Abstract
Noradrenergic (NE) excitatory drive maintains activity of hypoglossal (XII) motoneurons during wakefulness. In predisposed persons, sleep-related decrements of NE cell activity may contribute to hypotonia of upper airway muscles innervated by XII motoneurons. The goal of this study was to determine whether NE neurons of the pontine A7 group, an anatomically identified source of NE projections to the XII nucleus, provide significant, endogenous NE excitatory drive to XII motoneurons. In anesthetized rats, we microinjected clonidine (0.75 mM, 20–40 nl), an α2-adrenergic receptor agonist that inhibits pontine NE cells, aiming at the A7 region. Nine injections were placed within 0.4 mm from the A7 group identified using tyrosine hydroxylase immunohistochemistry: they reduced XII nerve activity by 31.3±2.8% (standard error) and decreased the central respiratory rate by 6%. Another 21 injections, including eight placed near NE cells of the subcoeruleus region, were made at distances over 0.5 mm from the A7 group and they did not alter either XII nerve activity or respiratory rate. In control experiments, clonidine injections into the A7 group preceded by injections of an α2-receptor antagonist, RS-79948, did not change XII nerve activity. Four experiments with unilateral clonidine injections into the A7 region and with Fos immunohistochemistry used as a marker of cell activity revealed that the percentage of Fos-positive A7 cells was significantly reduced on the injected side. There was also a significant positive correlation between Fos expression in A7 cells and XII nerve activity. Thus, decrements of NE excitatory drive from the A7 group may significantly reduce XII nerve activity. In obstructive sleep apnea patients, in whom the muscles innervated by XII motoneurons act as upper airway dilators, this may contribute to sleep-related respiratory disorders.
Keywords: locus coeruleus, norepinephrine, pons, sleep, upper airway, obstructive sleep apnea
In obstructive sleep apnea syndrome patients, activity of the genioglossus, an important airway dilator is higher during wakefulness than in healthy individuals to compensate for their anatomically compromised upper airway patency (Suratt et al., 1988; Mezzanotte et al., 1992; Hendricks et al., 1993; Katz and White, 2004). This activity decreases during non-rapid eye movement (REM) sleep and is often abolished during REM sleep, thus facilitating the occurrence of sleep-related obstructions of the upper airway (Remmers et al., 1978; Sauerland and Harper, 1976; Okabe et al., 1994; Mezzanotte et al., 1996; Ikeda et al., 2001; Katz and White, 2004). Recent studies in rats suggest that sleep-related suppression of genioglossal muscle activity is mainly caused by sleep-related withdrawal of noradrenergic (NE) activation of hypoglossal (XII) motoneurons that innervate the muscles of the tongue (Fenik et al., 2005b; Chan et al., 2006).
Norepinephrine excites XII motoneurons (Funk et al., 1994; Parkis et al., 1995; Al-Zubaidy et al., 1996). Pontine NE neurons have highest levels of activity during wakefulness, reduce activity during non-REM sleep and cease firing during REM sleep (Aston-Jones and Bloom, 1981; Reiner, 1986; Kubin, 2001; Fenik et al., 2002). Since antagonism of NE excitatory receptors (α1-adrenergic) located in XII nucleus region causes a profound decrease of XII motoneuronal activity (Fenik et al., 2005b; Chan et al., 2006), sleep-related decrements of NE cell activity probably also contribute to decrements of upper airway motor tone.
NE cells of the pontine A7, subcoeruleus (SubC) and A5 groups, as well as the medullary A1/C1 group, have axonal projections to the XII nucleus (Aldes et al., 1992; Rukhadze and Kubin, 2007), but the relative contribution of silencing of cells in different NE nuclei to the decrements of XII motoneuronal activity is unknown. The numbers of NE neurons projecting to the XII nucleus from each NE group cannot be used to infer about the impact of the loss of activity in that cell group on the activity of XII motoneurons because cells of different groups may have different densities and strengths of synaptic connections. In our earlier study (Fenik et al., 2002), we observed that silencing of cells in the ventrolateral pontine A5 group did not significantly alter the activity of XII motoneurons despite anatomical evidence for NE projections from this group to the XII nucleus (Aldes et al., 1992; Rukhadze and Kubin, 2007). Data showing that the dorsolateral pontine NE group designated as A7 has the highest percentage of cells with axonal projections to the XII nucleus (Rukhadze and Kubin, 2007) and that activity of these cells is reduced in a pharmacological model of REM sleep (Rukhadze et al., 2008), prompted us to test whether NE cells located in the pontine A7 group are a source of significant endogenous NE excitatory drive to XII motoneurons. We used local microinjections of clonidine, an α2-adrenergic receptor agonist that powerfully inhibits activity of pontine NE neurons (Reiner, 1985; Huangfu and Guyenet, 1997), into and around the A7 group and measured the resulting changes of XII nerve activity. We found that unilateral clonidine injections into the A7 group significantly reduced XII nerve activity whereas those made at a distance from the A7 region had no effect. A preliminary report has been published (Fenik et al., 2007).
EXPERIMENTAL PROCEDURES
Animal preparation
Experiments were performed on 20 adult male Sprague–Dawley rats (body weight: 325–470 g, mean: 393±9.5 g (standard error (S.E.)) obtained from Charles River Laboratories (Wilmington, MA, USA). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the guidelines established by the National Institutes of Health. The number of animals used was the lowest necessary to achieve satisfactory power of statistical analysis. Anesthesia was carefully monitored and maintained at adequate level at all time to avoid animal suffering.
The animals were pre-anesthetized with isoflurane (2%) and then anesthetized with urethane (1 g/kg, i.p., supplemented by 50 mg i.v. injections, as needed). The trachea was intubated and a femoral artery and vein were catheterized for arterial blood pressure monitoring and fluid injections, respectively. The right XII nerve was cut and its central end placed inside a cuff electrode for recording (Fenik et al., 2001). The cervical vagi were cut to enhance XII nerve activity and eliminate its reflex modulation by pulmonary afferents. The head of the animal was placed in a stereotaxic holder, two screws were attached to the skull to record the electroencephalogram (EEG) and a bipolar recording electrode was inserted into the hippocampus, as described previously (Fenik et al., 2005b; Lu et al., 2007). Openings were made bilaterally in the interparietal bone and the dura removed for inserting a drug-containing pipette into the pontine A7 region.
