Abstract
The c-Fes protein-tyrosine kinase (Fes) has been implicated in the differentiation of vascular endothelial, myeloid hematopoietic, and neuronal cells, promoting substantial morphological changes in these cell types. The mechanism by which Fes promotes morphological aspects of cellular differentiation is unknown. Using COS-7 cells as a model system, we observed that Fes strongly colocalizes with microtubules in vivo when activated via coiled-coil mutation or by coexpression with an active Src family kinase. In contrast, wild-type Fes showed a diffuse cytoplasmic localization in this system, which correlated with undetectable kinase activity. Coimmunoprecipitation and immunofluorescence microscopy showed that the N-terminal Fes/CIP4 homology (FCH) domain is involved in Fes interaction with soluble unpolymerized tubulin. However, the FCH domain was not required for colocalization with polymerized microtubules in vivo. In contrast, a functional SH2 domain was essential for microtubule localization of Fes, consistent with the strong tyrosine phosphorylation of purified tubulin by Fes in vitro. Using a microtubule nucleation assay, we observed that purified c-Fes also catalyzed extensive tubulin polymerization in vitro. Taken together, these results identify c-Fes as a regulator of the tubulin cytoskeleton that may contribute to Fes-induced morphological changes in myeloid hematopoietic and neuronal cells.
The human c-fes gene encodes a 93-kDa nonreceptor protein-tyrosine kinase that is predominantly expressed in myeloid hematopoietic cells, the vascular endothelium, and several types of neurons (9, 24). Activated forms of Fes can influence morphological differentiation in multiple cell types. For example, Fes overexpression induces growth arrest and terminal differentiation in the myeloid leukemia cell line K-562, leading to cell attachment and spreading (2, 31). Active Fes has also been shown to promote differentiation of the cytokine-dependent myeloid leukemia cell line TF-1, leading to the extension of long cellular processes in some cases (3). In PC12 cells, overexpression of wild-type Fes accelerates neurite outgrowth in response to NGF, while kinase-active Fes induces spontaneous neurite formation (13, 22). These results implicate Fes in signaling pathways that control the morphological differentiation of myeloid hematopoietic and neuronal cells.
Structurally, c-Fes consists of a C-terminal kinase domain, a central SH2 domain, and a long N-terminal region (9, 24). While the SH2 and kinase domains are closely related to those found in other cytoplasmic tyrosine kinases, such as c-Src and c-Abl, the N-terminal region is unique to Fes and the closely related kinase Fer (6). Previous work from our laboratory has identified at least two regions of coiled-coil homology within the unique N-terminal region and has shown that these coiled-coil motifs contribute to the regulation of kinase activity in vivo (2, 3, 17). Unlike its transforming viral counterparts, wild-type c-Fes tyrosine kinase activity is strictly regulated in mammalian cells. However, mutation or deletion of the more N-terminal coiled-coil motif is sufficient to release c-Fes tyrosine kinase and transforming activities in rodent fibroblasts and to induce morphological differentiation of hematopoietic and neuronal cell lines as described above (2, 3). The N-terminal region also includes a Fes/CIP4 homology (FCH) domain, which was first described as a region of homology between Fes/Fer and the Cdc42-interacting protein, CIP4 (1). The FCH domains of CIP4 and Fes have been reported to bind to microtubules (25, 27). Although the CIP4 FCH domain has been implicated in cytoskeletal rearrangement, a functional role for the Fes FCH domain has not been identified.
Microtubules (MTs) are dynamic polymers of α-β tubulin heterodimers that play an essential role in cell division, cytoplasmic organization, and organelle mobility, as well as the generation and maintenance of cell polarity, which often characterizes cellular differentiation (7). At least two populations of MTs, called dynamic and stable according to their rates of turnover, are readily distinguishable in cells. In proliferating and undifferentiated cells, dynamic MTs with a half-life of minutes prevail in the cytoplasm (20, 21). In contrast, stable MTs predominate in differentiated and polarized cell types and have a half-life of an hour or more. The MT-associated proteins are among the best-known factors that regulate MT dynamics and stability by suppressing catastrophes and increasing rescues (7). However, growing evidence suggests that MT stability may also be regulated by tyrosine phosphorylation. For example, tyrosine phosphorylation of tubulin by the Src family kinases Lyn and Fyn alters tubulin assembly and monocyte differentiation in the HL-60 myeloid leukemia cell line (11).
In this study, we provide direct evidence that the c-Fes tyrosine kinase influences MT dynamics both in vitro and in vivo. Activation of Fes led to strong colocalization with the MT network in COS-7 cells, including association with the mitotic spindle during metaphase. While the FCH domain was required for Fes binding to soluble tubulin in vitro, Fes mutants lacking the domain still colocalized with MTs in cells. In contrast, both kinase activity and a functional SH2 domain were required for colocalization of Fes and MT. Purified Fes efficiently catalyzed the tyrosine phosphorylation of tubulin dimers in vitro and promoted the assembly of long tubulin polymers in the absence of other regulatory proteins. Together, these data support a role for Fes as an MT regulator and suggest a mechanism for Fes-induced morphological differentiation in multiple cell types.
MATERIALS AND METHODS
Construction of Fes expression vectors.
The kinase-active coiled-coil domain mutant of human c-Fes (L145P), as well as a kinase-dead mutant (K590E), have been described elsewhere (3, 19). Wild-type and mutant Fes cDNAs were subcloned downstream and in frame with the coding sequence for an enhanced yellow-shifted variant of green fluorescent protein (EYFP) in the plasmid vector pEYFP-C1 (BD Bioscience-Clontech). For simplicity, EYFP is referred to as green fluorescent protein (GFP) here. Fes FCH domain deletion mutants lacking N-terminal amino acids 1 to 58 were generated using a PCR-based approach in the context of both wild-type Fes and the active L145P mutant (FesΔFCH and L145PΔFCH, respectively). The coding sequence of the Fes FCH domain was also amplified by PCR and subcloned into pEYFP-C1. The SH2 domain of Fes L145P was inactivated by replacing the conserved arginine residue in the phosphotyrosine binding pocket with leucine at position 483 (L145P-RL). All Fes constructs used in this study encode a C-terminal FLAG epitope tag.
