Abstract
Objective
Assay interference could be the cause of abnormal thyroid function tests. Early recognition prevents inappropriate patient management. The objective of this report is to present a case with discordant thyroid function tests in different thyroid assay platforms due to thyroid autoantibodies.
Methods
We present a case her family, laboratory data and methods that investigate immunoassay interference.
Results
A 21-year-old woman with autoimmune thyroid disease was treated for hypothyroidism with levothyroxine and noted to have elevated total and free thyroxine, free triiodothyronine but normal thyroid-stimulating hormone. Repeat thyroid function tests using different platforms revealed discrepant results. Further investigation showed that the patient had positive thyroid hormone autoantibodies (THAAbs).
Conclusion
We demonstrates abnormal thyroid function tests caused by THAAbs. The latter were the cause of interference with assays resulting in discrepant test results inconsistent with the clinical presentation. Early recognition would prevent inappropriate patient management.
Keywords: thyroid function test, thyroid hormone autoantibodies, euthyroid hyperthyroxinemia
INTRODUCTION
The prevalence of thyroid hormone autoantibodies (THAAbs) has been reported to be from 0 to 25% (1). This variability probably reflects populations with different prevalence of autoimmune thyroid disease (AITD) and different methods of iodothyronines determination (2). Almost all reported cases of THAAbs have discrepancies between physical findings and results of thyroid function tests. Failure to detect THAAbs may lead to unnecessary testing and treatment. Here we report a case with discordant thyroid function tests among assay platforms due to THAAbs.
CASE REPORT
A 21-year-old Croatian woman was brought to our attention for the possibility of resistance to thyroid hormone (RTH) of the beta type (3), based on high serum T3 levels with non-suppressed thyroid stimulating hormone (TSH). History obtained by one of us (J.J.) revealed that, at 13 years of age, she presented with goiter, had high titer of thyroperoxidase antibodies (TPOab) and thyroglobulin antibodies (TGab) and that her thyroid ultrasound was consistent with thyroiditis. She was placed on levothyroxine (L-T4) based on an elevated level of serum TSH, even though she had no symptom of hypothyroidism. There was strong history of AITD on both sides of her family but no consanguinity. Six years later (2013), while off L-T4, her thyroid function tests showed increased total and free T4 (TT4 and FT4) and total T3 (TT3) with normal TSH in the absence of clinical stigmata of thyroid hormone excess (Table 1). These tests abnormalities were still present in 2015 and concordant on two assay platforms, Immulite 2000 (Siemens) and Delfia (Wallace) (Table 1). As AITD has been shown to coexist with RTH-beta (4), the thyroid hormone receptor beta (THRB) gene was sequenced but no mutations were found.
Table 1.
Thyroid hormone value. *
| TSH mU/L | Total T4 (μg/dL) | Total T3 (ng/dL) | FT4 (ng/dL) | FT3 (ng/L) | |
|---|---|---|---|---|---|
|
| |||||
| April 2013 | |||||
| Immulite 2000 (Siemens) a | 2.95 | 18.10 | - | >5.98 | >39 |
|
| |||||
| April 2015 | |||||
| Immulite 2000 (Siemens) a | 5.80 | 15.22 | - | >5.98 | >39 |
| Delfia (Wallace) b | - | 13.51 | - | >5.98 | - |
|
| |||||
| June 2015 | |||||
| Elecsys 2010 (Roche) c | 9.1 | 8.9 | 209 | - | - |
| Elecsys 2010 + Ethanol extraction c | - | 8.5 | - | - | - |
| Immulite 2000 (Siemens) a | - | 14.20 | - | 5.75 | - |
| Immulite + ethanol extraction a | - | 6.56 | - | - | - |
| Architect 12000 SR (Abbott) d | - | 6 | - | 1 | - |
| DXI (Beckman) e | - | 14.6 | - | 1.03 | - |
| Cobas 6000 (Roche) f | - | 9.56 | 206 | 1.55 | - |
| Equilibrium dialysis g | - | - | - | 1.2 | - |
Patient results outside reference intervals are in bold type.
