Abstract
To understand high-risk (hr) human papillomavirus (HPV) epidemiology in mid-adulthood, we assessed whether associations between incident detection of hrHPV DNA and recent sexual behavior differed according to whether or not there was serologic evidence of prior infection. From 2011–2012, we enrolled 409 women aged 30–50 years into a 6-month longitudinal study. We collected health and sexual behavior histories, enrollment sera for HPV antibody testing, and monthly self-collected vaginal swabs for HPV DNA genotyping. Generalized estimating equations logistic regression identified risk factors for type-specific incident hrHPV DNA, stratified by type-specific hrHPV serostatus at enrollment. Population attributable risks of hrHPV due to prior and recent exposure were estimated. When type-specific hrHPV serology was negative, recent sexual risk behavior was positively associated with incident hrHPV DNA (odds ratio in women reporting ≥3 recent sexual risk behaviors [e.g., new or multiple partners] versus no recent sexual activity=9.8, 95%CI:2.4–40.6). No associations with recent sexual behavior were observed with positive type-specific hrHPV serology. Thirty percent of incident hrHPV DNA detection was attributable to prior infection (with positive serology) and 40% was attributable to recent sexual risk behavior (with negative serology). The proportion of incident hrHPV DNA detection attributable to recent sexual risk behavior decreased with increasing age. Among women with serologic evidence of prior infection, re-detection of the same hrHPV type is likely due to reactivation or intermittent detection of persistent infection. Without serologic evidence of prior infection, new detection is likely due to new acquisition or to intermittent detection of persisting infection.
Keywords: human papillomavirus, women, mid-adult, incident, serology
INTRODUCTION
Whereas incident human papillomavirus (HPV) infections in newly sexually active young women are likely to represent new acquisition (and show strong associations with recent sexual behavior),1 newly detected HPV DNA in mid-adult women may represent newly acquired infection or re-detection of a previous infection. However, the source of infection is usually unknown. While the majority of HPV infections are transient, a minority persist.2 Intermittent detection of persistent infection can occur when viral levels fluctuate below the detection limit of DNA assays.3 Reactivation of prior infection is also possible, with biologic evidence indicating that HPV can enter a latent state in the basal cells of the cervical epithelium.4, 5 Understanding the relative frequencies of new acquisition versus re-detection of prior infection has implications for both prophylactic HPV vaccination recommendations in this age group and clinical counseling for women who test HPV-positive during routine cervical cancer screening.1, 6
Differentiating between new infection versus intermittent persistent detection or reactivation of a previously acquired type is methodologically challenging without a reliable indicator of prior infection. Limitations of HPV serology as a marker of prior infection include limited sensitivity,7 lack of a standardized approach,8 and the fact that antibody responses are not uniformly detected9, 10, 11, 12 nor lifelong.9, 11 However, HPV serology can be combined with HPV DNA genotyping of current infection and sexual behavior data as a research strategy to better understand the likelihood of new infection given new HPV DNA detection. Using data from a cohort of mid-adult women followed for 6 months with HPV serology testing at enrollment, monthly vaginal self-sampling for HPV DNA testing, and detailed reporting of sexual behaviors, we assessed whether the incidence of, and risk factors for, newly detected type-specific high-risk (hr) HPV DNA varied depending on whether or not there was serologic evidence of prior infection with the same hrHPV type. We expected that newly detected hrHPV in women with serologic evidence of prior infection with the same type would be more likely due to reactivation or intermittent detection of persisting infection, rather than to re-infection with the same type from a new partner. On the other hand, we expected that newly detected hrHPV in women without serologic evidence of prior infection would be due to either new acquisition from a new partner or intermittent detection of persistent infection. Therefore, we hypothesized that new sexual exposures would only be associated with newly detected infections in the absence of serologic evidence of prior infection with the same type.
METHODS
Study Population
From March 2011 to January 2012, we enrolled 409 women affiliated with the University of Washington (UW) into a 6-month longitudinal study of HPV infections.13 Eligible women were aged 30–50 years, were not currently pregnant, had never had a hysterectomy, did not have a serious medical condition that would preclude study participation, and were willing to provide monthly self-collected vaginal samples. Informed consent was administered by the study coordinator at enrollment. The protocol was reviewed and approved by the UW Institutional Review Board.
Data Collection
In-clinic enrollment and six-month exit visits were held at an on-campus health clinic. At enrollment, women provided a venous blood specimen, self-collected a vaginal sample (two sequential swabs) into 1.5 mL specimen transport medium (STM), and filled out an online survey covering demographic characteristics, women’s health history, and sexual history. Updated information was gathered at exit. Between visits, women were asked to: 1) complete biweekly online sexual behavior diaries capturing daily frequency of vaginal intercourse and condom use and male sex partner characteristics; and 2) self-collect and return monthly vaginal samples to the clinic. Batched specimens were transported to the laboratory weekly and stored at −20°C or prepared for immediate testing.
HPV Genotyping
From self-collected vaginal samples, we isolated genomic DNA and conducted HPV genotyping using the Roche Linear Array assay.14 Briefly, the vaginal samples were digested with 20 μg/mL proteinase K at 37°C for one hour, and DNA isolated from 200 μL of the digested sample using QIAamp DNA blood mini kit, following the protocol of the manufacturer (Qiagen, Cat. No.51104). Samples were directly genotyped using the Roche Linear Array assay for 37 alpha-genus HPV types. Samples testing negative for β-globin were considered insufficient (n=2/2755 samples; 0.07%).
HPV Serology
We used a Luminex-based assay to test enrollment sera samples for antibodies, described in detail previously.15, 16 Briefly, HPV 16/18/31/33/35/39/45/51/52/56/58/59/68 L1 proteins and BKPyV VP1 proteins (a positive control)17 were expressed as GST-fusion proteins.18 Human sera were tested at a final dilution of 1:100. The median fluorescent intensity (MFI) values for each antigen (after subtracting the MFI for glutathione S-transferase–coated beads) were plotted as a histogram (data not shown). All histograms had a single large peak presumably representing values from subjects that had not been exposed to that HPV type. Cut points for each antigen were selected to exclude the entire peak, as we reasoned that there would not be a peak for the presumed exposed population unless a large fraction of subjects had been infected; instead, the presumed exposed subjects would exhibit a broad range of values.19–21 The cut points selected were: 2,000 for HPV-35; 1,000 for HPV-16/18/31/51/52/56/58/59; and 500 for HPV-33/39/45/68. The HPV-16 international standard (10 U/ml) (National Institute for Biological Standards and Controls)22 and a serum being evaluated by the World Health Organization as a potential HPV-18 standard were used as serologic controls. Details on reliability testing were described previously.23
Statistical Analyses
Each vaginal sample was numbered according to the sample window during which it was received, defined as a period of 14 to 45 days after the previous sample was received or within 30 days after the end of the previous sample window if no sample was received.13 This numbering system accounts for samples not submitted according to the monthly schedule so that samples would be spaced at least 14 days apart. Additional samples received during the same sample window were randomly dropped, and samples received ≥8 sample windows after enrollment were excluded.