Cell culture.
COS-7 and 293T cells were obtained through the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium supplemented with 10 or 5% fetal bovine serum, respectively, and 50 μg of gentamicin/ml. Transient transfections of COS and 293T cells were performed using standard calcium phosphate techniques as described elsewhere (19). In some experiments, cells were treated with Colcemid (desacetyl-N-methyl colchicine; Sigma) to a final concentration of 500 nM and returned to the incubator for 1 h to destabilize the MTs (16).
Immunoprecipitation and immunoblotting.
COS-7 or 293T cells were washed with phosphate-buffered saline (PBS) and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.4], 50 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 1% Triton X-100) supplemented with 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 50 μM Na2MoO4, 20 mM NaF, 50 μg of leupeptin/ml, and 25 μg of aprotinin/ml. The cells were then sonicated three times for 10 s each time at 4°C. The lysates were clarified by centrifugation and diluted with 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (125 mM Tris-HCl [pH 6.8], 20% glycerol, 4% SDS, 172 mM 2-mercaptoethanol, 0.05% bromophenol blue). For immunoprecipitation, clarified cell extracts were incubated with the antibodies described below and protein G-Sepharose (AP Biotech) for 2 h at 4°C. The precipitates were collected by brief centrifugation and washed by resuspending them three times with 1.0 ml of RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate). The precipitated proteins were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and visualized by immunoblotting. Immunoreactive bands were detected using an alkaline phosphatase-conjugated secondary antibody followed by colorimetric detection with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate or the chemiluminescent reagent CDP-Star (Perkin-Elmer Life Science) and autoradiography. Tubulin was both immunoprecipitated and immunoblotted using an anti-α-tubulin mouse monoclonal antibody (Molecular Probes). GFP-Fes expression was detected on immunoblots using an anti-GFP rabbit polyclonal antibody (Sigma).
Immunofluorescence microscopy.
Transfected COS-7 cells were fixed with 3.7% paraformaldehyde in PBS for 10 min followed by three washes with PBS and treated with 0.5% saponin in PBS for 15 min. The cells were then blocked in PBS containing 3% bovine serum albumin for 60 min and incubated with a mouse anti-α-tubulin antibody (Molecular Probes; 1:200 dilution) and an anti-Fes phosphospecific antibody (1:1,000 dilution) that recognizes phosphotyrosine 713 in the activation loop (26). Cells for confocal imaging (Fig. 1) were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 2% bovine serum albumin in PBS. The cells were incubated with the primary antibodies overnight at 4°C and visualized with secondary antibodies conjugated to Cy3 (Molecular Probes), Cy5 (Sigma), or Texas red (Molecular Probes). Fluorescent images were recorded using a Nikon TE300 inverted microscope with epifluorescence capability and a SPOT cooled charge-coupled device high-resolution digital camera and software (Diagnostic Instruments). The confocal images shown in Fig. 1 and 2 were acquired using a Zeiss LSM 5 laser scanning confocal microscope equipped with a 63× numerical aperture 1.4 oil immersion plan-apochromat objective. Fluorescence images were acquired sequentially using the 543- and 633-nm lines from an He-Ne laser to excite Cy3 and Cy5, respectively; a 488-nm Ar laser line was used to excite GFP.
FIG. 1.
Colocalization of kinase-active but not wild-type c-Fes with MTs. Wild-type GFP-Fes (WT) or a kinase-active GFP-Fes mutant (L145P) was transiently expressed in COS-7 cells. Forty-eight hours after transfection, the cells were fixed, permeabilized, and immunostained with both α-tubulin and Fes-tyrosine phosphospecific antibodies. Secondary antibodies conjugated with Cy3 or Cy5 were used to differentiate tubulin from active Fes (pFes) staining by confocal microscopy. Overall Fes protein distribution was visualized by GFP fluorescence. A merged image is shown on the far right. GFP, Cy5, and Cy3 fluorescences are represented as green, blue, and red, respectively, while merged colors appear as white.
FIG. 2.
Activation of wild-type Fes induces localization to MTs in vivo. COS-7 cells were transiently transfected with expression vectors for wild-type GFP-Fes (WT), kinase-active GFP-Fes (L145P), kinase-dead GFP-Fes (K590E), or the empty vector (vector) as a control. Cells were also cotransfected with a combination of wild-type or kinase-dead GFP-Fes and an activated form of the Src family kinase, Hck-YF. Forty-eight hours after transfection, the cells were fixed and stained with α-tubulin, Hck, and/or Fes-tyrosine phosphospecific antibodies. (A) Confocal images of a representative cell coexpressing wild-type GFP-Fes and Hck-YF. Overall Fes protein distribution was visualized by GFP fluorescence. Secondary antibodies conjugated with Cy3 or Cy5 were used to differentiate tubulin from active Fes (pFes) staining. GFP, Cy5, and Cy3 fluorescences are represented as green, blue, and red, respectively, while merged colors appear white. (B) (Top) Cells from each transfection condition were examined for the appearance of MT-like localization of each GFP-Fes protein. For cotransfected cultures, only cells exhibiting GFP-Fes and Hck protein expression by immunofluorescence were counted. At least 100 cells were counted for each condition. The error bars represent standard deviations. (Bottom) Immunoblot analysis of GFP-Fes phosphotyrosine content (pTyr; top), protein level (GFP; middle), and Hck protein expression (bottom).
Phosphorylation of tubulin in vitro and MT assembly assay.