The animals were paralyzed with pancuronium bromide (2 mg/kg i.v., supplemented with 1 mg/kg injections, as needed) and artificially ventilated with a mixture of air and oxygen (30–60% O2) at a rate 50–70 lung inflations/min. After paralysis, the level of anesthesia was assessed by intermittently applying a pinch to the hind limb while recording the arterial blood pressure, EEG, hippocampal and XII nerve activity. Absence of pinch-induced changes in respiratory rate or XII nerve activity and only transient changes in blood pressure, EEG and hippocampal signals, similar to those before paralysis, indicated adequate level of anesthesia. Rectal temperature was maintained at 35.5–36.5 °C with a heating pad. End-expiratory CO2 (Columbus Instruments capnograph, Columbus, OH, USA) was adjusted at the beginning of the experiment to obtain a steady respiratory modulation of XII nerve activity and then kept constant. The mean systolic blood pressure was 90.4±8.4 mm Hg.
Electrophysiological recordings
EEG (bandwidth 0.5–100 Hz), hippocampal activity (2–20 Hz) and XII nerve activity (30–2500 Hz) were amplified with AC amplifiers (N101, Neurolog System; Digitimer, Hertfordshire, UK). The signals were continuously monitored on an eight-channel chart recorder (TA-11; Gould Instruments, Valley View, OH, USA) and recorded on a 16-channel digital tape recorder (C-DAT; Cygnus Technology, Delaware Water Gap, PA, USA) together with tracheal pressure, end-expiratory CO2, arterial blood pressure and an event marker. XII nerve activity was fed into a moving average circuit with a time constant of 100 ms (MA-821 RSP; CWE, Inc., Ardmore, PA, USA).
Experimental and histological procedures
The glass pipettes used for the injections (A-M Systems, Carlsborg, WA, USA) had tip diameters of 25–30 μm and were filled with 0.75 mM clonidine hydrochloride, an α2-adrenoceptor agonist (Sigma-Aldrich, St. Louis, MO, USA) in 0.9% NaCl. Pontamine Sky Blue dye (2%, ICN Biomedicals Inc., Aurora, OH, USA) was included in clonidine solution to mark the injection sites. Once the animal was prepared for recording, one unilateral, or up to two bilateral clonidine injections were made at 10–20 min intervals aiming at the A7 group(s). The injections (20 or 40 nl) were made over 30–60 s by applying pressure to the fluid in the pipette. In two control experiments, 80 nl of RS-79948 hydrochloride (2 mM in 0.9% NaCl; Tocris, Ellisville, MO, USA), an α2-adrenoceptor antagonist, were injected at the same site 13–19 min prior to clonidine injections. The injected volume was monitored with 2 nl resolution by observing movement of the meniscus through a calibrated microscope.
The experiments were terminated 40 min after the last clonidine injection with additional i.v. injections of urethane (1 g/kg) and heparin (100 units). The animal was intra-arterially perfused with cold (4–6 °C) phosphate-buffered saline (PBS, pH 7.4, with 5 USP units/ml of heparin and 0.004% lidocaine) followed by 4% phosphate-buffered formalin. The pons was removed, post-fixed in 4% phosphate-buffered formalin, cryoprotected in 30% sucrose and cut into three series of 35 μm coronal sections. One series was stained with Neutral Red and used for localization of the injection site and the other two were used for immunohistochemistry. Sections from the animals with unilateral clonidine injections were immunohistochemically processed for Fos, the protein product of the immediate early gene c-fos used as a marker of cell activity, and then for tyrosine hydroxylase (TH), a marker of catecholaminergic neurons, as previously described (Lu et al., 2007). Sections from the animals that received two or more clonidine injections were processed for TH only. The double-labeling procedures were conducted successively, first using Fos antibodies (1:100,000; catalog number PC38; lot D14211; Oncogene, Temecula, CA, USA) and then TH antibodies (1:35,000; catalog number T-1299; lot 41K4829; Sigma-Aldrich). Incubations with primary antibodies were followed by appropriate biotinylated secondary antibodies, avidin-biotin reaction (Vector, Burlingame, CA, USA), and diaminobenzidine–horseradish peroxidase (DAB-HRP) reaction. The DAB-HRP step was heavy metal-intensified for Fos, resulting in black staining of neuronal nuclei, but conducted without the intensification for TH, resulting in golden-brown staining of catecholaminergic cells. Photomicrographs were taken using an upright microscope (Leica DML, Wetzlar, Germany) and a digital camera (DMC Ie, Polaroid, Cambridge, MA, USA). Image processing was limited to brightness, contrast and color adjustments to most faithfully represent the appearance of the specimen under direct microscopic observation (Photoshop CS software, Adobe, San Jose, CA, USA).
Data analysis
The amplitude of the moving average of XII nerve activity and central respiratory rate were measured during a 30–60 s baseline period preceding the injection and during 30–60 s 4.4–15 min following each clonidine injection. This delay was empirically selected because clonidine injections often produced short-lasting changes of arterial blood pressure that were occasionally accompanied by transient changes of XII nerve activity after which the activity stabilized.
Tyrosine hydroxylase–immunopositive (TH+) A7 cells were present in brain cross-sections corresponding to the antero-posterior (AP) levels from −8.72 mm to −9.30 mm from bregma (B) according to a rat brain atlas (Paxinos and Watson, 1997). From the caudal to rostral levels, A7 cell initially appeared as isolated TH+ neurons, then, near the AP level of B-8.90, they became more numerous but were loosely scattered near the ventral tip of the brachium conjunctivum. Further rostrally, they formed a compact cluster. Based on this pattern, we distinguished a “loose” and a “compact” division of the A7 group. As our main anatomical reference for localization of microinjections, we used the center of the “loose” A7 group because it contained more A7 cells than the “compact” group. The AP coordinates of the injection sites were measured relative to the reference point by multiplying the section thickness by the number of sections separating the injection site from the landmark. The distance between the center of the “loose” A7 region and the injection site was then calculated as the square root of A2+R2, where A was the AP distance and R was the distance between the center of the injection site and the center of the “loose” A7 group projected onto the section containing the injection site. TH+ cells with Fos-stained nuclei (Fos-immunopositive, Fos+) and TH+ cells without Fos were separately counted in left and right A7 groups in every third 35 μm-thick section.
Statistical analysis
After verification that the data were normally distributed, two-tailed Student’s t-test and two-way repeated measures ANOVA with Bonferroni’s correction were used for statistical analyses (SigmaStat, Jandel, Inc., San Rafael, CA, USA). Two-tailed Fisher exact test was used to assess significance of the difference between proportions. Linear regression analysis was applied to assess statistical significance of the relationship between clonidine-induced changes in Fos expression in A7 cell and XII nerve activity. The variability of the means is characterized by the S.E. throughout the report. Differences were considered significant when P<0.05.
