Abstract
Background:
The development of flap necrosis distally remains a concern during microsurgical flap transfers because, at least in part, of decreased perfusion. Microvascular fragments (MVFs) are microvessels isolated from adipose tissue that are capable of improving tissue perfusion in a variety of tissue defects. The aim of this study was to determine whether the transplantation of MVFs in a dorsal rat skin flap model can improve flap survival.
Methods:
A 10 × 3 cm flap was raised in a cranial to caudal fashion on the dorsal side of 16 Lewis rats, with the caudal side remaining intact. The rats were equally divided into a treatment group (MVFs) and a control group (sterile saline). At the time of surgery, sterile saline with or without MVFs was injected directly into the flap. Microvessel density was determined after harvesting flap tissue by counting vessels that positively stained for Griffonia simplicifolia lectin I-isolectin B4. Laser Doppler was used to measure blood flow before and after surgery and 7 and 14 days later. Flap survival was evaluated 7 and 14 days after surgery by evaluating the percentage of viable tissue of the flap with photodigital planimetry.
Results:
Despite the lack of a significant difference in microvessel density and tissue perfusion, flap survival increased 6.4% (P < 0.05) in MVF-treated animals compared with controls.
Conclusions:
The use of MVFs may be a means to improve flap survival. Future studies are required to delineate mechanisms whereby this occurs and to further optimize their application.
Astrategy for soft-tissue reconstruction is the direct introduction of tissue (grafts or flaps) extracted from local (rotational flaps) or distant (free flaps) sites.1,2 The use of flaps (often referred to as microsurgical free tissue transfer) is common in the area of plastic surgery for a wide variety of ailments including the repair of extremity trauma defects, burned tissue, or restoration of cancer extirpative defects.3,4 The importance of an early perfusion for the survival of flaps has been clearly demonstrated. A delay in anastomosis and/or decreased vascularity is associated with a decrease in flap survival,5 whereas improving vascularity improves flap viability.6 Therefore, strategies that have proven to be a particularly effective means for improving flap viability are those that increase tissue perfusion and promote a regenerative environment.
Microvascular fragments (MVFs) consist of a heterogeneous mixture of arterioles, capillaries, and venules that have previously been suspended in a 3-dimensional collagen matrix where they spontaneously form developed vascular networks.7 Their potential for improving tissue perfusion is well recognized because they are a rich source of mesenchymal stem cells (MSCs) and have the ability to rapidly develop into blood-perfused networks. Their ability to promote tissue perfusion in vivo without the addition of additional growth factors has been well documented. MVFs have been used as a means to (1) improve tissue perfusion after myocardial infarction,8 (2) improve vascularity and tissue perfusion for the treatment of large muscle defects,9 (3) aid in islet implant survival,10 and (4) provide an implant that retains its native structure when implanted into a dorsal skinfold chamber in mice.11 The findings that they inosculate within a day when implanted subcutaneously in collagen12 and that they are capable of extensive remodeling in vivo over time8,12 make them particularly suited for improving flap survival. We hypothesized that application of MVFs into the flap would serve as a means for rapid improvements in tissue perfusion and thereby improve flap survival.
MATERIALS AND METHODS
This study has been conducted in compliance with the Animal Welfare Act and the Implementing Animal Welfare Regulations and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals and was conducted in the animal facility at the US Army Institute of Surgical Research. Rats were housed individually in a temperature-controlled environment with a 12-hour light/dark cycle.
MVF Isolation
MVFs were isolated from the epididymal fat pads of wild-type male Lewis rats (350+ g), using procedures similar to those previously described.7 Briefly, adipose tissue from the epididymal fat pads of rats was subjected to a limited collagenase (Worthington Biochemical Corporation; Lakewood, N.J.) digestion (~8 min) at 37°C with agitation and centrifugation (400g × 4 min), resulting in a floating layer of adipocytes and a pellet containing a heterogeneous mixture of cells and MVFs (Figs. 1A, B). The pellet was resuspended in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (Sigma; St. Louis, Mo.) and filtered through 500 μm and 30 μm filters to remove large debris and minimize cell contamination, respectively (Fig. 1C). MVFs (Fig. 1D) were then counted, centrifuged, and resuspended in PBS at a concentration of 75 to 100,000/ml, and up to 100 μl was injected into 10 evenly dispersed sites in the dorsal half underneath the flap (Fig. 1E).
