Summary
The sarcomeric cytoskeleton is a network of modular proteins that integrate mechanical and signaling roles. Obscurin, or its homolog obscurin-like-1, bridges the giant ruler titin and the myosin crosslinker myomesin at the M-band. Yet, the molecular mechanisms underlying the physical obscurin(-like-1):myomesin connection, important for mechanical integrity of the M-band, remained elusive. Here, using a combination of structural, cellular, and single-molecule force spectroscopy techniques, we decode the architectural and functional determinants defining the obscurin(-like-1):myomesin complex. The crystal structure reveals a trans-complementation mechanism whereby an incomplete immunoglobulin-like domain assimilates an isoform-specific myomesin interdomain sequence. Crucially, this unconventional architecture provides mechanical stability up to forces of ∼135 pN. A cellular competition assay in neonatal rat cardiomyocytes validates the complex and provides the rationale for the isoform specificity of the interaction. Altogether, our results reveal a novel binding strategy in sarcomere assembly, which might have implications on muscle nanomechanics and overall M-band organization.
Keywords: muscle, M-band, myomesin, obscurin, obscurin-like-1, protein complexes, X-ray crystallography, SAXS, atomic force microscopy, immunoglobulin domain
Graphical Abstract
Highlights
-
•
The structure of the human obscurin-like-1:myomesin complex has been determined
-
•
A myomesin sequence complements an immunoglobulin fold of obscurin-like-1
-
•
This binding mechanism provides mechanical stability up to forces of ∼135 pN
-
•
Possible implications on muscle nanomechanics and M-band organization are discussed
Pernigo et al. analyze the myomesin-dependent integration of obscurin/obscurin-like-1 at the muscle M-band. They discover a mechanism of structural trans-complementation whereby an incomplete immunoglobulin-like domain of obscurin-like-1 assimilates an isoform-specific myomesin interdomain sequence providing mechanical stability.
Introduction
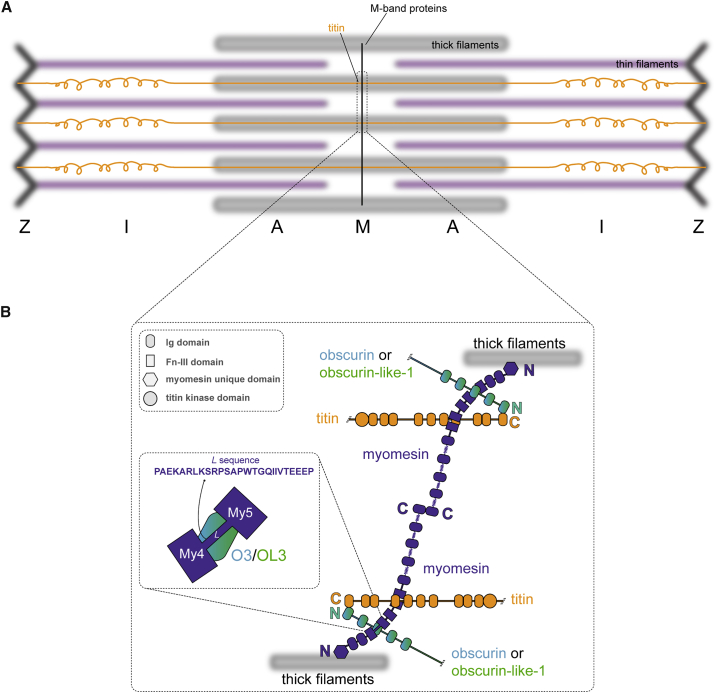
Sarcomeres, the basic contractile units of striated muscles, specialize in force generation through cyclic interactions of myosin and actin filaments. This fundamental activity requires the correct positioning of hundreds of proteins assembled in an overall functional architecture that need to respond to mechanical force in a cooperative, orchestrated way, as well as providing key integration of regulatory signals. The Z-disc and M-band sarcomeric regions (Figure 1A), although not directly involved in the actomyosin complex, are hubs where multiple structural and regulatory proteins are linked (Gautel and Djinovic-Carugo, 2016). In particular, the central M-band, where titin filaments entering from opposite half-sarcomeres overlap, has been proposed as a structural safeguard of sarcomere integrity during force-generation cycles (Agarkova et al., 2003).
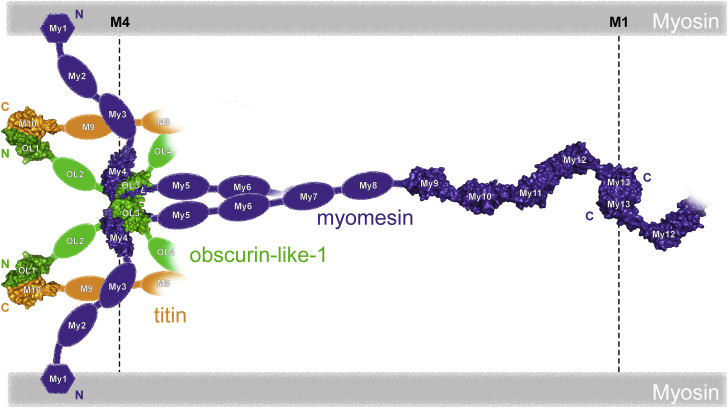
Figure 1.
Schematic of the M-Band Network
(A) Principal sarcomere regions are marked by the letters Z, I, A, and M.
(B) Modular myomesin, titin, and obscurin/obscurin-like-1 proteins form an intricate M-band network with C-terminal myomesin dimers crosslinking myosin filaments. The inset highlights the interaction between myomesin and obscurin/obscurin-like-1, which has been mapped to linker sequence (L) located between the myomesin fibronectin (Fn-III) domains My4 and My5 and the third immunoglobulin (Ig) domain of obscurin/obscurin-like-1 (O3/OL3, respectively) (Fukuzawa et al., 2008).
Myomesin is a 185 kDa modular protein that localizes exclusively at the M-band, where anti-parallel dimers cross link myosin filaments (Figure 1B). It is expressed in all muscle types and its knockdown by siRNA results in a general failure in M-band assembly and the formation of disordered sarcomeres (Fukuzawa et al., 2008). Long interdomain α-helices at the protein's C-terminus have been suggested to act as strain absorbers enabling myomesin to buffer mechanical forces between molecules during muscle work (Pinotsis et al., 2012, Xiao and Grater, 2014). In addition to a mechanical role, myomesin is also needed for the integration of obscurin and its smaller obscurin-like-1 homolog at the M-band (Fukuzawa et al., 2008). Together with titin's C-terminus, a hotspot for disease-related mutations (Carmignac et al., 2007, Pollazzon et al., 2009), myomesin recruits obscurin and obscurin-like-1 N-termini at the myofibril periphery and core, respectively, establishing a ternary complex (Figure 1B).
Obscurin and obscurin-like-1 share a common immunoglobulin (Ig)-rich modular structure, which, in the case of obscurin, is more extended, featuring additional signaling and protein-binding domains absent in obscurin-like-1 (Fukuzawa et al., 2008). The presence of a non-modular C-terminus able to interact with small ankyrin-1 isoform 5 and ankyrin-2 led to the suggestion that obscurin plays a role in establishing the sarcomere-sarcoplasmic reticulum connection (Bagnato et al., 2003, Kontrogianni-Konstantopoulos et al., 2003). The pathophysiological roles of these proteins are only beginning to emerge. Ablation of obscurin in mice results in changes in longitudinal sarcoplasmic reticulum architecture with alterations in several SR-associated proteins (Lange et al., 2012) as well as marked sarcolemma fragility and reduced muscle exercise tolerance (Randazzo et al., 2013), while its depletion in zebrafish leads to disturbances in the extracellular matrix organization during skeletal muscle development (Raeker and Russell, 2011). The founding member of the obscurin family of proteins is UNC-89 in Caenorhabditis elegans (Benian et al., 1996). unc-89 loss-of-function mutant worms display reduced locomotion, disorganized myofibrils, and lack M lines (Small et al., 2004, Waterston et al., 1980). unc-89 mutants show disorganization of myosin thick filaments by immunostaining (Qadota et al., 2008, Wilson et al., 2012). Drosophila expresses a protein more similar to nematode UNC-89 than to vertebrate obscurin. In Drosophila, RNAi experiments indicate that obscurin is needed for the formation of normal symmetrical sarcomeres (Katzemich et al., 2015). However, fundamental differences exist in the domain patterns and likely functions of the signaling domains in vertebrate, insect, and nematode obscurins/unc-89 members. All obscurin/UNC-89 members contain a constitutively expressed Rho-type GDP/GTP exchange factor domain (GEF) with a preceding Src-homology-3 (SH3) domain, which in insect and nematode obscurin/UNC-89 are situated at the N-terminal end of the proteins, while in vertebrate obscurin, the GEF domain is at the C-terminus. In addition, obscurin/UNC-89 isoforms can contain up to two serine/threonine kinase domains (Katzemich et al., 2012, Spooner et al., 2012). In insect and nematode obscurin, these are catalytically inactive pseudokinases that form scaffolds for the interactions with regulators of sarcomere assembly and/or maintenance (Katzemich et al., 2012), while the two differentially spliced kinases in vertebrate obscurin contain all canonical residues required for catalysis (Fukuzawa et al., 2005) and were reported to be catalytically active in vitro (Hu and Kontrogianni-Konstantopoulos, 2013). Analyzing the molecular interactions and signaling functions therefore requires dedicated approaches for each of these presumptive homologs. From a pathological viewpoint, obscurin polymorphisms has been linked to hypertrophic cardiomyopathy (Arimura et al., 2007) and dilated cardiomyopathy (Marston et al., 2015), while mutations in obscurin-like-1 have been linked to the rare hereditary growth retardation 3-M syndrome (Huber et al., 2009) with a role in the maintenance of cullin-7 levels (Hanson et al., 2009). Understanding obscurin/UNC-89 functions thus also bears relevance to understanding the impact of pathogenic variants in human.
