Abstract
Acute inflammation is accompanied from its outset by the release of specialized pro-resolving mediators (SPMs), including resolvins, that orchestrate the resolution of local inflammation. We showed earlier that, in rats with subcutaneous inflammation of the back induced by carrageenan, stretching for 10 minutes twice daily reduced inflammation and improved pain, two weeks after carrageenan injection. In this study, we hypothesized that stretching of connective tissue activates local pro-resolving mechanisms within the tissue in the acute phase of inflammation. In rats injected with carrageenan and randomized to stretch vs. no stretch for 48 hours, stretching reduced inflammatory lesion thickness and neutrophil count, and increased resolvin (RvD1) concentrations within lesions. Furthermore, subcutaneous resolvin injection mimicked the effect of stretching. In ex vivo experiments, stretching of connective tissue reduced the migration of neutrophils and increased tissue RvD1 concentration. These results demonstrate a direct mechanical impact of stretching on inflammation-regulation mechanisms within connective tissue.
Keywords: Stretching, SPM, Resolvin, Inflammation, Connective tissue
INTRODUCTION
It is now well established that acute inflammation is accompanied by an active program of resolution that begins in the first few hours after the onset of inflammation and involves the synthesis of specialized pro-resolving mediators (SPMs) derived from dietary n-3 polyunsaturated fatty acids including resolvins, protectins and maresins (Serhan, 2014; Serhan et al., 2000). While there is considerable interest in the clinical development of pharmacological agents and nutritional approaches that can promote the resolution of inflammation via these pathways and mediators (Cholkar et al., 2015; Norling et al., 2011; Souza and Norling, 2015), it is also important to understand naturally occurring mechanisms that might enhance the resolution of inflammation without the use of drugs, one of which being physical activity, particularly stretching.
Recent research in our laboratory using rodent models has begun to suggest a link between stretching and the resolution of inflammation within connective tissue. We developed a method by which rats and mice spontaneously stretch their whole body when they are partially lifted by the tail and allowed to grasp the edge of a surface with their front paws (Bouffard et al., 2008; Corey et al., 2012). When held in this position, the animals spontaneously extend both front and hind-limbs, which increases the distance between shoulders and hips by ~25%. This increase in shoulder-to-hip distance stretches the thoracolumbar fascia, which connects the shoulders and pelvis, and creates a shear plane deformation between its most superficial layer (aponeurosis of latissimus dorsi) and the subcutaneous tissues of the back (Benjamin, 2009). Injection of carrageenan into this subcutaneous connective tissue plane induces acute inflammation within 48 hours that progresses to a subacute monocytic inflammatory infiltrate within 2 weeks, accompanied by increased local pain sensitivity and altered gait (decreased stride length)(Corey et al., 2012). Using this model, we found that stretching for 10 minutes twice a day improved both gait and pain sensitivity and decreased inflammatory infiltration within the subcutaneous lesion (Corey et al., 2012). The mechanisms underlying the decrease in inflammation observed in this rodent model are currently unknown, but could involve either generalized systemic effects during the stretching procedure (e.g. stress-induced glucocorticoid release) or direct local effects on the stretched tissues. In the present report we tested the hypothesis that stretching of connective tissue has a direct, local pro-resolution effect on tissue inflammation that can be monitored both in vivo and ex vivo.
MATERIALS AND METHODS
Experimental design
Animals
Male Wistar rats (250–300g) where purchased from Charles River Laboratories, Newton, MA, and were used for all experiments except for those testing the effect of ex vivo stretching on RvD1 production in which male C57BL6 mice (20–24g), also from Charles River Laboratories, were used. All animal experiments were approved by the Animal Care and Use Committee at Harvard Medical School.
Carrageenan inflammation model
We used carrageenan to induce localized inflammation of subcutaneous tissue. Carrageenan is commonly used in models of musculoskeletal, subcutaneous and intradermal inflammation (Radhakrishnan et al., 2003). We used a non-gelling mixture of kappa and lambda carrageenan (Sigma Cat # C1867) diluted in sterile PBS, both types of Carrageenan are known to induce inflammatory responses (reviewed in(Bhattacharyya and Tobacman, 2012)) but because kappa carrageenan has the property of a hydrogel by itself, a mixture with lambda carrageenan results in a thicker preparation allowing for the compound to stay confined to the subcutaneous tissue as previously described (Corey et al., 2012).
Effect of active and passive stretching on subacute inflammation in vivo
Rats were injected with 3% carrageenan in sterile PBS on one side of the back, then randomized to active stretch (N=6), passive stretch under anesthesia (N=6), or anesthesia alone (N=5) once a day for 2 weeks. Rats were then euthanized and the inflammatory lesion thickness was measured with ultrasound.