Purified bovine brain tubulins (rhodamine tagged and unmodified) were purchased from Cytoskeleton, Inc. Rhodamine-labeled tubulin was mixed with unlabeled tubulin in a 1:9 ratio at a final concentration of 3.5 mg/ml in BRB80 MT stabilizing buffer [80 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8), 5 mM MgCl2, 1 mM EGTA, and 2 mM GTP] in the presence or absence of ATP (100 μM). Purified human c-Fes protein (5 ng/μl; Upstate Biotechnology) or Fes buffer (50 mM Tris-HCl, pH 7.5) was incubated with the tubulin dimers in a final volume of 12.5 μl for 60 min at 37°C. A 2-μl aliquot of each reaction mixture was diluted with 18 μl of BRB80 buffer and 20 μl of 2× SDS-PAGE sample buffer for immunoblot analysis. Tyrosine-phosphorylated tubulin was detected using the antiphosphotyrosine monoclonal antibody PY99 (Santa Cruz Biotechnology). The remaining mixture was combined with an equal volume of BRB80 buffer containing 60% glycerol and placed on ice. The reaction mixture was squashed between a glass microscope slide and a coverslip, and rhodamine fluorescence was visualized under the microscope. These methods are adapted from those of Desai et al. (8). Each reaction mixture contained 42 μg of tubulin protein (∼55 kDa) and 62 ng of His-tagged Fes kinase protein (∼94 kDa) for a Fes/tubulin molar ratio of roughly 1:1,000.
RESULTS
Fes colocalizes with the MT network in COS-7 cells.
Previous work from our laboratory demonstrated that a point mutation within the first coiled-coil domain stimulates Fes tyrosine kinase and biological activities in vivo (3, 13). Expression of this active form of Fes (L145P) in myeloid and neuronal cell lines, two physiological sites of Fes expression, produces dramatic changes in cellular shape and leads to the extension of long cellular processes in some cases. To determine whether Fes activation correlates with a change in subcellular localization, wild-type Fes and the active L145P mutant were transiently expressed as GFP fusion proteins in COS-7 cells. Confocal images of cells expressing the GFP-Fes fusion proteins are shown in Fig. 1. The kinase-active Fes mutant consistently showed a distribution strikingly similar to the prominent meshwork of MTs present in this cell line. In contrast, wild-type Fes demonstrated a diffuse cytoplasmic distribution consistent with previous work on rodent fibroblasts and myeloid cell lines (18).
To investigate whether this activated Fes mutant colocalizes with MTs, transfected COS-7 cells were stained with a tubulin antibody and with a phosphospecific antibody raised against a phosphopeptide corresponding to the Fes kinase domain activation loop sequence surrounding the autophosphorylation site at tyrosine 713 (26). The superimposed images shown in Fig. 1 reveal strong colocalization of the kinase-active Fes mutant with the MT network. In contrast, cells expressing wild-type GFP-Fes showed low levels of diffuse, nonspecific staining with the phosphospecific antibody, consistent with previous work showing that wild-type Fes adopts an inactive conformation in vivo (2, 3, 18, 26). These results demonstrate that Fes activation correlates with redistribution to the tubulin cytoskeleton in this cell type.
Activation of wild-type Fes induces redistribution to MTs in vivo.
The results presented in Fig. 1 strongly suggest that activation induces movement of Fes onto MTs. However, this localization may represent a consequence of the activating mutation and not a property of wild-type Fes. To distinguish between these two possibilities, wild-type GFP-Fes was expressed in the presence of an active form of Hck (Hck-YF), a member of the Src kinase family expressed in myeloid cells (5, 14). Previous work had established that Src family kinases can directly phosphorylate Fes on its activation loop as a possible mechanism of activation in vascular endothelial cells (10). Cotransfected cells were stained with the tubulin and Fes phosphospecific antibodies. The confocal images shown in Fig. 2A reveal that Hck-induced activation of wild-type GFP-Fes led to colocalization with the MT network. The resulting staining pattern radiates from the center of the cell and is very similar to that observed with the kinase-active form of Fes shown in Fig. 1.
Transfected cells were also stained with an Hck antibody, and the numbers of cells exhibiting MT-like localization of GFP-Fes in the presence and absence of Hck-YF were determined. As shown in Fig. 2B, GFP-Fes exhibited MT localization in approximately one-half of the cells that coexpressed Hck-YF; this number compares favorably to that observed with cells expressing GFP-Fes-L145P alone (75%). In contrast, <5% of cells expressing wild-type GFP-Fes alone exhibited MT localization, supporting the idea that Fes activation is required for localization to MT in vivo. The immunoblots shown in the lower part of Fig. 2B confirm that wild-type GFP-Fes is not active when expressed alone in COS-7 cells, consistent with results in other cell types. On the other hand, coexpression with Hck-YF induced strong GFP-Fes tyrosine phosphorylation; GFP-Fes-L145P also showed high phosphotyrosine content, in agreement with previous studies of this mutant (3, 13).
We also tested whether an active Fes kinase domain is required for MT association in COS-7 cells. For these experiments, a kinase-dead mutant of GFP-Fes (K590E) was expressed in the presence or absence of Hck-YF, and the cells were examined for the association of this mutant with MTs. Figure 2B shows that GFP-Fes K590E failed to localize to MTs in the presence or absence of Hck-YF, despite the phosphorylation of the mutant upon coexpression with Hck-YF. In addition, when this kinase-inactivating mutation was introduced into the context of GFP-Fes-L145P, the mutant lost its MT association and remained diffusely distributed in the cytoplasm (data not shown). These results show that the Fes kinase domain must adopt and maintain an active conformation in order for the protein to remain associated with the MT network.
Fes phosphorylates tubulin and promotes MT polymerization in vitro.
