subjects, analysis of the frequency of individual twitches in different muscles innervated by the facial motor nucleus revealed that, during REM sleep, twitches are more frequent in the corrugator and orbicularis muscles than in zygomatic muscles, whereas individual twitch durations did not differ among these muscles (Rivera-Garcia et al., 2011). The same study found a high correlation between REMs density and twitches in facial muscles but noted that REMs generally started earlier than twitching after the onset of REM sleep. Thus, measurements based on RMS values calculated over successive fixed intervals that integrate amplitude and frequency of twitching (our study), as well as those based on measurement of twitch frequency, can uncover quantitative differences among different muscles innervated by the same motor nucleus.
In another study on Wistar rats, EMGs of the dorsal neck and extensor digitorum muscles which were regarded as representative of non-respiratory muscles, and of the genioglossus, external intercostal muscles and the diaphragm, regarded as representative of respiratory muscles, were quantified during episodes of REM sleep (Fraigne and Orem, 2011). As we in our study, the authors observed that activity of all muscles except those of the neck, the respiratory frequency and ventilation gradually increased after the onset of REM sleep. It is important to note that, in that study, not only EMG activity but also the duration of REM sleep episodes was normalized by dividing all episodes into 20 segments regardless of their actual duration. Despite this approach that must have partially masked the distinct decline in phasic activity that we found prominent in long-lasting REM sleep episodes only, the authors noticed that phasic twitches declined near the end of the episodes and that this was accompanied by a decline of the respiratory rate and amplitude of respiratory modulation of diaphragmatic activity. Since the initial increase evident with a delay after the onset of REM sleep and subsequent decline near the end of the episodes was absent from the dorsal neck EMG and was barely visible in the extensor digitorum muscle, the authors concluded that the phasic activation they observed was unique to respiratory muscles, although they also reported that the twitching in the genioglossus was unrelated to respiration. This conclusion contradicted the one derived from observation of the relationship between middle-ear, masseter, digastric, laryngeal, neck and limb muscle activities in humans during REM sleep (Slegel et al., 1991). The authors of the latter study found a highly significant association between middle-ear muscle activity and twitches in other muscles innervated by both cranial and spinal motoneurons, although the degree of coincidence was considerably higher for orofacial (masseter/digastric, laryngeal) than for the tibialis or wrist flexors. These observations have led them to conclude that REMs, phasic twitches in orofacial and other cranial muscles, and phasic activation of upper and lower limb muscles all originate in a common central generator. This conclusion was consistent with that proposed by Gassel et al. (1964) based on observation of REMs and muscle twitches during REM sleep in cats.
Based on our results and data from the other studies discussed above, we propose that acceleration of the respiratory rate and its increased variability can be added to the list of phasic excitatory events that occur in different motor outputs with increasing intensity during the initial part of REM sleep episodes and then decline during the second part in those episodes that are not terminated prematurely. These excitatory phenomena manifest in different forms and may have different magnitudes in different outputs, but their common time course is consistent with the notion of them having one common central origin. In most studies to date, only the N EMG appears not to fit this scheme but this discrepancy may be related to stronger hyperpolarization of motoneurons that innervate proximal/axial than most other muscles, cranial and spinal, during REM sleep.
