Abstract
The causes of most cases of Parkinson’s disease (PD) are still poorly understood. Here we show that chronic stress and elevated corticosterone levels exaggerate motor deficits and neurodegenerative events in a Parkinson’s disease rat model. Animals were tested in skilled and non-skilled movement while being exposed to daily restraint stress or oral corticosterone treatment. Stress and corticosterone compromised normal motor function and exaggerated motor deficits caused by unilateral 6-hydroxydopamine lesion of the nigrostriatal bundle. Moreover, stress and corticosterone treatments diminished the ability to acquire compensatory strategies in limb use during skilled reaching and skilled walking. In contrast, lesion control animals were able to significantly improve in the ability of skilled limb use during the repeated test sessions. The exaggerated motor impairments in stress-treated animals were related to accelerated loss of midbrain dopamine-producing neurons during the first week postlesion. Correlation analysis revealed a significant connection between loss of tyrosine hydroxylase-positive cells and increase in Fluoro-Jade-positive cells only in stress- and corticosterone-treated animals. Furthermore, stress and elevated corticosterone levels caused greater permanent loss of midbrain neurons than found in non-treated lesion animals. These findings demonstrate that stress and elevated corticosterone levels can exaggerate nigral neuronal loss and motor symptoms in a rat analogue of PD. It is therefore possible that stress represents a key factor in the pathogenesis of human PD by impeding functional and structural compensation and exaggerating neurodegenerative processes.
Keywords: 6-OHDA, cell death, corticosterone, medial forebrain bundle, skilled movement, tyrosine hydroxylase
Introduction
Parkinson’s disease (PD) is a common neurological condition of the motor system caused by loss of dopaminergic neurons in the substantia nigra (Hornykiewicz, 1975; Wolters & Calne, 1989). The resulting loss of dopamine leads to rigidity, tremor, bradykinesia, and abnormalities in body posture and skilled movements. Although the pathology and symptoms of PD have been widely investigated, the factors involved in onset and course of this disease in the majority of cases are not understood. Individual physiological conditions, such as stress, are likely to account for the diversity in symptoms and course of PD among the patient population, as well as for the individual response to medication after the onset of PD (Foley et al., 2004).
Stress has been one of the earliest proposed causes of PD (Charcot, 1878; Gowers, 1888). It has been suggested that acute or chronic stress might lead to earlier onset or worsen the motor symptoms of PD (Gibberd & Simmons, 1980; Weiner & Lang, 1989; Goetz et al., 1990; Treves et al., 1990; Smith et al., 2002). Although there has been no study to investigate the causal relationship between stress and PD, an increasing body of evidence suggests that stress and stress hormones might represent critical cofactors in its pathogenesis. For instance, brain regions participating in motor control, such as motor cortex, cerebellum and the basal ganglia, show considerable density of glucocorticoid receptors (Ahima & Harlan, 1990; Ahima et al., 1991), rendering these areas susceptible to the effects of stress. Accordingly, stress and stress hormones were shown to affect the function of the intact motor system in both human (Maki & McIlroy, 1995) and rat (Metz et al., 2001a, 2005). Furthermore, one study indicated that cortisol levels are positively associated with gait deficits in Parkinson patients (Charlett et al., 1998). In line with findings showing that the dopaminergic system is particularly susceptible to the effects of stress (Finlay & Zigmond, 1997; Pani et al., 2000; Izzo et al., 2005), it has been demonstrated that oxidative stress caused by immobilization stress selectively damages the nigrostriatal dopaminergic system (Kim et al., 2005). These observations suggest that stress might be a critical variable influencing the neurodegenerative processes and symptoms of PD.
The purpose of the present study was to evaluate the effects of stress on motor impairments and neurodegenerative processes in the unilateral 6-hydroxydopamine (6-OHDA) rat model of PD. Two experiments were performed in order to examine the effects of chronic restraint stress and oral treatment with corticosterone, the main stress hormone in the rat, on both skilled and non-skilled movements and their relationship with markers of neurodegenerative events and plasticity after 6-OHDA-induced dopamine depletion. A battery of sensitive motor tasks with high clinical validity (Whishaw et al., 2002) and a combination of immunohistochemical assessments were used to assess the severity of 6-OHDA lesions and the consequences of physiological manipulations. The results demonstrate that stress and corticosterone exaggerate motor deficits and that stress is critically contributing to accelerated cell loss in dopamine-depleted animals.
Materials and methods
Subjects
A total of 71 adult female Long-Evans hooded rats were used. The animals were raised at the University of Lethbridge vivarium and housed in groups of two in standard polycarbonate shoebox cages. The housing room was maintained on a 12/12-h light–dark cycle, with lights on at 07:30 h. Behavioural training and testing took place during the light phase of the cycle. The temperature was kept at 22 °C and relative humidity at 30%.
To encourage participation in the skilled reaching task, animals of Experiment 1 were placed on a restricted feeding schedule to maintain body weights at 90–95% of their baseline weights. Animals were weighed daily. All procedures were performed according to standards set by the Canadian Council of Animal Care and approved by the University of Lethbridge Animal Welfare Committee.
Experimental design
Two experiments were performed. Experiment 1 investigated the behavioural correlates of stress and corticosterone on motor symptoms after dopamine depletion. Experiment 2 focused on neurodegenerative processes after dopamine depletion in stress- and corticosterone-treated rats.
Experimental groups
Following baseline training and testing, animals in Experiment 1 were matched for reaching success and assigned to the following groups: lesion + restraint stress (STRESS, n = 9), lesion + corticosterone treatment (CORT, n = 9) and lesion only (CONTROL, n = 6). Experiment 2 involved 47 animals that were divided into four groups: lesion + restraint stress (STRESS, n = 14), lesion + corticosterone (CORT, n = 15), lesion only (CONTROL, n = 14) and non-lesion controls (NON-LESION, n = 4).
Time course
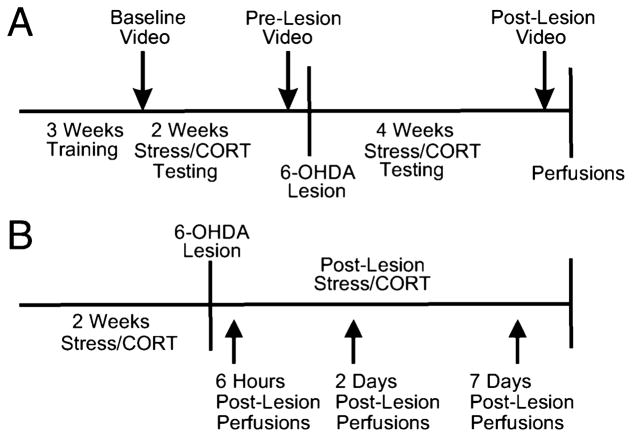
Animals in Experiment 1 (Fig. 1A) were pretrained in the skilled reaching task. Once reaching performance reached a plateau after 3 weeks of training, performance was video recorded for qualitative movement analysis. At this time, animals were also video recorded in skilled walking and open field tasks (‘baseline’). The STRESS and CORT groups were then exposed daily to their respective treatments for 17 days. During this period, all groups continued to be tested in the reaching task on a daily basis for 15 days. Video recordings of skilled reaching, skilled walking and open field tasks were made at the end of this period on day 16 (‘pre-lesion’). Unilateral 6-OHDA lesions were given and stress and CORT treatments continued for a further 29 days. Skilled reaching was tested daily for 27 days after lesion. Video recordings of skilled reaching, skilled walking and open field tasks were made at the end of this period on day 28 (‘post-lesion’). Apomorphine-induced rotations were also performed at the post-lesion time point. Blood samples were taken at baseline, 17 days pre-lesion and 29 days post-lesion after behavioural testing was completed.
Fig. 1.
Time lines illustrating the course of experimental manipulations, behavioural training and testing in (A) Experiment 1 investigating the effects of chronic stress on 6-OHDA-induced loss of motor function, and (B) Experiment 2 evaluating neurodegenerative processes after 6-OHDA lesion in stress-treated rats.
Animals in Experiment 2 (Fig. 1B) did not undergo behavioural testing or blood sampling. The STRESS group was subjected to 14 days of restraint stress prior to receiving unilateral 6-OHDA lesions and up to a maximum number of 7 days post-lesion. The CORT group was treated similarly, except that these animals received daily corticosterone doses. CONTROL animals were perfused at the respective time points as well. To determine a time course of neurodegenerative events, groups of animals were perfused at the following time points: 6 h (STRESS n = 4, CORT n = 5, CONTROL n = 4), 2 days (STRESS n = 5, CORT n = 5, CONTROL n = 5), and 7 days (STRESS n = 5, CORT n = 5, CONTROL n = 5) post-lesion. Four NON-LESION animals were perfused as well.
Physiological manipulations and stress procedures
Blood samples
Blood samples were always collected at the same time of day, between 08:30 and 11:00 h and approximately 50 min after restraint or corticosterone treatment. Blood was collected from the tail vein under isoflurane anaesthesia. After centrifuging at 1100 g for 8 min, the plasma samples were stored at −20 °C. Plasma CORT concentrations were determined by radioimmunoassay using commercial kits (Coat-A-Count, Diagnostic Products Corp., Los Angeles, CA, USA; Ishida et al., 2003).