To advance knowledge on M-band organization and function, we have previously established the molecular basis for titin:obscurin-like-1 (Pernigo et al., 2010) and titin:obscurin (Pernigo et al., 2015) connection at the M-band. Obscurin and obscurin-like-1 use their homologous N-terminal immunoglobulin-like (Ig) domains (O1 and OL1, respectively) to bind titin's most C-terminal Ig domain (M10) in a mutually exclusive manner and in a unique chevron-shaped anti-parallel Ig-Ig architecture (Pernigo et al., 2010, Pernigo et al., 2015, Sauer et al., 2010). Mechanically, the M-band titin:obscurin(-like-1) junction is labile, as in single-molecule force spectroscopy experiments both M10:O1 and M10:OL1 complexes yield at forces of around 30 pN (Pernigo et al., 2010). An obvious missing piece in the M-band structural puzzle is the molecular architecture of the obscurin(-like-1):myomesin complex, a key elusive element to understand the global geometry and mechanical stability defining the M-band. Using a multidisciplinary approach encompassing structural techniques, in vivo cellular competition assays, and single-molecule force spectroscopy experiments, we investigated here the myomesin-dependent mechanism of obscurin(-like-1) integration at the M-band.
Results
Human Obscurin/Obscurin-like-1:Myomesin Complex for Structural Analysis
Large muscle proteins are typically modular, featuring several Ig and fibronectin-type-III (Fn-III) domains interspersed by linkers of variable length and structural order. Yeast two-hybrid and biochemical analyses have mapped the obscurin/obscurin-like-1:myomesin interaction to the linker region (L) located between the fourth and fifth Fn-III domains of myomesin (My4 and My5, respectively) and the third Ig domain of either obscurin or obscurin-like-1 (O3 and OL3, respectively) (Figure 1B, inset) (Fukuzawa et al., 2008). O3 and OL3 are highly homologous, sharing 47.2% sequence identity. To produce protein complexes for structural analysis, we initially attempted the expression of isolated domains in Escherichia coli, but failed to obtain soluble O3 or OL3. We therefore decided to pursue a co-expression approach and cloned either O3 or OL3 C-terminal to a GST tag in the first expression cassette of a bicistronic vector, where the myomesin region encompassing the fourth and fifth Fn-III domains (My4LMy5) was cloned in the second expression cassette. This strategy readily produced soluble protein for both constructs and size-exclusion chromatography (SEC) analysis of GST-cleaved complexes is consistent with the formation of obscurin(-like-1):myomesin heterodimers with a 1:1 stoichiometry (Figure S1). Using a matrix microseeding (MMS) approach (D'Arcy et al., 2014), we successfully crystallized the OL3:My4LMy5 complex and solved its structure at the 3.1 Å resolution. X-ray data collection and refinement statistics are given in Table 1. The final model is characterized by excellent statistics and R/Rfree (%) values of 21.6/25.9. While the myomesin My4 domain and its C-terminal linker L as well as obscurin-like-1 OL3 are well defined in the structure, the entire myomesin My5 domain is invisible in electron density maps. Proteolysis during crystallization does not appear to be the reason for this, as SDS-PAGE analysis of dissolved crystals shows both My4LMy5 and OL3 components at the expected molecular weight (Figure S2). Thus, we conclude that the lack of electron density for My5 is due to its positional disorder in the crystal. As My5 is not visible in the structure, we hereafter refer to the crystallographic complex as to OL3:My4L.
Table 1.
X-Ray Data Collection and Refinement Statistics
| Data Collection | |||
| Dataset | OL3:My4L | My4LH (1) | My4LH (2) |
| Beamline | I04 (DLS) | I04 (DLS) | I04-1 (DLS) |
| Wavelength (Å) | 0.9778 | 0.97949 | 0.92819 |
| Resolution rangea (Å) | 67.19–3.10 (3.18–3.10) | 84.36–2.05 (2.11–2.05) | 39.21–2.80 (2.87–2.80) |
| Space group | C2 | P65 | P21 |
| Cell dimensions | |||
| (a, b, c) (Å) | 91.18, 134.38, 68.39 | 97.41, 97.41, 106.15 | 43.53, 156.84, 48.26 |
| (α, β, γ) (°) | 90, 92.50, 90 | 90, 90, 120 | 90, 94.38, 90 |
| Unique reflectionsa | 14,811 (1,064) | 35,371 (2,587) | 15,138 (1,048) |
| Overall redundancya | 4.5 (4.5) | 20.0 (20.0) | 3.1 (3.1) |
| Completenessa (%) | 99.1 (98.2) | 99.9 (98.7) | 95.3 (89.8) |
| Rmergea (%) | 9.8 (69.8) | 13.4 (242.1) | 9.0 (31.4) |
| Rp.i.m. (I)a (%) | 6.2 (44.3) | 3.6 (56.7) | 8.1 (24.5) |
| 〈I/σ(I)〉a | 12.7 (1.8) | 13.4 (1.6) | 10.8 (3.1) |
| Refinement | |||
| PDB code | 5FM5 | 5FM8 | 5FM4 |
| Rfactor (%)/Rfree (%) | 21.6/25.9 | 19.2/21.7 | 26.1/28.7 |
| Twin/twin fraction | no | k,h,−l/0.67 | no |
| No. of non-H atoms | |||
| Protein | 3,482 | 3,142 | 4,726 |
| Water | 84 | 140 | 37 |
| Average B value (Å2) | 85.5 | 50.1 | 35.2 |
| Root-mean-square bond lengths (Å) | 0.009 | 0.013 | 0.005 |
| Root-mean-square bond angles (°) | 1.46 | 1.72 | 1.07 |
Numbers in parentheses refer to the highest resolution bin.
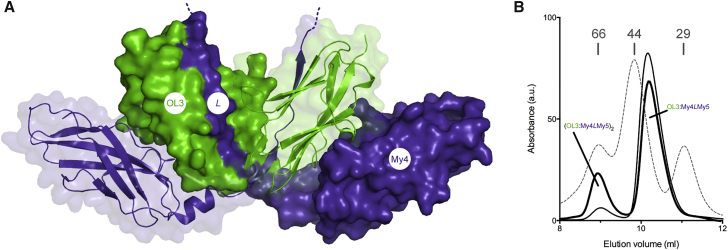
Overall Organization
The OL3:My4L complex is present as a (OL3:My4L)2 dimer of heterodimers in the crystal (Figure 2A). Individual OL3:My4L complexes display a bent dumbbell-shaped structure, in which the myomesin L linker extends away from the My4 domain integrating within the OL3 fold. Two OL3:My4L heterodimers then interlock around a non-crystallographic two-fold axis, giving rise to a dimeric assembly with overall dimensions of 105 Å × 48 Å × 26 Å. Large solvent channels running parallel to the molecular dyad axis are observed in the crystallographic packing (Figure S3). These are compatible with the presence of positionally disordered My5 domains.
Figure 2.
The (OL3:My4L)2 Complex Dimer
(A) In the crystal, two OL3:My4L heterodimers are arranged around a non-crystallographic two-fold axis (vertical in this view) forming a W-shaped (OL3:My4L)2 dimeric assembly. My4L and OL3 are in slate blue and green, respectively, with one heterodimer shown as a solid surface and the other in cartoon mode with a transparent surface. The dotted line at the C-terminus of L indicates the flexible region leading to My5 not visible in the structure.
(B) Superdex S75 10/300 SEC traces for the OL3:My4LMy5 complex injected in the column at 3 mg/mL (thin continuous line) and 8.2 mg/mL (thick continuous line). The OL3:My4LMy5 complex forms a mixture of monomers and dimers in a concentration-dependent manner. The elution profile of calibration markers is shown with a gray broken line. Molecular weights are indicated.
As the OL3:My4LMy5 complex typically elutes from SEC as a monomeric unit during purification (Figure S1), we analyzed its behavior at concentrations similar to that used for crystallization. In the accessible range of 3.0–8.2 mg/mL (0.082–0.225 mM), we observed the formation of complex dimers in a concentration-dependent manner with an approximately 30:70 dimer:monomer ratio at the highest protein concentration (Figure 2B). Thus, the oligomerization state observed in the crystal reflects that of a population in solution promoted by high protein concentration (∼15.9 mM in the crystal).
