Abstract
In 1948, Seymour S. Kety and Carl F. Schmidt published back-to-back papers in the JCI that are widely acknowledged as landmarks. Upon publication, the studies resolved a century-old debate, irrefutably demonstrating that cerebral blood flow is regionally regulated. The reported findings turned out to be so powerful in their implications that they provided the inspirational spark that illuminated a brand-new field: functional brain imaging. Thus these papers are landmarks of the rarest kind, not only ending a controversy, but also giving birth to one of the most exciting fields within modern day neuroscience.
During the 19th century, vascular physiologists began to show that blood flow to an organ is not solely regulated by extrinsic factors like cardiac output. Rather, a growing number of studies suggested that blood flow could be regulated intrinsically within the organ itself (1, 2). Chemically induced vascular dilatation was thought to underlie the intrinsic effect, such that regional expansion of vascular volume would then cause a subsequent increase in blood flow. Although this was proposed as a general mechanism, a controversy arose as to whether it applied in the unique case of cerebral blood flow (CBF). The prevailing view was that, because of the rigid skull, even subtle increases in cerebral volume would cause untenable elevations in intracranial pressure. This gave rise to a strong position, one that fired a century-long debate: that CBF cannot be regulated intrinsically and that any change in CBF must occur via extrinsic mechanisms such as changes in systemic blood pressure or cardiac output (3).
Those in the minority clung to the view that local regulation of CBF may occur, although admittedly this was based more on theoretical considerations then on empirical evidence. Toward the end of the 19th century, Charles S. Roy and Charles S. Sherrington provided the first evidence supporting this position (4). In their experiments, a novel monitoring device was placed on the brain surface of anesthetized dogs — essentially measuring fluctuations in volume — and shifts in intracranial volume were observed during chemical challenges. These fluctuations were presumed to reflect a change in vascular volume and therefore to represent a proxy measure of changing CBF. Nevertheless, this paper did not resolve the controversy of whether intrinsic or only extrinsic factors regulated CBF. Advocates of the opposition view had an easy time criticizing the paper, and much of this criticism was legitimate (5). For example, as critics pointed out, the monitoring device was only an indirect indicator of vascular volume and, in any case, the yielded measurements were not quantitative. Or, as was also noted, the experiments were performed in anesthetized subjects and required an invasive craniectomy, and so the observed effects did not necessarily reflect the natural environment (5). In fact, the debate over whether intrinsic factors can regulate CBF raged on for over 60 years more, until the 1948 JCI publications by Kety and Schmidt (Figure 1).
Figure 1.
Carl F. Schmidt (left; image courtesy of the National Library of Medicine) and Seymour S. Kety (right).
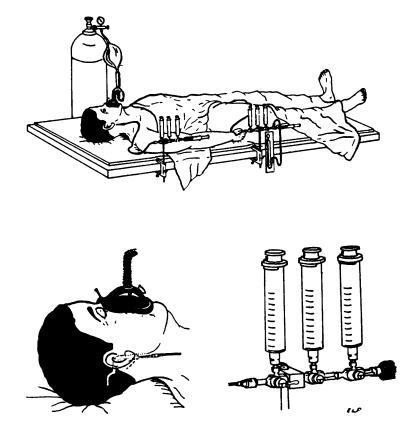
During his medical training, Kety became familiar with Fick’s principle and its successful application in quantifying cardiac blood flow (6). Fick’s principle states that blood flow to an organ must equal the rate in which the organ metabolizes a blood constituent, divided by the constituent’s vascular concentration — its arterial-venous difference. Defying the conventional approach, which relied on the endogenous gas oxygen, Kety had subjects inhale the exogenous gas nitrous oxide (Figure 2) (7). Kety realized that, because nitrous oxide is metabolically inert (relatively speaking), he could simplify Fick’s principle so that instead of three variables there were two and that, by measuring the arterial-venous concentration of nitrous oxide, he could solve the equation for CBF. In so doing, and by paying fastidious attention to technical details, Kety and Schmidt introduced, in their first JCI paper, a technique that, for the first time ever, generated a quantitative measure of CBF noninvasively in unanesthetized humans. As if this were not enough, in the same paper they then exploited CBF and the measurements of arterial-venous blood gases to quantify cerebral metabolic rates of oxygen consumption — again a first-time achievement in the conscious human.
Figure 2.
Experimental set-up for cerebral blood flow determination. Position of needles in internal jugular is shown as well as plastic tubing, manifolds, sampling and flushing syringes, and inhalation system. Mean arterial blood pressure is read from a mercury manometer attached to the arterial manifold. Only the expiratory valve on the mask is shown, the inspiratory valve is between the fluted tubing and the mask. Drawings by Dr. E. L. Foltz. Figure and legend reprinted from ref. 7.