Corticosterone administration
Five milligrams of corticosterone (Sigma-Aldrich, St. Louis, MO, USA) was mixed with crushed reaching pellets, cookie crumbs, peanut oil and water. Corticosterone was administered in the morning hours 60 min prior to behavioural training / testing or blood sampling (Metz et al., 2005).
Restraint stress
Animals were placed in Plexiglas tubes (5 cm inner diameter) for 20 min (García et al., 2000; Faraday, 2002; Mercier et al., 2003). Restraint stress took place in the morning hours 60 min prior to behavioural testing and blood sampling and was administered in a room different from the housing and testing rooms.
Nigrostriatal 6-OHDA lesions
Unilateral 6-OHDA lesions of the nigrostriatal bundle were performed in 46 animals. Animals in Experiment 1 were lesioned on the side contralateral to their preferred reaching paw. Animals in Experiment 2 were randomly assigned to either right or left side lesion. Thirty minutes prior to surgery, rats received desmethylimipramine (25 mg / kg i.p.; Sigma-Aldrich). Lesions of the nigrostriatal bundle were induced by infusing 2 μL of 4 mg / mL 6-OHDA hydrobromide (in saline ascorbic acid solution; Whishaw et al., 1986; Metz et al., 2001b) at the following coordinates: 4.0 mm posterior to bregma, 1.5 mm lateral to the midline, and 8.5 mm ventral to the skull surface with skull flat between lambda and bregma (Paxinos & Watson, 1998). Infusion took place over 5 min, with 5 min for diffusion (Metz & Whishaw, 2002b).
Behavioural training and testing
Skilled forelimb reaching
Apparatus
Animals were trained and tested in a transparent Plexiglas box (45 cm long × 14 cm wide × 34 cm tall) with a 1-cm-wide vertical opening in the front wall. Food pellets (45 mg, Bio-Serv, Frenchtown, NJ, USA) were placed on a shelf attached to the outside of the front wall (Whishaw et al., 1986; Metz et al., 2005). Two small indentations on the upper side of the shelf served to hold the food pellets. Pellets were placed in the indentation contralateral to the preferred forelimb (Fig. 2A). Animals were video recorded from a frontal view using a digital video camera.
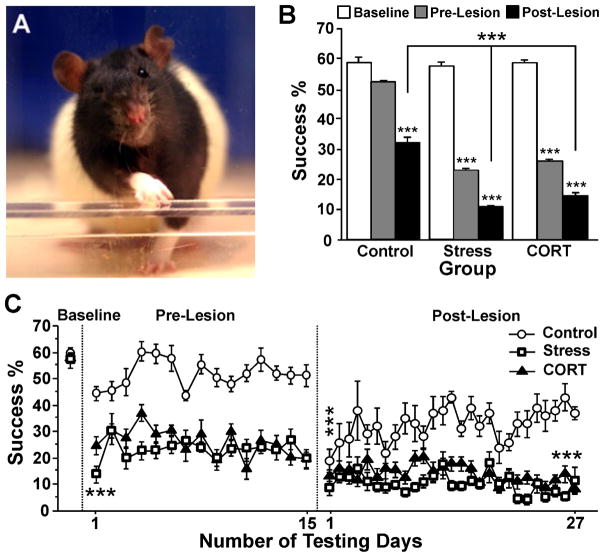
Fig. 2.
Skilled reaching task. (A) The apparatus with an opening in the front wall through which animals were required to reach and retrieve food pellets with the forelimb contralateral to the lesion. (B) Mean reaching success in each group during each experimental period. (C) Time course of daily reaching success in control, stress and corticosterone-treated animals prior to the treatment (baseline), during the treatment (15 days of pre-lesion testing) and after unilateral 6-OHDA lesion (27 days of post-lesion testing). Note that both stress and corticosterone reduced reaching success and abolished spontaneous improvement after lesion, although stress rats were most severely affected. Asterisks indicate significant differences: ***P = 0.001. (B) Paired t-test of mean success rate compared with baseline values. (C) Posthoc comparison with control animals on day 1 pre-lesion and day 27 post-lesion, and paired t-test with pre-lesion values on day 1 post-lesion.
Training and testing
The animals were pretrained daily for 3 weeks to extend their preferred forelimb through the slit to grasp and retrieve single food pellets (Metz & Whishaw, 2000). Success rates for 5 days were averaged and designated ‘baseline’ for the analyses. Limb preference was determined based on the first reaching attempts. Animals were presented with 20 pellets in each test session. Instances of defecation during a test session were also recorded. Success rates were recorded daily, and performance was video recorded at baseline, pre- and post-lesion periods. During video recording sessions, the apparatus was illuminated by a cold light source (Lowel Caselight, New York, USA).
Analysis
The number of successful reaches was recorded daily and converted to a percentage. A successful reach was defined as the animal obtaining a food pellet on the first attempt and withdrawing the pellet through the slit to consume it (Metz & Whishaw, 2000).
Videotapes were analysed frame-by-frame and the first three successful reaches were scored. Movement analysis was based on a scale described earlier (Whishaw & Pellis, 1990; Metz & Whishaw, 2000). For each of the 11 movement components, a score of 0 was given when the movement was absent, a score of 0.5 was given if the movement was present but abnormal, and a score of 1 was given if the movement was normal.
Skilled walking
Apparatus
Skilled walking was assessed with the ladder rung walking task apparatus, which consisted of two clear Plexiglas walls connected by metal rungs arranged in an irregular pattern (Metz & Whishaw, 2002a; Fig. 3A). The apparatus was placed on two animal transport tubs, with a neutral start tub and the familiar home tub at the end. A digital video camera was placed in a way to record all four limbs simultaneously. During video recording sessions, the apparatus was illuminated by a cold light source (Lowel Caselight).
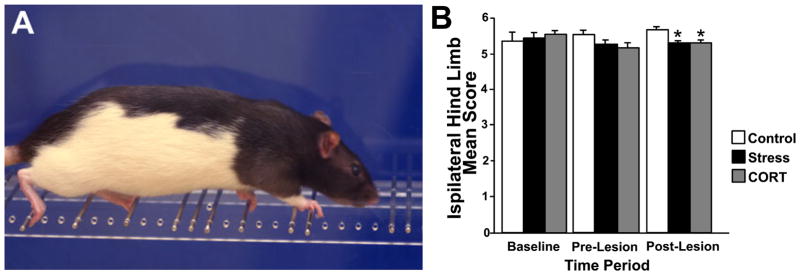
Fig. 3.
Skilled walking task. (A) Photograph showing a rat crossing the ladder rung walking apparatus. (B) Mean ipsilateral hind limb scores (out of 6 points) in control, stress and corticosterone-treated animals prior to treatment (baseline), after 16 days of treatment (pre-lesion) and 28 days after unilateral 6-OHDA lesion (post-lesion). Note that both stress and corticosterone treatments diminished interlimb coordination and ipsilateral limb placement accuracy, thus reducing the hind limb score. Asterisks indicate significances: *P = 0.05, unpaired t-test compared to control animals.
Training and testing
The animals received one training session the day before baseline testing in which they crossed the horizontal ladder five times. On testing days the animals were videotaped when crossing the ladder three times. Animals performed this task at baseline, pre-lesion and post-lesion time points. Three crossings per animal were video recorded.
Analysis
Video recordings were analysed frame-by-frame. Scores of both fore- and hind limbs were averaged for the three crossings. The type of limb placements and errors on the rungs were rated using a seven-point scale described earlier (Metz & Whishaw, 2002a). The number of errors of fore- and hind limbs was determined using the sum of the number of slips and misses (scores of 0, 1 or 2 points) divided by the number of steps. The time needed to cross the entire length of the ladder excluding the time an animal spent in a pause was also determined.
Open field behaviour
Apparatus
The open field apparatus was a 100 × 100-cm square box made of opaque black Plexiglas (Fig. 3A). The bottom of the box was divided into 16 (22 × 22 cm) fields. A digital video camera was positioned above the open field apparatus. The open field was illuminated at 380 lux by a cold light source (Lowel Caselight).
Testing
Each rat was placed in the middle of the open field apparatus and video recorded for 5 min. Animals were tested at baseline, pre-lesion and post-lesion testing time points. Open field testing was conducted in the afternoon hours to allow for the animals to recover from any fatigue from the reaching task.
Analysis
Video tapes were analysed to determine the total number of fields entered, the number of novel fields entered, the percentage of inside fields entered, the percentage of outside fields entered and the number of rears. In addition, the time spent in inside and outside fields, and rearing was evaluated. Times were expressed as percentages of the total time in the open field apparatus. An activity score was determined by dividing the number of fields visited by the amount of time spent in locomotion. The time spent rearing was considered vertical exploration whereas time spent in locomotion was considered horizontal exploration.
Apomorphine-induced rotation
To measure drug-induced turning, animals were injected s.c. with 0.05 mg / kg apomorphine-hydrochloride (Sigma-Aldrich). The animals were then placed individually into a round rotation bowl for 40 min. A cuff was wrapped around the trunk of each rat, which was connected to a lead and swivel that was connected to a computer. A custom-made computer program recorded the turns in the direction ipsilateral and contralateral to the lesion (Ungerstedt, 1971; Metz & Whishaw, 2002b).