RESULTS
Effect of dorsolateral pontine clonidine injections on XII nerve activity
In 18 rats, clonidine was injected into 35 sites in the dorsolateral pons aiming at the A7 region. Five animals received two bilateral injections each, two animals received one bilateral injection each, and the remaining 11 animals had unilateral injections aiming at the left A7 group. In 10 of these 11 animals, Fos expression was assessed in TH cells of the A7 group on the injected and control sides. In additional two animals, three clonidine injections into the A7 region of one or both sides were preceded by injections of an α2-adrenergic receptor antagonist.
Fig. 1 shows the locations of the injection sites superimposed on serial cross-sections of the pons stained for TH and counterstained with Neutral Red. Our preliminary analysis of the effects of clonidine on XII nerve activity revealed that the injections placed at distances shorter than 0.4 mm from the center of the “loose” part of the A7 group consistently decreased XII nerve activity, whereas those made at larger distances (over 0.5 mm) resulted in small and inconsistent changes. On this basis, we grouped the injection sites into those placed within 0.4 mm from the A7 group (filled circles in Fig. 1), those placed more than 0.5 mm away (open circles), and those made at intermediate distances of 0.4–0.5 mm (dotted circles).
Fig. 1.
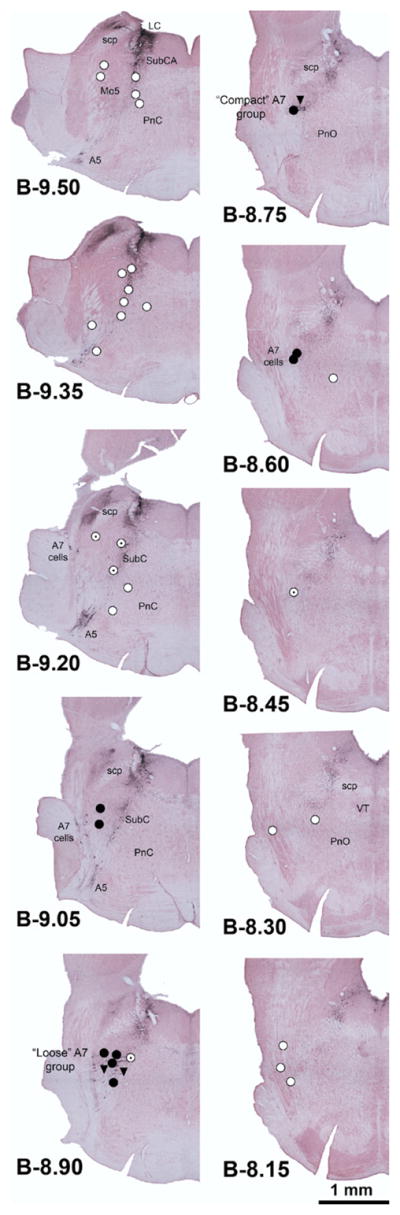
Distribution of clonidine injection sites superimposed onto evenly spaced serial cross-sections of the pons. The sections were stained with Neutral Red and immunostained for TH. Closed circles: clonidine injections placed within 0.4 mm from the center of the “loose” part of the A7 group; open circles: clonidine injections placed at a distance of 0.5–1.2 mm from the A7 group; dotted circles: injections placed at intermediate distances, 0.4–0.5 mm; triangles: control clonidine injections made into the A7 region following microinjections of RS-79948, an α2-adrenoceptor antagonist, placed at the same site. The injections were made on either the left or right side and their location was then redrawn at the corresponding sites on one side of the pons. Abbreviations: A5, NE A5 group; A7, NE A7 group; LC, locus coeruleus; Mo5, trigeminal motor nucleus; PnC, nucleus pontis caudalis; PnO, nucleus pontis oralis; scp, superior cerebellar peduncle; SubCA, nucleus subcoeruleus, alpha part; VT, ventral tegmental nucleus.
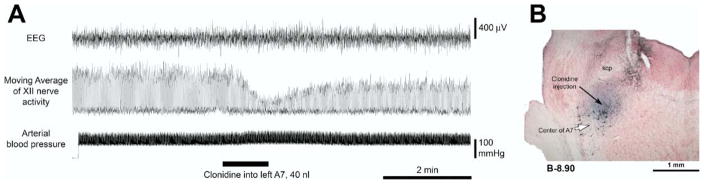
Following the nine clonidine injections that were placed at distances lesser than 0.4 from the A7 region, XII nerve activity was reduced to 55–80% of the pre-injection baseline (mean: 68.7±2.8%) with a mean latency of 88.3±22 s (range: 12–186 s). The effects were long-lasting, over 40 min in some cases, and only a partial recovery could be observed either due to superposition of the effects of additional injections or because our protocol required the animal to be perfused 40 min after the injection. Fig. 2A shows an example of a long-lasting reduction of XII nerve activity (by 34% in this case) that followed clonidine injection made at the site shown in Fig. 2B. A short-lasting disturbance of the XII nerve activity (Fig. 2A) sometimes occurred right after the injection (in three of the nine injections into the A7 region). Following seven of the nine injections, clonidine also elicited a transient increase of arterial blood pressure, by 8.63±3.1 mm Hg. Given the short-lasting nature of these changes (1.4–2.9 min for XII nerve activity and 2.2–7 min (mean: 3.64±0.68 min) for arterial blood pressure), they were unlikely to reflect genuine clonidine effects mediated by α2-adrenoceptors and were not analyzed.
Fig. 2.
Example of a long-lasting depressant effect of clonidine injected into the A7 group on XII nerve activity. (A) Moving average of XII nerve activity, arterial blood pressure and cortical EEG recorded for ~2.5 min before and ~6 min after unilateral clonidine injection. The injection initially elicited a strong but transient depression of XII nerve activity and a slight increase of arterial blood pressure. Because such initial effects were short-lasting and had variable magnitude and pattern among the injections placed within and outside the A7 group, they were not considered to be site- or receptor-specific. The subsequent, long-lasting depressions of XII nerve activity (to 66% of the pre-injection level in this case) that consistently occurred following clonidine injections into the A7 group were considered to be caused by prolonged inhibitory effect of clonidine on A7 neurons. (B) The site of clonidine injection in this experiment (filled arrow). The site was marked by Pontamine Sky Blue injected together with the drug and the section was stained with Neutral Red and immunohistochemically processed for TH to visualize A7 neurons. The hollow arrow points to the center of the A7 group which, at this level, represents its “loose” part. Abbreviation: scp, superior cerebellar peduncle.