Fig. 1.
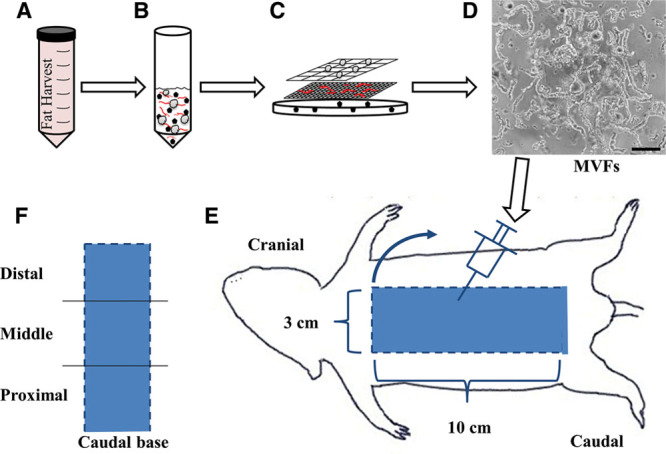
Schematic depicting the MVF isolation procedure and injection into the dorsal skin flap. A and B, Adipose tissue was harvested, minced, digested with collagenase, centrifuged (400g × 4 min), and (C) filtered through 500 μm and 30 μm filters to (D) isolate a heterogeneous mixture of MVFs. Black scale bar represents 100 μm. E, View of dorsal side of the rat with a diagram of the 10 × 3 cm skin flap. Dashed lines indicate edges that were cut and flap was raised (blue arrow) in a cranial to caudal fashion with the caudal side remaining intact. Injections were performed by injecting MVFs in 10 evenly dispersed sites in the distal half. Control animals underwent the identical surgery with sterile PBS injected alone as a control. F, Diagram of the 3 regions (distal, middle, and proximal) into which the flap was separated for perfusion and microvessel density measurements.
Dorsal Skin Flap Model
A total of 16 adult male Lewis rats (4–5 mo, ~350 g; Harlan Laboratories; Indianapolis, Ind.) were randomly divided into 2 groups, control or MVF treated. Buprenorphine SR LAB (1.0–1.2 mg/kg, ZooPharm; Windsor, Colo.) was subcutaneously administered as analgesia once preoperatively. Anesthesia was maintained at a vaporizer setting of 0.5% to 3% isoflurane delivered with a nose cone on a Bain circuit hooked to the rodent gas anesthesia machine (VetEquip, Inc.; Pleasanton, Calif.). The back was aseptically prepared by clipping the hair and sterilizing with betadine and 70% alcohol. In a manner similar to the method of McFarlane et al,13 the caudally based dorsal skin flap (10 × 3 cm), including intimately attached panniculus carnosus, was raised in a cranial to caudal fashion from the wound bed, and all perforator vessels were cauterized. The caudal side remained intact to provide blood flow to the flap. Each rat of the control group (n = 8) was injected with a total of 1 ml of PBS. MVFs were isolated from donor adipose tissue as described above, and up to 100,000 fragments resuspended in 1 ml of PBS were injected into each rat of the experimental group (n = 8). In all rats, the 1 ml volume was injected into the bottom side of the flap with a 22-gauge needle with up to 100 μl injected into 10 evenly dispersed sites underneath the flap (Fig. 1E). After hemostasis was established, the flap was returned to its wound bed and closed with running subcuticular sutures using 3-0 polydioxanone (Ethicon; Somerville, N.J.). On the 14th postoperative day, digital images of the flap were taken to measure flap survival. The rats were then euthanized with an intraperitoneal injection of pentobarbital sodium. The flaps were then harvested for later immunohistochemical analysis.
Measurement of Flap Survival
Images were acquired via digital camera (Canon; New York, N.Y.) on day 7 and after euthanasia on day 14. The survival of the flaps was assessed based on color and necrotic regions by 3 blinded reviewers. The flap survival area was calculated using image analysis software (ImageJ, NIH; Bethesda, Md.) and expressed as a percentage. Flap survival = Area of Viable Tissue/Total Area of Flap × 100.