Histology
Tyrosine hydroxylase (TH) immunohistochemistry
At the end of behavioural testing, the rats were deeply anaesthetized with an overdose of Euthansol (Schering Plough, Point Claire QB, Canada) and transcardially perfused with 0.1 m phosphate-buffer containing 0.9% NaCl (pH 7.4). This was followed by fixative solution containing 0.9% NaCl and picric acid (Lana’s fix). The brains were removed, weighed and stored in this fixative until sectioning. Adrenal glands were also weighed.
Brains were cut into 50-μm serial sections on a vibratome. Free-floating sections in Experiment 1 were processed for TH immunohistochemistry of dopamine-producing cells using the ABC method (Metz et al., 2001b). The number of TH-positive cell bodies was determined manually using a light microscope (Olympus). Cell counts were performed on three sections per rat in the medial tegmental area (MTA), ventral tegmental area (VTA), substantia nigra pars compacta (SNc), which also encompassed parts of substantia nigra pars reticulata, and substantial nigra lateralis (SNl) according to previous descriptions (see Fig. 5A; Metz et al., 2001b). All sections were located between 4.8 and 5.8 mm posterior to bregma (according to Paxinos & Watson, 1998).
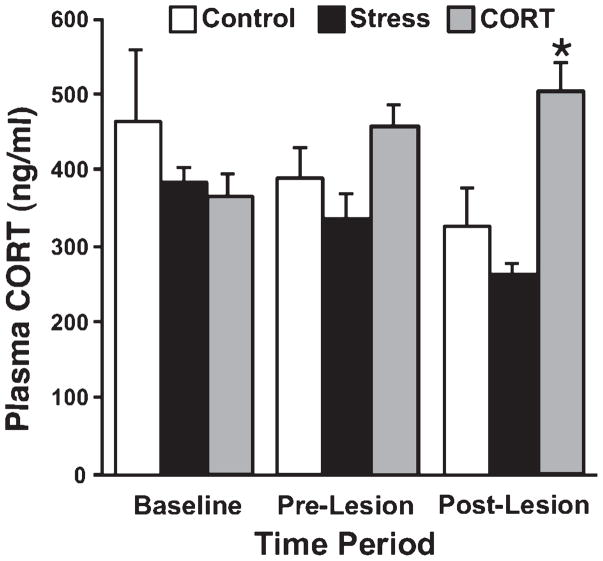
Fig. 5.
Circulating plasma CORT levels in control, stress and corticosterone-treated animals prior to treatment (baseline), after 17 days of treatment (pre-lesion) and 29 days after unilateral 6-OHDA lesion (post-lesion). Daily CORT treatment caused consistent elevation in plasma CORT level, while levels after daily restraint stress were close to baseline. Asterisks indicate significant differences: *P = 0.05, t-test compared with control animals.
Triple labelling
Animals in Experiment 2 were deeply anaesthetized and transcardially perfused with PBS followed by 4% paraformaldehyde. The brains were removed and stored in the fixative solution for 24 h and then transferred to cryoprotectant (PBS containing 30% sucrose and 0.02% sodium azide). The brains were cut frozen, in the coronal plane, on a freezing sliding microtome at a thickness of 40 μm to collect serial sections.
Brains were stained for TH, Fluoro-Jade B, a marker for neuronal degeneration (Schmued & Hopkins, 2000) and glial fibrillary acidic protein (GFAP) immunofluorescence. Sections taken at 240-μm intervals were incubated, free-floating, in a guinea-pig polyclonal anti-GFAP antibody (1 : 1000 in PBS and 3% Triton X; Advanced Immunochemical, Long Beach, CA, USA) and a mouse monoclonal anti-TH antibody (1 : 1000 in PBS and 3% Triton X; Sigma-Aldrich, Oakville, ON, Canada) for 24 h and incubated for 24 h in PBS containing a Cy3-conjugated donkey anti-guinea-pig IgG (1 : 500; Jackson ImmunoResearch, West Grove, PA, USA) and a Cy5-conjugated goat anti-mouse IgG (1 : 500; Chemicon, Temecula, CA, USA). The sections were then stained with Fluoro-Jade B (Chemicon; Schmued & Hopkins, 2000). Sections were viewed using an Axioskop 2 Mot Plus Epifluorescence microscope (Carl Zeiss, Jena, Germany), and images were collected using Northern Eclipse image analysis software (Empix Imaging Inc., Mississauga, ON, Canada). Images were analysed using Axiovision image analysis software (Carl Zeiss). Cell counts of TH-positive and Fluoro-Jade-positive cells were performed on the three sections per brain with the highest TH staining density in substantia nigra at 100× magnification in areas MTA, VTA, SNc and SNl. Optical density of GFAP immunoreactivity was evaluated using NIH Image analysis software (Version 1.63, National Institutes of Health, Bethesda, MD, USA). After binarization, the area of GFAP density was measured in a rectangular field (24034 μm pixel2 at 100× magnification) placed over SNc in the lesion and non-lesion hemispheres. To correct for individual differences in staining intensity, GFAP density was expressed as percentage of non-lesion hemisphere density. One CONTROL and one STRESS animal were removed from the analysis due to a lack of adequately stained sections.
Nissl staining
For each triple-labeled section in Experiment 2, an adjacent section was stained using a standard protocol for Nissl staining. On three sections, cell numbers in the VTA and SN (including SNc and SNl) were estimated according to adjacent TH-stained sections. Only cells with visible cytoplasm and nucleus were counted. Cell counts were performed in CONTROL (6 h n = 3, 2 days n = 5, 7 days n = 4), STRESS (6 h n = 3, 2 days n = 3, 7 days n = 5), CORT (6 h n = 5, 2 days n = 5, 7 days n = 5) and NON-LESION (n = 4) animals.
Statistical analysis
Statistical analyses were performed using JMP-IN (Version 3.2.1; SAS Institute Inc., Cary, NC, USA) and Statview (Version 5.0; Abacus Concepts Inc., Berkeley, CA, USA) software packages. Behavioural data were analysed using repeated-measures anovas, followed by Scheffe posthoc tests. Histological data, including cell counts and optical density, were analysed by anovas followed by paired and unpaired t-tests, respectively. Correlation analysis of cell numbers was performed by using Fisher’s R to Z transformation and a z-test to calculate the significance of the correlation coefficients. A P-value of 0.05 was chosen as the significance level for all analyses. Values given are means ± SE.
Results
Experiment 1
Stress and CORT treatments diminish skilled reaching success prior to and after lesion
Success rates
There were significant Group (F2,69 = 55.88, P ≤ 0.001) and Time Period (F2,69 = 293.43, P ≤ 0.001) effects for skilled reaching success. All pre-lesion and post-lesion success rates were significantly lower than those seen at baseline (all P ≤ 0.001). Post-lesion, success rates of all groups were lower than pre-lesion performance (all P ≤ 0.001).
Reaching success in STRESS and CORT animals dropped to on average less than half of baseline reaching success in the pre-lesion period (STRESS, 22.5%; CORT, 25.5%). In contrast, CONTROL animals maintained reaching success at an average of 52% throughout the pre-lesion period. Consequently, both the STRESS and the CORT animals were considerably less successful than CONTROL animals during the pre-lesion period (F2,21 = 78.26, P ≤ 0.001; posthoc P ≤ 0.001). Success rates in STRESS and CORT animals dropped even further during the post-lesion period (STRESS, 10.2%; CORT, 13.9%), which was significantly lower than the success rate (31.3%) that CONTROL rats were able to achieve (F2,20 = 32.16, P ≤ 0.001; CONTROL > CORT P ≤ 0.001; CONTROL > STRESS P ≤ 0.001). On all test days except two pre-lesion days, treated STRESS and CORT groups reached lower success rates than the CONTROL group (all posthoc P ≤ 0.05; Fig. 2B and C). The STRESS group was also significantly less successful than the CORT group on days 1 and 4 pre-lesion (posthoc P ≤ 0.05).
Although success rates equally dropped in all groups initially after lesion, only the CONTROL group was able to show significant spontaneous improvement compared with post-lesion day 1 (posthoc P ≤ 0.05; Fig. 2C). Thus, success rates in STRESS and CORT rats were significantly lower than those of CONTROL rats at 3 weeks after the lesion (both P ≤ 0.001). The STRESS group was less successful than the CORT group on post-lesion days 10, 11, 14, 15, 22 and 26 (all P ≤ 0.05). Thus, STRESS overall had a more severe effect on reaching success than CORT alone.
Reaching times
A significant effect of Time Period was found, with the animals needing twice as long to perform the task after surgery than prior to the lesion in all three groups (F2,69 = 147.06, P ≤ 0.001; all posthoc P ≤ 0.001). There were no significant group differences at any of the time periods tested, however.
Reaching movement performance
Group differences were found for advance (F2,67 = 3.93, P ≤ 0.05; CORT < CONTROL posthoc P ≤ 0.05); grasp (F2,67 = 3.07, P ≤ 0.05; posthoc not significant), supination I (F2,67 = 4.55, P ≤ 0.05; posthoc not significant) and release (F2,67 = 4.16, P ≤ 0.05; STRESS and CORT > CONTROL posthoc P ≤ 0.05). During the pre-lesion interval, CONTROL and STRESS groups showed higher scores than the CORT group in advance (posthoc P ≤ 0.05) and supination I (posthoc P ≤ 0.05).