Twenty-one injections were made at distances larger than 0.5 mm from the A7 group (open circles in Fig. 1). Following these injections, the mean level of XII nerve activity was 103±3.8% of the baseline (range: 78–167%). The remaining five injections (dotted circles in Fig. 1) were placed 0.4–0.5 mm from the A7 group and had variable effects on XII nerve activity that ranged from a decrease to 43% to an increase to 109% of the baseline. In 1 of the 21 and two of the five injections, clonidine elicited short-lasting decreases of XII nerve activity similar to those described above for the injections placed into the A7 group. Three of the 21 clonidine injections also caused transient increases of arterial blood pressure by 12.9±6.9 mm Hg that lasted 2.57±0.38 min. The frequency of occurrence of clonidine-induced blood pressure increases was not significantly different between the nine injections placed at distances less than 0.4 from the center of the A7 group and the 21 injections placed at distances larger than 0.5 mm from the A7 group (P=0.06, Fisher exact test).
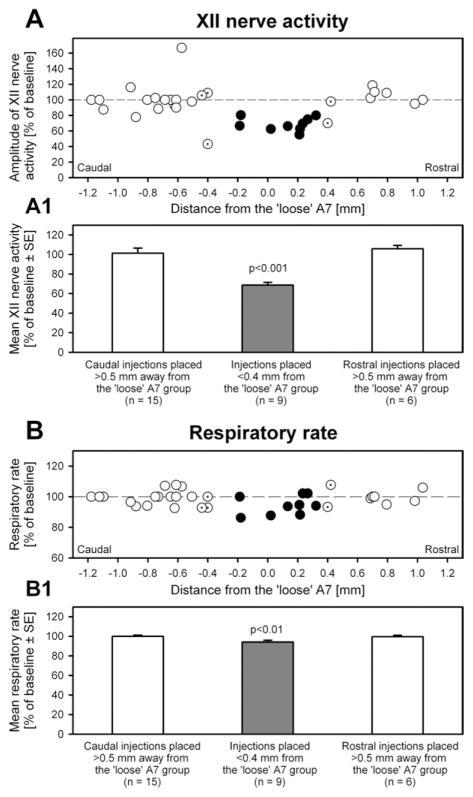
Fig. 3 shows the changes of XII nerve activity and respiratory rate following clonidine injections relative to the pre-injection baseline plotted versus the distance between injection site and the A7 group separately for the injections placed rostral and caudal to the center of the “loose” part of the A7 group. Compared with the 15 injections placed caudal, or the six injections placed rostral, to the A7 group, the injections into the nine sites located within 0.4 mm from the “loose” part of the A7 group significantly reduced XII nerve activity (Fig. 3A). There was a statistically significant interaction between the location of the injection site and clonidine effect on XII nerve activity (F1,2=12.1, P<0.001, two-way repeated measures ANOVA). The post hoc, pairwise comparisons revealed significant effect of clonidine only for the injections placed into the A7 group (t7=5.69, P<0.001) (Fig. 3A1). In one of the seven experiments in which clonidine was injected bilaterally, injections on both sides were placed within 0.4 mm from the A7 groups. This resulted in a combined suppression of XII nerve activity to 43.8% of the activity level prior to the injections. The injections placed farther than 0.5 mm away from the A7 group did not significantly change XII nerve activity. Following these injections, XII nerve activity stabilized at 101±5.2% of the pre-injection level (n=15) for the caudal injection sites, and at 106±3.4% (n=6) following the rostral injections (Fig. 3A1).
Fig. 3.
Changes of XII nerve activity and respiratory rate following clonidine injections within and outside the A7 group. (A) Effects of clonidine injections on XII nerve activity in individual experiments are shown as a function of the distance between the injection site and the center of the “loose” part of the A7 group. The sites located caudal to the A7 group are shown at negative AP coordinates. Closed circles: clonidine injections placed within 0.4 mm from the A7 group; open circles: injections placed at distances 0.5–1.2 mm from the A7 group; dotted circles: injections placed at intermediate distances of 0.4–0.5 mm from the A7 group. (A1) The filled and open bars show the mean values corresponding to the injections placed within and outside the A7 group, respectively. Only injections placed within 0.4 mm from the A7 group caused a significant depression of XII nerve activity (by ~30%). (B and B1) Clonidine-induced changes in the central respiratory rate shown in the same format as in A. There was a small but significant reduction of the respiratory rate following clonidine injections into the A7 group.
The central respiratory rate was only slightly reduced after the nine clonidine injections made within 0.4 mm from the A7 group (to 94.4±2.1% of the baseline, or from 43.8±2.4 min−1 before, to 41.1±1.8 min−1 after clonidine) (Fig. 3B). However, when compared with the 15 caudal or the six rostral injections, there was a statistically significant interaction between location of the injections and clonidine effect on the respiratory rate (F1,2=4.08, P<0.05, two-way repeated measures ANOVA). A post hoc, paired comparisons revealed significant effects only for the injections placed into A7 group (t7=3.69, P<0.01). Following the caudally placed injections, respiratory rate measured 99.9±1.2% of the baseline (n=15), and following the rostral injections, it was 99.5±1.5% (n=6) (Fig. 3B1).
Among the 21 injections made at distances larger than 0.5 mm from the A7 group, eight were placed within 0.4 mm from either the dorsal or ventral part of the SubC region containing a high density of TH+ neurons. These injections did not result in a significant change of either XII nerve activity (104±9.3% of the baseline) or the respiratory rate (to 100±1.8% of the baseline).
Following most (31 of 35) clonidine injections, changes of XII nerve activity were not accompanied by visible changes in the patterns of cortical EEG or hippocampal activity. Among the remaining four cases, in one, forebrain activation occurred following an injection into the A7 group; in one, activation followed an injection outside the A7 group; in one, forebrain activity was reduced following an injection within the A7 group; and, in one, forebrain deactivation occurred following an injection outside the A7 group.