Analyses were restricted to 13 hrHPV types (HPV-16/18/31/33/35/39/45/51/52/56/58/59/68)24 with both HPV genotyping and serology results. At enrollment, each hrHPV type within a woman (hereafter referred to as a “woman-type”) was classified as negative or positive by DNA and by serology (Figure 1). Women-types that were DNA-positive in the enrollment vaginal sample (i.e., prevalent) were excluded from analyses. Otherwise, type-specific incident hrHPV detection was defined as the first DNA-positive in a woman previously negative for that specific type.
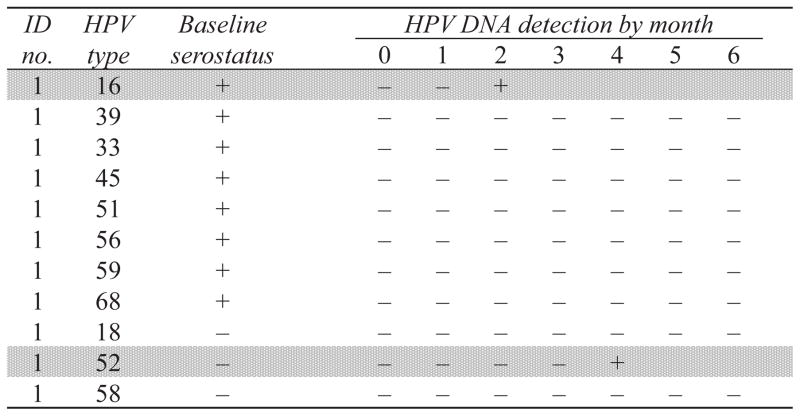
Figure 1. Example of infection-level analysis of type-specific incident high-risk (hr) HPV DNA detection in mid-adult women, stratified by enrollment serostatus.
This hypothetical study participant contributed 11 hrHPV types, or “woman-types”, to the analysis: 8 seropositive (HPV-16, 33, 39, 45, 51, 56, 59, and 68) and 3 seronegative (HPV-18, 52 and 58) at baseline. Two incident hrHPV DNA detections were observed over follow-up (shaded in light gray), 1 in a seropositive woman-type (HPV-16, in month 2) and 1 in a seronegative woman-type (HPV-52, in month 4). Two woman-types (HPV-31 and 35) were excluded from the analysis due to prevalent type-specific DNA detection at enrollment and are not shown.
Cumulative incidence of detecting type-specific hrHPV was calculated using Kaplan-Meier methods with 95% confidence intervals (bootstrapped to account for within-women correlation), with estimates stratified by type-specific hrHPV serostatus at enrollment. Each woman could contribute up to 13 women-types to the analysis, and could contribute to both the seropositive and seronegative analyses. Time-at-risk was represented by the discrete number of samples (an approximation of months) until first type-specific incident detection. Women-types were censored after incident detection or at the last follow-up visit. In a post-hoc analysis, we further generated stratified estimates by both type-specific hrHPV serostatus and age at enrollment (30 to 39 years versus 40 to 50 years) using a similar approach.
We used generalized estimating equations (GEE) with an independence working correlation structure, robust standard errors, and logit link function to estimate odds ratios for associations between potential risk factors and type-specific incident hrHPV detection, with separate models for seropositive versus seronegative types (Figure 1). Correlation within women due to multiple outcome measurements was accounted for. Sample numbers, a time surrogate, were included as indicator variables. Both time-fixed and time-dependent variables were assessed. Time-fixed factors assessed at enrollment included age, body mass index (BMI), marital status, history of pregnancy, history of non-HPV sexually transmitted diseases (STDs), history of genital warts, current hormonal contraceptive use, current immunosuppressive conditions, smoking status, lifetime number of male sex partners, and age at first vaginal intercourse. Time-dependent sexual behaviors during the 6 months prior to each sample were assessed by merging sexual behavior data from enrollment and exit surveys to daily sexual behavior recorded in biweekly diaries. Sexual behaviors were considered present if reported in either a survey or diary and absent if the subject reported sexual activity in the prior 6 months but did not report the particular sexual behavior. Time-dependent variables included the average number of sex acts per week and condom use, each restricted to women reporting sex with a male partner in the past 6 months (women reporting no recent male partners were coded to missing for these two variables). When multiple partners were reported, averages were summed. Condom use with all reported male sex partners was combined across partners, with “always” indicating always using a condom with every male partner and “not always” indicating not always using a condom with ≥1 male partner. In addition, to assess the overall impact of recent sexual behaviors, a composite variable was constructed ranging from not sexually active with male partners in the prior 6 months to sexually active with male partners with 0, 1, 2, or ≥ 3 risk factors (risk factors include: ≥2 sex partners, ≥1 new sex partner, ≥1 casual partner, ≥1 partner with other concurrent partners, and ≥1 partner with a history of STDs), with the variable considered missing only if information on all factors were missing.
Variables with p-values<0.10 in univariate analyses were included in multivariate models. When there was evidence of a monotonic trend across more than two categories of a variable, we fit a second model with the categorical variable as a linear term to test for a dose-response effect (a post-hoc analysis).
Since increased risk on the multiplicative scale does not necessarily translate to an appreciable risk difference on the additive scale, the effect of type-specific hrHPV seropositivity and recent sexual behaviors on incident hrHPV detection was assessed on the additive scale by the attributable risk percent (%AR) and population attributable risk percent (%PAR).25 The exposure of interest variable combined hrHPV serostatus and composite sexual behavior risk factors (seropositive; seronegative and either not sexually active or sexually active with no risk factors; or seronegative and sexually active with ≥1 risk factor). To determine covariates to include in a multivariate model with this combined exposure variable, time-fixed and time-dependent variables were assessed in univariate GEE logistic regression models with incident hrHPV detection as the outcome. Model terms with p-values<0.10 in the univariate analyses were included in the final multivariate model to estimate odds ratios used to calculate %AR and %PAR. In a post-hoc analysis, we further calculated %AR and %PAR stratified by age group (30 to 39 versus 40 to 50 years of age) using a similar approach.