Effect of active stretching on acute inflammation in vivo
Rats were injected with 100ul of 1% carrageenan in sterile PBS on one side of the back, then randomized to active stretch or no stretch twice a day for 48 hours (N=15/group). Rats were then euthanized and the inflammatory lesion thickness and cross sectional area was measured with ultrasound. The inflammatory lesion was then excised for measurement of inflammatory cell count and SPM concentration. In a subset of samples (N=18) cells were stained for flow cytometry analysis. In an additional group of rats, (N=3) Resolvin D2 (RvD2) was added to the carrageenan injection to compare the effect of stretching with that of exogenous resolvin injection.
Ex vivo neutrophil migration experiment
Rats (N=6) underwent unilateral injection of 3% carrageenan as described above. 48 hours after carrageenan injection, rats were euthanized by decapitation. The inflammatory lesion was excised and neutrophils were purified by density gradient centrifugation (Lymphocyte separation medium, MP Biomedicals) and counted as described below. A tissue sample including skin and subcutaneous tissue was excised from the back on the non-injected side of the rat. The sample was divided into two pieces which were randomized to stretch vs. no stretch ex vivo as described below, and the purified neutrophils from the same animal were used for the neutrophil migration assay.
Effect of stretching on RvD1 production ex vivo
Normal mice (N=9) were euthanized by decapitation and a tissue sample including skin and subcutaneous tissue was excised from the back of the mouse. The sample was divided into two pieces which were randomized to stretch vs. no stretch ex vivo as described below. Mouse tissue was used in this experiment (rather than rat) because the more compliant mouse dermis allows more precise control on the strain applied to the subcutaneous tissue.
Experimental procedures
Carrageenan and resolvin injection
The lumbar subcutaneous space, 1cm lateral of the spine at the L3 level, was injected with 100μl of 3% (for subacute inflammation) or 1% (for acute inflammation) carrageenan (diluted in sterile PBS). In rats injected with resolvin D2 (RvD2), 100ng (Cayman, Ann Arbor, MI, USA) was mixed with 1% carrageenan to a final volume of 100μl.
Active stretching
Rats were stretched by lifting them gently by the base of the tail until reaching ~45° angle with the table. While grasping onto the edge of the table with their front paws, the rats spontaneously extend their hind-limbs (illustrated in Fig. 1C). The stretching, which exerts traction on the thoracolumbar fascia and adjacent subcutaneous tissue, increases the distance between the shoulder and hip by ~25% (Corey et al., 2012). With minimal habituating, rats are able to hold this position comfortably for 10 minutes without struggling, vocalizing or other signs of distress.
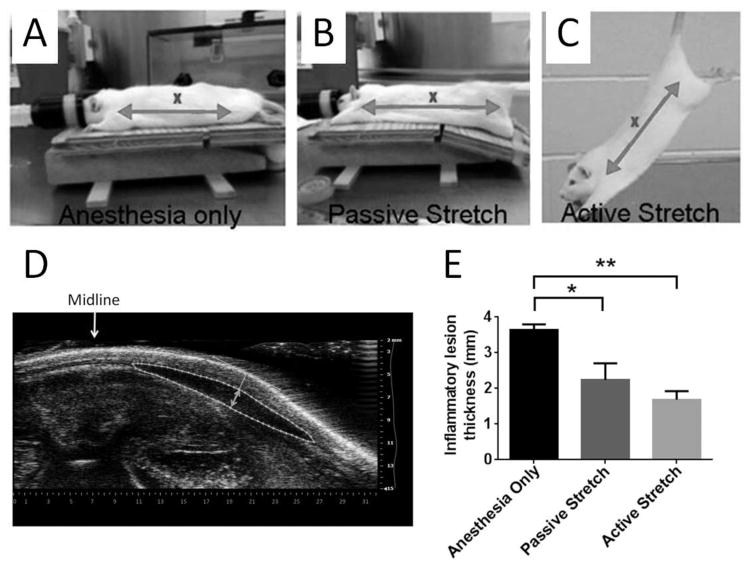
Figure 1. In vivo stretching and subacute inflammation.