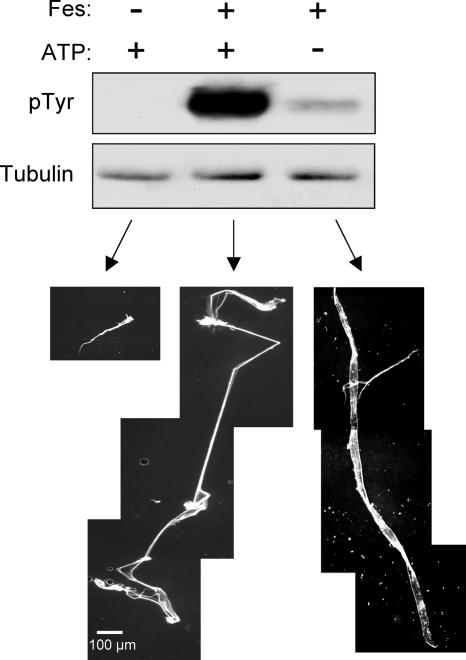
To investigate whether tubulin is a substrate for Fes, we incubated purified, rhodamine-labeled tubulin heterodimers in the presence or absence of purified Fes kinase protein at a Fes/tubulin molar ratio of ∼1:1,000. Following incubation, aliquots of each reaction mixture were analyzed by immunoblotting for the presence of phosphorylated tubulin. As shown in Fig. 3, Fes efficiently phosphorylated tubulin under these conditions. A control reaction run in the absence of added ATP revealed only a trace of tubulin phosphorylation, which might have been due to a low level of ATP bound to the active site of the purified Fes kinase.
FIG. 3.
Purified Fes catalyzes tubulin phosphorylation and polymerization in vitro. Purified Fes kinase was incubated with purified rhodamine-labeled bovine tubulin dimers in the presence (+) or absence (−) of ATP, as indicated. Tubulin was also incubated in the absence of Fes as a negative control. Reaction mixtures were incubated for 60 min at 37°C, and aliquots were analyzed by immunoblotting for evidence of tubulin tyrosine phosphorylation (pTyr) (top). Replicate blots were probed with a tubulin antibody to verify equivalent levels of tubulin in each reaction mixture. (Bottom) The remainder of the reaction mixture was squashed on a microscope slide with a coverslip, and polymerized tubulin was visualized as red fluorescence.
To determine if Fes affects MT assembly in vitro, Fes was incubated with purified rhodamine-tubulin as described above. Following incubation, the mixture was squashed between a glass slide and a coverslip and tubulin polymers were observed by fluorescence microscopy. As shown in Fig. 3, incubation of tubulin alone under these conditions resulted in the formation of short MT-like structures. However, the addition of Fes and ATP led to the dramatic appearance of MT-like polymers with long, fine strands. Interestingly, incubation of Fes with tubulin in the absence of ATP resulted in polymerized strands longer than those observed in the control reaction without Fes, although they were consistently shorter than those observed in the presence of both Fes and ATP. These results show that Fes can induce the polymerization of tubulin into stable MT-like assemblies in vitro and that Fes-mediated polymerization does not require stoichiometric tubulin phosphorylation by Fes (see Discussion).
Active Fes induces MT polarization in vivo.
To investigate whether Fes expression alters MT dynamics in vivo, COS-7 cells were transfected with either the active Fes mutant or GFP alone and treated with the MT-destabilizing agent Colcemid (16). Following Colcemid washout, the cells were allowed to recover for 120 min to permit MT reassembly. The cells were then fixed and stained with the tubulin antibody, and the results are shown in Fig. 4. In control cells expressing GFP alone, Colcemid induced MT collapse, resulting in the appearance of a speckled pattern of staining with the tubulin antibody (data not shown). Following washout, MTs returned to their starting morphology, showing a randomly oriented staining pattern that often included bundles that wrapped around the nucleus. In cells expressing GFP-Fes-L145P, Colcemid also induced a loss of tubulin fibers and the appearance of speckles (not shown). Interestingly, GFP-Fes fluorescence adopted this distribution as well, indicative of continued association with tubulin following depolymerization (see Fig. 6). Following washout, MTs again reappeared, but in this case they were more organized, forming fibers radiating from the center of the cell. These results suggest that Fes affects MT formation in vivo and is consistent with its ability to regulate MT assembly in vitro.
FIG. 4.
Active Fes reorients MT formation in vivo. COS-7 cells expressing either kinase-active GFP-Fes-L145P or GFP alone were treated with Colcemid for 60 min. The drug was washed out, and the cells were allowed to recover for 120 min prior to being fixed and stained for tubulin using a secondary antibody conjugated to Texas red. GFP and GFP-Fes-L145P fluorescence are shown on the left; tubulin staining is shown on the right.
FIG. 6.
Depolymerized tubulin remains associated with Fes in an FCH domain-dependent manner. COS-7 cells expressing either kinase-active GFP-Fes (L145P) or the kinase-active Fes FCH deletion mutant (LP-ΔFCH) were incubated in the absence (− Colcemid; upper six panels) or presence (+ Colcemid; lower six panels) of 500 nM Colcemid for 60 min. The cells were fixed and stained for α-tubulin using a secondary antibody conjugated to Texas red. Fes localization was followed as GFP fluorescence, while tubulin staining is pseudocolored red; merged images are shown on the right. This experiment was repeated twice with comparable results.
The FCH domain is involved in Fes interaction with tubulin but not with MTs.
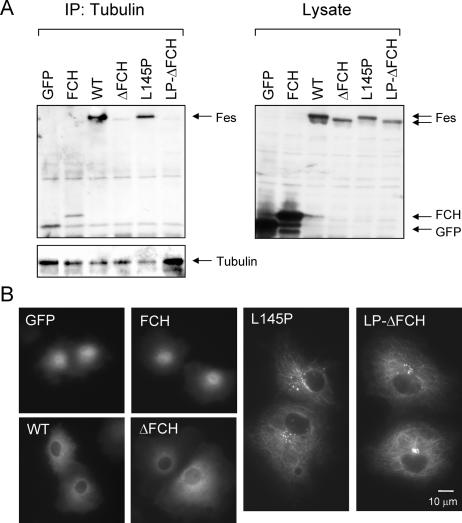
The Fes N-terminal region contains the FCH domain, so named because of its sequence homology with the Cdc42-interacting protein, CIP4 (1). The Fes FCH domain has been suggested to play a role in the interaction of Fes with both tubulin and MTs (9, 25). To test this possibility directly, we created GFP expression constructs with the FCH domain alone, as well as wild-type and active Fes with the FCH deletion (Fes-ΔFCH and L145P-ΔFCH, respectively). These constructs, along with wild-type Fes and the L145P mutant, were tested for the ability to bind endogenous tubulin in 293T cell extracts using a coimmunoprecipitation assay. Soluble tubulin was immunoprecipitated from transfected 293T cell lysates and tested for associated Fes proteins by immunoblotting. As shown in Fig. 5A, both wild-type and L145P Fes proteins were readily detected in the anti-tubulin immunoprecipitates, indicative of complex formation. However, the corresponding FCH deletion mutants failed to associate with tubulin in this assay, indicating a role for the domain in tubulin binding. Unexpectedly, the GFP-FCH fusion protein coprecipitated with tubulin at the same low level as the GFP control, suggesting that the FCH domain is necessary but not sufficient to bind soluble tubulin in vitro.