RESULTS
Of the 409 mid-adult women enrolled, 30 (7%) were excluded from analyses due to self-reported history of HPV vaccination. The mean age of the remaining 379 women at enrollment was 38.7 (standard deviation [SD]=6.1) years, and the median lifetime number of male sex partners was 7 (IQR:3–15) (Table 1). At enrollment, 68% of women were seropositive for ≥1 hrHPV type and 15% were DNA-positive for ≥1 hrHPV type. The mean follow-up time was 6.7 months (SD=1.8), and 354 women (93%) completed an exit visit.
Table 1.
Enrollment characteristics of mid-adult women in Seattle, Washington, 2011–2012 (N = 379a)
| Characteristic | ||
|---|---|---|
| Mean | (SD) | |
| Age at first sexual intercourse (years)b | 18.7 | (3.9) |
| Median | (IQR) | |
| Lifetime number of male sex partners | 7 | (3 – 15) |
| nc | (%) | |
| Age (years) | ||
| 30–39 | 229 | (60.4) |
| 40–50 | 150 | (39.6) |
| Body Mass Index (kg/m2) | ||
| 17 – 25 (underweight or normal) | 206 | (54.5) |
| 25 – 29.9 (overweight) | 104 | (27.5) |
| 30 – 51 (obese) | 68 | (18.0) |
| Race | ||
| African American | 11 | ( 2.9) |
| Asian | 42 | (11.1) |
| White | 299 | (78.9) |
| Otherd | 27 | ( 7.1) |
| Education | ||
| Some college or less | 64 | (16.9) |
| College bachelor’s degree | 137 | (36.1) |
| College master’s or doctoral degree | 178 | (47.0) |
| Marital status | ||
| Unmarried or separated | 147 | (38.9) |
| Married or living with a partner | 231 | (61.1) |
| Ever had a non-HPV-related sexually transmitted diseasee | ||
| No | 301 | (79.8) |
| Yes | 76 | (20.2) |
| Ever had genital warts | ||
| No | 337 | (88.9) |
| Yes | 42 | (11.1) |
| Ever had an abnormal Pap test | ||
| No | 221 | (58.3) |
| Yes | 158 | (41.7) |
| Ever been pregnant | ||
| No | 149 | (39.3) |
| Yes | 230 | (60.7) |
| Currently using hormonal birth control methodsf | ||
| No | 252 | (65.5) |
| Yes | 127 | (33.5) |
| Currently have an immunosuppressive conditiong | ||
| No | 371 | (97.9) |
| Yes | 8 | ( 2.1) |
| Smoking statush | ||
| Never | 278 | (73.5) |
| Former | 81 | (21.4) |
| Current | 19 | ( 5.0) |
| Sexual activity with male partners within 6 months prior to enrollment | ||
| Not sexually active | 82 | (21.8) |
| Sexually active – no risk factorsi | 140 | (37.2) |
| at least 1 risk factori | 154 | (41.0) |
| Seropositive for at least one high-risk HPV typej | ||
| No | 121 | (31.9) |
| Yes | 258 | (68.1) |
| DNA-positive for at least one high-risk HPV typej | ||
| No | 322 | (85.0) |
| Yes | 57 | (15.0) |
Of the 409 mid-adult women recruited, 30 women were excluded due to previous HPV vaccination history.
Restricted to 367 women who reported ever having had sex with a male partner
Numbers may not add up to total due to missing data.
Includes individuals indicating any of the following: American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, other race, or multiple races
Includes chlamydia, gonorrhea, genital herpes, and HIV
Includes birth control pills, hormonal patches, vaginal rings, implanted contraception, injectable contraception, and hormonal intrauterine devices
Includes HIV positivity (n=1) or currently taking immunosuppressive medications (n=7)
Smoking was defined as smoking at least one cigarette a day for one month or longer; former smokers reported ever smoking but not currently smoking, and current smokers reported currently smoking.
Risk factors include ≥1 new sex partner, multiple partners (>1 sex partners), any casual partner, any partner with other concurrent partners, and any partner with a history of STDs.
HPV-16/18/31/33/35/39/45/51/52/56/58/59/68
Out of 4,927 possible women-types evaluated, 78 women-types in 6 women completing only the baseline visit and 78 additional prevalent women-types were excluded. Of the remaining 4,771 women-types, 3,960 (83%) were seronegative at enrollment, and 811 (17%) were seropositive. 74 incident type-specific hrHPV infections were detected during follow-up; the majority (65%) were observed when there was no serologic evidence of type-specific prior infection with the same type. The most commonly detected incident hrHPV types included HPV-51 (23% of incident hrHPV detections), HPV-31 (11%), HPV-16 (8%), HPV-39 (8%), HPV-45 (8%), and HPV-59 (8%).
The 6-month cumulative incidence of type-specific hrHPV detection was higher when there was serologic evidence of prior infection with the same type (2.9%; 95%CI:1.7–4.2) than when there was not (1.2%; 95%CI:0.9–1.7). This trend was observed both among women 30 to 39 years of age (6-month cumulative incidence with serologic evidence of prior infection=3.4%, 95%CI:2.0–5.7 vs. without serologic evidence of prior infection = 1.4, 95%CI:1.0–2.2) and 40 to 50 years of age (6-month cumulative incidence with serologic evidence of prior infection=2.2%, 95%CI:1.1–4.5 vs. without serologic evidence of prior infection=0.8, 95%CI:0.5–1.5).
In univariate analyses, among women with serologic evidence of prior type-specific infection, being obese (OR=2.8; 95%CI:0.96–7.9) was associated with an increased likelihood of incident hrHPV DNA detection relative to being normal or underweight (Table 2). A borderline statistically significant linear categorical dose-response relationship between increasing BMI categories and likelihood of hrHPV detection was observed (p=0.07 by trend test). Lifetime number of male sex partners was also positively associated with an increased likelihood of incident hrHPV detection, but the associations across categories were inconsistent.
Table 2.