Reference intervals
Immulite:TSH; 0.4–5.5 mU/L, TT4; 4.5–12.5 μg/dL, TT3; 83–200 ng/dL, FT4; 0.77–1.94 ng/dL, FT3; 1.63–4.23 ng/L
Delfia: TT4; 5.36–11.0 μg/dL, FT4; 0.76–1.31 ng/dL
Elecsys: TSH; 0.4–3.6 mU/L, TT4; 5.1–13.5 μg/dL, TT3; 85–202 ng/dL
Architect: TT4; 4.87–11.72 μg/dL, FT4; 0.7–1.48 ng/dL
DXI: TT4; 6.09–12.23 μg/dL, FT4; 0.61–1.12 ng/dL
Cobas: TT4; 4.9–11.2 μg/dL, TT3; 94–182 ng/dL, FT4; 0.78–2.09 ng/dL
FT4, equilibrium dialysis; 0.8–2.7 ng/dL
Conversion traditional unit to SI unit
TT4 μg/dL × 12.87 = nmol/L, TT3 ng/dL × 15.36 = μmol/L, FT4 ng/dL × 12.87 = pmol/L, FT3 ng/ml × 1.536 = nmol/L
Blood samples were obtained, with written consent, from family members for investigation according to a protocol approved by the Institutional Review Board. Results are shown in Figure 1. While the presence of AITD in all family members studied was confirmed, the high iodothyronine levels of the proband were notably not observed by the Elecsys (Table 1, June 2015). The discrepancy of thyroid hormone measurements when compared to data originally transmitted, suggested that assay interference should be considered.
Figure 1.
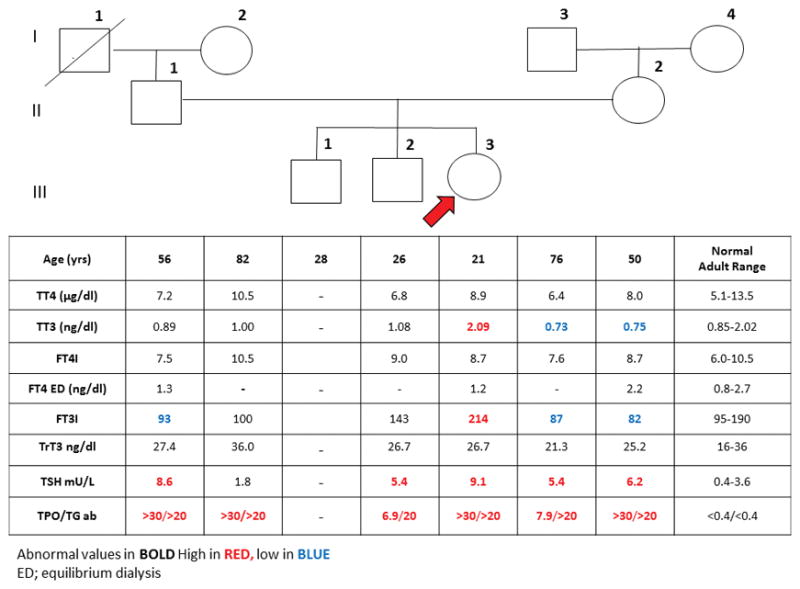
Pedigree of the family and results of thyroid function test. (Elecsys 2010) Roman numerals indicate each generation and number on the upper right of each symbol identify the subjects. Test results are aligned with each symbol. Abnormal values are in bold numbers. Arrow indicates the proposita. Abbreviations: TT4; total thyroxine, TT3; total triiodothyronine, FT4I; free thyroxine index, FT3I; free triiodothyronine index, ED; Equilibrium dialysis, TrT3; total reverse triiodothyronine, TSH, thyroid-stimulating hormone, TPOab; thyroperoxidase antibodies, TGab; thyroglobulin antibodies.
To verify the suspected assay interference the proband’s TT4 and FT4 were determined by four more platforms in addition to the two used earlier in 2015. As shown in Table 1, TT4 values were high in the Immulite 2000 (Siemens), DXI (Beckman) and Delfia (Wallace) but not Elecsys (Roche), Cobas 6000 (Roche) and Architect (Abbott). Of interest was the finding that TT4 but not FT4 was high in the DXI platform. FT4 measured by equilibrium dialysis was in the normal range (Table 1).
