Abstract
Background:
Originally designed for prosthetic control, regenerative peripheral nerve interfaces (RPNIs) prevent neuroma formation by providing free muscle grafts as physiological targets for peripheral nerve ingrowth. We report the first series of patients undergoing RPNI implantation for treatment of symptomatic postamputation neuromas.
Methods:
A retrospective case series of all amputees undergoing RPNI implantation for treatment of symptomatic neuromas between November 2013 and June 2015 is presented. Data were obtained via chart review and phone interviews using questions derived from the Patient Reported Outcomes Measurement Information System instruments. Statistical analyses were performed using dependent sample t tests with a significance threshold of P < 0.01.
Results:
Forty-six RPNIs were implanted into 16 amputees for neuroma relief (3 upper extremities and 14 lower extremities). Mean age was 53.5 years (6 females and 10 males). All patients participated in postoperative phone interviews at 7.5 ± 3.4 (range: 3–15) months. Patients reported a 71% reduction in neuroma pain and a 53% reduction in phantom pain. Most patients felt satisfied or highly satisfied with RPNI surgery (75%), reporting decreased (56%) or stable (44%) levels of analgesic use. Most patients would strongly recommend RPNI surgery to a friend (88%) and would do it again if given the option (94%). Complications included delayed wound healing (n = 4) and neuroma pain at a different site (n = 2).
Conclusions:
RPNI implantation carries a reasonable complication profile while offering a simple, effective treatment for symptomatic neuromas. Most patients report a significant reduction in neuroma and phantom pain with a high level of satisfaction. The physiological basis for preventing neuroma recurrence is an intriguing benefit to this approach.
More than 500 Americans sustain major amputations each day1 and almost 2 million people in the United States currently live with major extremity loss.2 Painful neuromas develop in 12.5% to 50% of cases,3,4 preventing or limiting prosthetic restoration and diminishing quality of life. Up to 30% of patients abandon their prostheses for reasons including neuroma pain.5 The fact that over 150 different methods of neuroma treatment have been described in the literature6 indicates the absence of a reliable and efficacious treatment modality.
Neuromas form when injured axons sprout in a discordant fashion, creating swellings of disorganized tissue containing axons, Schwann cells, endoneurial cells, perineurial cells, and fibroblasts in a dense collagenous matrix.7 The neuroma’s afferent fibers develop ectopic activity, mechanical sensitivity, and chemosensitivity to catecholamines.8 The altered expression of transduction molecules, upregulation of sodium channels, downregulation of potassium channels, and development of nonfunctional connections between axons all contribute to the hyperexcitability and spontaneous discharge witnessed within injured nerves.9 Nonsurgical treatment includes desensitization, chemical or anesthetic injections, transcutaneous electrical nerve stimulation, topical lidocaine, and adjuvant pain medications such as antidepressants and anticonvulsants.7 Recalcitrant cases are subject to various surgical treatments such as simple neuroma excision,4 nerve capping,10–12 and nerve relocation into bone,13 muscle,13–15 or vein.7 Fibrin patch application with placement of a local pain catheter has also been described.16 The long-term outcomes of such methods are mixed,6,8 likely because of the nonphysiological nature of these treatments.
We propose a simple, straightforward, and reproducible method of neuroma management using regenerative peripheral nerve interfaces (RPNIs). RPNIs are constructed by implanting severed peripheral nerve ends into free, devascularized muscle grafts,17,18 which serve as denervated targets for nerve ingrowth and survive through a process of degeneration, regeneration, revascularization, and reinnervation.18–25 Muscle graft survival has been demonstrated in numerous animal experiments in which RPNIs have been studied for use in prosthetic control.17,18,26 Muscle graft reinnervation occurs as early as 1 month postoperatively27 and can occur with sensory nerves28–30 in addition to motor nerves. By providing a physiological target for regenerating axons, RPNI surgery prevents neuroma formation by taking advantage of fundamental nerve and muscle biology.
Here, we describe our initial experience treating patients with symptomatic postamputation neuromas by neuroma excision and RPNI implantation.
METHODS
Patient Registry
With institutional review board approval, a retrospective registry was created, comprising all patients treated for postamputation symptomatic neuromas via neuroma excision and immediate RPNI implantation by the senior author (PSC) between November 2013 and June 2015. Demographic and clinical data were extracted from the electronic medical record. Inclusion criteria were the diagnosis of symptomatic neuromas within a residual limb and a postoperative follow-up greater than 3 months. Patients were excluded if RPNIs were performed prophylactically during primary amputation or if the neuroma was not associated with limb amputation.
Surgical Technique
An RPNI is constructed by excising the neuroma bulb and wrapping the proximal nerve end with a small free muscle graft (Fig. 1). Previous work from our laboratory has shown that any further manipulations are unnecessary, such as wrapping the RPNI with a biological tissue cover for insulation31 or anchoring the RPNI to surrounding tissues.32 The free muscle graft (approximately 3 × 1 × 1.5 cm) is harvested sharply from a muscle near the neuroma or from a muscle at a distant site. Care is taken to avoid harvesting muscle that would otherwise serve as crucial padding for the residual limb. Donor muscle fiber–type composition, whether slow-twitch or fast-twitch, has not be shown to make a significant difference in muscle graft reinnervation when implanted with a peripheral nerve.17 Grafts are harvested along the axis of the muscle fibers to avoid unnecessary tissue trauma, thereby optimizing the viability of muscle fibers within the graft. A single RPNI takes approximately 7 to 10 minutes to construct under 2.5× to 3.5× loupe magnification.