Methods used for anesthesia only (A), passive stretch (B) and active stretch (C). The distance between shoulder and hip (arrows) is ~25% greater in both passive and active stretch, compared with anesthesia only. “X” indicates location of carrageenan injection. (D) Method of inflammatory lesion measurement on ultrasound images. The inflammatory lesion is outlined with a dashed line and the thickness measurement (double arrow) is taken perpendicular to the skin tangent at the point of maximum subcutaneous tissue thickness. (E) Two weeks after subcutaneous carrageenan injection, there was a reduction in inflammatory lesion thickness with both passive and active stretching, compared with anesthesia alone, (* p<0.05, **p<0.01, N=6 rats/group for active and passive stretch and N=5 rats for anesthesia alone). The difference between active and passive stretching was not statistically significant (p=0.27).
No stretching
Rats were removed from their cage and handled gently for 10 minutes but were not lifted by the tail or stretched.
Passive stretching
Rats were anesthetized with 2% isoflurane then placed on an articulated table which, when lowered by 17°, increases the distance between the hip and shoulder by 25% (Fig. 1B). Rats with anesthesia alone were placed on the table which was not lowered (Fig. 1A).
Ultrasound imaging
The lesion thickness was measured with a VisualSonics ultrasound scanner (15MHz) under isoflurane anesthesia. The transducer was stabilized with a clamp, perpendicular to the skin of the back, mid-way between the ribcage and iliac crest, and centered transversely on the midline. Measurement of inflammatory lesion thickness and cross sectional area was performed as illustrated in Fig. 1D. We used ultrasound cross sectional area measurements for normalization of resolvin D1 (RvD1) and leukotriene B4 (LTB4), rather than the weight or volume of the dissected tissues, as it represents a measurement of the intact lesion in situ. Our measurements were made in the transverse orientation since this is the predominant direction of spreading of the inflammatory lesions within the subcutaneous plane. We also verified that there were no significant differences in the longitudinal extent of the lesions across groups.
Inflammatory cell count
Cells were released from the inflammatory lesion by chopping the tissue with a scalpel in PBS, passing through a cell strainer and centrifuging at 800g. The supernatant was collected for lipid mediator analysis and cells were counted using a hemocytometer. In a subset of samples (N=20) cells were stained with anti-rat CD45 (OX-1), anti-rat CD11b/c (OX-42) (both from BioLegend), anti-rat granulocytes (RP1; BD Bioscience). Stained cells were examined using a FACSCanto II Flow Cytometer BD Biosciences, San Jose, CA, with the FlowJo single cell analysis software. At the acquisition and analysis steps, leukocytes were selected in a gate based on the forward scatter (FCS)/side scatter (SSC) parameters. On this selected cell population, a subsequent region around the CD45+ population was drawn. Only the cells in this gate were selected for further analysis. Expression of cell markers was assessed by determining the relative number of positive cells.
Lipid Mediator –SPM metabololipidomics
Supernatant from the inflammatory lesions were placed in ice cold methanol containing deuterated internal standards (d8-5S-hydroxyeicosatetraenoic acid (HETE), d4-leukotriene (LT) B4, d4-prostaglandin (PG)E2 and d5-lipoxin (LX) A4; 500pg each) and homogenized using a PTFE dounce (Kimble Chase). Proteins were allowed to precipitate (4°C), and lipid mediators were extracted using C18 solid-phase cartridges and a Biotage RapidTrace®. Measurement of lipid mediators was carried out by liquid chromatography-tandem mass spectrometry using a QTrap 5500 (ABSciex, Framingham, MA) equipped with a Shimadzu LC-20AD HPLC and a Shimadzu SIL-20AC autoinjector (Shimadzu, Kyoto, Japan). An Agilent Eclipse Plus C18 column (100mm × 4.6 mm × 1.8 μm) maintained at 50°C was used with a gradient of methanol/water/acetic acid of 55:45:0.01 (v/v/v) to 100:0:0.01 at 0.4 ml/min flow rate. Multiple reactions monitoring (MRM) was used to monitor lipid mediator profiles with more than 60 bioactive products from specific biosynthetic pathway including their pathway markers. Identification was carried out with signature ion fragments for each target lipid mediator (pro-inflammatory mediators PG, LT as well as SPM) using a minimum of six diagnostic ions. Quantification was achieved using calibration curves (Colas et al., 2014).
ELISA measurements
Tissue levels of RvD1 and LTB4 were measured using an ELISA kit following the manufacturer’s instructions (Cayman Chemical, Ann Arbor, Michigan).