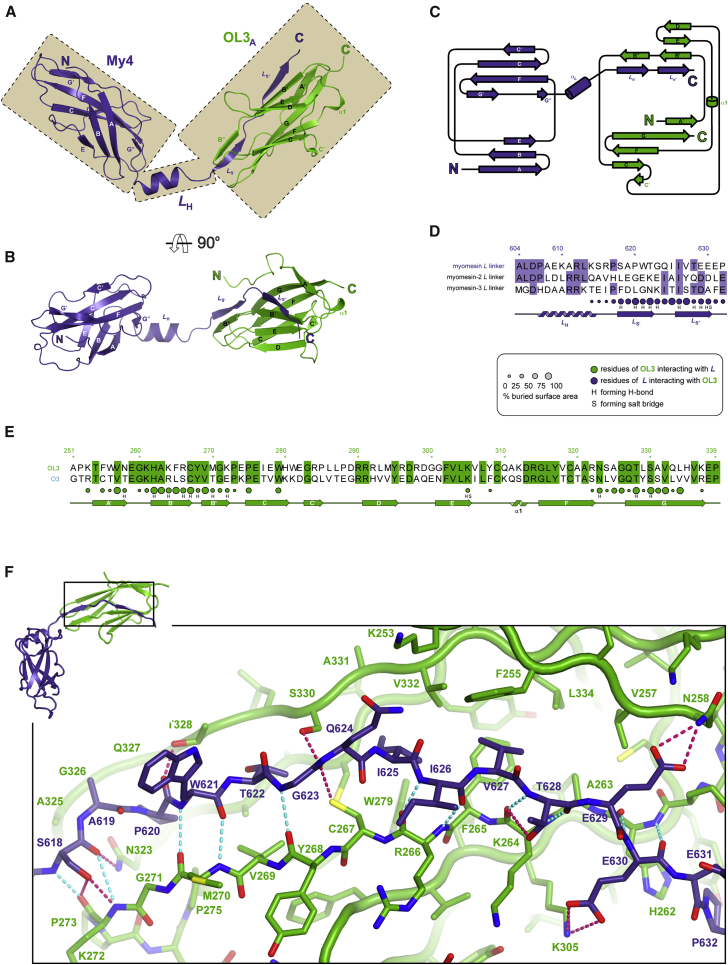
The OL3:My4L Heterodimer
Three distinct structural regions that we identify as My4, LH, and OL3A contribute to the architecture of individual OL3:My4L heterodimers (Figures 3A and 3B). The My4 domain displays the typical Fn-III fold made of seven anti-parallel β-strands organized in two separate β-sheets (A-B-E and C-C′-F-G) arranged in a β sandwich. C′ is rather short, while G is broken in two (G′ and G″) and interacts with the beginning and end of the long F β-strand. C-terminal to My4, the LH spacer region encompasses the first 11 amino acids of L and is formed by a 10.6-Å-long α-helix (Pro607-Lys614) followed by a short three amino acids peptide (Ser615-Pro617). LH leads the C-terminal portion of L, an 18-amino-acid-long extended stretch divided into two β-strands (LS′ and LS″) that integrate within the OL3 Ig fold. Similar to the Fn-III architecture, the Ig fold is also organized into a β sandwich formed by two β-sheets (A′-G-F-C-C′ and LS′/LS″-B′/B″-E-D). As OL3 integrates structural elements of L, we refer to this portion of the complex as the augmented OL3 (OL3A) domain. The distinctively bent geometry of the heterodimer is dictated by the principal axes of My4 and OL3A forming a ∼100° angle along the longest dimension of the complex. This, coupled with the 18.2-Å-long LH region (Pro607-Pro617, Cα-Cα distance) that acts as a spacer between the domains, allows for the positioning of the second OL3:My4L complex within the tetrameric assembly (Figure 2A).
Figure 3.
OL3:My4L Heterodimer
(A and B) Cartoon representation of the OL3:My4L heterodimer. The view in (B) is rotated by 90° around the x axis compared with (A). My4L and OL3 are shown in slate blue and green, respectively. In (A), the three main regions contributing to the complex are highlighted: the My4 Fn-III domain, its C-terminal helical spacer LH, and the OL3A (A for augmented) domain in which the Ig OL3 domain is stabilized by myomesin fold complementation in-trans.
(C) Topology diagram of the complex.
(D and E) Sequence alignment of the linker region for the different myomesin isoforms (D) and of the OL3 and O3 domains (E). Identical amino acids are highlighted. Colored circles above the secondary structure cartoons show residues of one domain contacting the other as indicated in the inset. Their radius is proportional to the buried area. The letter codes H and S indicate residues involved hydrogen bonds and salt bridges, respectively.
(F) Enlarged stick representation of the boxed region in the top left-hand corner (view rotated approximately 90° around the x axis compared with B) highlighting important myomesin:obscurin-like-1 interactions. Nitrogen, oxygen, and sulfur atoms are shown in blue, red, and yellow, respectively. Hydrogen bonds involving main-chain atoms only are shown as dotted cyan lines. H bonds and salt bridges involving side chains are indicated by dotted magenta lines.
OL3A is an example of fold complementation (Figure 3C), and the isolated OL3 domain is best described as an incomplete Ig of the intermediate-set (I-set) subfamily (Harpaz and Chothia, 1994). This type of Ig is often found in muscle proteins (Otey et al., 2009) and consists of a total of nine strands arranged into two distinct β-sheets (A-B-E-D and A′-G-F-C-C′), exhibiting the characteristic discontinuous A/A′ strand distributed over both β-sheets (Figure S4A). In OL3, the A β-strand that is hydrogen-bonded to B is missing and is replaced by myomesin LS′ (Ser618-Thr622), thus re-establishing a complete Ig architecture. A second myomesin strand (LS″, Ile626-Glu630) also hydrogen bonds to B″ at a position that is reminiscent of the A′ positioning found in a few deviant I-set Ig domains, identified as the I∗-set subtype (Pernigo et al., 2015). Members of this subtype feature a relocation of their A′ strand, resulting in the formation of an A/A′-B-E-D β-sheet (Figure S4B). Thus, OL3A is a complex trans-complemented hybrid I/I∗-set Ig fold.
Molecular Interfaces
Two sets of molecular interfaces are present in the crystallographic structure. The first one is involved in the formation of the OL3:My4L heterodimer. An additional set of interactions enables its dimerization. As SEC analysis indicates that in solution the formation of the (OL3:My4L)2 assembly is promoted by high concentration of the complex (Figure 2B), this implies that homodimerization is hierarchically secondary to the establishment of the OL3:My4L interface.
As highlighted in the contact maps in Figures 3D and 3E, the OL3:My4L heterodimer is held together by Ig-fold complementation. A mixture of hydrogen bonds and hydrophobic interactions stabilizes the heterodimer (Figure 3F). One edge of the mixed LS′/LS″-B′/B″-E-D β-sheet is engendered by the anti-parallel pairing of LS′-B″ and LS″-B′ β-strands mediated by a total of 11 main-chain hydrogen bonds (in cyan in Figure 3F) connecting LS′/LS″ residues to B′/B″ residues. Side chains also stabilize the complex by hydrogen bonding (in pink in Figure 3F). They typically involve hydroxyl groups of Thr and Ser residues (myomesin S618, T628 and OL3 T328, S330) interacting with main-chain carbonyl oxygen atoms. A single salt bridge connects the carboxylate of myomesin E630 to the amine side chain of OL3 K305. A number of hydrophobic residues are buried upon complex formation. For example, myomesin I625 points its aliphatic side chain in a tight cavity lined by OL3 F265, W279, L304, Y317, C319, V332. Together with myomesin P620, T622, and V627, this residue buries more than 90% of its surface in the interaction, representing a critical determinant for binding. Overall, the OL3:My4L interface area is 1,046 Å2.
OL3:My4L homodimerization further stabilizes the assembly, resulting in the establishment of an interface area of 2,320 Å2 (Figure S5). This is largely engendered by OL3∗:My4L (and symmetric OL3:My4L∗, where ∗ indicates that the domain belongs to the dimer partner), while My4L:My4L∗ and OL3:OL3∗ interactions are rather limited with interface areas of 122 Å2 and 278 Å2, respectively. PISA analysis (Krissinel and Henrick, 2007) indicates positive ΔG dissociation values of 14.5 kcal/mol and 12.75 kcal/mol for (OL3:My4L)2 and OL3:My4L stable assemblies, respectively.
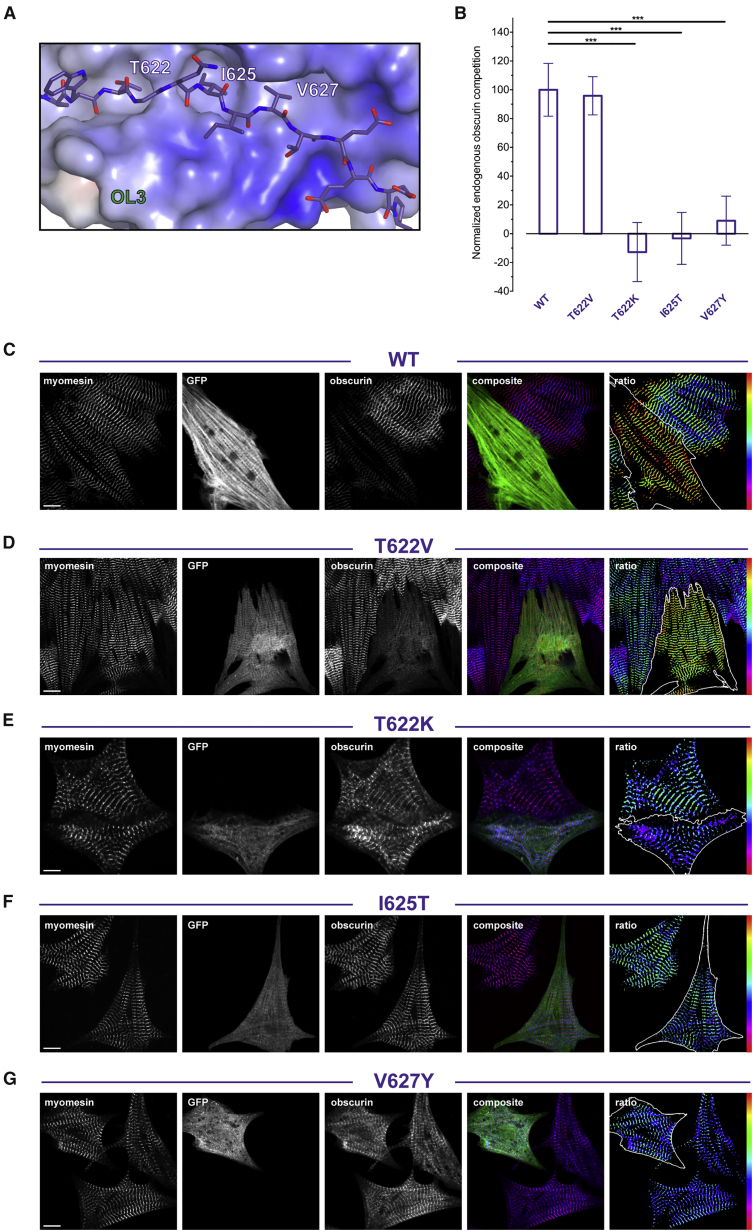
Molecular Basis for Myomesin Isoform Specificity
The myomesin gene family comprises three MYOM genes in humans (Schoenauer et al., 2008). MYOM1 encodes the ubiquitously expressed myomesin protein, while MYOM2 and MYOM3 encode a fast-fiber isoform called M-protein or myomesin-2 and myomesin-3, a recently identified isoform of slow fibers, respectively. The interaction with obscurin/obscurin-like-1 is limited to myomesin, as neither M-protein nor myomesin-3 shows any appreciable binding (Fukuzawa et al., 2008). Our X-ray structure explains the molecular basis for this specificity. Three myomesin residues mapping onto the L linker (T622, I625, and V627) display side chains that are complementary to the OL3 surface (Figure 4A). These are not conserved in either M-protein or myomesin-3 and occasionally exhibit rather dramatic amino acid substitutions. For example, myomesin T622 is replaced by a lysine in M-protein, while in myomesin-3 a more polar threonine takes the place of myomesin I625 (Figure 3E).