Fig. 1.
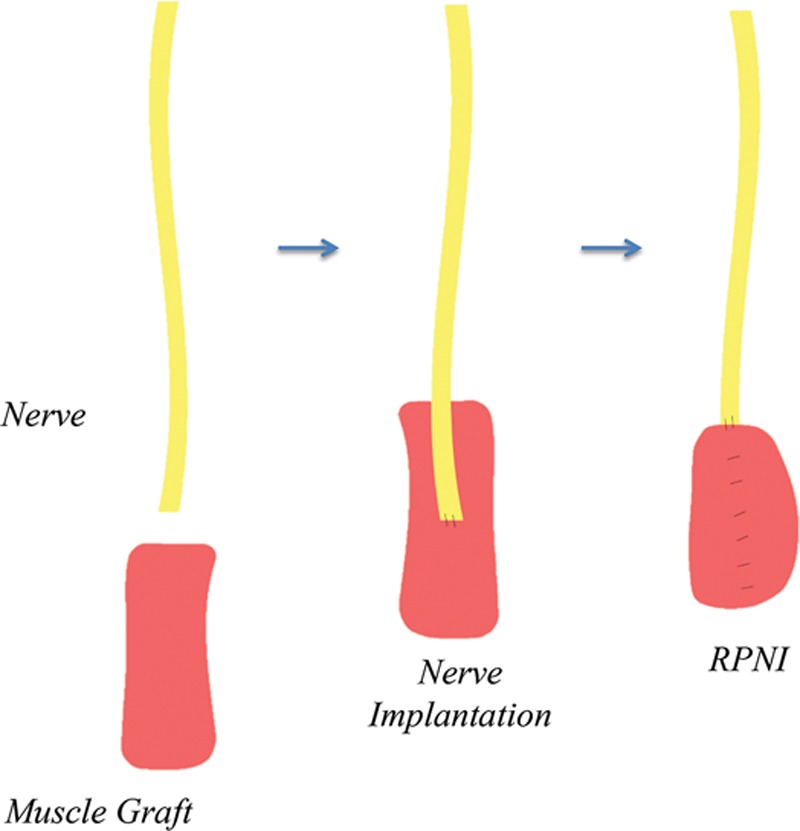
Illustration of RPNI construction.
In general, neuromas are exposed under tourniquet control through existing scars. In some cases, such as superficial and deep peroneal neuromas after below-knee amputations, a separate incision in normal tissue at the lateral knee can be used to identify the common peroneal nerve proximally. The common peroneal nerve is transected, and an intrafascicular dissection is performed to separate the nerve into 2 fascicles to construct 2 separate RPNIs. For sciatic neuromas in above-knee amputations, the sciatic nerve is split into 3 fascicles to construct 3 separate RPNIs (Fig. 2). In theory, splitting large nerves into separate fascicles for RPNI construction improves the axon number–to-muscle graft volume ratio, promoting muscle graft reinnervation and therefore neuroma prevention. Notably, nerve splitting is performed without the need for distinguishing between motor and sensory axons, as either type can reinnervate a denervated muscle graft.29,30 Furthermore, there is no practical way to distinguish between motor and sensory axons in a residual limb, as these axons lack normal end targets from prior amputation, precluding the ability to test by nerve stimulation.
Fig. 2.
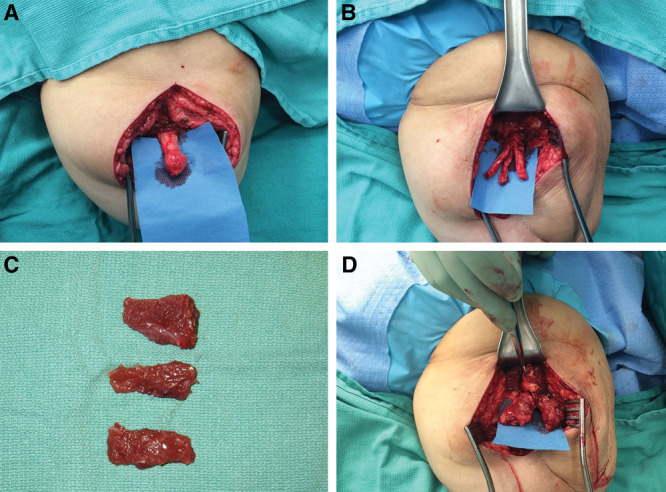
Excision of sciatic neuroma with construction of 3 RPNIs. A, Sciatic neuroma is identified. B, Sciatic nerve is split into 3 fascicles after neuroma excision. C, Three small muscle grafts are harvested from the adjacent biceps femoris muscle. D, Three RPNIs are constructed.
All suturing is performed with 6-0 nonabsorbable monofilament. The nerve end is first secured to the center of the muscle graft using 2 or 3 epineural-to-epimysial stitches. The muscle graft is then wrapped and secured around the nerve with additional epimysial stitches. Finally, 2 more stitches are placed for extra support from the proximal edge of the muscle graft to the adjacent epineurium, taking care to avoid kinking of the nerve or disruption of the axons within the epineurium. Once all RPNIs are constructed, primary closure of the surgical site is performed.