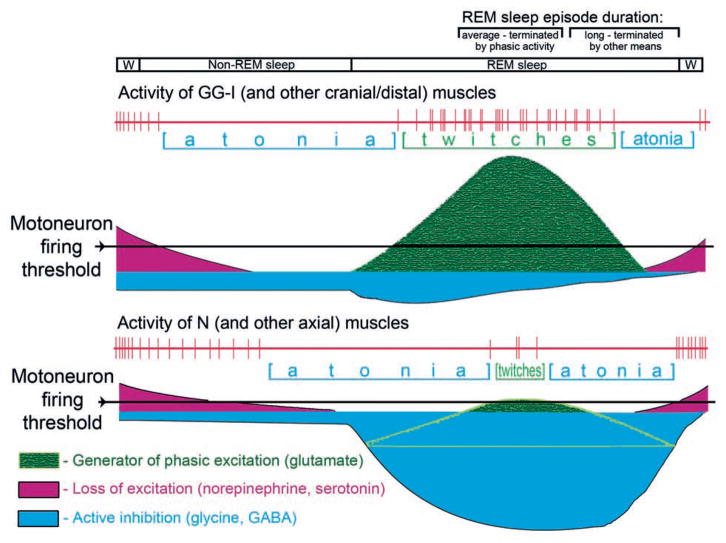
In the scheme shown in Fig. 7, we propose to account for different expression of phasic activity in different muscles on the basis of different mechanisms and magnitudes of tonic motoneuronal hyper-polarization during sleep. We assume that motoneurons that innervate orofacial (and other cranial and distal skeletal) muscles are more hyperpolarized than motoneurons that innervate N (and other axial) muscles because this is consistent with their mainly phasic and predominantly tonic patterns of activity, respectively. Following the onset of non-REM sleep, motoneurons are initially hyperpolarized due to the loss of wake-related excitatory inputs, especially those dependent on norepinephrine and serotonin. Then, during REM sleep, this process continues and is additionally strengthened by active inhibition that can effectively shunt phasic excitatory inputs and occurs with different magnitudes in motoneurons that innervate orofacial/distal and axial mucles. Our assumptions about the relative strength of these effects and their time course are based on the evidence that the loss of activation mediated by norepinephrine and serotonin is the main mechanism underlying sleep-related hyperpolarization of XII motoneurons (Kubin et al., 1992, 1998; Fenik et al., 2005; Chan et al., 2006; Neuzeret et al., 2009), whereas active postsynaptic inhibition minimally contributes to motoneuronal hyperpolarization during REM sleep in either XII or trigeminal motoneurons (Kubin et al., 1993; Morrison et al., 2003; Brooks and Peever, 2008). On the other hand, active inhibition may contribute more significantly to REM sleep-related hyperpolarization of some spinal motoneurons (Chase et al., 1989; Soja et al., 1991). Furthermore, recent data from mice show that brainstem noradrenergic neurons of the locus coeruleus cease firing early after non-REM sleep onset and resume firing before awakening (Takahashi et al., 2010). If this is also the case with those brainstem noradrenergic and serotonergic neurons that provide excitatory input to motoneurons (see Manaker and Tischler, 1993 and Rukhadze and Kubin, 2007 for sources of serotonergic and noradrenergic axonal projections to orofacial motoneurons), then a partial or total loss of aminergic excitation during non-REM sleep may explain why some muscles become atonic during non-REM sleep (e.g., GG-I in our study), whereas others maintain some activity during non-REM sleep and reach atonia only at the onset of REM sleep (e.g., N in our study). The scheme that we propose attempts to account for these differences on the basis of different magnitudes and time courses of disfacilitation and active inhibition, while assuming that one common phasic event generator is responsible for twitches in all muscles.
Fig. 7.
Schematic representation of the interaction between a common phasic event generator and the tonic dis-facilitatory and inhibitory inputs that decrease mononeuronal excitability during transitions from quiet W to non-REM sleep and then REM sleep. The scheme accounts for different dependence of orofacial (and probably also other cranial and distal skeletal) muscles and nuchal (and probably also other axial) muscles on the tonic wake-related excitation mediated by the aminergic systems and tonic active inhibition. Different magnitudes of twitching in different muscles are secondary to the magnitudes of tonic hyperpolarization imparted on different motoneuronal pools. In addition, the relatively stronger active inhibition of motoneurons that innervate axial muscles reduces membrane resistance, thereby reducing the effectiveness of phasic excitatory inputs. According to this scheme, there is one common central network that generates phasic events, a network that is gradually activated after the onset of REM sleep and then gradually becomes quiescent. The gradual waning of phasic activation is revealed during relatively long-lasting episodes of REM sleep, while the typical ones usually terminate around the time of most intense phasic activity. Accordingly, terminations of the typical episodes of REM sleep may be facilitated by intense activity in the central generator of phasic events, whereas the long episodes end as a result of gradual waning of the tonic disfacilitatory and inhibitory processes. Within the proposed framework, the scheme may be modified to account for altered activity in different motoneuronal pools after various pharmacological treatments and in disorders such as RBD or obstructive sleep apnea.