Figure 4.
Cellular Validation and Myomesin Isoform Specificity
(A) Close up of the OL3:My4L interface. OL3 is shown as surface representation and colored according to its electrostatic potential. The myomesin L linker interacting with OL3 is shown as stick representation.
(B) Quantification of endogenous obscurin displaced in neonatal rat cardiomyocytes expressing GFP-fused wild-type My4LMy5 (n = 11) and its T622V (n = 10), T622K (n = 9), I625T (n = 10), V627Y (n = 13) variants. Amino acid replacements in the L linker inspired by myomesin-2 (T622K and V627Y) and myomesin-3 (I625T) sequences abrogate competition. Error bars are SEM values. ∗∗∗p ≤ 0.001.
(C) Example of the competitive effect of overexpressed GFP-fused My4LMy5 (WT) on endogenous obscurin in NRCs. The separate channels for endogenous myomesin, GFP, endogenous obscurin, as well as the combined and ratiometric images with overlaid GFP mask for the outline of the transfected cell are shown. The false-color scale range indicator shows an increased obscurin/myomesin ratio. The scale bar represents 10 μm.
(D–G) Similar to (C) for overexpressed GFP-fused My4LMy5 T622V (D), T622K (E), I625T (F), and V627Y (G). The scale bar represents 10 μm.
To validate the interaction between myomesin and obscurin/obscurin-like-1 in the context of the sarcomere, we generated a number of myomesin variants targeting the L linker and tested them for their ability to compete endogenous obscurin from the M-band. A quantitative analysis of these results is summarized in Figure 4B, while immunofluorescence images of representative experiments are shown in Figures 4C–4G and S6. When overexpressed in neonatal rat cardiomyocytes (NRCs), GFP-My4LMy5 targets the M-band, in addition to other diffuse subcellular localizations, displacing endogenous obscurin (first bar in Figures 4B and 4C). In the case of T622, its replacement with an isosteric valine (T622V) does not significantly alter the wild-type behavior (second bar in Figures 5B and 4D). This is consistent with the lack of hydrogen bonding between the side chain of T622 and OL3 residues contributing to the small receptor cavity (Figures 3F and 4A). However, when T622 is replaced by a lysine (T622K) as in M-protein (third bar in Figures 4B and 4E), or alternatively when I625 is replaced by a threonine like in myomesin-3 (fourth bar in Figures 4B and 4F), competition is essentially abrogated. A similar effect is mediated by the V627Y replacement also found in M-protein (fifth bar in Figures 4B and 4G). As expected, control substitutions targeting myomesin regions not involved in the interface have no effect on the ability to compete endogenous obscurin (Figure S6).
Figure 5.
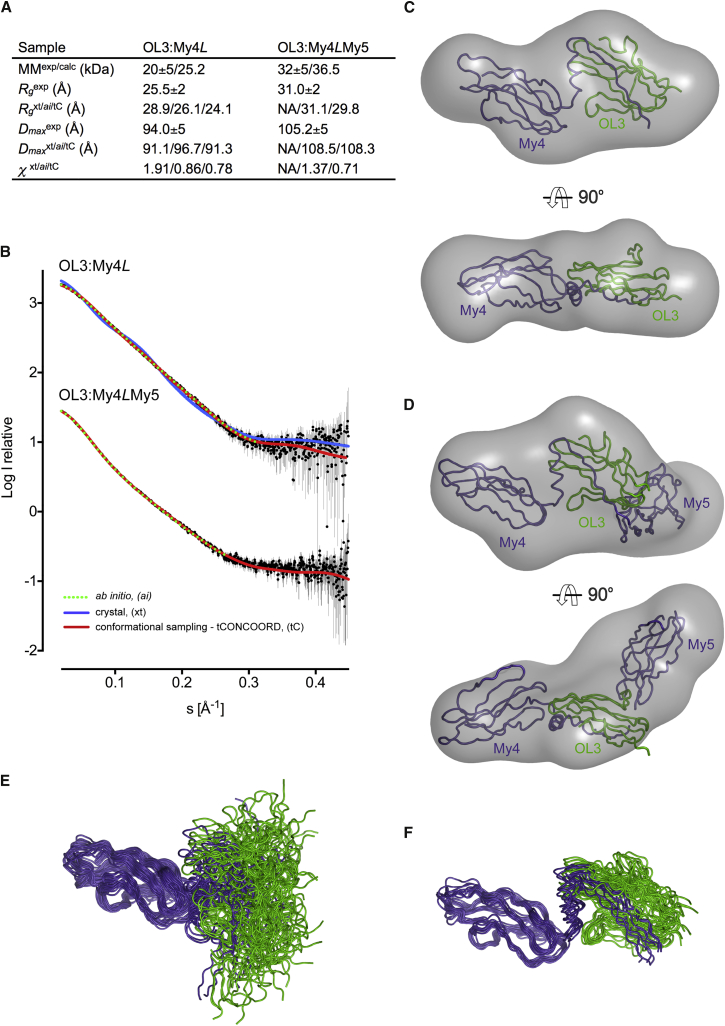
Small-Angle X-Ray Scattering Analysis of the Myomesin:Obscurin-Like-1 Complex
(A) Molecular parameters calculated from SAXS data. MM, Rg, Dmax are the molecular mass, radius of gyration, and maximum size, respectively. The superscript exp denotes experimental values while xt, ai, and tC refer to crystal, ab initio, and tCONCOORD fitted models, respectively. MMcalc is the theoretical MM computed from the protein sequence. χ is the discrepancy between experimental data and those computed from models.
(B) Experimental scattering intensities for OL3:My4L (upper curve) and OL3:My4LMy5 (lower curve) are displayed as dots with error bars. Curves computed from the crystallographic model and the best tC models are shown as solid blue and red lines, respectively, while the curve computed from the ab initio models is shown as a dotted green line.
(C) Cartoon-tube representation of a selection of 20 OL3:My4L tCONCOORD models (out of >30,000) aligned with respect to their My4 domain. My4L is in slate blue and OL3 is in green.
(D) Cartoon-tube representation of the ten OL3:My4L tCONCOORD models providing the best fit to the SAXS data (0.78 < χ < 0.81).
(E and F) OL3:My4L SAXS molecular envelope calculated from the ab initio model with a representative tCONCOORD model in two orthogonal orientations; (F) like (E) for OL3:My4LMy5.
The OL3:My4L Heterodimer Is a Flexible Structural Element
The bent dumbbell shape of OL3:My4L observed in the crystal is stabilized by its homodimeric assembly. As SEC analysis indicates that the complex is predominantly monomeric in solution, we explored whether this geometry is representative of the complex in solution using small-angle X-ray scattering (SAXS). The overall molecular parameters derived from scattering data on OL3:My4L and OL3:My4LMy5 are shown in Figure 5A. A comparison of the experimental radius of gyration Rg for OL3:My4L (25.2 ± 2 Å) with that calculated from the structure (28.9 Å) indicates that in solution, the complex adopts a less extended conformation than in the crystal. Accordingly, the scattering pattern computed from the crystallographic model yielded a suboptimal fit (χ = 1.91) to the SAXS data (Figure 5B, upper curve, blue line), suggesting differences in the relative domain arrangement. To investigate the structure in solution, we considered the complex composed of three rigid bodies defined by the My4, LH, and OL3A structural regions (Figure 3A). A good fit to the scattering curve was obtained with a model that is more compact than that seen in the crystal. We then used this structure as a starting template and, following energy minimization, generated >30,000 additional models (a selection shown in Figure 5C) using the tCONCOORD (Seeliger et al., 2007) algorithm, a computationally efficient method for sampling conformational transitions. Within this large pool, we found ∼500 models that provide an excellent fit (χ < 1.0) to the experimental curves (Figure 5B, upper panel, red line). These models all display the LH helix resting on the OL3A domain, resulting in a less extended conformation compared with the dimer-stabilized crystal structure (a selection shown in Figure 5D). Additional SAXS data measured on OL3:My4LMy5 reveal that inclusion of the My5 domain increases the Rg value to 31.0 ± 2 Å (Figure 5A). To model this complex, we started from the OL3:My4L solution model and again used tCONCOORD to sample the conformational space following addition of an additional Ig domain (My5). Several similar models provide an excellent fit (χ < 1.0) to the scattering curve (Figure 5B, lower curve). We find that the OL3:My4L portion of the complex remains largely invariant, with My5 approximately orthogonal to OL3A (Figure 5F).