Phone Interviews
Postoperative phone interviews were conducted by the same author (SLW) using a short questionnaire (Fig. 3) to elicit patient-reported pain scores both before and after surgery, while also distinguishing neuroma pain from phantom pain. Pain interference questions were adapted from the publically available Patient Reported Outcomes Measurement Information System (PROMIS) instruments.33 The PROMIS instruments facilitate patient communication regarding outcomes related to injury or illness34,35 and are useful in evaluating pain, disability, and quality of life in patients with amputations involving the upper extremity36 and lower extremity.37 Because shortened pain inventories have been shown to be roughly equivalent or slightly more effective than more exhaustive inventories in detecting patient-reported pain scores,38 our questionnaire was fashioned to simplify reporting, decrease interviewee fatigue, and optimize response rate. Although not validated, our questionnaire was effectual for the purposes of this study.
Fig. 3.
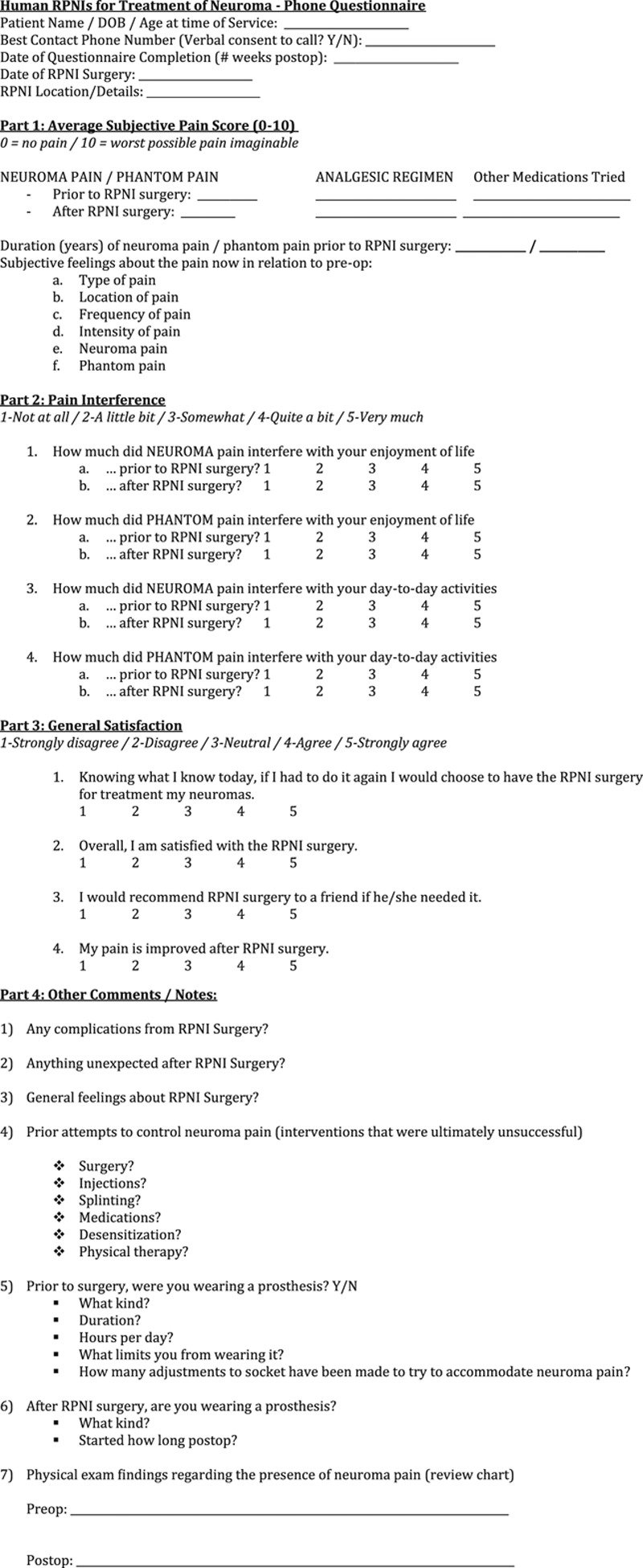
Phone interview questionnaire.
Statistical Analyses
Descriptive statistics were employed to quantitatively describe the characteristics of this patient population. Further analyses were performed using dependent sample t tests with a significance threshold of P < 0.01, where applicable.