In Vivo Perfusion Measurement by Laser Doppler
Animals were anesthetized, and cutaneous flap perfusion was determined using an Oxylab LDF microvascular perfusion monitor (Oxford Optronix; Abingdon, United Kingdom). The flap was divided into thirds for perfusion measurements: distal (farthest from caudal base), middle, and proximal (closest to caudal base, Fig. 1F). Blood perfusion units were measured from 3 different points of all 3 zones (distal, middle, and proximal) along the center of the flap and were recorded for all rats before surgery, immediately after surgery, and then 7 and 14 days postoperatively.
Microvessel Density Analysis
After the rats were euthanized, the dorsal skin flap was excised and divided into 3 parts (distal, middle, and proximal, Fig. 1F) for tissue processing. Tissues were fixed in 4% paraformaldehyde for up to 2 hours and cryoprotected by immersion in increasing concentrations of sucrose (10%, 12.5%, and 15%; Sigma; St. Louis, Mo.) for up to 1 hour each at room temperature (RT), and then in 20% sucrose overnight at 4°C. Skin samples were placed in a mold containing a 1:1 mixture of 20% sucrose/Tissue-Tek OCT embedding medium (Sakura Finetek USA; Torrance, Calif.) and rapidly frozen using 2-methylbutane cooled with liquid nitrogen (−100°C). Longitudinal sections were cut at 10 μm using a cryostat (−20°C, Leica Microsystems; Buffalo Grove, Ill.). All sections were stored at −80°C until staining. Cryosections were thawed for 30 minutes at RT, washed with Hank’s balanced salt solution (HBSS, Gibco; Grand Island, N.Y.), and blocked in HBSS containing 0.05% tween and 0.1% bovine serum albumin for 1 hour at RT. Vessels were identified by incubating the sections with fluorescein-labeled (excitation: 495 nm/emission: 515 nm) Griffonia simplicifolia lectin (GSL) I-isolectin B4 (1:200; Vector Laboratories; Burlingame, Calif.) 2 hour at RT. Sections were washed with HBSS and mounted using Dapi-Fluoromount-G (Electron Microscopy Sciences; Hatfield, Pa.). Images were taken using a Zeiss Axio Scan.Z1 Digital Slide Scanner (Carl Zeiss Microscopy; Thornwood, N.Y.) at 10× magnification. Two sections per sample were labeled, and at least 5 randomly chosen fields per section were captured and analyzed using ImageJ 1.44p (NIH; Bethesda, Md.). Images were converted to 8-bit, binarized, and vessels were counted. The microvessel density could be calculated by dividing the number of GSL I-isolectin B4 positive vessels by the area of the tissue to determine the number of vessels per mm2. Microvessel density = (number of GSL I-isolectin B4 positive vessels)/area.
Statistical Analysis
One-way or 2-way analysis of variance procedures with repeated measures were used to analyze experimental results using SigmaPlot 12.0 (Systat Software; San Jose, Calif.) followed by Tukey post hoc analyses where appropriate. Differences were considered significant when P < 0.05. All values are presented as means ± SEM.
RESULTS
Flap Survival
Representative digital images of the dorsal skin flap at 7 and 14 days for both the PBS and MVF groups are presented in Figure 2A. In every flap, the regions of necrosis were clearly demarcated by the skin color change from white/tan to dark brown/black. Given this clear demarcation, flap survival rates could be calculated based on the amount of viable tissue compared to the total area of the flap as previously described.14 MVF-treated flaps were not different from saline-injected controls at 7 days postoperatively (MVF: 62.9% ± 1.31% vs PBS: 58.9% ± 1.38%, P < 0.066). However, MVF-treated flaps survived better than did PBS-treated flaps on postoperative day 14 (MVF: 61.0% ± 1.89% vs PBS: 54.6% ± 1.52%, P < 0.05; Fig. 2B). No statistical differences were observed comparing PBS from day 7 to day 14 or MVF-treated flaps from day 7 to day 14.
Fig. 2.