Defecation rate
There was a significant Group effect for the number of fecal pellets deposited during skilled reaching testing (F2,45 = 7.99, P ≤ 0.001). The CORT animals defecated more than the CONTROL (posthoc P ≤ 0.01) and STRESS groups (posthoc P ≤ 0.05).
Stress reduces skilled walking performance
Group effects were found for ipsilateral forelimb scores (F2,68 = 3.33, P ≤ 0.05), ipsilateral forelimb errors (F2,68 = 3.44, P ≤ 0.05), contralateral forelimb score (F2,68 = 3.19, P ≤ 0.05), time to cross (F2,68 = 4.34, P ≤ 0.05) and pauses (F2,68 = 7.84, P ≤ 0.001). Overall, STRESS animals had lower ipsilateral forelimb scores than CONTROL animals (score of 5.1 compared with 5.5; posthoc P ≤ 0.05). Furthermore, STRESS animals overall made more errors with the ipsilateral forelimb than CONTROL rats (11.1 vs. 4.2 errors; posthoc P ≤ 0.05). Moreover, STRESS and CORT groups showed lower ipsilateral hind limb scores than the CONTROL group throughout the post-lesion period (average score of 5.4 as compared with 5.7; F2,20 = 4.22, P ≤ 0.05). During the post-lesion period both STRESS and CORT animals showed significantly lower scores than CONTROL rats (t-test P ≤ 0.05; Fig. 3B). Accordingly, STRESS animals made twice as many ipsilateral forelimb errors than CONTROL animals after lesion (t-test P ≤ 0.05). STRESS and CORT animals paused about four times more often than CONTROL rats after the lesion. CORT animals overall paused more than STRESS animals (posthoc P ≤ 0.01) and STRESS animals paused less often than the other two groups (t-tests P ≤ 0.05).
Stress and cort treatments elevate activity in the open field
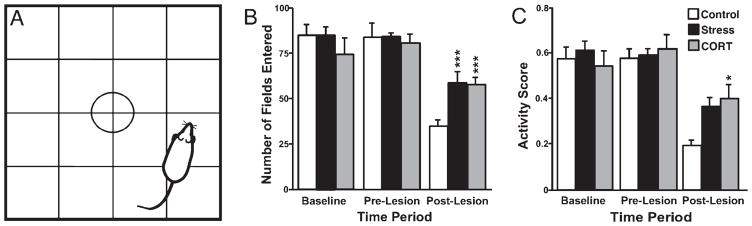
Animals spent 50% less time in the inside fields pre-lesion than at baseline (posthoc P ≤ 0.05) and post-lesion (posthoc P ≤ 0.01). The lesion caused animals to visit fewer fields than at baseline and pre-lesion (posthoc P ≤ 0.001) and to rear 50% less often (posthoc P ≤ 0.001). Furthermore, animals groomed more frequently post-lesion than during the other time periods (t-test P ≤ 0.5). Thus, activity scores during the post-lesion interval were significantly reduced (0.59 pre-lesion vs. 0.39 post-lesion; posthoc P ≤ 0.001). The most significant differences were found in STRESS animals post-lesion, which visited fewer fields (F2,24 = 9.56, P ≤ 0.001; posthoc P ≤ 0.01), reared less (F2,24 = 23.24, P ≤ 0.001; posthoc P ≤ 0.001), and had lower horizontal activity scores (F2,24 = 12.75, P ≤ 0.001; posthoc P ≤ 0.001) than at baseline and pre-lesion. However, STRESS and CORT groups visited more fields than CONTROL animals (58 as compared with 34 fields; posthoc P ≤ 0.05; Fig. 4B). Moreover, activity scores in STRESS and CORT animals were twice as high as in CONTROL animals (0.38 as compared with 0.19), which reached significance for CORT animals (posthoc P = 0.05, Fig. 4C). CORT animals spent more time in the inside fields and visited more inside fields than the STRESS and CONTROL groups (206 vs. 129 fields; posthoc P ≤ 0.05). Animals with more severe dopamine depletion, as indicated by enhanced contralateral rotation bias, were showing less exploration (r = −0.57, d.f. = 23, P ≤ 0.01).
Fig. 4.
Open field test. (A) The open field with a floor divided into 16 squares to measure motor activity and exploration. (B) Number of fields entered during a test session. (C) Activity score in control, stress and corticosterone-treated animals prior to treatment (baseline), after 16 days of treatment (pre-lesion) and 28 days after unilateral 6-OHDA lesion (post-lesion). Note that the 6-OHDA lesion reduced locomotor activity in all animals; however, chronic stress and CORT treatment significantly increased exploratory activity as compared with untreated control animals. Asterisks indicate significant differences: *P = 0.05, ***P = 0.001, t-test compared with control animals.
Apomorphine-induced rotation
Neither the total number of rotations nor the percentage of contralateral rotations was influenced by any of the treatments. The STRESS group, however, deposited more fecal pellets during rotations than the CORT group (posthoc P ≤ 0.05).
Oral cort treatment chronically elevates plasma corticosterone
There was a significant effect of Group for plasma corticosterone levels (F2,64 = 5.47, P ≤ 0.01; Fig. 5), with the STRESS group demonstrating lower levels than the CORT group (posthoc P ≤ 0.01). At 454 ng / mL, the CORT group had higher corticosterone levels than the STRESS group during the pre-lesion interval (posthoc P ≤ 0.05) and higher plasma corticosterone levels than both the STRESS (posthoc P ≤ 0.01) and the CONTROL (posthoc P ≤ 0.05) groups during the post-lesion interval (500 ng / mL).
Stress and cort treatments prevent gain in body weight
Animals showed a significant overall effect of Group (F2,69 = 3.94, P ≤ 0.05), with the CORT group weighing more than the CONTROL group (posthoc P ≤ 0.05). Group differences were found during the baseline period, at which time both the STRESS and the CORT groups weighed more than the CONTROL group (posthoc P ≤ 0.05). This difference disappeared during the pre-lesion period as a result of weight gain in the CONTROL group, which did not occur in STRESS or CORT animals while they were treated. In spite of regulated food rations, CONTROL animals gained on average 3% of body weight, while CORT and STRESS animals lost an average of up to 2.3% of body weight.
Brain and adrenal gland weights
There were no significant group differences in either gross brain weight or brain weight when corrected for body weight. Due to suppression of adrenal activity, CORT animals had lighter adrenal glands than STRESS and CONTROL animals (t-test P ≤ 0.05).
TH cell counts
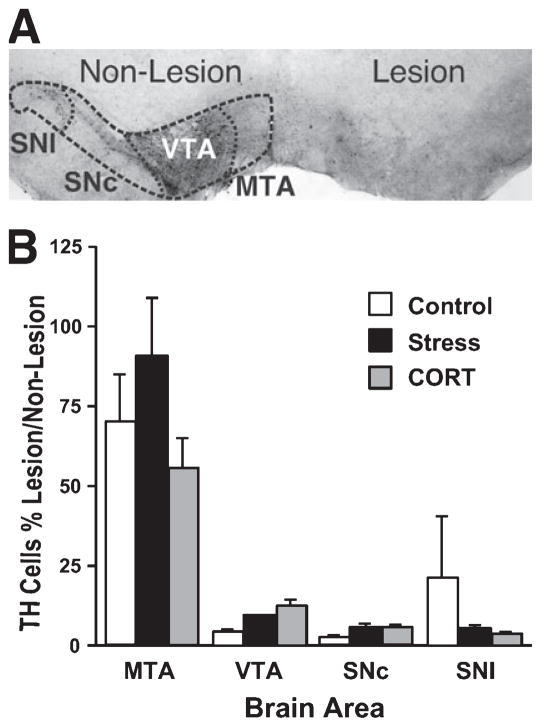
TH-positive cells were counted on both the lesion and the non-lesion sides of the brain. As illustrated in Fig. 6A, the unilateral 6-OHDA infusion caused loss of TH-positive cells in the SNc with considerable sparing in the VTA. The number of TH-positive cells in the SNc and SNl was reduced to about 5–10% that of the non-lesion side (Fig. 6B). The CONTROL group appeared to have more sparing in the SNl area than the other two groups, but this trend was not significant.
Fig. 6.
Number of TH-positive cells 3 weeks after lesion. (A) Photograph of a section through the midbrain, illustrating a representative unilateral dopamine depletion in substantia nigra as reflected by TH immunohistochemistry. The four areas used for cell counts are delineated. (B) TH cell counts in Experiment 1. Although there was no significant difference between groups after chronic CORT or stress treatment, that there was a trend towards reduced numbers of dopamine-positive cells in the sustantia nigra pars compacta in treated animals. Magnification: 25×. SNl, substantia nigra lateral segment; SNc, substantia nigra pars compacta; VTA, ventral tegmental area; MTA, medial tegmental area.