Neutrophil migration
Immediately after euthanasia, the carrageenan-induced lesion was excised and neutrophils were isolated by density gradient centrifugation. Two tissue samples including skin and subcutaneous tissue were excised from the other side of the back and placed horizontally into metal grips with 1.8cm×1.5cm of the tissue between them. A 1×1 cm “well” was made by dissecting off the dermis and subcutaneous muscle, leaving intact the subcutaneous tissue layer (Fig. 4A). The grips were submerged in a bath containing HEPES physiologic buffer at 37ºC for 20 minutes before initiating stretch or no-stretch. For stretched samples, the tissue was elongated at 1 mm/sec until reaching a load of 58 mN (6g), and then maintained at that length for 120 minutes (Figure 4B). Non-stretched samples were handled in identical manner without tissue elongation. At 90 minutes, the buffer solution was replaced with HEPES buffer containing 100 nM N-formyl-methionyl-leucyl-phenylalanine (fMLF) up to, but not exceeding the level of the tissue. HEPES buffer (200ul) containing the purified neutrophils was placed on top of the tissues in the “well” (Fig. 4C) for the final 30 minutes of stretching. After 2 hours of stretching, the bottom bath buffer was harvested for neutrophil cell count.
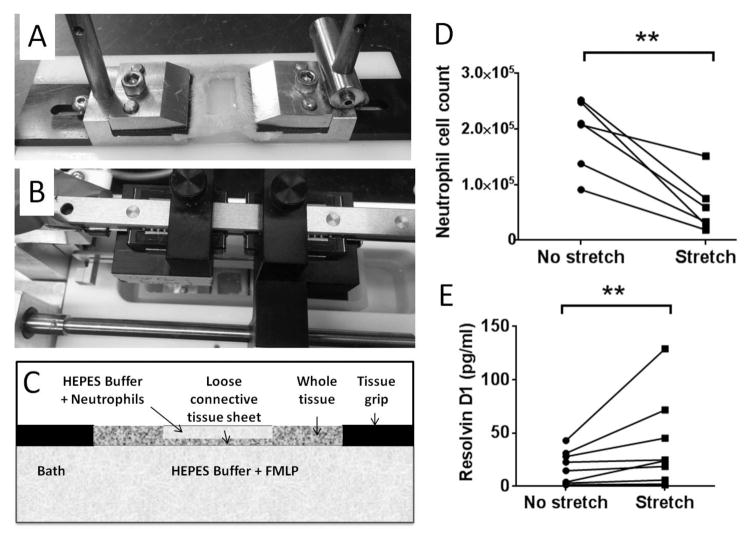
Figure 4. Ex vivo stretching, neutrophil migration and RvD1 production.
A: Tissue sample (including skin and subcutaneous tissue) showing cut out “window” exposing the subcutaneous tissue layer. B: Tissue sample mounted in stretching apparatus. C: Schematic of tissue with HEPES buffer+neutrophils above the tissue, and HEPES buffer+chemoattractant (fMLF) in the bath below. D: Neutrophil cell count in the lower bath was smaller in stretched vs. non-stretched tissue (*p<0.05, N=6 experiments). (E) In subcutaneous tissue samples stretched ex vivo, tissue RvD1 concentration was greater in stretched vs. non-stretched samples (**p<0.01, N=9 experiments).
Ex vivo tissue stretching
Connective tissue samples were stretched ex vivo as previously described (Langevin et al., 2005). For stretched samples, the tissue was elongated a 1 mm/sec until reaching a load of 19 mN (2g), then maintained at that length for 4 hours. Non-stretched samples were handled in identical manner without tissue elongation. At the end of incubation, the connective tissue was dissected and minced in PBS, followed by centrifugation at 800g and the supernatants were collected for RvD1 measurements.
Statistical Methods
A one way ANOVA was used to compare mean lesion thickness in rats randomized to active stretch, passive stretch or anesthesia alone. Fisher’s LSD was used to perform pairwise comparisons. One-way ANOVA was also used to compare lesion thickness between rats undergoing in vivo stretch, no stretch and exogenous RvD2 injection. Analyses of variance corresponding to a randomized block design were used to compare rats randomized to stretch and no stretch conditions within carrageenan batch. Dependent variables were lesion thickness, cross-sectional area, total cell count and neutrophil count. Subsequently, RvDI concentrations, LTB4, and RvD1/LtB4 ratios based on ELISAs were also analyzed in the subset of samples for which ultrasound measurement of lesion cross-sectional area was available for normalization. Nonparametric Wilcoxon Signed Rank tests were used to examine neutrophil migration and RvD1 production in tissue explants randomized to ex vivo stretch or no-stretch as dependent variables were non-normal. Analyses of lipidomic screening data in stretch and no-stretch rats were primarily descriptive. All statistical analyses were performed using SAS statistical software Version 9.3 (SAS Institute, Cary NC)
RESULTS
Stretching and subacute inflammation in vivo
We first tested whether passive stretching under anesthesia would have effects similar to that of active stretching, 2 weeks after carrageenan injection. As shown in Figure 1, the thickness of the inflammatory lesion was smaller with both active stretching (p=0.001) and passive stretching (p=0.012) compared with anesthesia alone (Figure 1E). There was no significant difference between active and passive stretching (p=0.24). Thus, active stretching was performed in the remainder of experiments to avoid repeated anesthesia.