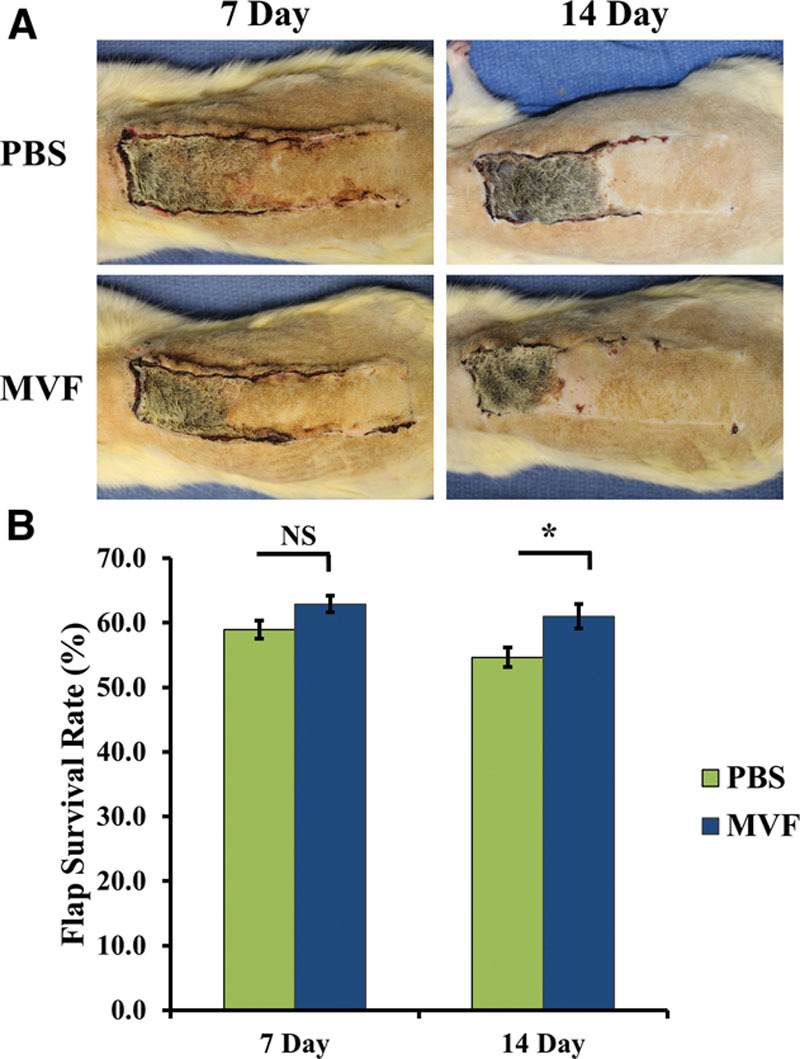
A, Digital images of PBS- and MVF-treated dorsal skin flaps 7 and 14 days after surgery. B, Injection of MVFs into the dorsal skin flaps significantly improved flap survival when compared to PBS control animals at 14 days. Results are expressed as mean ± SEM; *statistical significance in comparison to PBS-treated animals (P < 0.05).
Blood Perfusion Measurements via Laser Doppler
Perfusion in MVF-treated flaps did not differ from PBS-treated controls at any time-matched region (e.g., day 7 distal MVF vs PBS: 30.0 ± 2.5 vs 23.1 ± 1.5, respectively). The blood perfusion measurements in all regions at all 4 time points were not different in any region at any time point: before surgery (Pre), after surgery (Post), 7 days postoperatively, and 14 days postoperatively for both the PBS- (light gray line) and MVF-treated flaps (dark gray line, Fig. 3).
Fig. 3.