Animals with lesions made within the dorsomedial pontine reticular formation have a major loss of tonic motoneuronal hyperpolarization and generate greatly enhanced phasic motoneuronal activity during REM sleep (Jouvet and Delorme, 1965; Henley and Morrison, 1974; Anaclet et al., 2010). As such, their motor activity during REM sleep is similar to that of humans with REM sleep behavior disorder (RBD) who also often have degenerative lesions that involve the mesopontine reticular formation (e.g., Mahowald and Schenck, 1992; Fantini et al., 2005; Iranzo et al., 2009). It is of interest that patients affected by both RBD and obstructive sleep apnea (OSA) have reduced severity of obstructive episodes during REM sleep when compared to control patients affected by OSA only (Huang et al., 2011). This suggests that the enhanced phasic activity in the upper airway muscles innervated by XII and trigeminal motoneurons has a beneficial effect on airway patency. Data also indicate that, in RBD patients, intensity of phasic twitches during REM sleep is enhanced in different muscles proportionally to the baseline levels of twitching in these muscles in healthy persons (Bliwise et al., 2006; Frauscher et al., 2008). Quantification of this effect has been conducted in humans using methods other than those used in our study and without attention to the time course of twitching intensity within the episodes of REM sleep. We would suggest that application of our methodology to the study of enhanced phasic twitches in RBD patients and the animal models of the disorder may help further test whether one common central generator is responsible for phasic muscle twitches and other phasic events of REM sleep in functionally and anatomically different motor outputs.
Acknowledgments
The study was supported by grants HL-092962 and HL-071097 from the National Institutes of Health.
References
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- Anaclet C, Pedersen NP, Fuller PM, Lu J. Brainstem circuitry regulating phasic activation of trigeminal motoneurons during REM sleep. PLoS One. 2010;5:e8788. doi: 10.1371/journal.pone.0008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnulf I. The ‘scanning hypothesis’ of rapid eye movements during REM sleep: a review of the evidence. Arch Ital Biol. 2011;151:367–382. doi: 10.4449/aib.v149i4.1246. (this issue) [DOI] [PubMed] [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, He L, Ansari FP, Rye DB. Quantification of electromyographic activity during sleep: a phasic electromyographic metric. J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokroverty S. Phasic tongue movements in human rapid-eye-movement sleep. Neurology. 1980;30:665–668. doi: 10.1212/wnl.30.6.665. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Fantini ML, Ferini-Strambi L, Montplaisir J. Idiopathic REM sleep behavior disorder: toward a better nosologic definition. Neurology. 2005;64:780–786. doi: 10.1212/01.WNL.0000152878.79429.00. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus III. Lingual muscle motor systems. Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. 2011;34:425–434. doi: 10.1093/sleep/34.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauscher B, Iranzo A, Högl B, Casanova-Molla J, Salamero M, Gschliesser V, Tolosa E, Poewe W, Santamaria J SINBAR (Sleep Innsbruck Barcelona group) Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–731. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel MM, Marchiafava PL, Pompeiano O. Phasic changes in muscular activity during desynchronized sleep in unrestrained cats. An analysis of the pattern and organization of myoclonic twitches. Arch Ital Biol. 1964;102:449–470. [PubMed] [Google Scholar]
- Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp, (Warsaw) 1974;34:215–232. [PubMed] [Google Scholar]