Univariate analysis of factors associated with incident type-specific high-risk HPV detection among mid-adult women by enrollment type-specific HPV-serostatusa, Seattle, WA, 2011–2012
| Characteristic | HPV-seropositive women-types (N = 811b)
|
HPV-seronegative women-types (N = 3,960b)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
No. incident HPV detections |
nc | OR | (95% CI) | p-value |
No. incident HPV detections |
nc | OR | (95% CI) | p-value | |
| Time-fixed variables from enrollment survey | ||||||||||
|
| ||||||||||
| Age (years) | ||||||||||
| 30–39 | 17 | 451 | 1.0 | -- | -- | 35 | 2,407 | 1.0 | -- | -- |
| 40–50 | 9 | 360 | 0.7 | (0.3, 1.7) | 0.38 | 13 | 1,553 | 0.5 | (0.3, 1.1) | 0.09 |
| Body Mass Index (kg/m2)d | ||||||||||
| 17 – 25 | 9 | 403 | 1.0 | -- | -- | 27 | 2,193 | 1.0 | -- | -- |
| 25 – 29.9 | 8 | 246 | 1.5 | (0.6, 3.8) | 0.35 | 13 | 1,067 | 1.0 | (0.5, 2.2) | 0.97 |
| 30 – 51 | 9 | 162 | 2.8 | (0.96, 7.9) | 0.06 | 8 | 687 | 1.0 | (0.3, 3.2) | 0.95 |
| Marital status | ||||||||||
| Unmarried or separated | 14 | 369 | 1.0 | -- | -- | 24 | 1,455 | 1.0 | -- | -- |
| Married or living with a partner | 12 | 441 | 0.7 | (0.3, 1.5) | 0.36 | 23 | 2,493 | 0.5 | (0.3, 1.1) | 0.08 |
| Ever been pregnant | 16 | 526 | 0.9 | (0.4, 2.1) | 0.77 | 33 | 2,381 | 1.5 | (0.7, 2.9) | 0.27 |
| Ever had non-HPV-related STDse | 8 | 256 | 1.0 | (0.4, 2.3) | 0.98 | 16 | 710 | 2.2 | (1.2, 4.3) | 0.01 |
| Ever had genital warts | 2 | 97 | 0.7 | (0.2, 2.9) | 0.62 | 8 | 443 | 1.7 | (0.6, 4.3) | 0.30 |
| Current use of hormonal birth controlf | 11 | 256 | 1.5 | (0.7, 3.5) | 0.31 | 15 | 1,318 | 0.9 | (0.5, 1.8) | 0.76 |
| Current immunosuppressive conditiong | 1 | 20 | 1.6 | (0.2, 12.9) | 0.66 | 2 | 82 | 1.9 | (0.5, 7.3) | 0.36 |
| Smoking statush | ||||||||||
| Never | 22 | 599 | 1.0 | -- | -- | 37 | 2,934 | 1.0 | -- | -- |
| Former | 3 | 170 | 0.5 | (0.1, 1.8) | 0.29 | 9 | 841 | 0.9 | (0.4, 2.0) | 0.72 |
| Current | 1 | 42 | 0.7 | (0.1, 5.0) | 0.71 | 2 | 185 | 0.9 | (0.2, 3.8) | 0.92 |
| Lifetime number of sex partnersij | ||||||||||
| 0 – 4 | 3 | 170 | 1.0 | -- | -- | 8 | 1,496 | 1.0 | -- | -- |
| 5 – 11 | 14 | 239 | 3.3 | (0.9, 11.7) | 0.07 | 14 | 1,247 | 2.1 | (0.8, 5.8) | 0.13 |
| 12+ | 9 | 395 | 1.3 | (0.3, 4.9) | 0.71 | 25 | 1,172 | 4.0 | (1.8, 9.1) | <0.01 |
| Age at first sexual intercourse (years) | 26 | 781 | 1.1 | (1.0, 1.2) | 0.20 | 46 | 3,782 | 0.9 | (0.8, 0.98) | 0.02 |
|
| ||||||||||
| Time-dependent variables from enrollment/exit surveys and diaries (within six months before each sample)k | ||||||||||
|
| ||||||||||
| Average weekly number of sex actsl | 19 | 573 | 1.1 | (0.8, 1.6) | 0.63 | 41 | 2,822 | 1.2 | (0.9, 1.6) | 0.17 |
| Condom usel | ||||||||||
| Not always | 21 | 626 | 1.0 | -- | -- | 41 | 2,861 | 1.0 | -- | -- |
| Always | 1 | 35 | 0.9 | (0.1, 6.7) | 0.95 | 2 | 259 | 0.6 | (0.2, 2.4) | 0.47 |
| Composite variable for additive sexual behavior risk factorsm | ||||||||||
| Not sexually active with male partners | 4 | 162 | 1.0 | -- | -- | 5 | 939 | 1.0 | -- | -- |
| Sexually active – no risk factors | 5 | 268 | 0.7 | (0.2, 2.8) | 0.57 | 5 | 1,488 | 0.7 | (0.2, 2.3) | 0.53 |
| 1 risk factor | 8 | 262 | 1.2 | (0.3, 5.2) | 0.79 | 14 | 1,322 | 2.2 | (0.8, 6.0) | 0.14 |
| 2 risk factors | 4 | 153 | 1.0 | (0.2, 4.4) | 0.97 | 10 | 531 | 4.4 | (1.5, 12.5) | 0.01 |
| 3 risk factors | 5 | 164 | 1.2 | (0.3, 5.5) | 0.82 | 14 | 466 | 6.7 | (2.4, 18.6) | <0.01 |
At enrollment, each woman-type was classified as either positive or negative by HPV DNA and serology. Incident type-specific high-risk HPV detection was defined as the first DNA-positive sample for an HPV type not detected at enrollment; samples after the first type-specific positive detection were censored. Analyses were conducted separately by enrollment type-specific HPV serostatus.
249 women contributed a total of 811 women-types to the seropositive analysis, and 367 women contributed 3,960 women-types to the seronegative analysis.
Women-types could change categories throughout follow-up for time-dependent variables, therefore the number of women-types at risk (n) may exceed total N for time-dependent variables.
For HPV-seropositives, there was a borderline statistically significant linear categorical dose-response relationship between increasing BMI categories and likelihood of hrHPV detection (p=0.07 by trend test).