Stretching and acute inflammation in vivo
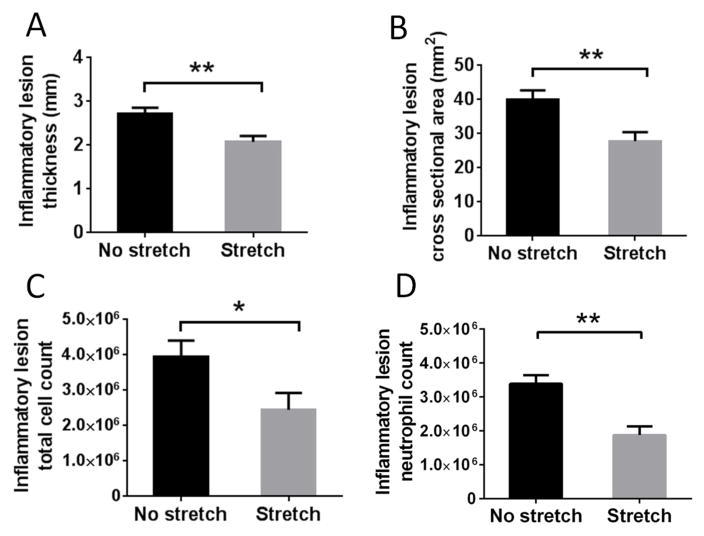
We then tested whether stretching could decrease local acute inflammation, compared with no stretching, 48 hours after carrageenan injection. Ultrasound measurements of inflammatory lesion thickness (p=0.0017) and cross sectional area (p=0.002) were significantly smaller in stretched rats compared with non-stretched rats (Figure 2A, B). Total cell counts within the inflammatory lesion were also reduced in the stretch, compared with non-stretch group (p=0.011) (Figure 2C). Flow cytometry analysis demonstrated that the cellular infiltrate was mainly composed of neutrophils, as expected in acute inflammation. Rats in the stretch group had a lower relative amount of neutrophils (p=0.002) (Figure 2D) compared with the non-stretched group. There was a significant positive correlation between the neutrophil count and ultrasound measurement of inflammatory lesion cross sectional area (r=0.51, p=0.01).
Figure 2. In vivo stretching and acute inflammation.
48 hours after subcutaneous carrageenan injection, stretched rats had a smaller inflammatory lesion thickness (A) (N=30), cross sectional area (B) (N=27), total cell count (C) (N=30) and neutrophil count (D) (N=18) compared with the non-stretched rats (* p<0.05, ** p<0.01).
Comparison of in vivo stretching and exogenous resolvin administration
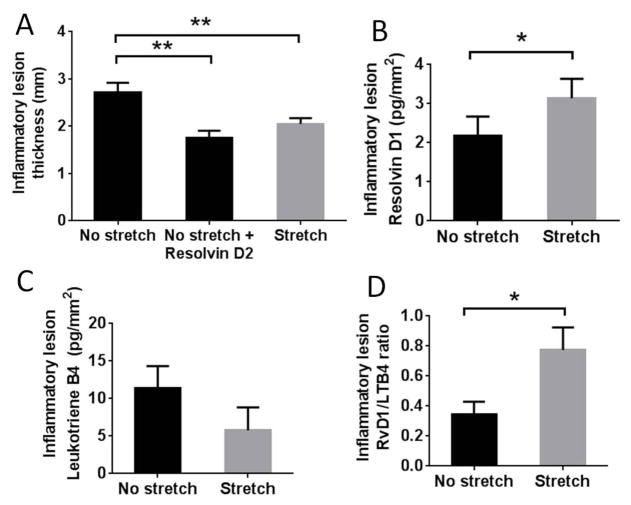
In order to test whether stretching activates inflammation-resolution mechanisms, we first compared the effect of stretching with the injection of 100 ng RvD2 at the site of inflammation. Both exogenous resolvin injection (p<0.01) and stretching (p<0.01) reduced the thickness of the inflammatory lesion, compared with no stretching, and there was no significant difference between the impact of resolvin injection and stretching (p=0.39) (Figure 3A).
Figure 3. In vivo stretching and SPM production.