In order to determine which of the platforms gave the correct values, the iodothyronines were extracted using three volumes alkalinized (2% ammonium hydroxide) ethanol. The extract was dried, redissolved in the appropriate matrix and TT4 was measured in both Elecsys and Immulite platforms. As shown on Table 1, assay interference cause the higher produced the high values.
To demonstrate the presence of THAAbs, radioiodine-labeled T4 or T3 was added to the patient’s serum and the IgG fraction subsequently precipitated by addition of 15% polyethylene glycol. Results showed that 8% and 20% of the respective labeled iodothyronines were precipitated compared to <3% in control sera.
DISCUSSION
Differential diagnosis for increased concentration of serum iodothyronines with normal TSH level in an apparently euthyroid patient includes (a) abnormal binding protein such as T4-binding globulin, (TBG) transthyretin (TTR), or albumin in familial dysalbuminemia hyperthyroxinemia (FDH), (b) RTH-beta, (c) a TSH-secreting pituitary adenoma (TSHoma) when the magnitude of hormone hyper-secretion is insufficient to produce thyrotoxicosis, (d) assay interference from THAAbs or human anti-mouse antibodies, (e) a drug effect such as amiodarone and (f) acute psychiatric disorder (5).
The patient was clinically euthyroid, was on no medications and had no psychiatric disorder. The presence of a TSHoma, was unlikely given the magnitude of T4 and T3 elevation with absence of stigmata of thyroid hormone excess. TBG excess was excluded given the elevated free T4 and T3. Moreover, the combined elevation of both T4 and T3 were inconsistent with FDH and TTR. RTH-beta was a distinct possibility given the lack of clinical manifestations, even in the presence of AITD. It is for this reason that THRB gene sequencing was done with an outcome. The normal thyroid hormone levels in family members (Figure 1), was consistent with the foregoing result as RTH-beta is dominantly inherited (6). In contrast to the absence of familial source of THRB, our investigation demonstrated a strong family history of AITD with subjects of the patient’s generation inheriting the trait from both parents. Critical for the direction of further investigation, the discrepancy of thyroid hormone measurements in the Elecsys when compared to data originally transmitted, suggested that interference in the assay might be a factor for assessment.
Methods to investigate immunoassay interference include (a) repeat analysis with different methods, (b) demonstration of nonlinear response to sample dilution, (c) demonstration of iodothyronine binding to the patient’s IgG by electrophoresis, or precipitation with anti-IgG or with polyethylene glycol, (d) block heterophile antibody with nonimmune serum or using blocking tubes and (e) suppression of patient antibodies with immunosuppressive therapy (7). In this case, we were able to confirm immunoassay interference by repeat analysis on different platforms and demonstration of THAAbs with PEG precipitation.
Moderate method-dependent differences of thyroid hormone measurements are common and normalized by variations in the normal ranges. The latter adjustment does not correct for specific assay interferences. It has been suggested that two-step methods are less prone to THAAbs interference, because the procedure ensures that there is no contact between serum components and the analog tracer (8, 9).
The Immulite assay is based on one-step analog-based immunoassay in which serum and labeled hormone analog are introduced together into the reaction tube and compete for a solid phase antibody. Unbound material is then washed out and the bound analog is measured. During single incubation, THAAbs present in the serum sample may bind the analog preventing its association to the solid phase antibody. This would reduce the signal and cause falsely increased estimates of thyroid hormone. Both Cobas and Elecsys instruments use a staggered incubation without an intervening washing step: The serum and capture antibody are mixed, then biotinylated T4 analog and streptavidin-coated microparticles are added. There is shorter duration of contact between THAAbs and the T4 analog in this assay. Beckman Coulter is one-step assay for TT4 and two-step assay for FT4 which may explain for discrepant result of TT4 but not FT4, the latter showing no interference. Architect (Abbott Diagnostics) and Delfia (Wallac) are two-step assay in which labeled analogs (T3 acridinium labeled tracer and europium-labeled T4, respectively) are introduced only after the unbound material from the sample has been washed, thereby precluding or reducing interaction between THAAbs and hormone analog. The result from Architect showed no interference but the results from Delfia showed high TT4 and FT4. This situation may be explained if the antibody in the patient’s serum has relatively higher affinity for T4 (both labeled and unlabeled) than the anti-T4 IgG present in the Delfia assay reagents. Unfortunately we were unable to test this hypothesis. The magnitude of immunoassay interference would be dependent on the assay format, the relative affinities/avidities of interfering antibodies and their titer (10).