- Huang J, Zhang J, Lam SP, Li SX, Ho CKW, Lam V, Yu MW, Wing YK. Amelioration of obstructive sleep apnea in REM sleep behavior disorder: implications for the neuromuscular control of OSA. Sleep. 2011;34:909–915. doi: 10.5665/SLEEP.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. CR Soc Biol. 1965;159:895–899. [Google Scholar]
- Krammer EB, Rath T, Lischka MF. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res. 1979;170:533–537. doi: 10.1016/0006-8993(79)90970-3. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Mechanisms of upper airway hypotonia. In: Pack AI, editor. Sleep Apnea Pathogenesis, Diagnosis, and Treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol. 1991;70:1655–1664. doi: 10.1152/jappl.1991.70.4.1655. [DOI] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Progr Neurobiol. 1981;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lu JW, Kubin L. Electromyographic activity at the base and tip of the tongue across sleep-wake states in rats. Respir Physiol Neurobiol. 2009;167:307–315. doi: 10.1016/j.resp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald MW, Schenck CH. Dissociated states of wakefulness and sleep. Neurology. 1992;42(Suppl 6):44–52. [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260:378–386. doi: 10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res. 2002;950:321–324. doi: 10.1016/s0006-8993(02)03240-7. [DOI] [PubMed] [Google Scholar]
- Megirian D, Cespuglio R, Jouvet M. Rhythmical activity of the rats’s tongue in sleep and wakefulness. EEG Clin Neurophysiol. 1978;44:8–13. doi: 10.1016/0013-4694(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzeret P-C, Sakai K, Gormand F, Petitjean T, Buda C, Sastre J-P, Parrot S, Guidon G, Lin J-S. Application of histamine and serotonin to the hypoglossal nucleus increases genioglossus activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Richard CA, Harper RM. Respiratory-related activity in hypoglossal neurons across sleep-waking states in cats. Brain Res. 1991;542:167–170. doi: 10.1016/0006-8993(91)91014-r. [DOI] [PubMed] [Google Scholar]
- Rivera-Garcia AP, Ramirez-Salado I, Corsi-Cabrera M, Calvo JM. Facial muscle activation during sleep and its relation to the rapid eye movements of REM sleep. J Sleep Res. 2011;20:82–91. doi: 10.1111/j.1365-2869.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Slegel DE, Benson KL, Zarcone VP, Jr, Schubert ED. Middle-ear muscle activity (MEMA) and its association with motor activity in the extremities and head in sleep. Sleep. 1991;14:454–459. [PubMed] [Google Scholar]
- Soja PJ, López-Rodríguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff AJ. Activity of tongue muscles during respiration: it takes a village? J Appl Physiol. 2004;96:438–439. doi: 10.1152/japplphysiol.01079.2003. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–1126. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Tobler I, Achermann P, Borbély AA. Sleep continuity and REM - non-REM cycle in the rat under baseline conditions and after sleep deprivation. Physiol Behav. 1991;49:575–580. doi: 10.1016/0031-9384(91)90283-t. [DOI] [PubMed] [Google Scholar]
- Travers JB, Jackson LM. Hypoglossal neural activity during licking and swallowing in the awake rat. J Neurophysiol. 1992;67:1171–1184. doi: 10.1152/jn.1992.67.5.1171. [DOI] [PubMed] [Google Scholar]
- Uemura-Sumi M, Itoh M, Mizuno N. The distribution of hypoglossal motoneurons in the dog, rabbit and rat. Anat Embryol. 1988;177:389–394. doi: 10.1007/BF00304735. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nakayama Y, Tanaka M, Tanaka M, Mori R, Furusawa K. The distribution of respiration-related and swallowing-related motoneurons innervating the rat genioglossus muscle. Somatosens Motor Res. 2002;19:30–35. doi: 10.1080/08990220120113020. [DOI] [PubMed] [Google Scholar]