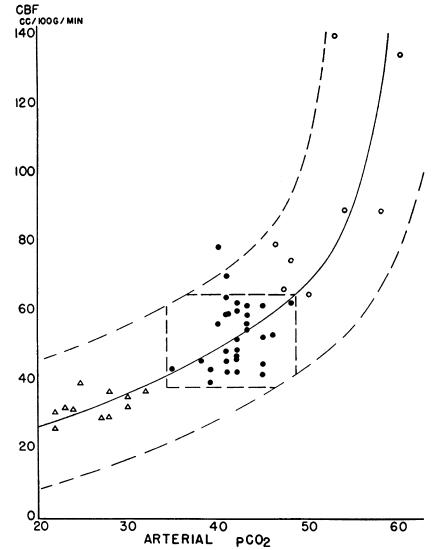
Although a technical tour de force, the first paper simply set the stage for the decisive findings presented in the second paper (8). In this definitive paper, Kety and Schmidt used the innovative nitrous oxide approach to show that inhaled carbon dioxide or oxygen has a predictable affect on CBF (Figure 3). Most importantly, they established that mechanisms intrinsic to the brain must regulate the effect, finally and forevermore laying to rest the controversy surrounding CBF regulation.
Figure 3.
The relationship between cerebral blood flow and arterial CO2 tension. Arterial CO2 tension was varied from the normal (filled circles) by hyperventilation (triangles) or by inhalation of 5–7% CO2 (open circles). The broken curves bound 98% of the observations while the central polygon encloses 94% of the normal values. Figure reprinted and legend adapted from ref. 8.
At the time of these publications, Schmidt and even Kety were considered vascular physiologists more than brain scientists. Nevertheless, the ability to measure CBF, a proven correlate of brain metabolism, opened up the remarkable possibility of studying brain function in humans. Although correlating behavior defects with structural lesions detected after death had its successes, this approach severely limited the ability to localize brain function and disease states in a systematic fashion. Thus, Kety and Schmidt’s approach brought with it the tantalizing realization that mapping brain dysfunction in living subjects was within reach. Of course, a single measure that reflects the CBF of the whole brain was inadequate for investigating brain function in all its complexity. The next goal became obvious: Build on these primary findings to develop techniques that can record CBF from many individual brain regions.
During subsequent decades, responding directly to this challenge and exploiting many of the principles introduced in these landmark papers (6), researchers ultimately met this goal. The effort was spearheaded by Kety’s own work published during the 1950s — yet more landmarks for this unstoppable investigator — and through a community-wide series of technical achievements. A string of chemical tracers was identified with which to estimate regional CBF and, through sheer technical wizardry, an assembly line of “brain cameras” was introduced that kept on improving the spatial resolution with which these tracers could be visualized. Thus, conceived out of Kety and Schmidt’s landmark papers, the field of functional brain imaging was born.
As a field, functional brain imaging has maintained its innovative spirit, unveiling its latest technique, functional magnetic resonance imaging (fMRI), during the 1990s. Although developed a half century later, even fMRI owes much of its intellectual heritage to Kety and Schmidt’s original papers. For example, one version of fMRI relies on the endogenous tracer deoxyhemoglobin — the blood oxygen level–dependent (BOLD) signal— to estimate regional metabolism (9). Here again, a modified version of Fick’s principle is invoked to account for why deoxyhemoglobin correlates with CBF and oxygen metabolism. fMRI presents many advantages over earlier techniques, including superior spatial and temporal resolution, greater safety to the subject, and easier use for the experimenter. Because of these attractions, psychologists in particular have flocked to BOLD fMRI in their efforts to map normal brain function.
Nevertheless, in the shadow of the high standards set by Kety and Schmidt, fMRI still needs to be perfected. In contrast to functional measures generated by other imaging techniques — such as positron emission tomography or single photon emission computerized tomography — the BOLD signal is unfortunately not quantitative. As stated explicitly in the title of Kety and Schmidt’s first JCI paper, generating a quantifiable measure was a key achievement of their original efforts. The absence of quantification is one of the main reasons that BOLD fMRI has yet to make significant contributions to clinical neuroscience, such as reliably mapping neurologic or psychiatric illness. It is this potential clinical utility, one that emerged from Kety’s lifelong interests, that motivates the current crop of fMRI investigators. The combination of innovation and rigor that characterized Kety and Schmidt’s landmark papers serves as a model to us all.
Footnotes
Nonstandard abbreviations used: BOLD, blood oxygen level–dependent; CBF, cerebral blood flow; fMRI, functional magnetic resonance imaging.Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Roy CS, Brown JG. The blood pressure and its variation in the arterioles, capillaries and smaller veins. J. Physiol. 1879;2:323–359. doi: 10.1113/jphysiol.1880.sp000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskell TWH. On the tonicity of the heart and blood vessels. J. Physiol. 1880;3:38–75. doi: 10.1113/jphysiol.1880.sp000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill, L. 1896. The physiology and pathology of the cerebral circulation: an experimental research. J. & A. Churchill. London, United Kingdom. 208 pp.
- 4.Roy CS, Sherrington CS. On the regulation of the blood supply of the brain. J. Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedland RP, Iadecola C. Roy and Sherrington (1890): a centennial reexamination of “On the regulation of the blood-supply of the brain”. Neurology. 1991;41:10–14. doi: 10.1212/wnl.41.1.10. [DOI] [PubMed] [Google Scholar]
- 6.Sokoloff L. In memoriam: Seymour Kety, M.D. 1915–2000. J. Cereb. Blood Flow Metab. 2000;20:1271–1275. doi: 10.1097/00004647-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J. Clin. Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast I magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]