FIG. 5.
The Fes FCH domain is required for tubulin binding in vitro but not for MT localization of Fes in vivo. (A) 293T cells were transiently transfected with expression vectors for GFP alone, a GFP-FCH domain fusion protein (FCH), wild-type GFP-Fes (WT), a GFP-Fes FCH deletion mutant (ΔFCH), kinase-active GFP-Fes (L145P), and the active mutant with the FCH deletion (LP-ΔFCH). Soluble tubulin was immunoprecipitated (IP) from clarified cell extracts, and associated GFP-Fes fusion proteins were detected with the GFP antibody. (Left) GFP blot of associated GFP-Fes proteins. The GFP blot was stripped and reprobed with the tubulin antibody to verify the presence of tubulin in each immunoprecipitate. (Right) Immunoblot of cell lysates showing expression of all GFP-Fes fusion proteins and GFP alone. Note that GFP and GFP-FCH were expressed at very high levels, leading to low levels of nonspecific binding in the coimmunoprecipitation experiment. This experiment was repeated twice with comparable results. (B) COS-7 cells were transfected with the same GFP-Fes constructs described in the legend to panel A. Localization of all constructs was determined in at least two independent experiments; representative live-cell images of GFP fluorescence are shown.
We next explored the role of the FCH domain in the association of active Fes with MTs in COS-7 cells. Surprisingly, live-cell images showed that the active Fes mutant with the FCH deletion localized to MTs in a manner indistinguishable from that of the same mutant with an intact FCH domain (Fig. 5B). The GFP-FCH fusion also failed to localize to MTs in vivo, suggesting that other Fes domains are involved. Deletion of FCH from wild-type Fes did not impact its diffuse cytoplasmic localization. Together with data shown in Fig. 5A, these results show that while the FCH domain is required for the interaction of Fes with soluble tubulin, it is not required for binding to the MT network in COS-7 cells.
Disruption of Fes-MT association by Colcemid treatment.
The data presented in Fig. 5 demonstrate that the FCH domain may allow Fes interaction with depolymerized forms of tubulin, but it is dispensable for stable association with polymerized MTs. To address this issue further, COS-7 cells were transfected with GFP-Fes-L145P and the corresponding ΔFCH mutant and treated with the MT-disrupting agent Colcemid. The cells were then stained with the tubulin antibody, and the localizations of Fes and tubulin were recorded by fluorescence microscopy. As shown in Fig. 6, both GFP-L145P and the ΔFCH mutant colocalized with MTs in the absence of Colcemid treatment, consistent with Fig. 5B. Dissociation of MTs with Colcemid resulted in a dramatic change in the localization of GFP-Fes-L145P, from the MT-like staining pattern to a series of small speckles. About 60% of the GFP-Fes-L145P-positive cells exhibited this speckled pattern of localization following Colcemid treatment, with the remainder showing a more diffuse Fes distribution (data not shown). As expected, tubulin staining revealed that MTs were disrupted by Colcemid treatment. Interestingly, tubulin staining produced an identical speckled pattern in Colcemid-treated cells expressing the Fes-L145P mutant, suggesting that depolymerized tubulin remained bound to active Fes following Colcemid treatment (Fig. 6, merged image).
Colcemid treatment also disrupted GFP-Fes-L145P-ΔFCH localization to MTs, resulting in a speckled localization pattern. However, this pattern was not observed upon staining of the same cells with the tubulin antibody. This observation suggests that association of Fes with depolymerized tubulin following Colcemid treatment requires FCH function and may have implications for the ability of Fes to nucleate MT formation (see Discussion).
The Fes SH2 domain is required for colocalization with MTs in vivo.
Because the FCH domain is dispensable for Fes association with MTs in vivo, we investigated the roles of other Fes domains in this localization. Previous reports had suggested that SH2 domains from Src family kinases might interact with tubulin (11, 15). To investigate the possible role of the Fes SH2 domain in this regard, we replaced the conserved Arg residue in the phosphotyrosine binding pocket of the Fes SH2 domain with Leu (R483L), a mutation known to block the SH2 phosphotyrosine binding function. This mutation was introduced into the GFP-Fes-L145P construct, and its localization was recorded in live cells by fluorescence microscopy. As shown in Fig. 7, the SH2 domain R483L mutation completely reversed the MT association of L145P. Whereas >60% of cells expressing GFP-Fes-L145P demonstrated MT-associated localization, this number dropped to <5% with the L145P-R483L mutant (Fig. 7B). The immunoblots shown in Fig. 7B demonstrate that this mutant was still kinase active, indicating that the SH2 domain is required for stable association with MTs in vivo. This finding suggests a role for tyrosine phosphorylation of tubulin by Fes and SH2 engagement in its stable association with MT.
FIG. 7.
Localization of Fes to MTs requires a functional SH2 domain. COS-7 cells were transfected with expression plasmids for wild-type GFP-Fes (WT), kinase-active GFP-Fes (L145P), or active Fes with a Leu substitution of Arg 483 in the SH2 domain phosphotyrosine binding pocket (LP-RL). (A) Transfected cells were visualized by GFP fluorescence, and digital images were recorded. (B) Cells from each transfection condition were examined for the appearance of MT-like localization of each GFP-Fes protein. At least 150 cells were counted for each condition. The results are presented as the mean plus standard deviation of three separate experiments. (Bottom) Immunoblots from transfected cell lysates showing phosphotyrosine content of GFP-Fes proteins (pTyr; top) and relative expression levels using a GFP antibody (Fes; bottom). The positions of the GFP-Fes proteins are indicated by the arrows.