A: 48 hours after carrageenan injection, inflammatory lesion thickness was smaller with both stretching and subcutaneous RvD2 injection (without stretching) compared with no stretching. The difference between RvD2 injection and stretching was not statistically significant (p=0.39) (N=3 for or RvD2 injection and N=10 per group for stretch and no stretch). B: RvD1 concentration was greater in the stretch vs. no stretch group (*p<0.05, N=27). C: Leukotriene B4 (LTB4) was not significantly different between stretched and non-stretched groups (p=0.19). D: the ratio of RvD1 to LTB4 was on average two-fold greater in the stretched compared with non-stretched rats (*p<0.05).
In vivo stretching and endogenous SPM production in acute inflammation
We next tested whether stretching could stimulate endogenous SPM production within the tissue. We first performed a metabolo-lipidomic screening of connective tissue in a subset of animals and observed a widespread increase in EPA and DHA-derived pro-resolving mediators in the stretch group, including both RvD1 and RvD2, as well as a decrease in most pro-inflammatory arachidonic-acid-derived mediators (Table 1). Based on these results, we measured RvD1 (using a commercially available ELISA) and found greater concentration of RvD1 in stretched compared with non-stretched rats (p=0.039) (Figure 3B). We also measured LTB4 concentration, because it is a potent neutrophil chemoattractant. The level of LTB4 was lower on average in the stretched group, but the difference between groups was not statistically significant (p=0.19, Figure 3C). However, the ratio of RvD1 to LTB4 was increased two-fold in the stretched compared with non-stretched rats (p=0.024) (Figure 3D).
Table 1.
Lipid mediator analysis in acute inflammation model
| No Stretch | Stretch | |
|---|---|---|
| DHA bioactive metabolome | ||
| Rv D1 | 0.04±0.007 | 0.06±0.01 |
| RvD2 | 0.04±0.01 | 0.06±0.02 |
| RvD3 | 0.01±0.0005 | 0.02±0.004 |
| RvD5 | * | * |
| RvD6 | * | * |
| AT-RvD1 | 0.01±0.004 | 0.01±0.009 |
| AT-RvD3 | 0.004±0.0007 | 0.006±0.001 |
| PD1 | * | * |
| 10S, 17S-diHDHA | 0.02±0.01 | 0.03±0.005 |
| MaRr1 | 0.04±0.009 | 0.04±0.005 |
| 7S,14S-diHDHA | * | * |
| EPA bioactive metabolome | ||
| RvE1 | * | * |
| RvE2 | * | * |
| RvE3 | 0.01±0.002 | 0.02±0.01 |
| AA bioactive metabolome | ||
| LXA4 | * | * |
| LXB4 | 0.2±0.04 | 0.2±0.1 |
| 5S, 15S-diHETE | 0.5±0.1 | 0.4±0.1 |
| AT-LXA4 | 0.9±0.02 | 0.1±0.06 |
| AT-LXB4 | * | * |
| LTB4 | 0.5±0.2 | 0.2±0.09 |
| 5S,12S-diHETE | * | * |
| 20-OH-LTB4 | * | * |
| 20-COOH-LTB4 | * | * |
| PGD2 | 7.7±2.5 | 7.7±5.3 |
| PGE2 | 17.2±2.6 | 14.8±5.9 |
| PGF2a | 1.5±0.5 | 1.3±0.9 |
| TXB2 | 8.6±3.1 | 11.7±8.2 |
Results are expressed as mean ± SE, normalized to the inflammatory lesion cross sectional area (n=4/group).
, Below Limit, limit ~0.1pg.
Ex vivo tissue stretching, neutrophil migration and RvD1 production
To investigate whether stretching could have direct effects on immune responses within connective tissue, we conducted ex vivo experiments on subcutaneous connective tissue explants incubated with or without stretch for 90 minutes. We first tested whether stretching could reduce the migration of neutrophils through the tissue in response to a chemoattractant. Stretching significantly reduced the movement of neutrophils from above to below a sheet of connective tissue, with a smaller number of neutrophils recovered in the lower incubation bath in stretched compared with non-stretched tissue (p=0.03) (Figure 4D).
We then tested whether 4 hours of stretching could increase resolvin concentration within a sheet of connective tissue incubated for 4 hours ex vivo and found greater amounts of RvD1 in stretched vs. non-stretched tissue (p=0.004) (Figure 4E).