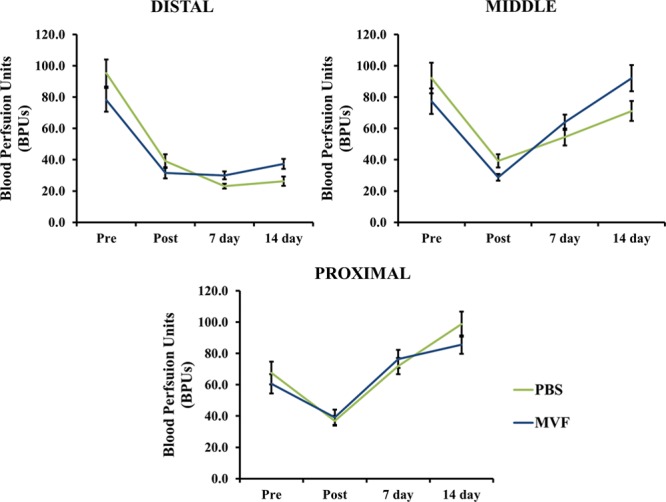
Laser Doppler measurements were recorded before surgery (Pre), after surgery (Post), 7 days postoperatively, and 14 days postoperatively. The values represented here were taken from all 3 regions of the flap. No significant differences were found when comparing MVF treated versus PBS treated at any time point for each location. Results are expressed as mean ± SEM.
Effect of MVFs on Microvessel Density
Within each region, skin flap–associated microvessel density did not differ between MVF- and PBS-treated rats (Fig. 4). However, for both control and treated rats, flap microvessel density differed (P < 0.001) among all regions (Fig. 4).
Fig. 4.
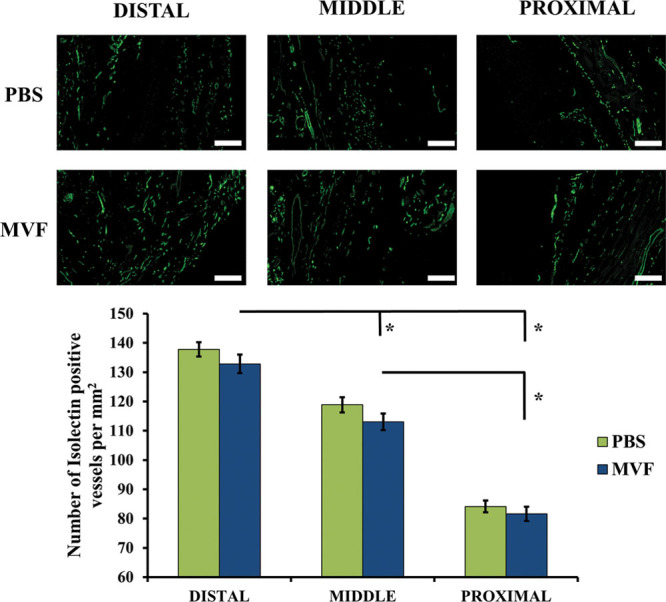
A, Representative images of microvessel detection by fluorescein-labeled GSL I-isolectin B4 immunofluorescence. Each image is from a different animal and shows the microvessel density in different regions of both PBS- and MVF-treated flaps. White scale bars represent 250 μm. B, Quantitation of the GSL I-isolectin B4 immunofluorescence to determine microvessel density. Microvessel density is the number of GSL I-isolectin B4 positive vessels per mm2. A significant difference in microvessel density is shown between the regions but not between the treatments. Results are expressed as mean ± SEM; *statistical significance in comparison to other regions (P < 0.001).
DISCUSSION
Strategies to improve flap survival involve use of preconditioning (methods used before surgery), postconditioning (methods used after surgery), pharmaceutical agents (used before, during, or after surgery), and regenerative medicine approaches by the use of stem cells (used at flap site or injected intravascular).15–26 These maneuvers have been attempted with the intent of making the tissue more resistant to prolonged ischemia27 or to improve perfusion by stimulating neovascularization. MVFs consist of adipose tissue–derived arterioles, venules, and capillaries,7 which improve tissue perfusion in a variety of tissue injury models.8,9,11,12,28 An additional factor that provides significant motivation for understanding the potential of MVFs for flap survival is that they can be delivered without prior culturing: that is, fragments can be harvested, implanted on the day of surgery, and inosculated with the host within days.12 In fact, a recent publication demonstrated the superiority of utilizing freshly isolated MVFs compared with those cultured in vitro.11 In agreement with our hypothesis, we show that MVFs did improve flap survival 14 days after surgery; however, the mechanisms whereby this improvement occurred remain to be determined.