Experiment 2
Stress leads to earlier presence of Fluoro-Jade-positive cells
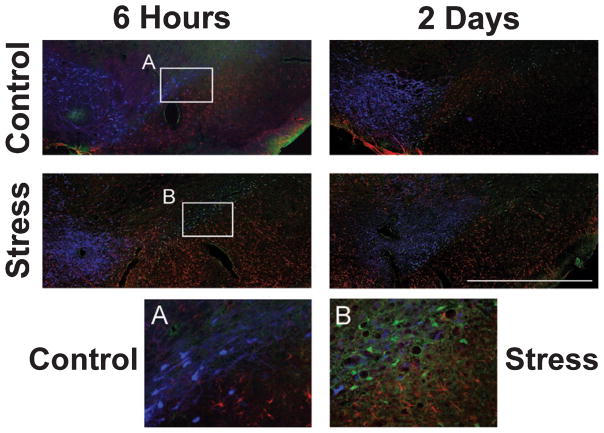
Figure 7 shows representative triple-labeled sections through the lesion midbrain of STRESS and CONTROL animals at 6 h and 2 days post-lesion. The sections illustrate a declining number of TH-positive cells from 6 h to 2 days post-lesion while the number of Fluoro-Jade-positive cells grew. In particular, Fluoro-Jade-positive cells were detected 6 h after 6-OHDA infusion only in STRESS animals, but not in CONTROL rats (see Fig. 7A and B).
Fig. 7.
Markers of dopaminergic neurons, cell death and astrogliosis in the lesion ventral midbrain. Upper panel: the lesion hemisphere in control animals at 6 h and 2 days after nigrostriatal bundle 6-OHDA lesion. Lower panel: the lesion hemisphere of stress-treated animals at 6 h and 2 days after lesion. Inserts: (A) control animal and (B) stress-treated animal at 6 h after lesion. Note that Fluoro-Jade-positive neurons at 6 h after lesion are absent in the control brain, but present in the stress-treated rat. Fluoro-Jade-positive neurons were present in both groups at later time points. Blue, TH-positive neurons; green, Fluoro-Jade B-positive neurons; red, GFAP-positive astrocytes. SN, substantia nigra; VTA, ventral tegmental area. Scale bar: 1 mm. Magnification of inserts: 200×.
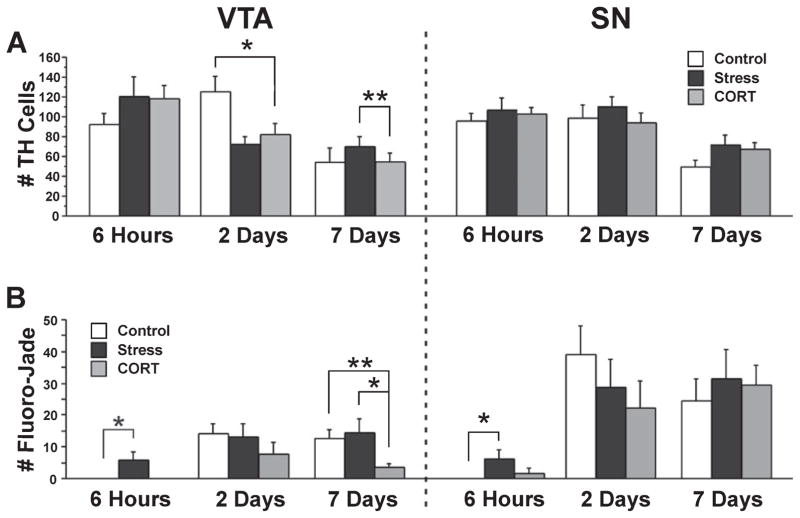
As shown in Fig. 8A and B, the number of TH- and Fluoro-Jade-positive neurons was dependent on the post-lesion interval. The number of TH-positive neurons was highest at 6 h (in SN: STRESS 107 ± 12 cells, CORT 102 ± 7 cells, CONTROL 102 ± 8 cells) and 2-day intervals and lowest at 7 days post-lesion (F3,143 = 21.7, P ≤ 0.001). All lesion groups showed a significant reduction in TH-positive cells in VTA and SN when compared with NON-LESION animals with the CORT group being most severely affected (VTA: t = −2.84, P ≤ 0.01; SN: t = −4.17, P ≤ 0.001). CORT animals showed a reduced number of TH-positive cells in VTA at 2 days when compared with CONTROL rats (t = −2.15, P ≤ 0.05) and at 7 days when compared with STRESS rats (t = −3.06, P ≤ 0.01; Fig. 8A).
Fig. 8.
Markers for 6-OHDA-induced neurodegeneration in control and stress-treated animals. (A) Number of TH-positive neurons in VTA and SN at 6 h, 2 days and 7 days after 6-OHDA lesion in control, stress and CORT-treated animals. (B) Number of Fluoro-Jade positive neurons in VTA and SN at different time points after lesion and in the three groups. Note the significant elevation in Fluoro-Jade-positive cell numbers in stress-treated rats early after lesion. Asterisks indicate significant differences: *P < 0.05, **P < 0.01; unpaired t-test compared between groups as indicated by brackets. VTA, ventral tegmental area; SN, substantia nigra pars compacta.
The number of Fluoro-Jade-positive neurons in the lesion hemisphere was lowest at 6 h and equally high at 2 and 7 days post-lesion (Fig. 8B; F3,143 = 14.55, P ≤ 0.001). The number of Fluoro-Jade-positive neurons increased significantly by 2 days in VTA (STRESS 13 ± 4 cells, CORT 7 ± 4 cells, CONTROL 14 ± 3 cells; t = −4.1, P ≤ 0.001) and SN (STRESS 28 ± 9 cells, CORT 22 ± 8 cells, CONTROL 39 ± 9 cells; t = 5.08, P ≤ 0.001). STRESS and CONTROL animals showed significantly higher numbers of Fluoro-Jade-positive cells compared with NON-LESION controls in VTA (STRESS: t = −2.63, P ≤ 0.05; CONTROL: t = 2.77, P ≤ 0.01) and SN (STRESS: t = −2.52, P ≤ 0.05; CONTROL: t = 2.41, P ≤ 0.05). CORT animals were higher than NON-LESION controls in the SN (t = 2.35; P ≤ 0.05). Most notably, STRESS animals were the only ones with Fluoro-Jade-positive cells present in VTA at 6 h post-lesion (6 ± 3 cells). Thus, they had a higher number of Fluoro-Jade-positive neurons at 6 h than CONTROL (t = −5.58, P ≤ 0.05) and CORT (t = −5.58, P ≤ 0.05) animals in VTA, and a higher number than CONTROL rats in SN (t = −2.6, P ≤ 0.05; Fig. 8B). At 7 days post-lesion, CORT animals showed a lower number of Fluoro-Jade-positive cells in VTA when compared with CONTROL and STRESS animals (CONTROL: t = 3.61, P ≤ 0.01; STRESS: t = −2.51, P ≤ 0.05).
Only in STRESS and CORT animals was the loss of TH-positive cells accompanied by a significant increase in Fluoro-Jade-positive cells in the SN, as revealed by correlation analysis (STRESS, r = 0.48, P ≤ 0.001; CORT r = 0.29, P ≤ 0.05, correlation analysis).
Stress and CORT treatments lead to greater cell loss in VTA and SN
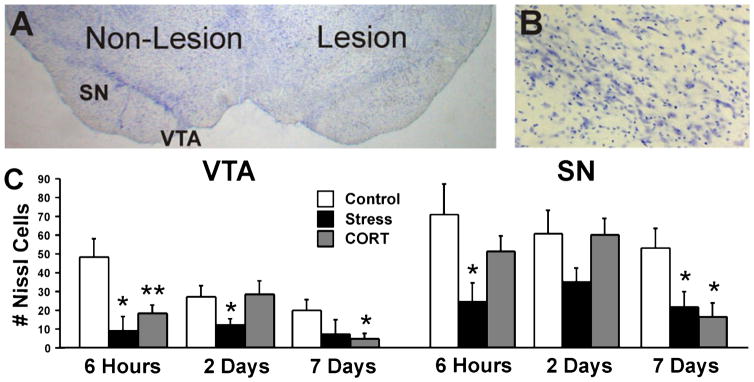
A representative section of a lesion brain with Nissl staining is shown in Fig. 9A. There was significant degeneration and loss of Nissl-stained cell bodies in the lesion hemisphere in all experimental groups (see Fig. 9B). The number of Nissl-positive cells in the VTA and SN is illustrated in Fig. 9C. There was a significant effect of Group (F3,120 = 4.48, P ≤ 0.05) in the lesion VTA and SN with STRESS showing the overall lowest cell numbers. There were no differences between groups in the non-lesion hemisphere.
Fig. 9.
Nissl staining in VTA and SN. (A) Representative Nissl-stained section illustrating the non-lesion and lesion hemispheres. (B) Higher magnification of lesion substantia nigra (100×). (C) The number of Nissl-positive cells in VTA and SN in the lesion hemisphere. There was a significant acceleration of cell loss in stress-treated rats and reduced nigral cell numbers at 7 days post-lesion. Asterisks indicate significant differences: *P = 0.05; **P = 0.01; unpaired t-test compared with lesion control animals. VTA, ventral tegmental area; SN, substantia nigra pars compacta.