CONCLUSION
One individual of a family with AITD and subclinical hypothyroidism had falsely elevated serum iodothyronine levels due to the presence of THAAbs. Recognition of assay interferences should be considered in patients who present with discrepancies in test and clinical presentation to prevent inappropriate patient management.
Acknowledgments
This work is supported in part by grant R37DK15070 from the National Institutes of Health and the Seymour J. Abrams fund for thyroid research (to S.R.). We are grateful to Dr. James Dohnal from NorthShore University Health System, Chicago and Dr. Shannon Haymond from Lurie Children’s Hospital of Chicago for measurement of iodothyronines by the Beckman DXI system and the Roche Cobas 6000, respectively; to the following colleagues for advice about the investigation: Dr. Roy E. Weiss, Dr. Alexandra M. Dumitrescu and Dr. Theodora Pappa.
List of abbreviations
- TSH
thyroid-stimulating hormone
- FT4
free thyroxine
- FT3
free triiodothyronine
- TT4
total thyroxine
- TT3
total triiodothyronine
- THAAbs
Thyroid hormone autoantibodies
- TPOab
thyroperoxidase antibodies
- TGab
thyroglobulin antibodies
- ED
Equilibrium dialysis
- AITD
Autoimmune thyroid disease
- TBG
T4-binding globulin
- TTR
transthyretin
- FDH
familial dysalbuminemia hyperthyroxinemia
- TSHoma
TSH-secreting pituitary adenoma
- RTH
resistance to thyroid hormone
Footnotes
Financial Disclosures: None
References
- 1.Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44(3):440–54. [PubMed] [Google Scholar]
- 2.Sakata S, Nakamura S, Miura K. Autoantibodies against thyroid hormones or iodothyronine. Implications in diagnosis, thyroid function, treatment, and pathogenesis. Ann Intern Med. 1985;103(4):579–89. doi: 10.7326/0003-4819-103-4-579. [DOI] [PubMed] [Google Scholar]
- 3.Refetoff S, Bassett JH, Beck-Peccoz P, Bernal J, Brent G, Chatterjee K, et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. J Clin Endocrinol Metab. 2014;99(3):768–70. doi: 10.1210/jc.2013-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkoff MS, Kocherginsky M, Anselmo J, Weiss RE, Refetoff S. Autoimmunity in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 2010;95(7):3189–93. doi: 10.1210/jc.2009-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745–62. doi: 10.1016/j.beem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830(7):3987–4003. doi: 10.1016/j.bbagen.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AM, Honour JW. Unusual results from immunoassays and the role of the clinical endocrinologist. Clin Endocrinol (Oxf) 2006;64(3):234–44. doi: 10.1111/j.1365-2265.2006.02439.x. [DOI] [PubMed] [Google Scholar]
- 8.Zouwail SA, O’Toole AM, Clark PM, Begley JP. Influence of thyroid hormone autoantibodies on 7 thyroid hormone assays. Clin Chem. 2008;54(5):927–8. doi: 10.1373/clinchem.2007.099770. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowski KC, Dabrowska K, Lewinski A. Case report: When measured free T4 and free T3 may be misleading. Interference with free thyroid hormones measurements on Roche(R) and Siemens(R) platforms. Thyroid Res. 2012;5(1):11. doi: 10.1186/1756-6614-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail AA. A radical approach is needed to eliminate interference from endogenous antibodies in immunoassays. Clin Chem. 2005;51(1):25–6. doi: 10.1373/clinchem.2004.042523. [DOI] [PubMed] [Google Scholar]