The ability of OL3:My4L to transition from the solution conformation to that observed crystallographically suggests that the LH helix might have a degree of flexibility. We explored this by solving the crystal structure of My4LH (myomesin residues 510–618) in two different space groups (data collection and refinement statistics in Table 1). In space group P65 (2.05 Å resolution), all four My4LH independent molecules in the a.u. display clear electron density until residue A608, while residues E609-S618 (LH) cannot be modeled (Figure S7A). The same applies for four of six My4LH independent molecules in the alternative P21 space group (2.80 Å resolution). However, in the latter crystal structure, crystal contacts stabilize the C-terminal region in the remaining two other My4LH molecules. While in one molecule, LH folds into an α-helix as in the My4L:OL3 complex (Figure S7B), in the other molecule the C-terminus is in a more extended conformation (Figure S7C). Overall, SAXS and crystallographic analyses support a model in which interdomain freedom allows the transition (see Movie S1) from a relatively compact solution conformation to an open one that can be stabilized by homodimerization.
Mechanical Stability of the Complex
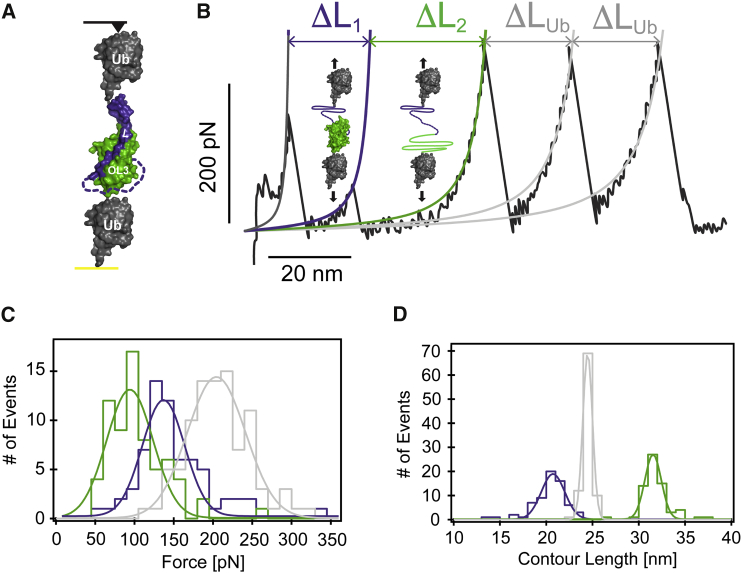
It is enticing to speculate that the physical connection via swapped secondary structure elements might act as the molecular glue necessary for the mechanical stability of the obscurin(-like-1):myomesin assembly. To probe this, we employed single-molecule force spectroscopy using atomic force microscopy (AFM), and guided by the structure, we fused the C-terminus of the myomesin L linker to the N-terminus of OL3 by an unstructured 43 amino acids connector. This single-chain L-(connector)43-OL3 complex was then sandwiched between two ubiquitin (Ub) domains that serve as well-characterized handles (Carrion-Vazquez et al., 2003) (Figure 6A). The engineered polyprotein enables the unambiguous characterization of the forces required to break the molecular interactions that hold the complex together.
Figure 6.
Fold Complementation by the Myomesin L Linker Protects the Mechanically Labile OL3 Domain
(A) Schematics of the ubiquitin-L-(connector)-OL3-ubiquitin polyprotein used in the single-molecule mechanical experiment. The 43 amino acid flexible connector covalently joining L to OL3 is depicted as a dashed line. The color code for L and OL3 is as in previous images. Ubiquitin (Ub) molecules bracketing the complex are in gray while the gold substrate and the atomic force microscopy (AFM) cantilever tip are shown in yellow and black, respectively.
(B) Typical force-extension trace for the ubiquitin-L-(connector)43-OL3-ubiquitin polyprotein exhibiting a saw-tooth pattern of unfolding events that does not follow a hierarchy in the mechanical stability: the first unfolding event occurs at higher forces than the second unfolding event. The unfolding of the ubiquitin modules, occurring at forces of ∼200 pN and characterized by an increment of contour length of ΔLUb ∼24.5 nm, ensures the single-molecule nature of the experiment.
(C) Histogram of unfolding forces. The first two events can be readily identified in light of their different mechanical stability and increment in contour length. While the first peak occurs at forces as high as 129.4 ± 27.0 pN (n = 66), the second peak unfolds at the markedly lower force of 86.6 ± 29.1 pN (n = 68).
(D) Histogram of contour length increase: ΔL1 = 20.2 ± 1.2 nm (n = 66) and ΔL2 = 31.0 ± 0.9 nm (n = 68). In (C) and (D), colored curves are Gaussian fits.
When stretched in our AFM setup at the constant velocity of 400 nm s−1 often employed in these types of studies (del Rio et al., 2009, Garcia-Manyes et al., 2012, Perez-Jimenez et al., 2006), the polyprotein unfolded displaying a saw-tooth pattern with peaks of alternating mechanical stability (Figure 6B). At the beginning of the trace, we identified two mechanical events with associated contour length increments of ΔL1 = 20.2 ± 1.2 nm (n = 66) and ΔL2 = 31.0 ± 0.9 nm (n = 68), respectively, followed by the unfolding of the two ubiquitin monomers (ΔLUb ∼24.5 nm), which serve as internal molecular calibration fingerprints (Figure 5B). Interestingly, the observed unfolding pattern does not follow the expected hierarchy of mechanical stability (Li and Fernandez, 2003). The first event occurs at a force value of 129.4 ± 27.0 pN (n = 66) while the second one only requires 86.6 ± 29.1 pN (n = 68) (Figure 5C). Both mechanical events are followed by the unfolding of the two Ub monomers, occurring at a higher force ∼200 pN (Carrion-Vazquez et al., 2003). Such unfolding scenario is hence reminiscent of a molecular mechanism whereby a mechanically labile domain is mechanically protected from the pulling force by a more resilient protein structure (Peng and Li, 2009).
The crystal structure shows that a stretch of 15 amino acids belonging to L lies within the OL3 domain. As the engineered protein connector is 43 residues long, the predicted length increase as a result of L detachment from OL3 is ΔL1=((15 + 43) residues × 0.36 nm/residue) = 20.88 nm. This value is in agreement with the experimental measurement (ΔL1 = 20.2 ± 1.2 nm, Figure 5D). The second unfolding event (ΔL2 = 31.0 ± 0.9 nm) corresponds to the unfolding and stretching of OL3 (89 amino acids). Thus, the single-molecule unfolding trajectories support an unfolding scenario whereby the first high-force event corresponds to the removal of the L linker from the OL3 domain, followed by the unfolding of OL3, occurring at a significantly lower force. To further confirm our molecular hypothesis, we constructed a second polyprotein in which the flexible connector length was lengthened to 64 residues. This new construct confirmed forces of 143 ± 29 pN (ΔL1 = 27.9 ± 1.5 nm) and 81 ± 22 pN (ΔL2 = 31.7 ± 1.2), for the detachment of the L latch and OL3 unfolding, respectively. As expected, while ΔL2 is invariant in the two polyproteins, the longer ΔL1 is fully consistent with the predicted extension of 28.4 nm ((15 + 64) residues × 0.36 nm/residue) for the longer connector (Figure S8). Our single-molecule nanomechanical experiments thus unambiguously support a molecular organization in which the mechanically labile OL3 domain is protected from force by a more resilient architecture afforded by myomesin L complementation (Peng and Li, 2009).
Discussion
The reason why muscle sarcomeres do not self-destruct during contraction lies in the intricate yet poorly understood cytoskeletal protein networks coordinated by titin at the Z-disk and M-band, which link actin and myosin filaments transversally and longitudinally (Horowits et al., 1986). The M-band network at the center of the myosin filaments is believed to play a key role as a mechanical safeguard during force-generating cycles and as a signaling hub (Agarkova et al., 2003). Compared with the Z-disk, there is currently limited knowledge of this sarcomeric region. The reason for this is 2-fold. On the one hand, although the identity of some key M-band proteins is well established, new components are steadily emerging, suggesting that a much richer complement resides either stably or transiently at this region. For example, cardiomyopathy associated 5 protein (Cmya5 or myospryn) has been recently shown to bind to M-band titin and calpain-3 (Capn3) protease (Sarparanta et al., 2010). Mutations in Capn3 lead to limb girdle muscle dystrophy (LGDM) type 2A, and secondary Capn3 deficiency occurs in LGMD type 2J. Also, a novel leucine-rich protein named myomasp ([myosin-interacting, M-band-associated stress-responsive protein]/LRRC39) has been detected as an interactor of myosin heavy chain (MYH7), and knockdown of the myomasp/LRRC39 ortholog in zebrafish resulted in severely impaired heart function and cardiomyopathy in vivo (Will et al., 2010). On the other hand, even for known M-band protein components, their complexity is such that their detailed molecular organization is still largely unknown. Thus, advances in our understanding of M-band biology need to address its dynamic proteome, and the mechanical and architectural aspects underpinning its function.