In the lesion VTA, both STRESS and CORT animals showed an overall larger loss of cells than NON-LESION animals (STRSS: t = 2.36, P ≤ 0.05; CORT: t = −2.3, P ≤ 0.05). Furthermore, STRESS animals showed a lower cell number in the VTA when compared with CONTROL animals (t = 2.17, P ≤ 0.05). At 6 h post-lesion, there were fewer Nissl-stained cells present in the VTA of STRESS and CORT groups when compared to CONTROL rats (STRESS: t = 2.54, P ≤ 0.05; CORT: t = 3.33, P ≤ 0.01). At 2 days post-lesion, STRESS animals showed a lower cell number than CONTROL and NON-LESION animals (t = 2.08, P ≤ 0.05; t = 2.25, P ≤ 0.05, respectively). Finally at 7 days post-lesion, CORT and CONTROL animals had lower cell numbers than NON-LESION animals (t = −3.53, P ≤ 0.01; t = −2.76, P ≤ 0.05, respectively). CORT animals also had a lower cell number than CONTROL rats (t = 2.59, P ≤ 0.05).
As shown in Fig. 9B, cell numbers were also significantly reduced in the SN. Both STRESS and CORT animals showed fewer cells than NON-LESION animals (STRESS: t = 2.8, P ≤ 0.01; CORT: t = −2.24, P ≤ 0.05). Moreover, STRESS animals showed a reduced cell count as compared with CONTROL animals (t = 2.1, P ≤ 0.05). At 2 days post-lesion, STRESS animals had the lowest cell number of all groups and showed about half as many cells in SN as compared with NON-LESION animals (t = 2.25, P ≤ 0.05). At 7 days post-lesion, both STRESS and CORT animals showed fewer cells than NON-LESION animals (STRESS: t = 2.07, P ≤ 0.05; CORT: t = −3.77, P ≤ 0.001) and fewer than CONTROL rats (STRESS: t = 2.07, P ≤ 0.05; CORT: t = 2.58, P ≤ 0.05). Moreover, STRESS had lower cell numbers than CONTROL animals (t = 2.08, P ≤ 0.05). There were no differences between CONTROL and NON-LESION animals.
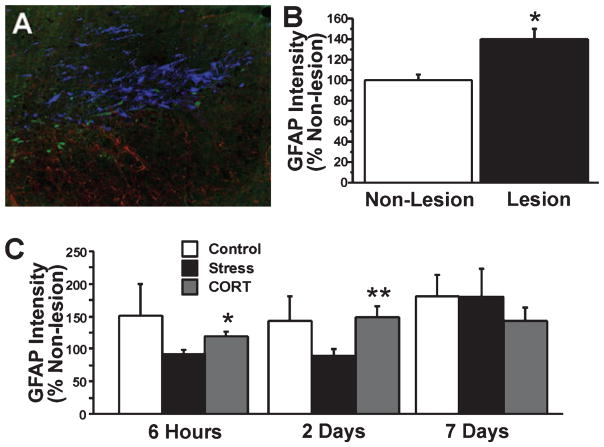
Stress and cort treatments enhance GFAP intensity
Figure 10A shows GFAP density in a representative section through the substantia nigra of a lesion animal. Absolute GFAP and relative GFAP values (percentage of non-lesion hemisphere) showed an elevation in the lesion hemisphere (t = 2.0, P ≤ 0.05; Fig. 10B), indicating reactive gliosis. Overall GFAP values were elevated in all lesion groups (STRESS: 22%; CORT: 32%; CONTROL: 62%) as compared with NON-LESION animals. Both STRESS and CORT groups showed elevated GFAP intensity in the non-lesion (t < −1.93, P ≤ 0.05) and the lesion (t < 2.0, P ≤ 0.05) SN when compared with NON-LESION values. The overall density of GFAP staining did not change significantly between 6 h and 7 days post-lesion (Fig. 10C). There were significant group differences, however. A significant increase in GFAP staining intensity as percentage of the intact hemisphere was found in the lesion SN of CORT animals 6 h post-lesion (t = 2.23, P ≤ 0.05) and 2 days post-lesion (t = 2.86, P ≤ 0.01) when compared with STRESS animals (Fig. 10C). There also was a significant increase in GFAP staining density in STRESS animals from day 2 to day 7 post-lesion (t = −2.1, P ≤ 0.05).
Fig. 10.
GFAP immunoreactivity. (A) Photomicrograph of the lesioned substantia nigra at day 2 post-lesion. (B) The area of GFAP immunoreactivity indicated by optical density (pixels × 102) expressed as percentage of non-lesion hemisphere in non-lesion and lesion animals. (C) GFAP intensity at three different time points after 6-OHDA lesion and in control, stress and corticosterone-treated animals. The lesion caused an overall elevation in GFAP staining. Stress animals were not different from controls, although they showed a lower GFAP intensity than corticosterone-treated rats. Blue, TH-positive neurons; green, Fluoro-Jade B-positive neurons; red, GFAP-positive astrocytes. Magnification: 250×. Asterisks indicate significant differences: *P < 0.05, unpaired t-test compared between non-lesion and lesion animals (B), and *P < 0.05, **P < 0.01, unpaired t-test between stress- and corticosterone-treated rats (C).
Discussion
The present study shows that chronic psychological stress, as produced by daily restraint, and elevated corticosterone levels impair skilled limb use in naïve and 6-OHDA dopamine-depleted rats. Stress and corticosterone treatments reduced reaching success immediately upon their introduction and impeded spontaneous recovery and compensation after dopamine depletion. In contrast, control rats showed asymptotic improvement in skilled motor function after lesion. Furthermore, stress and corticosterone treatments disturbed limb coordination in 6-OHDA lesion animals and altered exploratory behaviour. Motor impairments in stress-treated animals were accompanied by acceleration of neurodegenerative processes in the substantia nigra. Although there was no significant difference in nigral TH cell counts between groups at chronic post-lesion time points, stress-treated animals displayed higher numbers of Fluoro-Jade-positive cells in substantia nigra and VTA 6 h post-lesion. Furthermore, the loss of midbrain TH-labeled cells in stress- and corticosterone-treated rats was accompanied by a significant increase in cell death and by greater cell loss at 1 week post-lesion. These findings demonstrate that stress exaggerates functional deficits and accelerates loss of dopamine-producing neurons. Thus, stress might represent a critical variable in the progression of neurodegenerative events underlying PD.
Stress impedes recovery and compensation in skilled limb use
Although the effects of stress and stress hormones, in particular corticosterone, on the limbic system have been widely investigated (Lupien & McEwen, 1997; Gregus et al., 2005), only few studies focused on motor system function and performance (Maki & McIlroy, 1995; Metz et al., 2001a, 2005). Previous studies in rats, demonstrating stress- and stress hormone-induced impairments in skilled reaching and walking (Metz et al., 2001a, 2005), support the present observations of deficiency in rotatory limb movement and movement accuracy. These findings are not surprising, however, given the observation that receptors for corticosterone are found throughout the brain including motor areas such as motor cortex and basal ganglia (Ahima & Harlan, 1990; Ahima et al., 1991; Marlier et al., 1995). The design of the present study allows dissociation of performance changes based on stress vs. elevated corticosterone levels, showing that both treatments can alter normal motor performance with a lasting effect. Although corticosterone might be causally involved in stress-associated motor modulation, the present findings and previous notes (Metz et al., 2005) suggest that more complex physiological and emotional aspects, such as anxiety, were involved. Consequently, chronic stress impaired motor function independently of elevated corticosterone levels. The psychological nature of restraint stress strengthens the ecological validity of the present findings for human patients with Parkinson’s disease.
Aside from modulating normal performance, stress had an added effect on 6-OHDA-induced motor impairments and limited recovery as indicated by permanently altered reaching movement patterns. Although stress- and corticosterone-treated animals received daily training while being tested in the skilled reaching task, a comparison with non-treated lesion rats shows that this exercise failed to promote acquisition of alternative successful movements. Thus, aside from reducing genuine recovery, these treatments also limited compensatory adjustments. Furthermore, one would assume that exercise, either forced or voluntary, prior to or after lesion will elevate the availability of neurotrophic factors, including GDNF, and thus ameliorate behavioural asymmetry (Cohen et al., 2003; Smith & Zigmond, 2007) and rotational bias (Mabandla et al., 2007). Hence behavioural training, as used in Experiment 1, might potentially affect the vulnerability of dopamine neurons to 6-OHDA. Our results and previous observations (O’Dell et al., 2007), however, indicate no sparing of TH-positive cells by exercise. Lastly, it could also be argued that stress suppresses motivation or hedonic responses, thus causing loss of reaching success. Nevertheless, Macht et al. (2007) found a stress-induced loss in hedonic responses in PD patients not to be associated with reach-to-grasp impairments. A loss of motivation in motor performance may also be reflected by prolonged time measurements needed to complete a task. No alterations in time measurements were found, however, suggesting that stress-associated changes in success rates and movement patterns represent primary motor deficits.