DISCUSSION
The results of this study show that stretching decreases carrageenan-induced inflammation, and the similar effects of active and passive stretching suggest a mechanical effect on the tissues. This is further supported by our ex vivo experiments in which stretching of connective tissue reduced neutrophil migration within the tissue, a central aspect of the acute inflammatory response. Although it is possible that the anti-inflammatory effects of in vivo stretching may have been due to changes in muscle activity, blood or lymphatic flow around the lesions, our data ex vivo shows that neutrophil migration was inhibited by stretching of connective tissue in a setting independent of vascular, lymphatic and neuromuscular systems. Finally, we show that stretching increases tissue SPMs both in vivo and ex vivo, demonstrating a direct mechanical effect of stretching on inflammation-regulation molecules within the connective tissue.
Humans and animals spontaneously stretch after waking, or spending time in a fixed position, and the reasons for this universal behavior are not fully known. Both stretching exercises, as well as exercises with a prominent stretching component (e.g. yoga, Tai Chi) have been found to decrease levels of circulating pro-inflammatory cytokines (Irwin and Olmstead, 2012; Irwin et al., 2014; Kiecolt-Glaser et al.; Morgan et al., 2014; Sarvottam et al., 2013). Although mechanisms investigated so far have focused on systemic and humoral factors, the results of the current study suggest that stretching exercises may have a direct pro-resolution impact within local connective tissues.
Connective tissue, or stroma, is increasingly recognized as an important player in both the transition from acute to chronic inflammation, as well as the resolution of acute inflammation, either through direct contact with immune cells or by inducing changes in cytokine profiles in the tissue (Dixon et al., 2014; McGettrick et al., 2009; Naylor et al., 2013; Van Linthout et al., 2014). The influence of mechanical forces within connective tissue is potentially far-reaching since connective tissue plays multiple roles in the body: as part of the musculoskeletal system, connective tissue forms continuous, compliant layers that can both stretch and bear loads; as part of the immune system, connective tissue is both the “container” for immune exchanges throughout the body, as well as the “conduit” through which water, proteins and immune cells return to the blood via lymphatics (Langevin et al., 2013; Malhotra et al., 2013). Given these multiple roles, it is plausible that body movements could influence immune-related processes through a cross-talk between resident stromal cells and circulating immune cells. A possible explanation for our results might be that stretching of connective tissue increases the production of SPMs by resident stromal cells (fibroblasts and/or monocytes), and that the resultant increase in tissue SPM levels reduces the recruitment of circulating neutrophils. Alternatively stretch-induced reduction of neutrophil infiltration of tissue (in vivo) and neutrophil migration (ex vivo) may be due to mechanisms independent of SPMs such as physical alteration of the connective tissue matrix. However, a mechanism involving SPMs would be consistent with several studies showing that SPMs reduce neutrophil recruitment during acute inflammation and directly regulate neutrophil chemotaxis to inflammatory stimuli (e.g., LTB4) in vitro (see ref 1 for review). This is further supported by our results showing that RvD2 is sufficient to reduce the thickness of the inflammatory lesion.
A limitation of our model is that the exact amount of force experienced by the tissue cannot be determined. However, we know that the tissue experiences ~25% strain, which may be a key factor in our stretching intervention, since this amplitude is low enough to minimize trauma to the tissue. Although it is possible that stretch-induced SPMs are products of local tissue damage caused by the stretching, such a scenario would have been expected to cause an increase in both pro-resolving and pro-inflammatory mediators. Markworth et al. reported that, in humans, a single bout of unaccustomed resistance exercise led to an acute increase (within 24 hours) in serum levels of EPA and DHA-derived resolvins (RvD1 and RvE1) as well as arachidonic acid (AA)-derived inflammatory mediators including prostaglandins and leukotrienes (Markworth et al., 2013). In contrast, in our experiments, we did not see a generalized increase in AA-derived mediators, and leukotrienes were on average lower in stretched, compared with non-stretched rats. This suggests that the stretch-induced production of SPMs results from deformation of the connective tissue matrix that is sufficient to create a biomechanical signal but not large enough to damage the tissue. An increase in tissue SPMs during non-injurious tissue deformation thus may be a protective biomechanically-transduced response that promotes healing of the tissue without causing further activation of the inflammatory response itself. Of note, in a similar model of carrageenan-induced inflammation, Xu et al. showed that that injection of resolvins had direct effects on nociception in addition to reducing inflammation, suggesting that SPMs may play multiple roles in reducing inflammatory pain (Xu et al., 2010).