In this work, we explored the myomesin-dependent anchoring of obscurin-like-1 to the M-band and found a mechanism that is new in the sarcomere context. The structure of the obscurin-like-1:myomesin complex reveals that the myomesin L linker between its fourth (My4) and fifth (My5) Fn-III domains integrates within the incomplete third Ig domain of obscurin-like-1 (OL3), resulting in a stable protein complex. The mechanism of fold complementation in-trans observed for the My4L:OL3 complex is somewhat reminiscent of that of subunit-subunit and chaperone-subunit interactions in bacterial pili assembled by the chaperone-usher pathway, whereby the binding partner inserts a β-strand into a partial Ig domain, thus restoring its fold (Remaut et al., 2006). In the case of OL3:My4L, this binding mode provides a surprisingly high mechanical stability to the complex (∼135 pN), a rupture force significantly higher than that required to unfold OL3 alone (∼85 pN) and quantitatively similar to that exhibited by the myomesin C-terminal dimer (∼137 pN) (Berkemeier et al., 2011) required for myosin crosslinking. The high force that the complex is able to withstand contrasts with the mechanical lability (∼30 pN) measured for the titin:obscurin/obscurin-like-1 complex between M10:OL1/O1 Ig domains (Pernigo et al., 2010). Such mechanically weaker interaction reflects a completely different structural architecture, based on a parallel β-strand augmentation in an Ig:Ig chevron-shaped zipper module (Pernigo et al., 2010, Pernigo et al., 2015, Sauer et al., 2010). Interestingly, the low rupture force of the latter interaction is on the order of only about six myosin crossbridges, thus stable anchoring of obscurin-like-1 to the M-band appears to be dependent on its binding to myomesin rather than to titin. Given the high sequence similarity between OL3 and obscurin O3, particularly for the residues involved in the molecular interface with myomesin (Figure 3E), we suggest that the same holds true for obscurin anchoring and that the obscurin:myomesin complex recapitulates OL3:My4L in its binding mode. This closely mirrors the behavior of N-terminal Ig domains OL1 and O1 that interact with titin M10 in a mutually exclusive manner using a common interface. However, as for OL1 and O1, where minor, yet significant, structural differences suggest different specificities for putative additional partners (Pernigo et al., 2015), we cannot exclude a similar unanticipated behavior for OL3/O3 as well. Interestingly, both OL3 and O3 are insoluble in bacteria when expressed in isolation, while co-expression in the presence of the myomesin L region results in biochemically well-behaved complexes. This suggests a chaperone effect by myomesin, effectively enabling the correct folding of the unconventional augmented O(L)3A Ig domain. Crucially, removal of the L linker from OL3A results in a semi-folded state with a significantly decreased mechanical stability, requiring only ∼85 pN to unfold.
A mechanism of β-strand complementation between linkers or non-structured regions with incomplete Ig domains has also been observed, both in-cis and in-trans, in Ig domains of the actin crosslinking protein filamin. Filamin A can interact with the cytoplasmic tail of integrin 3 via its Ig-like domain 21 (FLNa21), but FLNa21 can also bind to the linker between FLNa20 and 21 in an intramolecular complex that competes with integrin. Intriguingly, the removal of the trans-complemented β-strand from FLNa21 unmasks the binding site for integrin, which, when bound to filamin, engages integrin an inactive state (Heikkinen et al., 2009, Liu et al., 2015). This specific interaction can be opened by mechanical stretch and triggers integrin binding, filamin's partner in mechanosensing (Chen et al., 2009, Seppala et al., 2015). The intermolecular domain trans-complementation we observe here for the obscurin(-like-1):myomesin complex might therefore also play a role in mechanosensing, by freeing the O3/OL3 domain for binding to an alternative ligand. As obscurin-like-1 has been linked to ubiquitin-mediated turnover, such a mechanosensing pathway around the obscurin/obscurin-like-1:myomesin complex might feed into the turnover of sarcomere-associated structures (Lange et al., 2012). Myomesin crosslinks myosin filaments and therefore must be exposed at least to some of the shear forces developing transversally to the myosin filament axis, but the extent to which myomesin is directly exposed to mechanical force in vivo remains unknown, not least because the exact orientation with respect to the filament axis can currently be only indirectly inferred, and the geometry of force transmission is therefore unclear. It is also yet unclear in which directionality mechanical forces act on the titin-obscurin, which might be relevant based on recent molecular dynamics simulations (Caldwell et al., 2015). However, it is reasonable to speculate that the extremely stable anchoring of obscurin(-like-1) to myomesin is not only structurally important, but has evolved also as functionally relevant for nanomechanical necessity, supporting the notion that the M-band is a key strain sensor in muscle sarcomeres (Agarkova et al., 2003, Pinotsis et al., 2012, Xiao and Grater, 2014).
The MYOM gene family codes for three proteins sharing a similar Ig/Fn-III-rich domain organization. Our OL3:My4L structure offers a clear structural basis for the specificity of obscurin(-like-1) binding to the myomesin-1 isoform that was validated by competition assays in the relevant cellular context of NRCs. Interestingly, the OL3:My4L complex also reveals interdomain flexibility and the ability to dimerize. The dimeric arrangement observed in the crystal and in solution at high protein concentration opens the possibility that this geometry might reflect the local obscurin(-like-1):myomesin organization in the crowded environment of the sarcomere. The M4/M4′ lines typical of striated muscles define a hexagonal arrangement of myosin filaments in the super-lattices of most vertebrates. Antibody mapping experiments suggested that the N-terminal region of myomesin runs roughly perpendicular to the myosin filament, since My1 and the L loop are only 7 nm apart from the M1 line (Obermann et al., 1996). Thus, it is conceivable that myomesin molecules emanating from neighboring myosin filaments of the hexagonal lattice cross over at the level of the L linker as seen in the OL3:My4L dimer (Figure 7). The intrinsic flexibility of the complex monomer coupled with the presence of the helical spacer at the L N-terminus appears perfectly poised for this. This suggestion is compatible with previous M-band models (Lange et al., 2005) but adds a novel geometric constraint. In summary, our work provides a necessary structural and biomechanical reference to establish the geometrical context and mechanical hierarchies in M-band assembly, which will need to be reconciled with more highly resolved in situ information of this protein network and its response to mechanical stress.
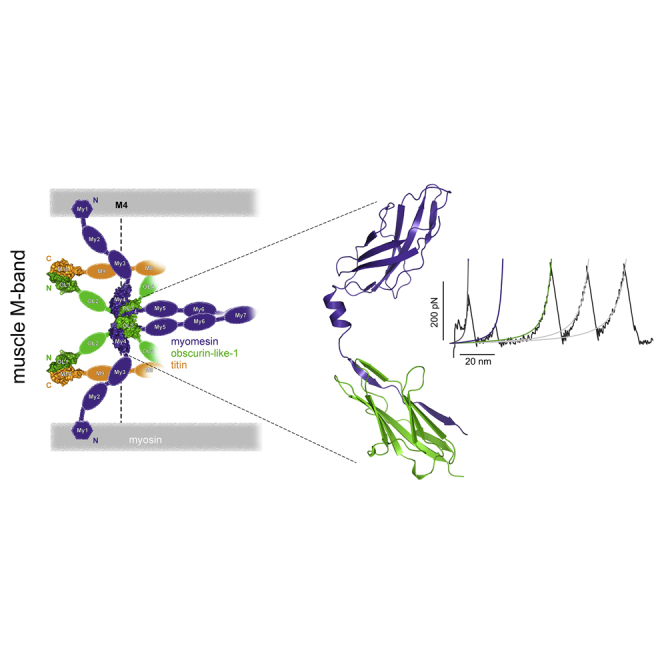
Figure 7.
Possible Model for the Organization of the Titin:Obscurin(-like-1):Myomesin Ternary Complex at the M-Band
Experimental complexes are shown as surface representation: OL3:My4L dimer (this work), M10:OL1 (Pernigo et al., 2010, Pernigo et al., 2015, Sauer et al., 2010), My9-M13 (Pinotsis et al., 2008, Pinotsis et al., 2012).
Experimental Procedures
Detailed methods used for cloning, protein expression, and protein purification are given in the Supplemental Experimental Procedures.
Crystallization
An initial vapor-diffusion sparse matrix screening performed using the sitting-drop setup with the aid of Mosquito crystallization robot (TTP LabTech) produced hundreds of OL3:My4LMy5 microcrystals in the presence of 1.1 M ammonium tartrate (pH 7.0) and a 1:2 protein:reservoir ratio. The protein concentration used in the screen was 4.0 mg/mL in storage buffer (20 mM HEPES, 150 mM NaCl, 1 mM DTT [pH 7.5]). A standard pH-precipitant grid optimization allowed us to obtain fewer marginally larger crystals in the presence of 0.8 M ammonium tartrate, 0.1 M sodium acetate (pH 5.5) using a 1:1 protein:reservoir ratio. These crystals, however, proved unsuitable for diffraction experiments. To further improve crystal quality, we employed the random MMS screening approach (D'Arcy et al., 2014). Crystals obtained in the optimization step were harvested and stored in a solution containing 0.9 M ammonium tartrate, 0.1 M sodium acetate (pH 5.5) (hit stock). A new sparse matrix screening was performed using various commercial screens using a hit stock:protein:reservoir ratio of 1:2:1. Few OL3:My4LMy5 single crystals were finally obtained in the presence of 20% PEG8000, 0.1 M Tris-HCl (pH 8.5), 0.2 M MgCl2 using the protein complex at 3.0 mg/mL. Crystallization of My4LH is described in the Supplemental Experimental Procedures.
X-Ray Data Collection and Structure Determination
Crystals were cryo-protected by soaking them in their reservoir solution supplemented with 20% glycerol. For OL3:My4LMy5 a 3.1 Å resolution dataset was collected in space group C2 while My4LH crystallized in the alternative space groups P65 and P2, yielding diffraction data at 2.05 Å and 2.8 Å resolution, respectively. All datasets were collected at Diamond Light Source synchrotron facility (Didcot, Oxfordshire, UK) and processed with the xia2 expert system (Winter et al., 2013) using XDS (Kabsch, 2010) and AIMLESS (Evans and Murshudov, 2013) packages. All X-ray structures were solved by the molecular replacement method with the package MOLREP (Vagin and Teplyakov, 2010) and refined using the programs REFMAC5 (Murshudov et al., 2011) and BUSTER (Bricogne et al., 2011). A summary of data collection and refinement statistics is shown in Table 1. Further details on the crystallographic methods are available in the Supplemental Experimental Procedures.