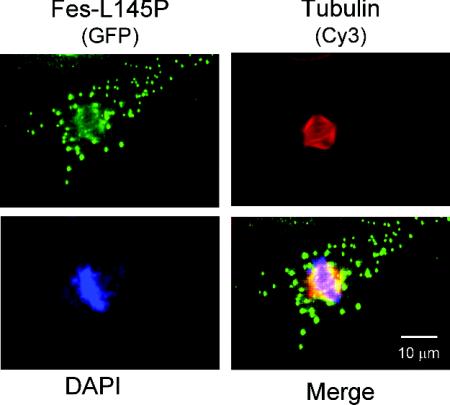
Fes binds to the mitotic spindle during cell division.
The data presented so far strongly suggest that Fes interacts directly with both tubulin and MTs, catalyzes tubulin tyrosine phosphorylation, and may regulate MT dynamics in vivo. Treatment of cells with Colcemid causes a loss of MT structure and associated Fes, although tubulin remains bound to Fes through its FCH domain (Fig. 6). To investigate whether Fes follows dynamic changes in the tubulin cytoskeleton under physiological conditions, we observed the changes in Fes-L145P localization that occur during M phase, when the cellular MT network breaks down and the mitotic spindle forms as the cell prepares to divide (4). Figure 8 shows the patterns of GFP-Fes-L145P and tubulin staining of a representative COS-7 cell in metaphase. Tubulin staining reveals that the cytoplasmic MT network has depolymerized, while the mitotic spindle is very prominent. Fes-L145P shows localization to two distinct sites in this cell. Prominent cytoplasmic speckles are present, very similar to the ones observed in Colcemid-treated cells and consistent with the depolymerization of cytoplasmic MTs. In addition, Fes is also associated with the mitotic spindle, suggesting a possible role for Fes in spindle assembly and chromosomal segregation.
FIG. 8.
Active Fes colocalizes with the mitotic spindle during metaphase. COS-7 cells were transfected with the GFP-L145P expression vector. Forty-eight hours later, the cells were fixed and immunostained for tubulin using a secondary antibody conjugated to Cy3 (red). DNA was visualized by DAPI (4′,6′-diamidino-2-phenylindole) staining (blue). Fes was localized as GFP fluorescence (green). A merged image is also shown.
DISCUSSION
Previous work from our laboratory established an important role for Fps/Fes kinases in regulating morphological aspects of differentiation in a variety of cell types. In myeloid hematopoietic and neuronal cells in particular, Fes can induce the extension of long cellular processes, suggesting a connection to remodeling of the cytoskeleton (3, 22). In a recent study, it was found that Fes can regulate the actin cytoskeleton in PC12 cells through a unique pathway involving the breakpoint cluster region protein (Bcr) and Rho family GTPases (13). In the present study, we focused on the tubulin cytoskeleton, based on the presence of the FCH domain in the Fes N-terminal region. We found that Fes has the ability to phosphorylate tubulin in vitro and strongly induces tubulin polymerization in the absence of other cellular proteins. Fes localized to MTs in transfected COS-7 cells, and localization required an active kinase domain, as well as a functional SH2 domain. While the FCH domain was involved in Fes binding to tubulin in solution, it was dispensable for stable association with MTs in vivo. Finally, Fes was shown for the first time to associate with the mitotic spindle, suggesting a function in the regulation of the M phase of the cell cycle.
The data presented here strongly suggest that Fes kinase activation is a prerequisite for association with the tubulin cytoskeleton in vivo. First, wild-type Fes, which exists in an inactive state in COS-7 and most other cell types examined, exhibits a diffuse cytoplasmic localization. In contrast, a mutant of Fes activated by a Pro-for-Leu substitution in the first N-terminal coiled-coil domain, showed strong autophosphorylation and constitutive localization to MTs (Fig. 1). In addition, activation of wild-type GFP-Fes by coexpression with an active Src family kinase (Hck) also resulted in MT localization (Fig. 2). Note that Hck itself did not associate with MTs in these experiments (data not shown). Interestingly, coexpression of kinase-dead Fes with Hck did not lead to MT localization, despite transphosphorylation of this mutant by Hck. This suggests that the kinase activity of Fes itself is essential for MT association, which may result from tubulin phosphorylation and binding through the SH2 domain (see below). In addition, double immunostaining of cells expressing active Fes with tubulin and phosphospecific Fes antibodies clearly showed that the active Fes molecules are associated with the MT network in vivo (Fig. 1). These findings suggest that activation of Fes by growth factors and cytokines may contribute to morphological aspects of differentiation through modulation of the tubulin cytoskeleton. One example is neuronal cells, in which overexpression of wild-type Fes leads to greater numbers and longer neurites following treatment with NGF (22). Note that kinase-active GFP-Fes localizes to MTs in the neuroblastoma cell line N1E-115 (C. Laurent and T. Smithgall, unpublished data), while endogenous Fes has been shown to associate with MTs in primary cultures of hippocampal neurons (25).
The association of kinase-active Fes with MTs in vivo prompted us to examine whether Fes can alter MT dynamics. Here, we show for the first time that Fes induces dramatic tubulin polymerization in vitro. Incubation of purified Fes kinase with tubulin dimers produced very long unbranched strands of polymerized tubulin (Fig. 3). One remarkable aspect of this experiment is the very low stoichiometry of Fes to tubulin required for polymerization to occur, suggesting that Fes acts in a catalytic fashion to promote MT assembly. Previous work has established that Fes is oligomeric, with the active form of the kinase consisting of at least four monomers held together through the N-terminal coiled-coil domains (17). This suggests that Fes may grab multiple tubulin dimers simultaneously, thus promoting their assembly by producing local areas of high tubulin concentration. Interestingly, tubulin polymerization assays run in the absence of ATP but in the presence of Fes still produced long polymerized strands. Anti-phosphotyrosine immunoblots show that very low levels of tubulin phosphorylation occurred despite the absence of added ATP (Fig. 3); this phosphorylation was probably due to small amounts of ATP that copurify with Fes. Nevertheless, these results suggest that stoichiometric phosphorylation of tubulin is not required for Fes-mediated MT assembly in vitro. Instead, tyrosine phosphorylation may promote stable association of Fes with assembled MTs through an SH2-mediated mechanism (see below).