Many recent studies with the dorsal skin flap rat model involve the use of different stem cell populations (bone marrow and adipose derived) that have a positive effect on flap survival.16,18,19,24,29–37 A possible contributing factor could be the multipotent properties inherent in either stem cell population that allow differentiation into endothelial cells required for angiogenesis.34 Any observed angiogenesis could also be explained by indirect effects of the stem cells via secreted protective growth factors or cytokines.34 With these concepts in mind, it is interesting to also consider whether additional bioactive factors (e.g., stem cells) that are associated with MVFs might have played a role, perhaps indirectly, in the beneficial effects on tissue perfusion that were observed. For example, recent studies have demonstrated the presence of stem cells within MVFs.11,28,38 This is intuitive because MSCs have been shown to reside within the vascular wall39 or in a perivascular location.38,40–42 Hence, it is possible that the MVFs may have produced beneficial effects that extend beyond their direct ability to support blood flow. This is particularly relevant to the dorsal flap model where MSCs provide beneficial effects for flap viability.16,18,19,24,31,37 Regardless of the mechanism, the beneficial effects observed for flap survival, and the clinical relevance of the procedure (i.e., same-day approach), provide the impetus for further exploration.
Although the results of this study are encouraging, it is important to point out that in previous work, the application of MVFs alone was unable to improve flap survival.43 Despite the fact that a different flap size was used (10 × 2 vs 10 × 3 cm) and that there are differences in the methodology for MVF isolation, the 2 studies are strikingly similar with regards to the percent flap survival in control animals (59.8% vs 58.9% in this study) and the percent survival at 7 days in the MVF-treated animals (63.1% vs 62.9% in this study).43 The previous study included an additional group that comprised both myofibroblasts (Mfs) and MVFs that demonstrated a superior flap survival at 7 days as compared to either MVFs or Mfs alone, which supports the notion that Mfs are necessary for the potential of MVFs to be realized. Although our results parallel Nakano et al43 in terms of the comparison between MVFs and control at 7 days after transplant, they differ in that we observed a significant improvement in flap survival with MVFs alone at 14 days after transplantation suggesting additional cell augmentation is not required. For MVFs to be more clinically relevant for flap survival, it is apparent that improvements in the therapy need to be made to realize an appreciable benefit in less than 14 days. Nonetheless, taken together, the 2 studies demonstrate the regenerative potential of MVFs and provide insight into the possible improvements that can be made to further improve their utility.
The delineation of a mechanism whereby MVFs improved flap survival is required and will help in the development of future MVF-based strategies. A logical speculation is that by applying freshly isolated MVFs directly to the flap, stem cells and vessels were applied to the entire area and allowed to migrate to the ischemic regions, secrete growth factors, and potentially form new vessels that reconnected the dorsal skin flaps’ blood supply. Our study shows a significant 6% increase in flap survival at 14 days that is similar in magnitude to other studies where stem cells were used.18,24 To further improve upon our initial findings, there are several areas to address: source of fat, number of MVFs/stem cells delivered, and method of delivery. Epididymal fat was chosen as the source of MVFs because it is a highly vascularized, easily accessible source of adipose tissue in the rodent model that allows us to build upon previous work. Utilizing the same tissue to isolate MVFs avoids potential confounding variables when comparing with other studies. In the future, we will be exploring other sources of adipose tissue. Given the size of the flap, we choose to inject 100,000 fragments as a starting amount. The actual number of total cells, or more importantly the number of stem cells per MVF, is difficult to confidently evaluate. However, if one could assume that at least 1 stem cell per fragment survived, migrated, proliferated, and/or differentiated, then based on the number of MVFs injected in this study, at least 100,000 stem cells were delivered. In this regard, it is also interesting to consider that implantation of a higher number of MVFs may result in a greater increase in vessel density and ultimately further improvements in flap survival. Other areas for improvement include the delivery methodology. Even though in vitro testing indicated that the fragments could safely pass through a needle intact, a better delivery method utilizing a biomaterial that allows for a more even distribution may facilitate inosculation between the flap and wound bed. Regardless, given the rapidly advancing field of biomaterials research, the many positive biological aspects of MVFs suggest that they are a good platform to build upon for improving flap tissue perfusion.