RESULTS
Patients
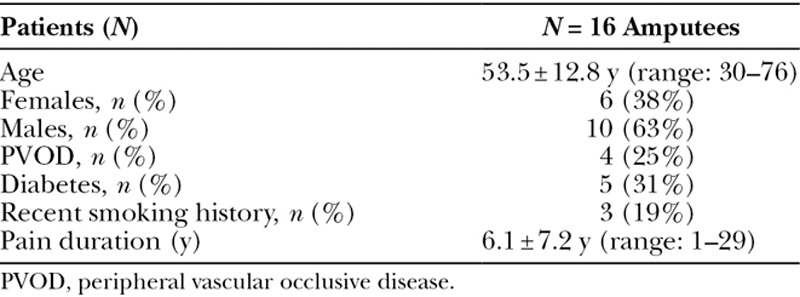
Sixteen patients (n = 16) met inclusion criteria during the study period (Table 1). All underwent RPNI implantation for postamputation neuroma treatment and had at least 3 months of postoperative follow-up documented in the medical record. Mean age was 53.5 ± 12.8 (range: 30–76) years, with 6 females (38%) and 10 males (63%). Four patients (25%) had documented peripheral vascular occlusive disease. Five patients (31%) had diabetes. Three patients (19%) were recent smokers who, after preoperative counseling, quit smoking 4 to 6 weeks before surgery. Ten patients had below-knee amputations, 4 patients had above-knee amputations, 2 patients had below-elbow amputations, and 1 patient had an above-elbow amputation. Duration of neuroma pain before RPNI surgery was an average of 6.1 ± 7.2 (range: 1–29) years. Patients reported that their neuroma pain was refractory to multiple prior treatments, including desensitization exercises, electrical stimulation, physical therapy, steroid injections, and surgical neurectomy.
Table 1.
Patient Characteristics
Operative Details
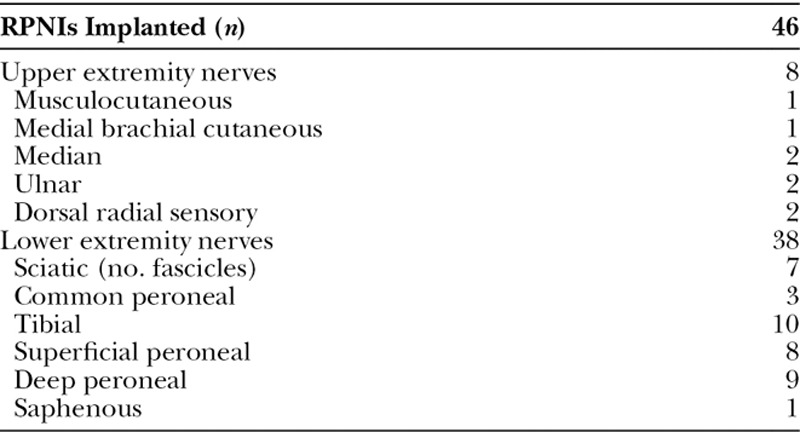
In these 16 patients, a total of 17 residual limbs were treated for symptomatic neuromas (3 upper extremity and 14 lower extremity). Forty-six RPNIs were constructed with an average of 2.7 RPNIs per limb (Table 2). All patients received preincisional intravenous antibiotics. Mean operative time was 132 ± 42 (range: 69–209) minutes. There were no intraoperative complications. The most commonly used donor muscles were the vastus lateralis (n = 14), gastrocnemius (n = 11), and biceps femoris (n = 10) muscles. Other donor sites included the gluteus maximus, semitendinosus, soleus, and flexor digitorum superficialis muscles.
Table 2.
Nerves Treated with RPNI Construction
Postoperative Course
Postoperatively, patients were admitted to the hospital for an average of 1.9 ± 1.2 (range: 0–4) days. Mean follow-up time for chart review was 11.3 ± 5.5 (range: 3–23) months. Before RPNI surgery, 9 of 16 patients (56%) reported being able to use prostheses. After RPNI surgery, 13 of 16 patients (81%) reported being able to wear prostheses. Average time to wearing prostheses after surgery was 4.6 ± 4.0 (range: 1–15) months.
Complications
Five of 16 patients (31%) experienced surgical complications (Table 3).
Table 3.
RPNI Surgery Complication Profile

One patient with a history of severe peripheral thromboembolic disease, due to a prothrombin G20210A mutation, developed acute bilateral lower extremity ischemia on postoperative day 1, requiring aortic and bilateral iliac thombectomies. Five days later while on a therapeutic heparin drip, she was diagnosed with an acute deep venous thrombosis of the contralateral lower extremity, and an inferior vena cava filter was placed. She was eventually discharged on warfarin for lifelong therapeutic anticoagulation. Two weeks later in clinic, a hematoma at the RPNI surgical site was detected, opened, and drained to allow for dressing changes. No further intervention was required.
Four patients (25%) experienced wound dehiscence and delayed wound healing. Three were in the setting of preoperative infection such as chronic osteomyelitis and/or recent cellulitis. One was in the setting of postoperative hematoma as described above. All were treated with topical dressings until fully healed
Two patients (13%) complained of new neuroma pain, each at a previously unaddressed site within the operated residual limb. One was confirmed by ultrasound at 7 months, involving a small branch of the peroneal nerve. This was treated with RPNI implantation at 9 months, with resolution of the neuroma pain documented at a follow-up of an additional 5 months. The other neuroma was detected during routine follow-up at 8 months and involved the lateral antebrachial cutaneous nerve. This patient elected to avoid further surgery and wished to proceed with prosthetic fitting.
Patient-reported Outcomes
All 16 patients were contacted for postoperative phone interviews with a 100% response rate. These interviews were conducted at an average of 7.5 ± 3.4 (range: 3–15) months after RPNI surgery.