Further support for stress-induced impediment of compensatory limb use is also provided by observations of reduced ipsilateral limb placement accuracy. Adjustments in ipsilateral limb placement might be made to overcome disturbed balance and misplacements of contralateral limbs. These findings complement a report by Snyder et al. (1985) showing that acute stressors precipitate neurological deficits and akinetic symptoms in 6-OHDA rats. Furthermore, it is possible that stress limits the recruitment of intact cortical and subcortical areas, including brain stem and cerebellum, to provide additional distal and proximal support. It is also possible that stress-induced anxiety affected skilled limb use by modulating sensory processing, as shown for other systems (Orr et al., 2002; Risbrough et al., 2004; Metz, 2007). In addition, the present changes in motor function in stress-treated 6-OHDA lesion rats were accompanied by prominent neurodegenerative events.
Stress accelerates neurodegenerative processes in 6-OHDA lesion rats
The present study employed a stressor of moderate severity to demonstrate that stress exaggerates motor deficits and neuronal loss in 6-OHDA dopamine-depleted rats. The main findings were that stress accelerates neuronal loss as reflected by elevated ventral midbrain Fluoro-Jade staining intensity 6 h after lesion. Furthermore, stress and corticosterone treatment both increased the loss of TH-positive cells on day 2 as compared with earlier time points. This finding is in line with Zuch et al. (2000), who showed that cell death is apparent in the substantia nigra pars compacta within 6 h after lesion while 6-OHDA neurotoxicity peaks at 48 h post-lesion. While our results suggest a stress-induced reduction in TH-positive cells at this time point, it is possible that compromised TH-positive neurons lose their TH phenotype without undergoing cell death. By contrast, Nissl staining indicates greater overall loss of midbrain neurons in stress- and corticosterone-treated animals at long-term intervals after lesion. These findings are in accordance with earlier studies combining TH- and Nissl-cell counts showing a dissociation of the two measures in 6-OHDA (Yuan et al., 2005) and MPTP (Seniuk et al., 1990; Tatton et al., 1990) lesion models. According to Yuan et al. (2005), Nissl staining provides a more suitable estimation of lesion size in the nigrostriatal 6-OHDA lesion than TH immunostaining. Although the present histological protocols did not permit the use of stereology, the data show a clear association between cell loss and permanent reduction in skilled limb function in stress- and corticosterone-treated animals.
Several mechanisms might account for stress-induced acceleration of dopamine loss and exaggeration of overall cell loss. Although one might expect that glucocorticoids are involved in stress-induced neurodegenerative effects (e.g. Marchetti et al., 2005), the present data suggest a mechanism independent of corticosterone based on the observation of absent chronic corticosterone elevation in stress-treated animals. The present study used oral corticosterone supplements to separate the effects of stress and glucocorticoids on 6-OHDA lesion pathology without stressful manipulations associated. The differences in the time course of behavioural and histological changes after stress and corticosterone treatment suggest distinct routes of action. While stress-induced motor loss appeared to be independent of elevated corticosterone levels, the accelerated neurodegenerative processes might be explained by a number of mechanisms. Previous studies suggested that in hippocampus, stress-associated changes in neurotrophic factor concentration can be regulated independently of glucocorticoids (Scaccianoce et al., 2004). Additionally, regulation of neurotrophin expression, including brain-derived neurotrophic factor, in striatum is not closely linked to density of glucocorticoid receptors (Schulte-Herbruggen et al., 2006). Stress was shown to disrupt expression of anti-apoptotic (DeVries et al., 2001) and neurotrophic factors (Adlard & Cotman, 2004) while increasing the expression of pro-apoptotic proteins (Scheff et al., 1980a, 1980b; Smith et al., 2002). Down-regulation of neurotrophic factor expression might explain the lack of compensatory behaviour found in stress-treated animals. Moreover, limited availability of trophic factors might explain the accelerated neuron loss in the lesion substantia nigra. The present findings might also derive from limitations in cellular functioning and lack of dopamine availability to exaggerate motor deficits in stressed animals. Support for this hypothesis comes from the lack of significant positive correlation between behavioural function and TH-positive cell numbers. Furthermore, it is possible that stress altered the metabolic activity of dopaminergic neurons, including dopamine uptake and storage, and so altered the susceptibility to 6-OHDA neurotoxic effects. Thus, the time course of neurotoxic events induced by 6-OHDA might have been shifted. Lastly, stress might exaggerate overall cell loss through promoting excitotoxicity generated by reactive oxygen species (Sapolsky, 1996; Kim et al., 2005).
In contrast to stress-induced exaggeration of neurodegenerative processes, corticosterone-treated rats rather showed attenuated cell death. This finding might be explained by modulation of inflammatory responses, which, depending on glucocorticoid concentration, might either promote or reduce cell death according to an inverted U-shape curve (Dhabhar & McEwen, 2001; Sapolsky et al., 2000; Dinkel et al., 2003). Thus, it is possible that the particular dose of oral corticosterone treatment used here exerted neuroprotective properties as described for other glucocorticoid treatments (Wojtal et al., 2006). It is possible though that cell death was delayed beyond 1 week post-lesion and thus was not assessed in the present study.
Stress as a potential factor in the pathogenesis of pd
Because of its direct and indirect influences on the dopaminergic system, stress is a candidate to modulate the pathology of PD. Both PD and 6-OHDA infusions lead to oxidative stress, cell loss and excitotoxicity (Mattson et al., 2002; Cohen et al., 2003; Gillies et al., 2004), all of which are processes that can be promoted by stress. Although so far the influence of stress on the pathology of PD has been hypothetical (Smith et al., 2002), the present data provide evidence of stress-induced neurodegenerative events that exaggerate the motor symptoms in a PD model.
It is possible that stress-associated modulation of motor function is mediated by the dopaminergic system (Dunn & File, 1983; Snyder et al., 1985; Abercrombie et al., 1989; Finlay & Zigmond, 1997; Pani et al., 2000; Kim et al., 2005). Whereas after lesion residual dopaminergic neurons continue to synthesize and release dopamine in response to stressors, this response may become inadequate under chronic stress (Abercrombie et al., 1989; Snyder et al., 1985; Keefe et al., 1990). The inability to compensate for stressors due to inadequate dopamine levels could account for the enhancement of movement deficits in dopamine-depleted animals. Furthermore, the present findings show that stress and glucocorticoids also restrict the use of compensatory movement strategies that would attenuate the neurological deficits associated with dopamine depletion. These findings lend support to observations in PD patients. Patients with preclinical PD may demonstrate symptoms characteristic of severe dopamine deficiency following stress (Schwab & England, 1966; Snyder et al., 1985) indicating at least temporary stress-induced acceleration of the disease (Gibberd & Simmons, 1980; Goetz et al., 1990; Treves et al., 1990). Furthermore, akinesia is often precipitated by emotionally distressing situations (Schwab & Zieper, 1965), indicating that stress affects PD pathology similarly to neurodegeneration in the rat model.
Conclusion
The present findings demonstrate that psychological stress and elevated corticosterone levels accelerate nigral neuronal loss and abolish adaptive behavioural responses in a rat analogue of PD. The observations suggest that stress represents a significant factor in the pathogenesis of PD. Based on the observations that stress accelerates nigral cell loss, it might lead to early onset of motor symptoms in the presymptomatic stage and worsen the symptoms of PD once the disease has been diagnosed. The observation that stress accelerates neurodegenerative processes of the motor system bears implications for prevention and treatment of PD and other neurodegenerative conditions and suggests a new therapeutic avenue for rehabilitative approaches.