Cellular Competition Assays in NRCs and Ratiometric Analysis
NRC isolation, culture, transfection, and staining were performed essentially as described previously (Pernigo et al., 2010). Briefly, NRCs were transfected with GFP-tagged transiently expressing constructs (pEGFPC2-, Clontech Laboratories) using Escort III (Sigma-Aldrich). After 48 hr culture to promote protein expression, cells were fixed with 4% paraformaldehyde/PBS, permeabilized with 0.1% Triton X-100/PBS, and then stained with the appropriate antibodies. The antibodies used for the current work were as follows: MyB4, a mouse monoclonal antibody to the myomesin domain My12 (Grove et al., 1984); and Ob5859, a rabbit polyclonal antibody to two consecutive Ig domains in obscurin, Ob58 and Ob59 (Fukuzawa et al., 2005, Young et al., 2001). All fluorescent-conjugated secondary antibodies were purchased from Jackson ImmunoResearch. All images for ratiometry analysis were collected on a Zeiss LSM510 confocal microscope as described previously (Fukuzawa et al., 2008). Image analysis was carried out as described in our previous work (Pernigo et al., 2015). Further details are available in the Supplemental Experimental Procedures.
Small-Angle X-Ray Scattering
Synchrotron SAXS data for OL3:My4L and OL3:My4LMy5 were collected at the BM29 BioSAXS beamline (ESRF, Grenoble) using a Pilatus 1M detector (Dectris) (Pernot et al., 2013). All samples were measured at four concentrations (0.5–4.5 mg/mL in 20 mM HEPES [pH 7.5], 500 mM NaCl, 1 mM DTT buffer) in the range of momentum transfer 0.005 < s < 0.608 Å−1 (s = 4πsinθ/λ, where the wavelength λ is 0.9919 Å and 2θ is the scattering angle). All experiments were performed at 18°C using a sample volume of 30 μL loaded into the flowing measurement cell. Individual frames were processed automatically and independently within the EDNA framework (Brennich et al., 2016). Merging of separate concentrations and further analysis steps were performed using a combination of tools from the ATSAS package (Petoukhov et al., 2012). Initial rigid body modeling of the complex was done with CORAL (Petoukhov et al., 2012) and domain dynamics of the protein complexes was further explored by generating conformational ensembles using the tCONCOORD (Seeliger et al., 2007) method. Further details are available in the Supplemental Experimental Procedures.
Single-Molecule Mechanical Experiments by Atomic Force Microscopy
cDNA was commercially synthesized (Genscript), which allowed the expression of a polyprotein in which the myomesin linker L and the obscurin-like-1 OL3 domain are connected by a flexible 43-amino-acid-long connector sandwiched between two ubiquitin (Ub) domains (Ub-L-connector-OL3-Ub). The synthetic gene was inserted into a pQE80L vector (QIAGEN) using standard molecular biology techniques. A PCR-based approach also allowed the extension of the connector length to 64 amino acids. Single proteins were picked up from the surface and pulled at a constant velocity of 400 nm s−1 (del Rio et al., 2009, Garcia-Manyes et al., 2012, Perez-Jimenez et al., 2006). Further details are available in the Supplemental Experimental Procedures.
Author Contributions
R.A.S. and M.G. designed the research. S.P. purified proteins and performed the crystallographic and SAXS work. A.R. analyzed SAXS data. A.P. generated models for SAXS analysis. A.F. and M.H. carried out the cellular competition assay and its analysis. A.E.M.B. and S.G.-M. performed and analyzed the AFM data. R.A.S. wrote the original draft. R.A.S., S.G.-M., and M.G. supervised the research and wrote the paper. All authors reviewed and contributed to the manuscript.
Acknowledgments
We thank the Diamond Light Source and the European Synchrotron Radiation Laboratory for access to MX and SAXS beamlines, respectively. This work was supported by a British Heart Foundation grant (PG/10/67/28527) awarded to R.A.S. and M.G. as well as MRC grant MR/J010456/1 to M.G. and a British Heart Foundation grant (PG/13/50/30426) and EPSRC Fellowship (K00641X/1) to S.G.-M.
Published: December 15, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, eight figures, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.str.2016.11.015.
Contributor Information
Sergi Garcia-Manyes, Email: sergi.garcia-manyes@kcl.ac.uk.
Mathias Gautel, Email: mathias.gautel@kcl.ac.uk.
Roberto A. Steiner, Email: roberto.steiner@kcl.ac.uk.
Accession Numbers
Atomic coordinates for the X-ray structures presented in this article have been deposited with the PDB under accession codes PDB: 5FM4, 5FM5, 5FM8.
Supplemental Information
References
- Agarkova I., Ehler E., Lange S., Schoenauer R., Perriard J.C. M-band: a safeguard for sarcomere stability? J. Muscle Res. Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- Arimura T., Matsumoto Y., Okazaki O., Hayashi T., Takahashi M., Inagaki N., Hinohara K., Ashizawa N., Yano K., Kimura A. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 2007;362:281–287. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- Bagnato P., Barone V., Giacomello E., Rossi D., Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 2003;160:245–253. doi: 10.1083/jcb.200208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benian G.M., Tinley T.L., Tang X., Borodovsky M. The Caenorhabditis elegans gene unc-89, required fpr muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J. Cell Biol. 1996;132:835–848. doi: 10.1083/jcb.132.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeier F., Bertz M., Xiao S., Pinotsis N., Wilmanns M., Grater F., Rief M. Fast-folding alpha-helices as reversible strain absorbers in the muscle protein myomesin. Proc. Natl. Acad. Sci. USA. 2011;108:14139–14144. doi: 10.1073/pnas.1105734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennich M.E., Kieffer J., Bonamis G., De Maria Antolinos A., Hutin S., Pernot P., Round A. Online data analysis at the ESRF bioSAXS beamline. BM29. J. Appl. Cryst. 2016;49:203–212. [Google Scholar]
- Bricogne G., Blanc E., Brandl M., Flensburg C., Keller P., Paciorek W., Roversi P., Sharff A., Smart O.S., Vonrhein C. Global Phasing; 2011. BUSTER Version 2.10.0. [Google Scholar]
- Caldwell T.A., Sumner I., Wright N.T. Mechanical dissociation of the M-band titin/obscurin complex is directionally dependent. FEBS Lett. 2015;589:1735–1739. doi: 10.1016/j.febslet.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Carmignac V., Salih M.A., Quijano-Roy S., Marchand S., Al Rayess M.M., Mukhtar M.M., Urtizberea J.A., Labeit S., Guicheney P., Leturcq F. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann. Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- Carrion-Vazquez M., Li H., Lu H., Marszalek P.E., Oberhauser A.F., Fernandez J.M. The mechanical stability of ubiquitin is linkage dependent. Nat. Struct. Biol. 2003;10:738–743. doi: 10.1038/nsb965. [DOI] [PubMed] [Google Scholar]
- Chen H.S., Kolahi K.S., Mofrad M.R. Phosphorylation facilitates the integrin binding of filamin under force. Biophys. J. 2009;97:3095–3104. doi: 10.1016/j.bpj.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcy A., Bergfors T., Cowan-Jacob S.W., Marsh M. Microseed matrix screening for optimization in protein crystallization: what have we learned? Acta Crystallogr. F Struct. Biol. Commun. 2014;70:1117–1126. doi: 10.1107/S2053230X14015507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J.M., Sheetz M.P. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa A., Idowu S., Gautel M. Complete human gene structure of obscurin: implications for isoform generation by differential splicing. J. Muscle Res. Cell Motil. 2005;26:427–434. doi: 10.1007/s10974-005-9025-6. [DOI] [PubMed] [Google Scholar]
- Fukuzawa A., Lange S., Holt M., Vihola A., Carmignac V., Ferreiro A., Udd B., Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J. Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- Garcia-Manyes S., Badilla C.L., Alegre-Cebollada J., Javadi Y., Fernandez J.M. Spontaneous dimerization of titin protein Z1Z2 domains induces strong nanomechanical anchoring. J. Biol. Chem. 2012;287:20240–20247. doi: 10.1074/jbc.M112.355883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M., Djinovic-Carugo K. The sarcomeric cytoskeleton: from molecules to motion. J. Exp. Biol. 2016;219:135–145. doi: 10.1242/jeb.124941. [DOI] [PubMed] [Google Scholar]
- Grove B.K., Kurer V., Lehner C., Doetschman T.C., Perriard J.C., Eppenberger H.M. A new 185,000-dalton skeletal muscle protein detected by monoclonal antibodies. J. Cell Biol. 1984;98:518–524. doi: 10.1083/jcb.98.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D., Murray P.G., Sud A., Temtamy S.A., Aglan M., Superti-Furga A., Holder S.E., Urquhart J., Hilton E., Manson F.D. The primordial growth disorder 3-M syndrome connects ubiquitination to the cytoskeletal adaptor OBSL1. Am. J. Hum. Genet. 2009;84:801–806. doi: 10.1016/j.ajhg.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz Y., Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J. Mol. Biol. 1994;238:528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- Heikkinen O.K., Ruskamo S., Konarev P.V., Svergun D.I., Iivanainen T., Heikkinen S.M., Permi P., Koskela H., Kilpelainen I., Ylanne J. Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin. J. Biol. Chem. 2009;284:25450–25458. doi: 10.1074/jbc.M109.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Kempner E.S., Bisher M.E., Podolsky R.J. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986;323:160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Hu L.Y., Kontrogianni-Konstantopoulos A. The kinase domains of obscurin interact with intercellular adhesion proteins. FASEB J. 2013;27:2001–2012. doi: 10.1096/fj.12-221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Fradin M., Edouard T., Le Merrer M., Alanay Y., Da Silva D.B., David A., Hamamy H., van Hest L., Lund A.M. OBSL1 mutations in 3-M syndrome are associated with a modulation of IGFBP2 and IGFBP5 expression levels. Hum. Mutat. 2009;31:20–26. doi: 10.1002/humu.21150. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzemich A., Kreiskother N., Alexandrovich A., Elliott C., Schock F., Leonard K., Sparrow J., Bullard B. The function of the M-line protein obscurin in controlling the symmetry of the sarcomere in the flight muscle of Drosophila. J. Cell Sci. 2012;125:3367–3379. doi: 10.1242/jcs.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzemich A., West R.J., Fukuzawa A., Sweeney S.T., Gautel M., Sparrow J., Bullard B. Binding partners of the kinase domains in Drosophila obscurin and their effect on the structure of the flight muscle. J. Cell Sci. 2015;128:3386–3397. doi: 10.1242/jcs.170639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Jones E.M., Van Rossum D.B., Bloch R.J. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol. Biol. Cell. 2003;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Lange S., Agarkova I., Perriard J.C., Ehler E. The sarcomeric M-band during development and in disease. J. Muscle Res. Cell Motil. 2005;26:375–379. doi: 10.1007/s10974-005-9019-4. [DOI] [PubMed] [Google Scholar]