Includes chlamydia, gonorrhea, genital herpes, and HIV
Includes birth control pills, hormonal patches, vaginal rings, implanted contraception, injectable contraception, and hormonal intrauterine devices
Includes HIV positivity or currently taking immunosuppressive medications
Smoking was defined as smoking at least one cigarette a day for one month or longer; former smokers reported ever smoking but not currently smoking, and current smokers reported currently smoking.
Lifetime number of sex partners categorized based on approximate tertiles
For HPV-seronegatives, there was a statistically significant dose-response relationship between increasing lifetime numbers of partners and likelihood of hrHPV detection (p<0.01 by trend test).
Sexual behavior data from enrollment and exit surveys and diaries were combined into summary variables. For each monthly HPV assessment, partner data from enrollment and exit surveys were included if the dates of first or last sex with the partners fell within 6 months prior to the sample collection date. Daily sexual behavior data in diaries were included for days falling within the 6-month period before each sample. Survey and diary data were merged to form the combined time-dependent sexual behavior variables used in our analysis. Sexual behaviors were considered present if reported in either a survey or diary and absent if the subject reported sexual activity in the prior 6 months but did not report the particular sexual behavior.
Restricted to women who reported sexual activity within the past 6 months
Risk factors include ≥1 new sex partner, multiple partners (>1 sex partner), ≥1 casual partner, ≥1 partner with other concurrent partners, and ≥1 partner with a history of STDs.
In the absence of serologic evidence of prior infection with the same type, older age (OR for age 40–50 years vs. age 30–39 years=0.5; 95%CI:0.3–1.1), being married or living with a partner (OR vs. being unmarried or separated=0.5; 95%CI:0.3–1.1), and older age at first sexual intercourse (OR for each one-year increase in age=0.9; 95%CI: 0.8–0.98) were each negatively associated with incident hrHPV detection (Table 2). Ever having had a non-HPV-related STD (OR=2.2; 95%CI:1.2–4.3) and reporting a lifetime number of male partners ≥12 (OR vs. 0–4 lifetime partners=4.0; 95%CI:1.8–9.1) were each associated with an increased likelihood of incident hrHPV detection (p<0.01 by trend test for a linear categorical dose-response relationship). Having 2 (OR=4.4; 95% CI: 1.5-12.5) or 3 (OR = 6.7; 95% CI: 2.4-18.6) sexual behavior risk factors within the prior 6 months was associated with significantly increased risk of incident hrHPV detection relative to not being sexually active.
Separate multivariate models were constructed for the seropositive and seronegative groups. In the seropositive group, obesity remained borderline statistically significantly associated with hrHPV detection after adjusting for lifetime number of partners (Table 3). The association between lifetime number of sex partners was slightly attenuated after adjusting for BMI. In the seronegative group, recent sexual behavior remained independently associated with an increased likelihood of hrHPV detection (Table 4). A linear categorical dose-response relationship was observed between increasing number of sexual behavior risk factors and increased likelihood of hrHPV detection (p<0.01 by trend test). Other individual measures of cumulative sexual behavior were attenuated in the multivariate model.
Table 3.
Multivariate analysis of factors associated with incident type-specific high-risk HPV infections among type-specific HPV-seropositive mid-adult women, Seattle, WA, 2011–2012a
| Characteristic | HPV-seropositive (N = 804 women typesb) | ||
|---|---|---|---|
|
| |||
| aORc | (95% CI) | p-value | |
| Time-fixed variables from enrollment survey | |||
|
| |||
| Body Mass Index (kg/m2)d | |||
| 17 – 25 | 1.0 | -- | -- |
| 25 – 29.9 | 1.5 | (0.6, 3.7) | 0.35 |
| 30 – 51 | 2.7 | (0.99, 7.1) | 0.05 |
| Lifetime number of sex partnerse | |||
| 0 – 4 | 1.0 | -- | -- |
| 5 – 11 | 3.3 | (0.9, 11.3) | 0.06 |
| 12+ | 1.3 | (0.3, 5.0) | 0.72 |
Incident type-specific high-risk HPV detection was defined as the first DNA-positive sample for a HPV type not detected at enrollment; samples after each type-specific first positive detection were considered censored.
246 women contributed 804 women-types to the seropositive analysis.
Adjusted odds ratio adjusted for all other variables in the table
There was a borderline statistically significant linear categorical dose-response relationship between increasing BMI categories and likelihood of hrHPV detection (p=0.06 by trend test).
Lifetime number of sex partners categorized based on approximate tertiles
Table 4.
Multivariate analysis of factors associated with incident type-specific high-risk HPV infection among type-specific HPV-seronegative mid-adult women, Seattle, WA, 2011–2012a
| Characteristic | HPV-seronegative (N = 3,748 women typesb) | ||
|---|---|---|---|
|
| |||
| aORc | (95% CI) | p-value | |
| Time-fixed variables from enrollment survey | |||
|
| |||
| Age (years) | |||
| 30–39 | 1.0 | -- | -- |
| 40–50 | 0.7 | (0.3, 1.3) | 0.25 |
| Marital status | |||
| Unmarried or separated | 1.0 | -- | -- |
| Married or living with a partner | 0.9 | (0.4, 2.5) | 0.89 |
| Ever had non-HPV-related STDsd | 1.8 | (0.9, 3.4) | 0.07 |
| Lifetime number of sex partnersef | |||
| 0 – 4 | 1.0 | -- | -- |
| 5 – 11 | 1.5 | (0.5, 4.9) | 0.51 |
| 12+ | 2.3 | (0.8, 7.1) | 0.14 |
| Age at first sexual intercourse (years) | 1.0 | (0.9, 1.1) | 0.98 |
|
| |||
| Time-dependent variables from enrollment/exit surveys and diaries (within six months before each sample)g | |||
|
| |||
| Composite variable for additive sexual behavior risk factorshi | |||
| Not sexually active with male partners | 1.0 | -- | -- |
| Sexually active – no risk factors | 1.3 | (0.2, 7.4) | 0.77 |
| 1 risk factor | 4.0 | (0.8, 19.5) | 0.09 |
| 2 risk factors | 5.8 | (1.2, 28.1) | 0.03 |
| ≥ 3 risk factors | 9.8 | (2.4, 40.6) | <0.01 |
Incident type-specific high-risk HPV detection was defined as the first DNA-positive sample for a HPV type not detected at enrollment; samples after each type-specific first positive detection were considered censored.
348 women contributed 3,748 women-types to the seronegative analysis.