Control experiments with α2-adrenergic receptor antagonist
In two control experiments, clonidine with Pontamine Sky Blue (40 nl) was injected within 0.4 mm from the center of the “loose” A7 group after an earlier 80 nl injection into the same site of a specific α2-adrenoceptor antagonist, RS-79948 (2 mM). In one rat, the injections were properly placed in the A7 region on one and then the other side. In the second rat, data from one injection placed near the A7 region were obtained (filled triangles in Fig. 1). Following injections of RS-79948, XII nerve activity was not significantly changed (93.1±5.7% of the pre-injection baseline). After the subsequent clonidine injections placed at the same sites, XII nerve activity was not changed either (97.4±4.6% of the pre-antagonist baseline). The lack of depression of XII nerve activity in these three trials was statistically different from the depressant effect of the nine clonidine injections into the A7 group made without prior injections of the antagonist, as described in the preceding section (P<0.001, t-test).
Suppression of Fos expression in A7 cells by clonidine
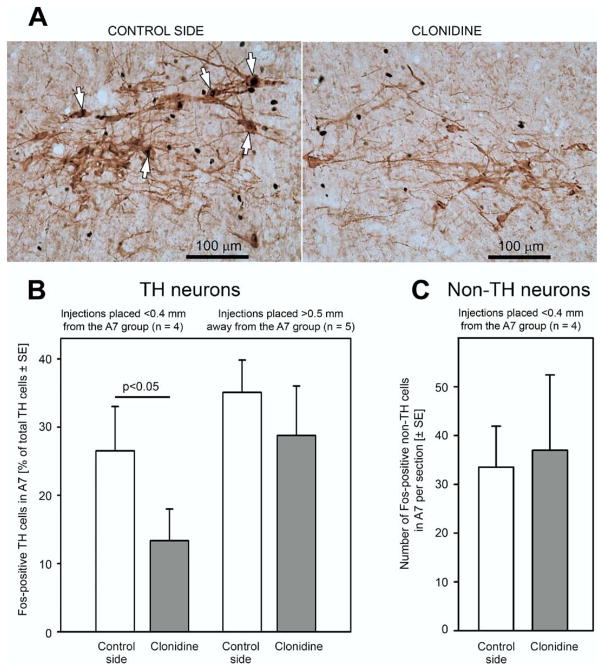
To verify whether clonidine microinjections suppressed activity of A7 neurons, we examined Fos expression in A7 cells on both sides in the 10 experiments with unilateral injection of clonidine. Of these 10 injections, four were placed within 0.4 mm from the A7 group, five more than 0.5 mm away from the A7 group, and the remaining one at an intermediate distance. The results with the four injections placed clearly within the A7 group and those with the five injections placed clearly outside the A7 group were averaged, whereas the injection placed at an intermediate distance was excluded to enhance the contrast. In the four rats with the injections made within the A7 group, the percentage of A7 cells with Fos+ nuclei was consistently lower on the injected side (4.8–25%) than on the control side (15–41%).
Fig. 4A shows an example of A7 neurons stained for TH and Fos on the control side (left panel) and on the injected side (right panel) in the same animal. In this section, none of the 16 TH+ cells had Fos-positive nuclei on the injected site, whereas on the opposite side, 5 of 22 TH+ cells were Fos+. Fig. 4B shows the mean percentages of Fos+ A7 neurons relative to all TH+ neurons in the A7 group on the injected and control sides in the four animals in which clonidine was injected within 0.4 mm from the center of the A7 group, and in the five animals with clonidine injections made farther than 0.5 mm away from the A7 group. In the first group, the percentage of Fos+ neurons was twice lower on the injected side than on the opposite side (13.4±4.7% vs. 26.5±6.5%; P<0.05, paired t-test). In the second group, there was no difference between the two sides, and these percentages were also not different from those on the control side in the first group.
Fig. 4.
Microinjections of clonidine into the A7 group reduced Fos expression in NE A7 neurons. (A) Example of Fos expression in A7 cells on the control side and the side injected with clonidine (0.75 mM, 40 nl) in the same experiment. On the control side, arrows point to each of the five Fos-positive TH cells of the 22 TH cells present in this frame; none of the 16 TH-positive cells present on the injected side had Fos-positive nuclei. (B) The mean percentages of Fos-positive TH cells in the A7 group on the control and injected sides in the four experiments with clonidine injections into the A7 group (bars on the left) and in another five experiments with clonidine injections placed more than 0.5 mm away from the A7 group. Only the injections placed within 0.4 mm from the A7 group significantly decreased Fos expression in A7 neurons. The percentage of Fos-positive TH cells on the injected side in the four experiments with clonidine injections into the A7 group was significantly lower than the percentages Fos-positive TH cells on the control side, whereas the latter was not different from the percentages of Fos-positive TH cells on either the control or injected side in the five experiments with clonidine injections placed more than 0.5 mm away from the A7 group. (C) Mean counts of Fos+ nuclei in non-TH cells located within a 0.5×0.5 mm counting box centered on the “loose” A7 group was not different between the injected and control side in the four experiments in which clonidine was injected less than 0.4 mm away from the A7 group.
To test whether non-TH cells located near the A7 group also were inhibited by clonidine and possibly contributed to the depression of XII nerve activity, we analyzed Fos expression in non-TH cells in A7 group on both the injected and control sides in the four experiments in which clonidine was unilaterally injected within 0.4 mm from the A7 group. In each rat, Fos+ nuclei in non-TH cells located within a 0.5×0.5 mm counting box centered on the A7 group were counted in one brain section. The average counts were not statistically different between the injected and control sides (37.0±15 vs. 33.5±8.4, n=4, P>0.7, paired t-test) (Fig. 4C). We also counted Fos+ nuclei in non-TH cells in a similarly anchored but larger counting box (1× 1 mm), and we found the average counts to be somewhat higher, but not significantly, on injected side when compared with the control side (115±52 vs. 78.0±9.7, n=4). This was in contrast to a significantly lower average number of Fos+/TH+ cells in the “loose” A7 region counted in the same sections on the injected side when compared with the control side (6.25±3.0 vs. 12.5±2.4, P<0.05).