Acknowledgments
We thank Scott Kirkland, Keon Habibi and Neal Melvin for helpful comments and assistance with the experiments. We are extremely grateful to Fiona Brown, Dr. Gerry Mears, and Dr. Karen Schwartzkopf-Genswein for assistance with the blood sample analyses. This research was supported by the Parkinson’s Disease Foundation (N.J.), the Canadian Institutes of Health Research (G.M.), the Alberta Heritage Foundation for Medical Research (G.M.), and the National Institutes of Health Grant no. NS043588 (G.M.).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- MTA
medial tegmental area
- PD
Parkinson’s disease
- SNl
substantia nigra lateral segment
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expession. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of Type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Tagoe CNB, Harlan RE. Type II corticosteroid receptor-like immunoreactivity in the rat cerebellar cortex: differential regulation by corticosterone. Neuroendocrinology. 1991;55:683–694. doi: 10.1159/000126188. [DOI] [PubMed] [Google Scholar]
- Charcot JM. In: Lectures on the Diseases of the Nervous System. Sigerson G, translator. Vol. 1. The New Sydenham Society; London: 1878. English Translation. [Google Scholar]
- Charlett A, Dobbs RJ, Purkies AG, Wright DJ, Peterson DW, Weller C, Dobbs SM. Cortisol is higher in parkinsonism and associated with gait deficit. Acta Neurol Scand. 1998;97:77–85. doi: 10.1111/j.1600-0404.1998.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Joh HD, Bernard O, Hattori K, Hurn PD, Traystman RJ, Alkayed NJ. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proc Natl Acad Sci USA. 2001;98:11824–11828. doi: 10.1073/pnas.201215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional effects of stress and glucocorticoid hormones on immune function: possible explanations for paradoxical observations. In: Ader R, Cohen N, Felten D, editors. Psychoneuroimmunology. 3. Academic Press; San Diego: 2001. pp. 301–338. [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Cold restraint alters dopamine metabolism in frontal cortex, nucleus accumbens and neostriatum. Physiol Behav. 1983;31:511–513. doi: 10.1016/0031-9384(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Faraday MM. Rat sex and strain differences in response to stress. Physiol Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochem Res. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Foley P, Gerlach M, Double KL, Riederer P. Dopamine receptor agonists in the therapy of Parkinson’s disease. J Neural Transm. 2004;111:1375–1446. doi: 10.1007/s00702-003-0059-x. [DOI] [PubMed] [Google Scholar]
- García A, Martí O, Vallès A, Dal-Zotto S. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration, and previous stress exposure. Neuroendocrinology. 2000;72:114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- Gibberd FB, Simmons JP. Neurological disease in ex-Far-East prisoners of war. Lancet. 1980;2:135–138. doi: 10.1016/s0140-6736(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol Biochem Behav. 2004;78:513–522. doi: 10.1016/j.pbb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tanner CM, Penn RD, Stebbins GT, 3rd, Gilley DW, Shannon KM, Klawans HL, Comella CL, Wilson RS, Witt T. Adrenal medullary transplant to the striatum of patients with advanced Parkinson’s disease: 1-year motor and psychomotor data. Neurology. 1990;40:273–276. doi: 10.1212/wnl.40.2.273. [DOI] [PubMed] [Google Scholar]
- Gowers WR. Diseases of the Nervous System. P. Blakiston, Son & Company; Philadelphia: 1888. American Edition. [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156:105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain monoamines and parkinsonism. Natl Institute Drug Abuse Res Monogr Series. 1975;3:13–21. doi: 10.1037/e472122004-001. [DOI] [PubMed] [Google Scholar]
- Ishida H, Mitsui K, Nukaya H, Matsumoto K, Tsuji K. Study of active substances involved in skin dysfunction induced by crowding stress. Effect of crowding and isolation on some physiological variables, skin function and skin blood perfusion in hairless mice. Biol Pharmaceut Bull. 2003;26:170–181. doi: 10.1248/bpb.26.170. [DOI] [PubMed] [Google Scholar]
- Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacol Biochem Behav. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Stricker EM, Zigmond MJ, Abercrombie ED. Environmental stress increases extracellular dopamine in striatum of 6-hydroxydopamine-treated rats: in vivo microdialysis studies. Brain Res. 1990;527:350–353. doi: 10.1016/0006-8993(90)91158-d. [DOI] [PubMed] [Google Scholar]
- Kim ST, Choi JH, Chang JW, Kim SW, Hwang O. Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem. 2005;95:89–98. doi: 10.1111/j.1471-4159.2005.03342.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BC. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Mabandla M, Kellaway L, St Clair Gibson A, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2007;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- Macht M, Brandstetter S, Ellgring H. Stress affects hedonic responses but not reaching-grasping in Parkinson’s disease. Behav Brain Res. 2007;177:171–174. doi: 10.1016/j.bbr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Influence of arousal and attention on the control of postural sway. J Vest Res. 1995;6:53–59. [PubMed] [Google Scholar]
- Marchetti B, Serra PA, Tirolo C, L’Episcopo F, Caniglia S, Gennuso F, Testa N, Miele E, Desole S, Barden N, Morale MC. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental parkinsonism: pivotal role for glia–neuron interactions. Brain Res Rev. 2005;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Marlier LN, Csikos T, Rebaudengo N, Borboni P, Patacchioli FR, Angelucci L, Privat A, Lauro R. Distribution of glucocorticoid receptor mRNA in the rat spinal cord. Neuroreport. 1995;6:2245–2249. doi: 10.1097/00001756-199511000-00034. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- Mercier S, Canini F, Buguet A, Cespuglio R, Martin S, Bourdon L. Behavioural changes after an acute stress: stressor and test types influences. Behav Brain Res. 2003;139:167–175. doi: 10.1016/s0166-4328(02)00265-6. [DOI] [PubMed] [Google Scholar]
- Metz GA. Stress as a modulator of motor system function and pathology. Rev Neurosci. 2007;18:209–222. doi: 10.1515/revneuro.2007.18.3-4.209. [DOI] [PubMed] [Google Scholar]
- Metz GA, Farr T, Ballermann M, Whishaw IQ. Chronic levodopa therapy does not improve skilled reach accuracy or reach range on a pasta matrix reaching task in 6-OHDA dopamine-depleted (hemi-Parkinson analogue) rats. Eur J Neurosci. 2001b;14:27–37. doi: 10.1046/j.0953-816x.2001.01615.x. [DOI] [PubMed] [Google Scholar]
- Metz GA, Jadavji NM, Smith LK. Modulation of motor function by stress: a novel concept of the effects of stress on behavior. Eur J Neurosci. 2005;22:1190–1200. doi: 10.1111/j.1460-9568.2005.04285.x. [DOI] [PubMed] [Google Scholar]
- Metz GAS, Schwab ME, Welzl H. The effects of acute and chronic stress on motor and sensory performance in male Lewis rats. Physiol Behav. 2001a;72:29–35. doi: 10.1016/s0031-9384(00)00371-1. [DOI] [PubMed] [Google Scholar]
- Metz GAS, Whishaw IQ. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav Brain Res. 2000;116:111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test. a new task to evaluate fore-and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002a;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Drug-induced rotation intensity in unilateral dopamine-depleted rats is not correlated with end point or qualitative measures of forelimb or hindlimb motor performance. Neuroscience. 2002b;111:325–336. doi: 10.1016/s0306-4522(02)00013-1. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6652. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system. The current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Mattei V, Del Bianco P, Gizzi C, Sorice M, Hiraiwa M, Misasi R. Hippocampal prosaposin changes during stress: a glucocorticoid-independent event. Hippocampus. 2004;14:275–280. doi: 10.1002/hipo.10192. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Bernardo LS, Cotman CW. Decline in reactive fiber growth in the dentate gyrus of aged rats compared to young adult rats following entorhinal cortex removal. Brain Res. 1980a;199:21–38. doi: 10.1016/0006-8993(80)90227-9. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Bernardo LS, Cotman CW. Hydrocortisone administration retards axon sprouting in the rat dentate gyrus. Exp Neurol. 1980b;68:195–201. doi: 10.1016/0014-4886(80)90077-1. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B. a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Chourbaji S, Ridder S, Brandwein C, Gass P, Hortnagl H, Hellweg R. Stress-resistant mice overexpressing glucocorticoid receptors display enhanced BDNF in the amygdala and hippocampus with unchanged NGF and serotonergic function. Psychoneuroendocrinology. 2006;31:1266–1277. doi: 10.1016/j.psyneuen.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Schwab RS, England AC. Parkinson syndromes due to various specific causes. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Wiley; New York: 1966. pp. 227–247. [Google Scholar]
- Schwab RS, Zieper I. Effects of mood, motivation, stress and alertness on the performance in Parkinson’s disease. Psychiat Neurol. 1965;150:345–357. doi: 10.1159/000127780. [DOI] [PubMed] [Google Scholar]
- Seniuk NA, Tatton WG, Greenwood CE. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990;527:7–20. doi: 10.1016/0006-8993(90)91055-l. [DOI] [PubMed] [Google Scholar]
- Smith AD, Castro SL, Zigmond MJ. Stress-induced Parkinson’s disease: a working hypothesis. Physiol Behav. 2002;77:527–531. doi: 10.1016/s0031-9384(02)00939-3. [DOI] [PubMed] [Google Scholar]
- Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol. 2007;184:31–39. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Snyder AM, Stricker EM, Zigmond MJ. Stress-induced neurological impairments in an animal model of Parkinsonism. Ann Neurol. 1985;18:544–551. doi: 10.1002/ana.410180506. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Kwan MM, Verrier MC, Seniuk NA, Theriault E. MPTP produces reversible disappearance of tyrosine-hydroxylase-sontaining retinal amacrine cells. Brain Res. 1990;527:21–31. doi: 10.1016/0006-8993(90)91056-m. [DOI] [PubMed] [Google Scholar]
- Treves TA, Rabey JM, Korczyn AD. Case-control study, with use of temporal approach, for evaluation of risk factors for Parkinson’s disease. Movement Dis. 1990;5(Suppl 1):11. [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigro-stiatal dopamine system. Acta Physiol Scand Suppl. 1971;367:89–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Weiner WJ, Lang AE. Movement Disorders: a Comprehensive Survey. Futura; Mount Kiso, New York: 1989. [Google Scholar]
- Whishaw IQ, O’Connor RB, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;7:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson’s disease (PD) reveals homology to deficits in animal models. Behav Brain Res. 2002;133:165–176. doi: 10.1016/s0166-4328(01)00479-x. [DOI] [PubMed] [Google Scholar]
- Wojtal K, Trojnar MK, Czuczwar SJ. Endogenous neuroprotective factors: neurosteroids. Pharmacol Rep. 2006;58:335–340. [PubMed] [Google Scholar]
- Wolters EC, Calne DB. Parkinson’s disease. CMAJ. 1989;140:507–514. [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Sarre S, Ebinger G, Michotte Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods. 2005;144:35–45. doi: 10.1016/j.jneumeth.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zuch CL, Nordstroem VK, Briedrick LA, Hoernig GR, Granholm AC, Bickford PC. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol. 2000;427:440–454. doi: 10.1002/1096-9861(20001120)427:3<440::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]