It is important to stress that the rodent model of “active” stretching used in this study was not intended to simulate a clinical stretching treatment that can be directly applied to humans. For example, the duration of stretching (10 minutes) is longer than typically used in physical therapy or yoga. It will therefore be important in further studies to determine the shortest duration of stretching that can have pro-resolution effects. On the other hand, the position adopted by the rats during active stretching, with pulling of the thoracolumbar fascia resulting from simultaneous extension of fore and hind-limbs, is remarkably similar to some basic yoga and “core” exercises. Furthermore, shear plane deformation of the thoracolumbar fascia has been shown to be reduced in human subjects with chronic low back pain (Langevin et al., 2011). It is thus plausible that the findings of our study could translate to humans once the optimal duration of stretch has been determined.
In summary, stretching decreased acute inflammation (in vivo), reduced neutrophil migration (ex vivo), and increased connective tissue pro-resolving mediators (in vivo and ex vivo). These results reveal important new interactions between musculoskeletal and immune systems that could potentially be used therapeutically.
Acknowledgments
Funding: This study was made possible by funds from the Bernard Osher Foundation. R.A.C. and experiments carried out in the CNS lab were supported by the National Institutes of Health GM Grant PO1GM095467 (C.N.S.)
The authors want to thank Dr. Bruce Levy for use of the flow cytometry equipment.
Footnotes
COMPETING FINANCIAL INTERESTS
C.N.S. is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. C.N.S. is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. C.N.S.’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- Benjamin M. The fascia of the limbs and back--a review. J Anat. 2009;214(1):1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Tobacman JK. Molecular signature of kappa-carrageenan mimics chondroitin-4-sulfate and dermatan sulfate and enables interaction with arylsulfatase B. J Nutr Biochem. 2012;23(9):1058–1063. doi: 10.1016/j.jnutbio.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Bouffard N, Cutroneo K, Badger G, White S, Buttolph T, Ehrlich H, Stevens-Tuttle D, Langevin H. Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: Evidence from ex vivo and in vivo models. Journal of cellular physiology. 2008;214(2):389–395. doi: 10.1002/jcp.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholkar K, Trinh HM, Vadlapudi AD, Wang Z, Pal D, Mitra AK. Interaction Studies of Resolvin E1 Analog (RX-10045) with Efflux Transporters. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2015;31(4):248–255. doi: 10.1089/jop.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology Cell physiology. 2014;307(1):C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey SM, Vizzard MA, Bouffard NA, Badger GJ, Langevin HM. Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PloS one. 2012;7(1):e29831. doi: 10.1371/journal.pone.0029831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon KO, Rossmann L, Kamerling SW, van Kooten C. Human renal fibroblasts generate dendritic cells with a unique regulatory profile. Immunology and cell biology. 2014;92(8):688–698. doi: 10.1038/icb.2014.41. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2012;20(9):764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, Yokomizo M, Lavretsky H, Carroll JE, Motivala SJ, Bootzin R, Nicassio P. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep. 2014;37(9):1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, Glaser R. Stress, inflammation, and yoga practice. Psychosom Med. 72(2):113–121. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. American journal of physiology Cell physiology. 2005;288(3):C747–756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Fox JR, Koptiuch C, Badger GJ, Greenan-Naumann AC, Bouffard NA, Konofagou EE, Lee WN, Triano JJ, Henry SM. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC musculoskeletal disorders. 2011;12:203. doi: 10.1186/1471-2474-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin HM, Nedergaard M, Howe AK. Cellular control of connective tissue matrix tension. Journal of Cellular Biochemistry. 2013;114(8):1714–1719. doi: 10.1002/jcb.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunological reviews. 2013;251(1):160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markworth JF, Vella L, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, Maddipati KR, Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1281–1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettrick HM, Smith E, Filer A, Kissane S, Salmon M, Buckley CD, Rainger GE, Nash GB. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. European journal of immunology. 2009;39(1):113–125. doi: 10.1002/eji.200838232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PloS one. 2014;9(7):e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AJ, Filer A, Buckley CD. The role of stromal cells in the persistence of chronic inflammation. Clin Exp Immunol. 2013;171(1):30–35. doi: 10.1111/j.1365-2249.2012.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186(10):5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104(3):567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvottam K, Magan D, Yadav RK, Mehta N, Mahapatra SC. Adiponectin, interleukin-6, and cardiovascular disease risk factors are modified by a short-term yoga-based lifestyle intervention in overweight and obese men. J Altern Complement Med. 2013;19(5):397–402. doi: 10.1089/acm.2012.0086. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PR, Norling LV. Implications for eicosapentaenoic acid- and docosahexaenoic acid-derived resolvins as therapeutics for arthritis. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.05.072. [DOI] [PubMed] [Google Scholar]
- Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102(2):258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature medicine. 2010;16(5):592–597. doi: 10.1038/nm.2123. 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]