We next investigated the structural domains of Fes that contribute to stable interaction with MTs in vivo. The unique Fes N-terminal region contains an FCH domain, which has been reported to function in MT binding in other systems (27). While removing this domain prevented Fes from associating with soluble tubulin in coimmunoprecipitation experiments, FCH deletion had no effect on active Fes association with MTs in COS-7 cells (Fig. 5). This result suggests that Fes binds to tubulin dimers and polymerized MT through distinct sites. Consistent with this hypothesis is our observation that active Fes lacking an FCH domain bound to intact MTs but failed to associate with tubulin following dissociation of MTs with Colcemid (Fig. 6). Distinct sites of interaction for tubulin and polymerized MTs have also been reported for the inner centromere protein INCENP, which has an essential role in the late stages of mitosis (28). Like Fes, INCENP also has coiled-coil domains, which in this case contribute to association with MTs. However, deletion of either of the c-Fes coiled-coil domains did not affect localization to MT, indicating that the Fes coiled coils are not directly involved in MT binding in vivo (data not shown).
Our observations that Fes strongly phosphorylates tubulin in vitro (Fig. 3) and that colocalization of Fes with MTs is kinase dependent suggested a possible role for the Fes SH2 domain in this association. A point mutation in the phosphotyrosine binding pocket of the SH2 domain completely reversed the localization of the kinase-active Fes mutant with MTs (Fig. 7). This observation suggests that Fes-induced tyrosine phosphorylation of tubulin may be responsible for stable association through its SH2 domain. Other nonreceptor tyrosine kinases, including the Src family member Fyn, have also been reported to interact with tubulin through their SH2 domains (11, 12, 15).
Not only does Fes associate with MTs in interphase cells, it also retains this association during M phase as the MT network reorganizes to form the mitotic spindle (Fig. 8). This observation suggests that Fes may regulate some aspect of spindle formation or chromosomal segregation. Dividing cells also showed prominent localization of GFP-Fes to cytoplasmic speckles, similar to those observed in Colcemid-treated cells. A similar speckled staining pattern was reported previously by Zirngibl et al. (32) in cells that express GFP-Fes and are blocked in M phase. These findings suggest that the clustering of Fes in cytoplasmic speckles may be important for its function at this phase of the cell cycle. Interestingly, tubulin staining of M-phase cells (Fig. 8) did not reveal the speckled pattern that colocalized with GFP-Fes in Colcemid-treated cells (Fig. 6). This observation suggests that either the tubulin speckles are unique to Colcemid-treated cells or the majority of cellular tubulin is tied up in spindle formation. In addition to the mitotic spindle, we have also observed colocalization of active Fes with the centrosome in transfected fibroblasts and other cell types, as revealed by staining with antibodies to γ-tubulin (data not shown).
While the impact of tyrosine phosphorylation on centrosome and mitotic-spindle functions has not been investigated in detail, general tyrosine kinase inhibitors have been shown to disrupt mitotic-spindle formation and to induce M-phase arrest (23, 29). Yamaguchi et al. (30) showed that overexpression of Chk, a negative regulator of Src family kinases, reduced mitotic-spindle formation and resulted in a multinucleated phenotype in myeloid cells. The mechanism involved Chk-induced suppression of the activity of Lyn tyrosine kinase, a nuclear member of the Src kinase family that associates with mitotic chromosome scaffolds and spindles. While clarification of the contribution of Fes to the regulation of spindle formation and function awaits the identification of selective inhibitors, it is tempting to speculate that Fes activation may couple morphological differentiation with growth arrest through tubulin-based mechanisms such as those reported here.
Acknowledgments
This work was supported by National Institutes of Health grant CA58667.
We thank Guillermo Romero, Department of Pharmacology, University of Pittsburgh School of Medicine, for assistance with the confocal microscopy.