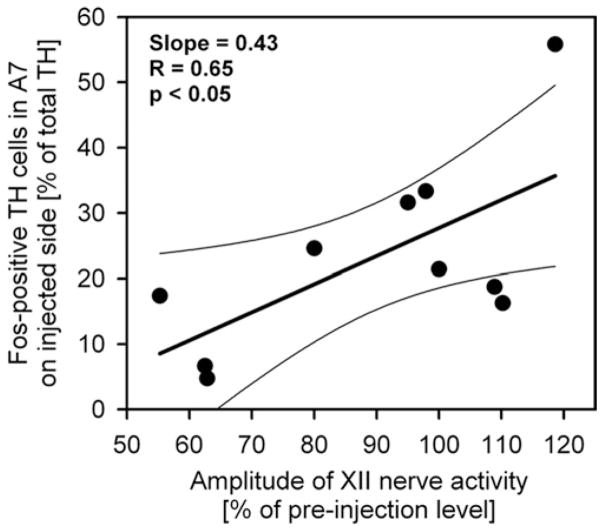
Since our results suggested that A7 neurons provide a significant excitatory NE drive to XII motoneurons, we tested whether there was a proportional relationship between the percentage of Fos+ A7 neurons on the injected side and the level of XII nerve activity after clonidine across all 10 experiments with unilateral clonidine injections. Fig. 5 shows that the level of Fos expression in A7 neurons was positively correlated with the level of XII nerve activity (P<0.05).
Fig. 5.
Relationship between the percentage of Fos-positive TH cells in the A7 group on the injected side and the level of XII nerve activity following 10 clonidine injections, of which four were made closer than 0.4 mm from the A7 group; one, 0.4–0.5 mm away, and the remaining five at distances larger than 0.5 mm from the A7 group. There was a significant positive correlation between the level of Fos expression in A7 cells after clonidine and XII nerve activity. R, linear regression coefficient; P, significance level for linear regression; thin lines, 95% confidence interval for the regression.
DISCUSSION
Unilateral injections of clonidine into the A7 region reduced XII nerve activity by about 30%, whereas injections placed farther away from the A7 group were ineffective. Clonidine injections into the A7 group also reduced Fos expression in TH-positive A7 cells, thus providing indirect evidence that A7 cell activity was reduced. Together, these results lead us to conclude that endogenous NE drive from A7 neurons significantly contributes to the level of activity in XII motoneurons. Consistent with this, we found a proportional relationship between XII nerve activity and A7 cell activity, as assessed by Fos expression.
Technical considerations
NE neurons of the A5 and A6 (locus coeruleus) groups are spontaneously active in anesthetized rats (Berridge et al., 1993; Kubin, 2001; Fenik et al., 2002, 2005c), and their activity is inhibited by local administration of the α2-adrenergic agonist, clonidine (Moore and Guyenet, 1983; Berridge et al., 1993). Clonidine also inhibits A5, A6 and SubC neurons following systemic administration (Reiner, 1985; Huangfu et al., 1991; Fenik et al., 2002). The effects of clonidine on the activity of A7 neurons have not been tested, but immunohistochemical studies show that A7 cells express α-adrenergic type 2A and 2C receptors similarly to A5, A6 and SubC neurons (Rosin et al., 1996; Lee et al., 1998). Our recent Fos expression study also suggested that A5, SubC and A7 cells are spontaneously active in urethane-anesthetized rats and that their activity is reduced during a pharmacologically-induced REM sleep-like state (Rukhadze et al., 2008), a finding consistent with previously demonstrated silencing of A5, A6 and SubC neurons during natural REM sleep or in urethane-anesthetized rat carbachol model of this state (Aston-Jones and Bloom, 1981; Reiner, 1986; Kubin, 2001; Fenik et al., 2002).
We used Fos expression to verify whether local micro-injections of clonidine suppress activity of A7 but not non-TH cells located near the A7 group. In these experiments, clonidine was injected on one side only into the A7 group located contralaterally to the recorded XII nerve activity. We used this approach because A7 projections to the XII nucleus are bilateral (Rukhadze and Kubin, 2007), and in this study we found similar effects of ipsi- and contralateral injections of clonidine, suggesting that A7 groups of both sides equally contribute to the control of XII motoneuronal activity. The percentage of A7 neurons expressing Fos was reduced twofold on the clonidine-injected side when compared with the opposite side (13% vs. 27%). This was a slightly stronger depression than the decrease in the percentage of Fos-expressing A7 neurons estimated to occur after a prolonged period of REM sleep-like state (to 15–20%, see Fig. 7 in the study of Rukhadze et al. (2008)). These findings, albeit based on Fos immunohistochemistry, suggest that A7 cells are spontaneously active in urethane-anesthetized rats and are inhibited by local microinjections of clonidine.
We found that Fos expression in non-TH cells located in A7 group was not affected by clonidine despite the possibility that they also may express α2-adrenergic receptors (Rosin et al., 1996; Talley et al., 1996). This suggests that non-TH neurons located in the A7 region had low, or no, activity under our experimental conditions and, therefore, could not be further inhibited by clonidine. The found absence of changes in Fos expression in non-TH cells suggests that they were unlikely to contribute to the decrease of XII nerve activity caused by clonidine injections in the A7 group.
In contrast to clonidine injections placed within the A7 group, the injections placed farther than 0.5 mm away from the A7 group did not decrease Fos expression in A7 neurons and had insignificant effects on XII nerve activity. After some injections, we observed transient perturbations of arterial blood pressure that usually dissipated within less than 4 min. Since clonidine displaces norepinephrine from α2-adrenergic receptors with a Ki in low nanomolar range (Chung et al., 2000) and systemic administrations of 10–15 μg/kg of clonidine caused a complete suppression of A5 cell activity lasting about 30 min (Huangfu et al., 1991), we regarded these transient effects as unlikely to be mediated by clonidine action on α2-adrenergic receptors located on A7 neurons. In support of this interpretation, these short-lasting effects occurred following some clonidine injections placed within and some outside the A7 group. It is likely that baroreceptor reflexes helped restore and stabilize arterial blood pressure following these transient perturbations. Given that blood pressure returned to the pre-injection level following clonidine injections within or outside the A7 region, it appears that dorsolateral pontine clonidine injections had no lasting effect on the set-point of the regulation of arterial blood pressure in these anesthetized rats. In contrast, XII nerve activity was suppressed for a prolonged period and this occurred only following those injections that were subsequently localized close to the A7 group. Thus, it appears that there were few spontaneously active and α2-adrenergic receptor-expressing cells in the lateral pontine reticular formation that could be inhibited by clonidine and elicit significant changes in blood pressure or other than XII nerve activity outputs monitored under our experimental conditions. Under different experimental conditions, additional effects of clonidine could perhaps occur because some non-NE neurons of the lateral pons also express α2-adrenergic receptors (Rosin et al., 1996; Talley et al., 1996).