Adjusted odds ratio adjusted for all other variables in the table
Includes chlamydia, gonorrhea, genital herpes, and HIV
Lifetime number of sex partners categorized based on approximate tertiles
No statistically significant dose-response relationship was observed between increasing lifetime numbers of sex partners and likelihood of hrHPV (p=0.12 by trend test).
Sexual behavior data from enrollment and exit surveys and diaries were combined into summary variables. For each monthly HPV assessment, partner data from enrollment and exit surveys were included if the dates of first or last sex with the partners fell within 6 months prior to the sample collection date. Daily sexual behavior data in diaries were included for days falling within the 6-month period before each sample. Survey and diary data were merged to form the combined time-dependent sexual behavior variables used in our analysis. Sexual behaviors were considered present if reported in either a survey or diary and absent if the subject reported sexual activity in the prior 6 months but did not report the particular sexual behavior.
Risk factors include ≥1 new sex partner, multiple partners (>1 sex partners), ≥1 casual partner, ≥1 partner with other concurrent partners, and ≥1 partner with a history of STDs.
A linear categorical dose-response relationship was observed between increasing number of sexual behavior risk factors and increased likelihood of hrHPV detection (p<0.001 by trend test).
While the risk of incident detection was 6.9-fold higher when there was serologic evidence of prior infection than when there was no serologic evidence of prior infection and no evidence of new sexual exposures (Table 5), only 26 of 74 incident hrHPV detections were among women seropositive for the same type at enrollment. Therefore, only 30% of incident detections in our cohort were attributable to prior infection with the same type based on positive serology. Forty percent of incident detections were attributable to recent sexual activity with ≥1 risk factor and negative serology for the same type. In our post-hoc analyses, we observed that the proportion of incident detections attributed to prior infection with the same type was similar for 30 to 39 and 40 to 50 year old women (29% versus 32%, respectively). However, the proportion attributable to recent sexual activity with ≥1 risk factor and negative serology for the same type was higher in the younger versus older age group (48% versus 21%, respectively).
Table 5.
Attributable risk of incident high-risk HPV DNA detection due to type-specific HPV serostatus and cumulative and recent sexual behavior among mid-adult women, Seattle, WA, 2011–2012
| Characteristic | Na | nb | aORc | (95% CI) | %ARd | %PARe |
|---|---|---|---|---|---|---|
| Overall | ||||||
|
| ||||||
| Seronegative, not sexually active OR sexually active and no risk factorsf | 10,727 | 10 | 1.0 | -- | ||
| Seronegative, sexually active and ≥ 1 risk factorf | 9,412 | 38 | 4.5 | (2.1, 9.6) | 77.6 | 39.8 |
| Seropositive | 4,093 | 26 | 6.9 | (3.0, 15.9) | 85.4 | 30.0 |
|
| ||||||
| Ages 30–39 | ||||||
|
| ||||||
| Seronegative, not sexually active OR sexually active and no risk factorsf | 6,110 | 5 | 1.0 | -- | ||
| Seronegative, sexually active and ≥ 1 risk factorf | 5,924 | 30 | 5.9 | (2.1–16.6) | 83.0 | 47.9 |
| Seropositive | 2,236 | 17 | 8.3 | (2.8–24.4) | 87.9 | 28.7 |
|
| ||||||
| Ages 40–50 | ||||||
|
| ||||||
| Seronegative, not sexually active OR sexually active and no risk factorsf | 4,617 | 5 | 1.0 | -- | ||
| Seronegative, sexually active and ≥ 1 risk factorf | 3,488 | 8 | 2.3 | (0.7–8.3) | 57.4 | 20.9 |
| Seropositive | 1,857 | 9 | 4.6 | (1.2–17.9) | 78.3 | 32.0 |
Indicates the total number of women-types * number of samples included in each stratum
Indicates the number of women-types * number of samples with incident HPV detected
Adjusted for age, marital status, ever had non-HPV-related STDs, and lifetime number of sex partners in tertiles for overall results; not adjusted for age in age-stratified results.
Attributable risk percent calculated as [(OR – 1) / OR] * 100
Population attributable risk percent calculated as {pd * [(OR – 1) / OR]} * 100, where pd is the proportion of cases exposed to the risk factor
Risk factors include ≥1 new sex partner, multiple partners (>1 sex partners), ≥1 casual partner, ≥1 partner with other concurrent partners, and ≥1 partner with a history of STDs.
DISCUSSION
In our study of mid-adult women, cumulative incidence of detecting hrHPV DNA was higher when there was serologic evidence of prior infection with the same type compared to when there was not. Similarly, in 35–45 year old women enrolled in the placebo arm of the quadrivalent HPV vaccine trial, HPV incidence (types 6, 11, 16, or 18) was higher among seropositive women (2.8 per 100 person-years) than among seronegative women (2.1 per 100 person-years).1 In our study, the observed cumulative incidence of hrHPV was higher in 30 to 39 versus 40 to 50 year old women, but cumulative incidence remained higher in the seropositive versus seronegative groups within both age groups. In contrast, in the placebo arm of the vaccine trial, incidence was higher in seronegative vs. seropositive women between the ages of 24–34 years (5.7 vs. 1.0 per 100 person-years).1 Other studies in cohorts of younger women (mostly ≤25 years of age) also showed protective effects of HPV antibodies against type-specific re-infection,26–29 suggesting that the protective effect of antibodies against type-specific re-infection may wane in older women.5, 30 However, it is important to note that apparent lack of protection from re-infection by naturally acquired HPV antibodies may actually reflect reactivation of prior infection; therefore, firm conclusions about the development of protective HPV type-specific immunity is very difficult to ascertain from observational studies. In fact, in our study, we observed that incident type-specific hrHPV DNA detection was not associated with recent sexual behavior in women seropositive for the same type, suggesting that these detections likely represented reactivation of a previous infection or intermittent shedding of virus from a persistent infection, rather than re-infection with the same type. Among women without serologic evidence of prior HPV infection, we did observe a positive association between recent sexual behaviors and incident hrHPV DNA detection. Trottier et al31 reported conflicting findings in a cohort of 18–59 year-old Brazilian women, whereby reports of new sex partners were associated with incident HPV-16 detection among women with higher levels of HPV-16 antibodies at baseline, but not among those with low levels. Differences in serologic methods, population characteristics, HPV types evaluated, or definitions of recent sexual behaviors could explain the inconsistent results between studies. In our study, in addition to recent new partners, we considered other risk factors (i.e., multiple partners, partners with other concurrent partners, and partners with a history of STDs) in a composite variable to assess the overall effect of recent sexual behavior on incident hrHPV detection. Using this composite variable, we estimated that the majority of new detections in seronegative women likely did represent new acquisitions. Rositch et al32 observed that among 35–60 year-old women, newly detected HPV was attributable to both past and recent sexual exposures. Similarly, in our univariate analyses, both cumulative (e.g. lifetime number of male sex partners) and recent sexual risk behaviors were associated with incident hrHPV in seronegative women. After adjusting for recent sexual behaviors, however, cumulative sexual behavior risk factors were attenuated and no longer statistically significantly associated with incident hrHPV, suggesting that recent sexual behaviors were a stronger risk factor than past sexual behaviors among seronegative women.