Using the numeric pain rating scale (0–10), patients reported an average reduction of neuroma pain score 71%, from 8.7 ± 1.4 preoperatively to 2.5 ± 2.1 postoperatively (P = 0.000001) (Fig. 4A). Twelve of the 16 patients (75%) reported at least a 50% decrease in neuroma pain score after surgery. Patients also reported an average reduction of phantom pain score by 53%, from 8.0 ± 2.1 preoperatively to 3.8 ± 3.3 postoperatively (P = 0.009). Five of 11 patients (45%) presenting with phantom pain reported at least a 50% decrease in phantom pain score after surgery (Fig. 4B). There was no association detected between preoperative duration of symptoms and reduction in pain scores.
Fig. 4.
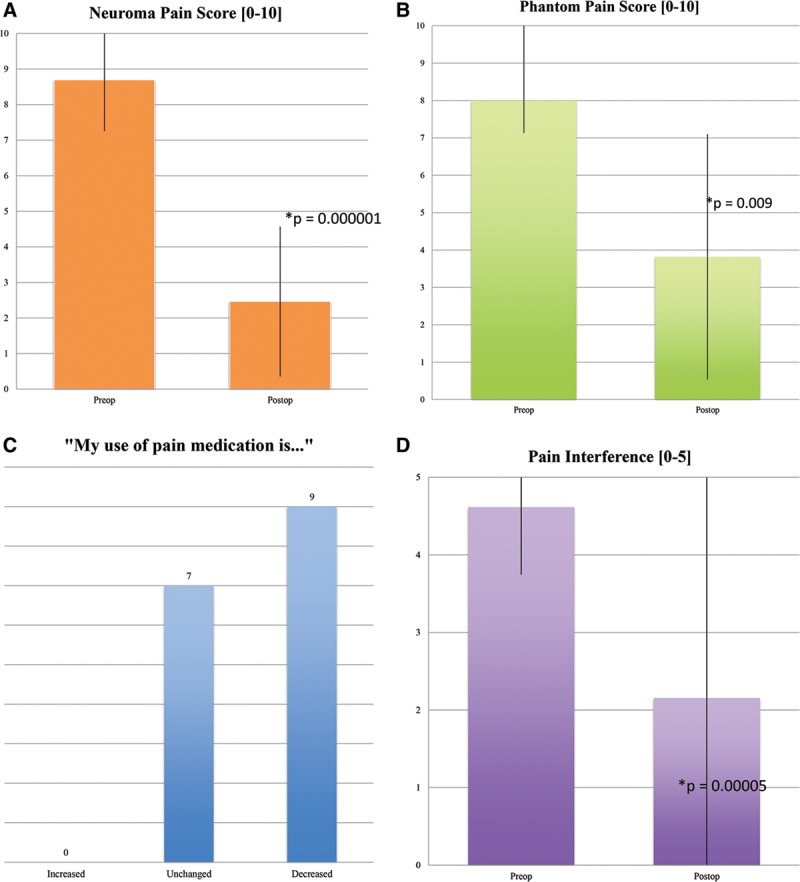
Patient-reported outcomes: A, neuroma pain score; (B) phantom pain score; (C) pain medication use; (D) pain interference.
Nine patients (56%) reported a reduction in pain medication use postoperatively, whereas 7 patients (44%) reported no change. No patients required an increased use of pain medications (Fig. 4C).
Aggregate pain interference scores, for both neuroma and phantom pain, dropped from 4.6 ± 0.9 preoperatively to 2.15 ± 1.3 postoperatively (P = 0.00005) (Fig. 4D). An interference score of 5 indicates that pain interferes “very much” with enjoyment of life and daily activities, whereas a score of 1 indicates that pain interferes “not at all” with enjoyment of life and daily activities.
Most patients (75%) felt satisfied or highly satisfied with RPNI surgery (Fig. 5A), and 94% would choose to undergo surgery again if given the option (Fig. 5B). Eighty-eight percent would strongly recommend the surgery to a friend (Fig. 5C).
Fig. 5.
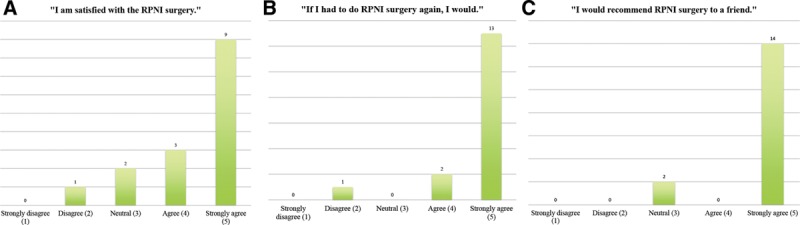
Patient-reported outcomes: A, satisfaction; (B) do it again?; (C) recommend to a friend?
DISCUSSION
General Considerations
We propose the novel use of RPNIs for the treatment of symptomatic neuromas in amputation patients. RPNI construction is simple to perform, by wrapping the distal end of a severed peripheral nerve with a free muscle graft. Free muscle grafts survive by a process of degeneration followed by regeneration and concomitant reinnervation in the presence of nerve implantation,24,39 hence the “regenerative” nature of RPNIs.17,40–42 Unlike the many existing methods to surgically treat neuromas, the RPNI is a physiological technique: the free muscle graft serves as a denervated target for regenerating axons, and reinnervation occurs through a well-described series of neurotrophin-mediated events.20,25,43 This process results in the formation of new neuromuscular junctions21–23 and precludes the formation of neuromas.