We also found that inhibition of A7 neurons had a small (6%) but significant negative effect on the central respiratory rate. This is in accordance with a stimulating role of pontine catecholaminergic neurons on breathing frequency in behaving rats (Errchidi et al., 1990; Li and Nattie, 2006). In other studies in rats, microinjections of inhibitory drugs into the pontine Kölliker-Fuse nucleus, which overlaps with the A7 group, reduced respiratory rate and attenuated respiratory responses to noxious stimuli (Jiang et al., 2004), injections of stimulants such as glutamate into the Kölliker-Fuse and the adjacent intertrigeminal region elicited apneic responses (Chamberlin and Saper, 1998), and lesions of this area reduced the occurrence of apneic episodes during sleep (Radulovacki et al., 2004). Thus, cells in this region clearly can have access to the respiratory rhythm-generating regions of the brainstem.
A7 and other potential sources of NE excitatory input to XII motoneurons
Retrograde tracing studies identified four NE cell groups as having axonal projections to the XII nucleus: the A7, SubC and A5 groups of the pons, and the A1 group of the rostral ventrolateral medulla (Aldes et al., 1992; Rukhadze and Kubin, 2007). Of those, at least A7, SubC and A5 cells have state-dependent activity, highest during wakefulness and lowest during REM sleep (Reiner, 1986; Fenik et al., 2002; Rukhadze et al., 2008), so their decreased activity during sleep may contribute to sleep-related decrements of XII motoneuronal activity (Fenik et al., 2005b; Chan et al., 2006). Our present experiments reveal that it is so for the A7 group, whereas clonidine injections into the A5 or SubC region did not yield significant results. In our earlier study in the same animal model and mode of clonidine injections, we found that clonidine microinjected into the central part of the A5 region did not affect XII nerve activity (Fenik et al., 2002). In the present study, eight injections were placed into the SubC region and they also had negligible effects. Thus, anatomical evidence of appropriate axonal projections does not provide sufficient basis for inferring about functional effects from a given afferent input.
Unilateral clonidine injections into the A7 region depressed XII nerve activity to about 70% of its pre-injection level. Based on this, one may estimate that bilateral suppression of A7 cell activity could reduce XII nerve activity to about 50% (0.70×0.70=0.49). Consistent with this prediction, in one animal, when clonidine was successfully injected into the A7 groups on both sides, XII nerve activity was reduced to 44% of its pre-injection level. Thus, A7 cells may be a major source of state-dependent NE drive to XII motoneurons. Removal of all NE excitatory drive to XII motoneurons by means of antagonizing α1-adrenergic receptors in the XII nucleus region in urethane-anesthetized rats reduced XII nerve activity to less than 20% of the baseline (Fenik et al., 2005b). Thus, there must be additional sources of significant endogenous NE activation besides the A7 group that contribute to the maintenance of activity in XII motoneurons. Such sources may have the typical for NE cells, sleep-related decrements of activity or may be state-independent. For example, ventrolateral medullary A1 cells may have axonal projections to the XII nucleus (Rukhadze and Kubin, 2007), but our recent Fos data suggest that they do not have REM sleep-related decrements of activity (Rukhadze et al., 2008). A source of NE excitatory drive to XII motoneurons that does not have sleep-related decrements of activity is interesting as one that may mediate the adaptive increase of upper airway motor tone observed in obstructive sleep apnea patients (Suratt et al., 1988; Mezzanotte et al., 1992; Hendricks et al., 1993; Katz and White, 2004).
In the 10 experiments in which we analyzed Fos expression in A7 cells, XII nerve activity varied from 55 to 119% of the baseline following clonidine injections. We found a significant proportional relationship between Fos expression and XII nerve activity. This strengthens our conclusion that A7 cells provide an important portion of NE excitatory drive to XII motoneurons.
Fifteen percent of A7 cells were retrogradely labeled from the XII motor nucleus, the highest percentage of all NE groups projecting to the XII nucleus (Rukhadze and Kubin, 2007). However, A7 cells also project to the nucleus of the solitary tract (Rukhadze and Kubin, 2007), ventral, dorsal and intermediolateral regions of the spinal cord (Lyons and Grzanna, 1988; Fritschy and Grzanna, 1990; Clark and Proudfit, 1991) and the cerebellum (Pasquier et al., 1980). The spinally projecting A7 neurons may play a role in the control of pain by acting on pre- and postsynaptic α1- and α2-adrenergic receptors in the dorsal horn (see Pertovaara, 2006 for a review), although their endogenous, tonic contribution to transmission of nociceptive stimuli under normal conditions remains to be demonstrated (Nuseir and Proudfit, 2000). The presence of A7 projections to both motor (XII nucleus and spinal ventral horn) and sensory (NTS and spinal dorsal horn) regions would be consistent with the modulation of sensorimotor systems in relation to behavioral state being an important function of A7 neurons. A7 neurons also could contribute to the reduced NE release in the XII nucleus and spinal ventral horn regions detected when muscle atonia was elicited by electrical stimulation of the dorsomedial pontine reticular formation, a model of REM sleep-related atonia (Lai et al., 2001). Recent antagonist studies show that a reduced release of NE may importantly contribute to the characteristic depression of motor tone during REM sleep (Fenik et al., 2005b; Chan et al., 2006).
A7 neurons may provide NE drive to XII motoneurons through their direct synaptic connections with XII motoneurons (Aldes et al., 1990; 1992; Rukhadze and Kubin, 2007) or indirectly, through interneurons located adjacent to the XII nucleus. The latter possibility is suggested by a study in which microinjections of an α1-adrenergic antagonist into XII nucleus depressed XII nerve activity with a time course that, based on diffusion analysis, involved a spread of the antagonist over a radius of 1.4 mm, thus beyond the boundaries of the XII nucleus (Fenik et al., 2005a,b). Whether an interneuronal relay is involved in mediation of the effects of A7 group to XII motoneurons remains to be determined.
Implications for state-dependent control of the upper airway
Our study suggests that A7 neurons provide a major NE excitatory drive to XII motoneurons. In unanesthetized, behaving animal, this drive may be highest during wake-fulness, reduced during non-REM sleep and abolished during REM sleep (Rukhadze et al., 2008). Therefore, A7 neurons may be responsible, at least in part, for sleep-dependent withdrawal of NE excitation from XII motoneurons, which has been proposed to be a major cause of sleep-related depression of upper airway motor tone (Fenik et al., 2005b; Chan et al., 2006). XII motoneurons innervate the genioglossus, a muscle of the tongue that has an important role in the maintenance of upper airway patency in patients with obstructive sleep apnea syndrome (Remmers et al., 1978; reviewed by Kubin and Davies, 2002). The upper airway anatomy of these patients is a major predisposing factor for the disorder but an adaptive elevation of upper airway motor tone helps them to keep the airway open at least during wakefulness (Suratt et al., 1988; Mezzanotte et al., 1992; Hendricks et al., 1993; Katz and White, 2004). When the genioglossus and other upper airway dilators become hypotonic during sleep, and especially REM sleep, the negative pressure inside the airways generated during inspiration can precipitate airway obstructions. Based on our study, the level of activity in the A7 group may importantly determine the level of upper airway motor tone during wakefulness.