- Lange S., Perera S., Teh P., Chen J. Obscurin and KCTD6 regulate cullin-dependent small ankyrin-1 (sAnk1.5) protein turnover. Mol. Biol. Cell. 2012;23:2490–2504. doi: 10.1091/mbc.E12-01-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Fernandez J.M. Mechanical design of the first proximal Ig domain of human cardiac titin revealed by single molecule force spectroscopy. J. Mol. Biol. 2003;334:75–86. doi: 10.1016/j.jmb.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Liu J., Das M., Yang J., Ithychanda S.S., Yakubenko V.P., Plow E.F., Qin J. Structural mechanism of integrin inactivation by filamin. Nat. Struct. Mol. Biol. 2015;22:383–389. doi: 10.1038/nsmb.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Montgiraud C., Munster A.B., Copeland O., Choi O., Dos Remedios C., Messer A.E., Ehler E., Knoll R. OBSCN mutations associated with dilated cardiomyopathy and haploinsufficiency. PLoS One. 2015;10:e0138568. doi: 10.1371/journal.pone.0138568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov G.N., Skubak P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann W.M., Gautel M., Steiner F., van der Ven P.F., Weber K., Furst D.O. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J. Cell Biol. 1996;134:1441–1453. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C.A., Dixon R., Stack C., Goicoechea S.M. Cytoplasmic Ig-domain proteins: cytoskeletal regulators with a role in human disease. Cell. Motil. Cytoskeleton. 2009;66:618–634. doi: 10.1002/cm.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Li H. Domain insertion effectively regulates the mechanical unfolding hierarchy of elastomeric proteins: toward engineering multifunctional elastomeric proteins. J. Am. Chem. Soc. 2009;131:14050–14056. doi: 10.1021/ja903589t. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez R., Garcia-Manyes S., Ainavarapu S.R., Fernandez J.M. Mechanical unfolding pathways of the enhanced yellow fluorescent protein revealed by single molecule force spectroscopy. J. Biol. Chem. 2006;281:40010–40014. doi: 10.1074/jbc.M609890200. [DOI] [PubMed] [Google Scholar]
- Pernigo S., Fukuzawa A., Bertz M., Holt M., Rief M., Steiner R.A., Gautel M. Structural insight into M-band assembly and mechanics from the titin-obscurin-like-1 complex. Proc. Natl. Acad. Sci. USA. 2010;107:2908–2913. doi: 10.1073/pnas.0913736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernigo S., Fukuzawa A., Pandini A., Holt M., Kleinjung J., Gautel M., Steiner R.A. The crystal structure of the human titin:obscurin complex reveals a conserved yet specific muscle M-band zipper module. J. Mol. Biol. 2015;427:718–736. doi: 10.1016/j.jmb.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Pernot P., Round A., Barrett R., De Maria Antolinos A., Gobbo A., Gordon E., Huet J., Kieffer J., Lentini M., Mattenet M. Upgraded ESRF BM29 beamline for SAXS on macromolecules in solution. Journal of Synchrotron Radiation. 2013;20(Pt 4):660–664. doi: 10.1107/S0909049513010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov M.V., Franke D., Shkumatov A.V., Tria G., Kikhney A.G., Gajda M., Gorba C., Mertens H.D., Konarev P.V., Svergun D.I. New developments in the program package for small-angle scattering data analysis. J. Appl. Crystallogr. 2012;45:342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotsis N., Lange S., Perriard J.C., Svergun D.I., Wilmanns M. Molecular basis of the C-terminal tail-to-tail assembly of the sarcomeric filament protein myomesin. EMBO J. 2008;27:253–264. doi: 10.1038/sj.emboj.7601944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotsis N., Chatziefthimiou S.D., Berkemeier F., Beuron F., Mavridis I.M., Konarev P.V., Svergun D.I., Morris E., Rief M., Wilmanns M. Superhelical architecture of the myosin filament-linking protein myomesin with unusual elastic properties. PLoS Biol. 2012;10:e1001261. doi: 10.1371/journal.pbio.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollazzon M., Suominen T., Penttila S., Malandrini A., Carluccio M.A., Mondelli M., Marozza A., Federico A., Renieri A., Hackman P. The first Italian family with tibial muscular dystrophy caused by a novel titin mutation. J. Neurol. 2009;257:575–579. doi: 10.1007/s00415-009-5372-3. [DOI] [PubMed] [Google Scholar]
- Qadota H., Blangy A., Xiong G., Benian G.M. The DH-PH region of the giant protein UNC-89 activates RHO-1 GTPase in Caenorhabditis elegans body wall muscle. J. Mol. Biol. 2008;383:747–752. doi: 10.1016/j.jmb.2008.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeker M.O., Russell M.W. Obscurin depletion impairs organization of skeletal muscle in developing zebrafish embryos. J. Biomed. Biotechnol. 2011;2011:479135. doi: 10.1155/2011/479135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo D., Giacomello E., Lorenzini S., Rossi D., Pierantozzi E., Blaauw B., Reggiani C., Lange S., Peter A.K., Chen J. Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity. J. Cell Biol. 2013;200:523–536. doi: 10.1083/jcb.201205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut H., Rose R.J., Hannan T.J., Hultgren S.J., Radford S.E., Ashcroft A.E., Waksman G. Donor-strand exchange in chaperone-assisted pilus assembly proceeds through a concerted beta strand displacement mechanism. Mol. Cell. 2006;22:831–842. doi: 10.1016/j.molcel.2006.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarparanta J., Blandin G., Charton K., Vihola A., Marchand S., Milic A., Hackman P., Ehler E., Richard I., Udd B. Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies. J. Biol. Chem. 2010;285:30304–30315. doi: 10.1074/jbc.M110.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F., Vahokoski J., Song Y.H., Wilmanns M. Molecular basis of the head-to-tail assembly of giant muscle proteins obscurin-like 1 and titin. EMBO Rep. 2010;11:534–540. doi: 10.1038/embor.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenauer R., Lange S., Hirschy A., Ehler E., Perriard J.C., Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. J. Mol. Biol. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Seeliger D., Haas J., de Groot B.L. Geometry-based sampling of conformational transitions in proteins. Structure. 2007;15:1482–1492. doi: 10.1016/j.str.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Seppala J., Tossavainen H., Rodic N., Permi P., Pentikainen U., Ylanne J. Flexible structure of peptide-bound filamin a mechanosensor domain pair 20-21. PLoS One. 2015;10:e0136969. doi: 10.1371/journal.pone.0136969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small T.M., Gernert K.M., Flaherty D.B., Mercer K.B., Borodovsky M., Benian G.M. Three new isoforms of Caenorhabditis elegans UNC-89 containing MLCK-like protein kinase domains. J. Mol. Biol. 2004;342:91–108. doi: 10.1016/j.jmb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Spooner P.M., Bonner J., Maricq A.V., Benian G.M., Norman K.R. Large isoforms of UNC-89 (obscurin) are required for muscle cell architecture and optimal calcium release in Caenorhabditis elegans. PLoS One. 2012;7:e40182. doi: 10.1371/journal.pone.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin A., Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 2010;66:22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- Waterston R.H., Thomson J.N., Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans. Dev. Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Will R.D., Eden M., Just S., Hansen A., Eder A., Frank D., Kuhn C., Seeger T.S., Oehl U., Wiemann S. Myomasp/LRRC39, a heart- and muscle-specific protein, is a novel component of the sarcomeric M-band and is involved in stretch sensing. Circ. Res. 2010;107:1253–1264. doi: 10.1161/CIRCRESAHA.110.222372. [DOI] [PubMed] [Google Scholar]
- Wilson K.J., Qadota H., Mains P.E., Benian G.M. UNC-89 (obscurin) binds to MEL-26, a BTB-domain protein, and affects the function of MEI-1 (katanin) in striated muscle of Caenorhabditis elegans. Mol. Biol. Cell. 2012;23:2623–2634. doi: 10.1091/mbc.E12-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Lobley C.M., Prince S.M. Decision making in xia2. Acta Crystallogr. D Biol. Crystallogr. 2013;69:1260–1273. doi: 10.1107/S0907444913015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Grater F. Molecular basis of the mechanical hierarchy in myomesin dimers for sarcomere integrity. Biophys. J. 2014;107:965–973. doi: 10.1016/j.bpj.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Ehler E., Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J. Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.