RPNIs were originally designed to harness voluntary motor nerve signals for neuroprosthetic control.26,41 Indeed, RPNIs can conduct and amplify nerve signals44 to support real-time intuitive control of advanced myoprostheses.45,46 More recent studies have demonstrated muscle reinnervation by sensory nerves as well, which may prove useful in achieving bioprosthetic somatosensory feedback.28 In general, RPNIs take advantage of basic nerve sprouting behavior, which favors the organized reinnervation of denervated targets. Without a target for nerve ingrowth, injured axons embark on an uncoordinated path toward neuroma formation, which may explain why other methods for neuroma treatment such as nerve relocation7,13–15 or nerve capping10–12 have not achieved widespread success.
In our case series, RPNI surgery was associated with reasonably positive outcomes. Sixteen patients were treated for symptomatic postamputation neuromas. On average, patients reported a 71% reduction in their neuroma pain scores and a 53% reduction in their phantom pain scores, along with a significant improvement in pain interference scores. More patients were able to use their prosthesis postoperatively, indicating an improvement in quality of life. Furthermore, the overwhelming majority patients felt satisfied or highly satisfied with the RPNI surgery, would do it again if given the option, and would recommend the surgery to a friend. Although subjective, these patient-reported outcomes highlight the promise that RPNIs hold in the treatment of amputation neuromas.
In addition to RPNI surgery, other neuroma treatments more recently described in the literature include targeted nerve implantation (TNI)47 and targeted muscle reinnervation (TMR).48 TNI involves securing nerve endings to transected intramuscular motor nerve branches within surgically denervated muscle in the residual limb. TMR involves coapting nerve endings to recipient motor nerve stumps or performing direct muscle neurotization. Based on the published case series, both methods show great promise in both neuroma treatment and prevention by taking advantage of basic nerve sprouting behavior. Still, these methods do require microsurgical techniques that may not be available to all surgeons, may prove challenging to perform, and may lead to longer operative times. Furthermore, both TNI and TMR require living muscle targets at the site of nerve coaptation, which may not be available in the presence of significant scar tissue or if faced with preoperative soft-tissue deficiencies. In contrast, RPNIs can easily be constructed without specialized microsurgical or peripheral nerve training. Furthermore, if suitable tissue is not present at the recipient site, muscle grafts can be harvested from a distant donor site with minimal morbidity.17,18 Finally, RPNIs may potentially be used for downstream control of advanced myoprostheses in the future17,18; RPNIs have the ability to harness and transduce discrete motor nerve signals that would otherwise be lost in a neuroma.
Future Directions
As this is a pilot study, further studies are warranted to better understand the outcomes and implications of RPNI surgery for neuroma treatment and prevention.
First, from a technical standpoint, the optimal ratio of axons-to-muscle graft volume has yet to be determined. This would affect the size of the nerve fascicles and/or the size of the muscle grafts used for RPNI construction.
Second, the impact of RPNI surgery on upper extremity versus lower extremity neuromas should be more closely evaluated, as there may be response differences that cannot be detected in this study because of small sample size.
Third, despite relatively short operative times, patients stayed an average of 2 days in the hospital after RPNI surgery. We have observed that these chronic pain patients require intensive postoperative pain management strategies to ensure that they are comfortable before discharge. Oftentimes, help from the Acute Pain Service is solicited. Further study regarding the mechanisms driving pain perception after RPNI surgery and regarding perioperative pain management is warranted.
Fourth, further investigation is needed regarding reasons why only half of all patients decreased their use of pain medications after surgery. Although an association between duration of symptoms and pain reduction was not detected in this study, likely because of small sample size, it is possible that patients with longer symptom duration possessed a larger central component to their pain that was unaddressed by peripheral nerve surgery. Indeed, the 6-year average duration of preoperative neuroma pain in this sample of patients may be indicative of this phenomenon. In addition, patients can be reluctant to change their habits of taking pain medication out of fear of more pain. Finally, as follow-up time was only 7.5 months, any medication changes that would occur beyond that time frame could not be captured. Regardless, patient quality of life still appeared to improve as indicated by reduced pain interference scores, independent of medication adjustments or lack thereof.
Fifth, 2 patients developed new neuroma pain at different sites at 7 and 8 months. These new sites were likely unmasked after RPNI treatment of more dominant painful neuromas in the area. By better understanding the incidence of new neuroma formation versus delayed presentation of less symptomatic neuromas, an improved algorithm can be developed to avoid such pitfalls.
Finally, RPNI surgery may also be beneficial for neuroma prophylaxis during primary amputations and for treatment of painful end neuromas and neuromas-in-continuity in patients without amputations. Studies focused on these particular patient populations are needed to explore the potential indications for RPNI surgery.
Limitations
This study is limited in that it is a retrospective, descriptive case series with a small number of patients, no control group, and relatively short follow-up time. Furthermore, the patient questionnaire was administered postoperatively, subjecting the data to recall bias. A more optimal approach for future study would be to administer both pre- and postoperative validated PROMIS instruments.
Despite its limitations, this pilot study has provided valuable data necessary to lay the foundation for a larger prospective study, which is now underway. While demonstrating the feasibility of performing such a study in this particular patient population,49 the study has also raised new, important questions pertaining to the optimization of RPNI design and its potential use not only for neuroma treatment but also for prophylaxis.