REFERENCES
- 1.Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, H. Y., J. A. Rogers, N. A. Dunham, and T. E. Smithgall. 1999. Regulation of c-Fes tyrosine kinase and biological activities by N-terminal coiled-coil oligomerization domains. Mol. Cell. Biol. 19:8335-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, H. Y., A. P. Schiavone, and T. E. Smithgall. 2001. A point mutation in the N-terminal coiled-coil domain releases c-Fes tyrosine kinase activity and survival signaling in myeloid leukemia cells. Mol. Cell. Biol. 21:6170-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton, D. A. 2000. Spindle assembly in animal cells. Annu. Rev. Biochem. 69:95-114. [DOI] [PubMed] [Google Scholar]
- 5.Corey, S. J., and S. M. Anderson. 1999. Src-related protein tyrosine kinases in hematopoiesis. Blood 93:1-14. [PubMed] [Google Scholar]
- 6.Craig, A. W., R. Zirngibl, and P. Greer. 1999. Disruption of coiled-coil domains in Fer protein-tyrosine kinase abolishes trimerization but not kinase activation. J. Biol. Chem. 274:19934-19942. [DOI] [PubMed] [Google Scholar]
- 7.Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13:83-117. [DOI] [PubMed] [Google Scholar]
- 8.Desai, A., S. Verma, T. J. Mitchison, and C. E. Walczak. 1999. Kin I kinesins are microtubule-destabilizing enzymes. Cell 96:69-78. [DOI] [PubMed] [Google Scholar]
- 9.Greer, P. A. 2002. Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell. Biol. 3:278-289. [DOI] [PubMed] [Google Scholar]
- 10.Kanda, S., E. C. Lerner, S. Tsuda, T. Shono, H. Kanetake, and T. E. Smithgall. 2000. The non-receptor protein-tyrosine kinase c-Fes is involved in FGF-2-induced chemotaxis of murine brain capillary endothelial cells. J. Biol. Chem. 275:10105-10111. [DOI] [PubMed] [Google Scholar]
- 11.Katagiri, K., T. Katagiri, K. Kajiyama, T. Yamamoto, and T. Yoshida. 1993. Tyrosine-phosphorylation of tubulin during monocytic differentiation of HL-60 cells. J. Immunol. 150:585-593. [PubMed] [Google Scholar]
- 12.Klein, C., E. M. Kramer, A. M. Cardine, B. Schraven, R. Brandt, and J. Trotter. 2002. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J. Neurosci. 22:698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurent, C. E., and T. E. Smithgall. 2004. The c-Fes tyrosine kinase cooperates with the breakpoint cluster region protein (Bcr) to induce neurite extension in a Rac- and Cdc42-dependent manner. Exp. Cell Res. 299:188-198. [DOI] [PubMed] [Google Scholar]
- 14.Lowell, C. A., and P. Soriano. 1996. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 10:1845-1857. [DOI] [PubMed] [Google Scholar]
- 15.Marie-Cardine, A., H. Kirchgessner, C. Eckerskorn, S. C. Meuer, and B. Schraven. 1995. Human T lymphocyte activation induces tyrosine phosphorylation of alpha-tubulin and its association with the SH2 domain of the p59fyn protein tyrosine kinase. Eur. J. Immunol. 25:3290-3297. [DOI] [PubMed] [Google Scholar]
- 16.Peterson, J. R., and T. J. Mitchison. 2002. Small molecules, big impact: a history of chemical inhibitors and the cytoskeleton. Chem. Biol. 9:1275-1285. [DOI] [PubMed] [Google Scholar]
- 17.Read, R. D., J. M. Lionberger, and T. E. Smithgall. 1997. Oligomerization of the Fes tyrosine kinase: evidence for a coiled-coil domain in the unique N-terminal region. J. Biol. Chem. 272:18498-18503. [DOI] [PubMed] [Google Scholar]
- 18.Rogers, J. A., H. Y. Cheng, and T. E. Smithgall. 2000. Src homology 2 domain substitution modulates the kinase and transforming activities of the Fes protein-tyrosine kinase. Cell Growth Differ. 11:581-592. [PubMed] [Google Scholar]
- 19.Rogers, J. A., R. D. Read, J. Li, K. L. Peters, and T. E. Smithgall. 1996. Autophosphorylation of the Fes tyrosine kinase: evidence for an intermolecular mechanism involving two kinase domain tyrosine residues. J. Biol. Chem. 271:17519-17525. [DOI] [PubMed] [Google Scholar]
- 20.Schulze, E., and M. Kirschner. 1986. Microtubule dynamics in interphase cells. J. Cell Biol. 102:1020-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulze, E., and M. Kirschner. 1987. Dynamic and stable populations of microtubules in cells. J. Cell Biol. 104:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata, A., C. E. Laurent, and T. E. Smithgall. 2003. The c-Fes protein-tyrosine kinase accelerates NGF-induced differentiation of PC12 cells through a PI3K-dependent mechanism. Cell Signal 15:279-288. [DOI] [PubMed] [Google Scholar]
- 23.Shimokado, K., K. Umezawa, and J. Ogata. 1995. Tyrosine kinase inhibitors inhibit multiple steps of the cell cycle of vascular smooth muscle cells. Exp. Cell Res. 220:266-273. [DOI] [PubMed] [Google Scholar]
- 24.Smithgall, T. E., J. A. Rogers, K. L. Peters, J. Li, S. D. Briggs, J. M. Lionberger, H. Cheng, A. Shibata, B. Scholtz, S. Schreiner, and N. A. Dunham. 1998. The c-Fes family of protein-tyrosine kinases. Crit. Rev. Oncog. 9:43-62. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi, S., R. Inatome, A. Hotta, Q. Qin, R. Hackenmiller, M. C. Simon, H. Yamamura, and S. Yanagi. 2003. Role for Fes/Fps tyrosine kinase in microtubule nucleation through its Fes/CIP4 homology domain. J. Biol. Chem. 278:49129-49133. [DOI] [PubMed] [Google Scholar]
- 26.Takashima, Y., F. J. Delfino, J. R. Engen, G. Superti-Furga, and T. E. Smithgall. 2003. Regulation of c-Fes tyrosine kinase activity by coiled-coil and SH2 domains: analysis with Saccharomyces cerevisiae. Biochemistry 42:3567-3574. [DOI] [PubMed] [Google Scholar]
- 27.Tian, L., D. L. Nelson, and D. M. Stewart. 2000. Cdc42-interacting protein 4 mediates binding of the Wiskott-Aldrich syndrome protein to microtubules. J. Biol. Chem. 275:7854-7861. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley, S. P., S. E. Kandels-Lewis, R. R. Adams, A. M. Ainsztein, and W. C. Earnshaw. 2001. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp. Cell Res. 262:122-127. [DOI] [PubMed] [Google Scholar]
- 29.Wright, S. J., and G. Schatten. 1995. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil. Cytoskeleton 30:122-135. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi, N., Y. Nakayama, T. Urakami, S. Suzuki, T. Nakamura, T. Suda, and N. Oku. 2001. Overexpression of the Csk homologous kinase (Chk tyrosine kinase) induces multinucleation: a possible role for chromosome-associated Chk in chromosome dynamics. J. Cell Sci. 114:1631-1641. [DOI] [PubMed] [Google Scholar]
- 31.Yu, G., T. E. Smithgall, and R. I. Glazer. 1989. K562 leukemia cells transfected with the human c-fes gene acquire the ability to undergo myeloid differentiation. J. Biol. Chem. 264:10276-10281. [PubMed] [Google Scholar]
- 32.Zirngibl, R., D. Schulze, S. E. Mirski, S. P. Cole, and P. A. Greer. 2001. Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp. Cell Res. 266:87-94. [DOI] [PubMed] [Google Scholar]