A strong relative association does not necessarily translate into an increase in the number of affected cases if the frequency of the risk factor in the population is low. For example, while Rositch et al32 reported a 5.6-fold increase in incident HPV detection associated with new sex partners, the prevalence of new partners in their mid-adult cohort was low (10%), and thus only 13% of the detected infections in their cohort were attributable to new partners. In our cohort, new partners and other recent high-risk sexual behaviors were more common, and we estimated that 40% of all incident hrHPV DNA detections were attributable to recent sexual activity with at least one high-risk behavior and negative serology for the same hrHPV type. Our post-hoc analysis showed differences by age, with both the attributable risk and population attributable risk for recent high-risk behavior (and negative serology) higher in 30 to 39 versus 40 to 50 year old women. While the proportions of incident hrHPV DNA detections attributable to prior infection (based on positive serology) were similar between the two age groups, a higher proportion of all incident detections were detected in the absence of either positive serology or recent high-risk sexual behavior in the older age group (5 out of 22; 23%) than in the younger age group (5 out of 52; 10%). A subset of these incident detections in the absence of either positive serology or recent high-risk sexual behavior were likely due to either reactivation of prior infection or intermittent detection of persistent infection, as not all hrHPV infections yield levels of antibodies that can be detected by current serological assays and antibodies may wane over time. Similarly, even among seronegative women with recent high-risk sexual behaviors, some incident hrHPV DNA detections likely represented re-detection of prior infection rather than new acquisition. Recent sexual behavior may be reflective of past behavior (e.g. women who choose risky partners in mid-adulthood may have also chosen risky partners as young adults), and challenges exist in disentangling the effects of recent and past sexual behaviors on apparent new infection.33
Among women with serologic evidence of prior infection, we observed that obesity was borderline statistically significantly associated with incident hrHPV. Obesity has been shown to be associated with increased risk for other viral pathogens such as herpes simplex virus-1 and -2, adenoviruses, enteroviruses, and influenza.34, 35 Increased susceptibility to viral pathogens may be due to changes in adipokine production, especially leptin, which may be involved in activating inflammation.35, 36 Baker et al37 detected increased levels of adipokines among older women with persistent HPV infection, but Liu et al38 did not find an association between obesity and incident HPV infection among a cohort of perimenopausal women. Future studies are needed to further explore the relationship between obesity, immune response, and HPV infection. Among seropositive women, we also observed that a higher lifetime number of sex partners was borderline statistically significantly associated with incident hrHPV detection (although women with 5–11 partners were actually at higher risk than women with ≥12 partners). A possible explanation for this finding is that positive serology may be more likely to reflect cleared versus intermittent persistent infections in women with low versus high lifetime numbers of partners (if women with higher lifetime numbers of partners are more likely to harbor more recent infections).
Several limitations should be noted. First, follow-up was only 6 months, and therefore, results may not generalize to new hrHPV DNA detected after a longer period of observable negativity. Second, the accuracy of serologic assays as measures of prior HPV infection is limited. Compared to other common viral infections, hrHPV infection tends to generate relatively low or even undetectable levels of antibodies, thereby resulting in a higher percentage of hrHPV-infected individuals wrongly classified as negative for prior infection. For example, studies show that only about 60% of women with cervical HPV infection develop detectable type-specific antibodies,9, 11, 12 and that antibodies may wane and become undetectable with time.9, 11 In addition to falsely negative serological results, falsely positive serological results for evidence of prior genital hrHPV infection may occur when the infection is only present at a non-genital site(s).39
In conclusion, our results suggest that among mid-adult women with serologic evidence of prior infection, new hrHPV DNA detection is more likely due to reactivation or intermittent detection of persisting infection than to re-infection from new partners. This observation is consistent with findings from other longitudinal studies suggesting that it is uncommon for a woman to become infected with more than one genetic variant of a specific HPV type.40 In comparison, among mid-adult women without serologic evidence of prior infection, incident hrHPV DNA detection is most likely due to new acquisition or to intermittent detection of persisting infection. Among those without serologic evidence of prior infection, the likelihood of new acquisition versus intermittent detection of persisting infection may be somewhat age dependent, as the proportion of infections attributable to recent sexual risk behavior decreased with increasing age. Future studies are needed to generate data pertinent to determining age-specific HPV vaccine strategies and cost-effectiveness of prophylactic vaccination in mid-adult women.6 Better estimates of the absolute risk of new acquisition are needed to effectively estimate the benefit of vaccination in this age group. Lastly, our study demonstrates that a substantial proportion of test-positive hrHPV infections in 30–50 year-old women are likely due to reactivation or intermittent detection of persistent infection rather than to new acquisition. This information may be reassuring for women in monogamous relationships who test hrHPV positive during routine cervical cancer screening.
Novelty and impact.
By combining HPV serology with HPV DNA genotyping and sexual behavior data, we attributed incident HPV detection in mid-adult women to either probable new acquisition or re-detection of prior infection. Recent sexual behavior was associated with incident HPV only without serologic evidence of prior infection. Results indicating that a substantial portion of incident HPV was likely due to prior infection may reassure women in monogamous relationships who test HPV positive during routine cervical cancer screening.
Acknowledgments
This work was financially supported by a National Institutes of Health P01 grant (AI083224-01A1) to LAK and RLW. We thank Sandra O’Reilly and Kathleen O’Connell for their contributions as research coordinators, and John Lin for his support in study management.