CONCLUSIONS
This pilot study serves to introduce a safe, novel, and potentially effective surgical technique in the form of RPNIs that may benefit patients with symptomatic postamputation neuromas who have otherwise failed all other treatment options. By providing free muscle grafts as targets for peripheral nerve ingrowth, RPNI implantation is physiological in nature by taking advantage of basic nerve sprouting behavior. The surgery is straightforward to perform, appears to be associated with a significant reduction in neuroma and phantom pain scores, results in a high level of patient-reported satisfaction, and has a reasonable complication profile. Further study is warranted, as RPNI implantation may also be indicated for the treatment of refractory neuromas in patients without amputations and for neuroma prophylaxis at the time of primary amputation.
Footnotes
Presented at the American Society for Peripheral Nerve (ASPN) Annual Meeting in Scottsdale, Ariz., on January 17, 2016, and earned the Best Basic Research Paper Resident Award.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Owings M, Kozak LJ. National Center for Health Statistics. 1998. National Center for Health S. Ambulatory and Inpatient Procedures in the United States, 1996. Hyattsville, Md: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 2.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Soroush M, Modirian E, Soroush M, et al. Neuroma in bilateral upper limb amputation. Orthopedics. 2008;31:1193 doi: 10.3928/01477447-20081201-26. [DOI] [PubMed] [Google Scholar]
- 4.Sehirlioglu A, Ozturk C, Yazicioglu K, et al. Painful neuroma requiring surgical excision after lower limb amputation caused by landmine explosions. Int Orthop. 2009;33:533–536. doi: 10.1007/s00264-007-0466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFarland LV, Hubbard Winkler SL, Heinemann AW, et al. Unilateral upper-limb loss: satisfaction and prosthetic-device use in veterans and servicemembers from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:299–316. doi: 10.1682/jrrd.2009.03.0027. [DOI] [PubMed] [Google Scholar]
- 6.Elliot D. Surgical management of painful peripheral nerves. Clin Plast Surg. 2014;41:589–613. doi: 10.1016/j.cps.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Stokvis A, van der Avoort DJ, van Neck JW, et al. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151:862–869. doi: 10.1016/j.pain.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. doi: 10.2147/JPR.S32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolajsen L. Postamputation pain: studies on mechanisms. Dan Med J. 2012;59:B4527. [PubMed] [Google Scholar]
- 10.Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg Am. 1977;2:70–78. doi: 10.1016/s0363-5023(77)80013-0. [DOI] [PubMed] [Google Scholar]
- 11.Robbins TH. Nerve capping in the treatment of troublesome terminal neuromata. Br J Plast Surg. 1986;39:239–240. doi: 10.1016/0007-1226(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Zhang F, Kolkin J, et al. Mechanisms of nerve capping technique in prevention of painful neuroma formation. PLoS One. 2014;9:e93973. doi: 10.1371/journal.pone.0093973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78:714–719. doi: 10.3171/jns.1993.78.5.0714. [DOI] [PubMed] [Google Scholar]
- 14.Ducic I, Mesbahi AN, Attinger CE, et al. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121:908–914. doi: 10.1097/01.prs.0000299281.57480.77. discussion 915–907. [DOI] [PubMed] [Google Scholar]
- 15.Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427–438. doi: 10.1097/00006534-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Prantl L, Schreml S, Heine N, et al. Surgical treatment of chronic phantom limb sensation and limb pain after lower limb amputation. Plast Reconstr Surg. 2006;118:1562–1572. doi: 10.1097/01.prs.0000233048.15879.0e. [DOI] [PubMed] [Google Scholar]
- 17.Woo SL, Urbanchek MG, Zheng X, et al. Partial skeletal muscle grafts for prosthetic control. Plast Reconstr Surg. 2014;134:55–56. doi: 10.1097/PRS.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 18.Woo SL, Urbanchek MG, Cederna PS, et al. Revisiting nonvascularized partial muscle grafts: a novel use for prosthetic control. Plast Reconstr Surg. 2014;134:344e–346e. doi: 10.1097/PRS.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 19.Gutmann E, Carlson BM. A comparison between the free grafting of sliced and intact muscles in the rat-1. Experientia. 1975;31:848–850. doi: 10.1007/BF01938500. [DOI] [PubMed] [Google Scholar]
- 20.Mong FF. Histological and histochemical studies on the nervous influence on minced muscle regeneration of triceps surae of the rat. J Morphol. 1977;151:451–462. doi: 10.1002/jmor.1051510309. [DOI] [PubMed] [Google Scholar]
- 21.Bader D. Reinnervation of motor endplate-containing and motor endplate-less muscle grafts. Dev Biol. 1980;77:315–327. doi: 10.1016/0012-1606(80)90477-7. [DOI] [PubMed] [Google Scholar]
- 22.Hakelius L, Nyström B, Stålberg E. Histochemical and neurophysiological studies of autotransplanted cat muscle. Scand J Plast Reconstr Surg. 1975;9:15–24. doi: 10.3109/02844317509022851. [DOI] [PubMed] [Google Scholar]
- 23.Killer H, Müntener M. Time course of the regeneration of the endplate zone after autologous muscle transplantation. Experientia. 1986;42:301–302. doi: 10.1007/BF01942513. [DOI] [PubMed] [Google Scholar]