CONCLUSION
There is a significant positive relationship between the level of activity in NE A7 cells and XII nerve activity. Many A7 neurons have axonal projections to the XII nucleus (Aldes et al., 1992; Rukhadze and Kubin, 2007) and similarly to other pontine NE neurons, may have reduced activity during sleep (Rukhadze et al., 2008). Therefore, sleep-related decrements of A7 cell activity may importantly contribute to state-dependent changes in the activity of the muscles of the tongue innervated by XII motoneurons.
Acknowledgments
We thank Ms. Jennifer Branconi and Ms. Janet Lee for technical assistance. The study was supported by grant HL-47600 from the Heart, Lung and Blood Institute of the National Institutes of Health.
Abbreviations
- AP
antero-posterior
- B
bregma
- DAB-HRP
diaminobenzidine–horseradish peroxidase
- EEG
electroencephalogram
- Fos+
Fos-immunopositive
- NE
noradrenergic
- REM
rapid eye movement
- SubC
subcoeruleus
- TH
tyrosine hydroxylase
- TH+
tyrosine hydroxylase–immunopositive
- XII
hypoglossal
References
- Aldes LD, Shaw B, Chronister RB, Haycock JW. Catecholamine-containing axon terminals in the hypoglossal nucleus of the rat: an immuno-electron microscopic study. Exp Brain Res. 1990;81:167–178. doi: 10.1007/BF00230113. [DOI] [PubMed] [Google Scholar]
- Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and nor-adrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflügers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. A brainstem network mediating apneic reflexes in the rat. J Neurosci. 1998;18:6048–6056. doi: 10.1523/JNEUROSCI.18-15-06048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chung SH, Yook J, Min BJ, Lee JY, Lee YS, Jin C. Pharmacological characterization of (10bS)-1,2,3,5,6,10b-hexahydropyrrolo [2,1-a]isoquinoline oxalate (YSL-3S) as a new alpha2-adrenoceptor antagonist. Arch Pharm Res. 2000;23:353–359. doi: 10.1007/BF02975447. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;547:279–288. doi: 10.1016/0006-8993(91)90972-x. [DOI] [PubMed] [Google Scholar]
- Errchidi S, Hilaire G, Monteau R. Permanent release of nor-adrenaline modulates respiratory frequency in the newborn rat: an in vitro study. J Physiol (Lond) 1990;429:497–510. doi: 10.1113/jphysiol.1990.sp018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Methods. 2001;116:147–150. doi: 10.1016/s0165-0270(01)00340-5. [DOI] [PubMed] [Google Scholar]
- Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005a;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005b;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Ogawa H, Davies RO, Kubin L. Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats. Sleep. 2005c;28:551–559. doi: 10.1093/sleep/28.5.551. [DOI] [PubMed] [Google Scholar]
- Fenik V, Rukhadze I, Branconi J, Kubin L. Microinjections of α2-adrenergic agonist, clonidine into the noradrenergic A7 cell region reduce hypoglossal (XII) nerve activity in urethane-anesthetized rats. Sleep. 2007;30(Suppl):A19. [Google Scholar]
- Fritschy JM, Grzanna R. Demonstration of two separate descending noradrenergic pathways to the rat spinal cord: Evidence for an intragriseal trajectory of locus coeruleus axons in the superficial layers of the dorsal horn. J Comp Neurol. 1990;291:553–582. doi: 10.1002/cne.902910406. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am Rev Respir Dis. 1993;148:185–194. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Guyenet PG. Alpha 2-adrenergic autoreceptors in A5 and A6 neurons of neonate rats. Am J Physiol. 1997;273:H2290–H2295. doi: 10.1152/ajpheart.1997.273.5.H2290. [DOI] [PubMed] [Google Scholar]
- Huangfu DH, Koshiya N, Guyenet PG. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol. 1991;261:R393–R402. doi: 10.1152/ajpregu.1991.261.2.R393. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ogura M, Oshima T, Suzuki H, Higano S, Takahashi S, Kurosawa H, Hida W, Matsuoka H, Takasaka T. Quantitative assessment of the pharyngeal airway by dynamic magnetic resonance imaging in obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol. 2001;110:183–189. doi: 10.1177/000348940111000215. [DOI] [PubMed] [Google Scholar]
- Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep apnea. Pathogenesis, diagnosis, and treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- Lai YY, Kodama T, Siegel J. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of α2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–1096. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol (Lond) 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates nor-adrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol (Lond) 2007;582:52–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Grzanna R. Noradrenergic neurons with divergent projections to the motor trigeminal nucleus and the spinal cord: a double retrograde neuronal labeling study. Neuroscience. 1988;26:681–693. doi: 10.1016/0306-4522(88)90174-1. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Moore SD, Guyenet PG. Alpha-receptor mediated inhibition of A2 noradrenergic neurons. Brain Res. 1983;276:188–191. doi: 10.1016/0006-8993(83)90563-2. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Proudfit HK. Bidirectional modulation of nociception by GABA neurons in the dorsolateral pontine tegmentum that tonically inhibit spinally projecting noradrenergic A7 neurons. Neuroscience. 2000;96:773–783. doi: 10.1016/s0306-4522(99)00603-x. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- Pasquier DA, Gold MA, Jacobowitz DM. Noradrenergic perikarya (A5–A7, subcoeruleus) projections to the rat cerebellum. Brain Res. 1980;196:270–275. doi: 10.1016/0006-8993(80)90737-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, compact. 3. San Diego: Academic Press; 1997. [Google Scholar]
- Pertovaara A. Noradrenergic pain regulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Pavlovic S, Carley DW. Pontine intertrigeminal region attenuates sleep apneas in rats. Sleep. 2004;27:383–387. doi: 10.1093/sleep/27.3.383. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Clonidine inhibits central noradrenergic neurons in unanesthetized cats. Eur J Pharmacol. 1985;115:249–257. doi: 10.1016/0014-2999(85)90697-1. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following REM sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience. 2008;152:208–222. doi: 10.1016/j.neuroscience.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]