Abbreviations used
- HPV
human papillomavirus
- hr
high-risk
- UW
University of Washington
- STM
specimen transport medium
- MFI
median fluorescent intensity
- GEE
generalized estimating equations
- BMI
body mass index
- STDs
sexual transmitted diseases
- AR%
attributable risk percent
- PAR%
population attributable risk percent
- SD
standard deviation
- IQR
interquartile range
- CI
confidence interval
- OR
odds ratio
Footnotes
CONFLICT OF INTEREST STATEMENT
DAG is a member of Merck’s Global Advisory Board for HPV.
References
- 1.Velicer C, Zhu X, Vuocolo S, Liaw KL, Saah A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. 2009;36:696–703. doi: 10.1097/OLQ.0b013e3181ad25ff. [DOI] [PubMed] [Google Scholar]
- 2.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):S42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–6. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 4.Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–63. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gravitt PE. Evidence and impact of human papillomavirus latency. The open virology journal. 2012;6:198–203. doi: 10.2174/1874357901206010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant LA, Dunne EF, Chesson H, Markowitz LE. Considerations for human papillomavirus (HPV) vaccination of mid-adult women in the United States. Vaccine. 2011;29:2365–70. doi: 10.1016/j.vaccine.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Strickler HD, Schiffman MH, Shah KV, Rabkin CS, Schiller JT, Wacholder S, Clayman B, Viscidi RP. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev. 1998;7:305–13. doi: 10.1097/00008469-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Mollers M, Vossen JM, Scherpenisse M, van der Klis FR, Meijer CJ, de Melker HE. Review: current knowledge on the role of HPV antibodies after natural infection and vaccination: implications for monitoring an HPV vaccination programme. J Med Virol. 2013;85:1379–85. doi: 10.1002/jmv.23616. [DOI] [PubMed] [Google Scholar]
- 9.Carter JJ, Koutsky LA, Wipf GC, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–36. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Schiffman M, Herrero R, Carreon J, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Clayman B, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2004;91:1269–74. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 12.Ho GY, Studentsov YY, Bierman R, et al. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004;13:110–6. doi: 10.1158/1055-9965.epi-03-0191. [DOI] [PubMed] [Google Scholar]
- 13.Fu TC, Xi LF, Hulbert A, et al. Short-term natural history of high-risk human papillomavirus infection in mid-adult women sampled monthly. Int J Cancer. 2015;137:2432–42. doi: 10.1002/ijc.29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Holmes KK, Koutsky LA. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 15.Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, Lemos BD, Lee S, Warcola AH, Iyer JG, Nghiem P, Galloway DA. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:1510–22. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 17.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. Journal of Immunological Methods. 2001;253:153–62. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 19.Madeleine MM, Carter JJ, Johnson LG, Wipf GC, Davis C, Berg D, Nelson K, Daling JR, Schwartz SM, Galloway DA. Risk of squamous cell skin cancer after organ transplant associated with antibodies to cutaneous papillomaviruses, polyomaviruses, and TMC6/8 (EVER1/2) variants. Cancer Med. 2014;3:1440–7. doi: 10.1002/cam4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, Becker JC, Madeleine MM, Nghiem P, Galloway DA. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–97. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, Galloway DA, Koutsky LA. Serum Antibody Response Following Genital α9 Human Papillomavirus Infection in Young Men. Journal of Infectious Diseases. 2011;204:209–16. doi: 10.1093/infdis/jir242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson M, Wilkinson DE, Zhou T. WHO meeting on the standardization of HPV assays and the role of the WHO HPV Laboratory Network in supporting vaccine introduction held on 24–25 January 2008, Geneva, Switzerland. Vaccine. 2009;27:337–47. doi: 10.1016/j.vaccine.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 23.Sadate-Ngatchou P, Carter JJ, Hawes SE, Feng Q, Lasof T, Stern JE, Fu TC, Galloway DA, Koutsky LA, Winer RL. Determinants of High-Risk Human Papillomavirus Seroprevalence and DNA Prevalence in Mid-Adult Women. Sex Transm Dis. 2016;43:192–8. doi: 10.1097/OLQ.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ Group IAfRoCMCCS. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 25.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho GY, Studentsov Y, Hall CB, Bierman R, Beardsley L, Lempa M, Burk RD. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186:737–42. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- 27.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn LJ, Sherman ME, Xhenseval V, Herrero R, Hildesheim A. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–62. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson L, Pawlita M, Castle PE, Waterboer T, Sahasrabuddhe V, Gravitt PE, Schiffman M, Wentzensen N. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210:448–55. doi: 10.1093/infdis/jiu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik ZA, Hailpern SM, Burk RD. Persistent antibodies to HPV virus-like particles following natural infection are protective against subsequent cervicovaginal infection with related and unrelated HPV. Viral Immunol. 2009;22:445–9. doi: 10.1089/vim.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012;72:6183–90. doi: 10.1158/0008-5472.CAN-12-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winer RL, Hughes JP, Feng Q, Xi LF, Lee SK, O’Reilly SF, Kiviat NB, Koutsky LA. Prevalence and risk factors for oncogenic human papillomavirus infections in high-risk mid-adult women. Sex Transm Dis. 2012;39:848–56. doi: 10.1097/OLQ.0b013e3182641f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring) 2007;15:245–52. doi: 10.1038/oby.2007.541. [DOI] [PubMed] [Google Scholar]
- 35.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–46. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 36.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 20. [DOI] [PubMed] [Google Scholar]
- 37.Baker R, Dauner JG, Rodriguez AC, Williams MC, Kemp TJ, Hildesheim A, Pinto LA. Increased plasma levels of adipokines and inflammatory markers in older women with persistent HPV infection. Cytokine. 2011;53:282–5. doi: 10.1016/j.cyto.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu SH, Rositch AF, Viscidi RP, Silver MI, Burke AE, Gravitt PE. Obesity and human papillomavirus infection in perimenopausal women. J Infect Dis. 2013;208:1071–80. doi: 10.1093/infdis/jit297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter JJ, Madeleine MM, Shera K, Schwartz SM, Cushing-Haugen KL, Wipf GC, Porter P, Daling JR, McDougall JK, Galloway DA. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61:1934–40. [PubMed] [Google Scholar]
- 40.Xi LF, Koutsky LA, Castle PE, Edelstein ZR, Hulbert A, Schiffman M, Kiviat NB. Human papillomavirus type 16 variants in paired enrollment and follow-up cervical samples: implications for a proper understanding of type-specific persistent infections. J Infect Dis. 2010;202:1667–70. doi: 10.1086/657083. [DOI] [PMC free article] [PubMed] [Google Scholar]