- 24.Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc. 1983;15:187–198. [PubMed] [Google Scholar]
- 25.Carlson BM, Gutmann E. Contractile and histochemical properties of sliced muscle grafts regenerating in normal and denervated rat limbs. Exp Neurol. 1976;50:319–329. doi: 10.1016/0014-4886(76)90007-8. [DOI] [PubMed] [Google Scholar]
- 26.Kung TA, Bueno RA, Alkhalefah GK, et al. Innovations in prosthetic interfaces for the upper extremity. Plast Reconstr Surg. 2013;132:1515–1523. doi: 10.1097/PRS.0b013e3182a97e5f. [DOI] [PubMed] [Google Scholar]
- 27.Urbanchek MG, Wei B, Baghmanli Z, et al. Long-term stability of regenerative peripheral nerve interfaces (RPNI) Plast Reconstr Surg. 2011;128:88–89. [Google Scholar]
- 28.Nghiem BT, Sando IC, Gillespie RB, et al. Providing a sense of touch to prosthetic hands. Plast Reconstr Surg. 2015;135:1652–1663. doi: 10.1097/PRS.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 29.Larson JV, Urbanchek MG, Moon JD, et al. Abstract 17: prototype sensory regenerative peripheral nerve interface for artificial limb somatosensory feedback. Plast Reconstr Surg. 2014;133:26–27. doi: 10.1097/01.prs.0000445040.57505.da. [DOI] [PubMed] [Google Scholar]
- 30.Nedic A, Moon JD, Kung TA, et al. Von Frey monofilament testing successfully discriminates between sensory function of mixed nerve and sensory nerve regenerative peripheral nerve interfaces.. 6th International IEEE/EMBS Conference on Neural Engineering (NER); 2013. pp. 255–258. [Google Scholar]
- 31.Larson JV, Kung TA, Cederna PS, et al. Surgical model for analysis of signal transfer through biologic and synthetic materials. Ann Plast Surg. 2015;75:55–61. doi: 10.1097/SAP.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 32.Woo SL, Urbanchek MG, Cederna PS, et al. Abstract P2: short-term electrophysiological changes in muscle after injury by tenotomy and partial sectioning: a pilot study. Plast Reconstr Surg. 2014;133:186–187. [Google Scholar]
- 33.PROMIS. Pain – interference. Available at: http://nihpromis.org/measures/instrumentoverview. Accessed December 2015.
- 34.Cella D, Riley W, Stone A, et al. PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortlever JT, Janssen SJ, van Berckel MM, et al. What is the most useful questionnaire for measurement of coping strategies in response to nociception? Clin Orthop Relat Res. 2015;473:3511–3518. doi: 10.1007/s11999-015-4419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheesborough JE, Souza JM, Dumanian GA, et al. Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y) 2014;9:253–257. doi: 10.1007/s11552-014-9602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amtmann D, Morgan SJ, Kim J, et al. Health-related profiles of people with lower limb loss. Arch Phys Med Rehabil. 2015;96:1474–1483. doi: 10.1016/j.apmr.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook KF, Cella D, Boespflug EL, et al. Is less more? A preliminary investigation of the number of response categories in self-reported pain. Patient Relat Outcome Meas. 2010;2010:9–18. doi: 10.2147/PROM.S7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson N. Autogenous free grafts of skeletal muscle. A preliminary experimental and clinical study. Plast Reconstr Surg. 1971;48:11–27. doi: 10.1097/00006534-197107000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Woo SL, Urbanchek M, Cederna PS, et al. Revisiting nonvascularized partial muscle grafts: a novel use for prosthetic control. Plast Reconstr Surg. 2014;134:344e–346e. doi: 10.1097/PRS.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 41.Langhals NB, Woo SL, Moon JD, et al. Electrically stimulated signals from a long-term regenerative peripheral nerve interface. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2014;2014:1989–1992. doi: 10.1109/EMBC.2014.6944004. [DOI] [PubMed] [Google Scholar]
- 42.Kung TA, Langhals NB, Martin DC, et al. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133:1380–1394. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 43.Wu P, Chawla A, Spinner RJ, et al. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen Res. 2014;9:1796–1809. doi: 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kung TA, Cederna PS, Langhals NB, et al. Augmented signal transduction from regenerative peripheral nerve interfaces. Plast Reconstr Surg. 2013;132:89. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 45.Frost CM, Ursu DC, Nedic A, et al. Abstract 49: neuroprosthetic hand real-time proportional control by rodent regenerative peripheral nerve interfaces. Plast Reconstr Surg. 2014;133:1012–1013. doi: 10.1097/01.prs.0000445051.00569.56. [DOI] [PubMed] [Google Scholar]
- 46.Ursu DC, Urbanchek MG, Nedic A, et al. In vivo characterization of regenerative peripheral nerve interface function. J Neural Eng. 2016;13:026012. doi: 10.1088/1741-2560/13/2/026012. [DOI] [PubMed] [Google Scholar]
- 47.Pet MA, Ko JH, Friedly JL, et al. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop Relat Res. 2014;472:2991–3001. doi: 10.1007/s11999-014-3602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Souza JM, Cheesborough JE, Ko JH, et al. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472:2984–2990. doi: 10.1007/s11999-014-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