CONCLUSIONS
The results of this study highlight the potential for MVFs to be used as a means to improve flap survival. Future studies identifying the mechanism underlying this increase are required to further develop this therapy to effectively cause greater increased tissue perfusion.
ACKNOWLEDGMENTS
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the US Government. We express our sincere gratitude to LTC Owens, DVM, and Marcello Pilia, PhD, for rodent surgical training, Monica Jalomo for assistance during rodent surgeries, and Jennifer McDaniels, PhD, for MVF isolation training.
Footnotes
Supported by US Army Medical Research and Materiel Command, Clinical and Rehabilitative Medicine Research Program; Grant number: F_015_2013. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the US Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and USAISR and by the US Army Medical Research and Materiel Command of the Department of Defense.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the US Army Medical Research and Material Command.
REFERENCES
- 1.Maciel-Miranda A, Morris SF, Hallock GG. Local flaps, including pedicled perforator flaps: anatomy, technique, and applications. Plast Reconstr Surg. 2013;131:896e–911e. doi: 10.1097/PRS.0b013e31828bd89f. [DOI] [PubMed] [Google Scholar]
- 2.Park YK, Seel DJ. Myocutaneous flaps in general surgery. J Korean Med Sci. 1987;2:1–6. doi: 10.3346/jkms.1987.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lineaweaver W, Akdemir O, Schleich A. Management strategies following microsurgical flap failure. Microsurgery. 2010;30:61–63. doi: 10.1002/micr.20682. [DOI] [PubMed] [Google Scholar]
- 4.Roehl KR, Mahabir RC. A practical guide to free tissue transfer. Plast Reconstr Surg. 2013;132:147e–158e. doi: 10.1097/PRS.0b013e3182910fba. [DOI] [PubMed] [Google Scholar]
- 5.Chen KT, Mardini S, Chuang DC, et al. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007;120:187–195. doi: 10.1097/01.prs.0000264077.07779.50. [DOI] [PubMed] [Google Scholar]
- 6.Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia- reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011;128:685e–692e. doi: 10.1097/PRS.0b013e318230c57b. [DOI] [PubMed] [Google Scholar]
- 7.Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol Anim. 1996;32:409–419. doi: 10.1007/BF02723003. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871–2879. doi: 10.1089/ten.2007.0025. [DOI] [PubMed] [Google Scholar]
- 9.Pilia M, McDaniel JS, Guda T, et al. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater. 2014;28:11–23. doi: 10.22203/ecm.v028a02. [DOI] [PubMed] [Google Scholar]
- 10.Hiscox AM, Stone AL, Limesand S, et al. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 11.Laschke MW, Kleer S, Scheuer C, et al. Pre-cultivation of adipose tissue-derived microvascular fragments in porous scaffolds does not improve their in vivo vascularisation potential. Eur Cell Mater. 2015;29:190–200. doi: 10.22203/ecm.v029a14. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd BR, Chen HY, Smith CM, et al. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 13.McFarlane RM, Deyoung G, Henry RA. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast Reconstr Surg. 1965;35:177–182. doi: 10.1097/00006534-196502000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Reichenberger MA, Heimer S, Schaefer A, et al. Adipose derived stem cells protect skin flaps against ischemia-reperfusion injury. Stem Cell Rev. 2012;8:854–862. doi: 10.1007/s12015-012-9368-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Yang D, Wang P, et al. Microencapsulated myoblasts transduced by the vascular endothelial growth factor (VEGF) gene for the ischemic skin flap. Aesthetic Plast Surg. 2011;35:326–332. doi: 10.1007/s00266-010-9610-y. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbeck ST, Senghaas A, Komatsu I, et al. Tissue engraftment of hypoxic-preconditioned adipose-derived stem cells improves flap viability. Wound Repair Regen. 2012;20:872–878. doi: 10.1111/j.1524-475X.2012.00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim DW, Jun I, Lee TJ, et al. Therapeutic angiogenesis by a myoblast layer harvested by tissue transfer printing from cell- adhesive, thermosensitive hydrogels. Biomaterials. 2013;34:8258–8268. doi: 10.1016/j.biomaterials.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Jeon YR, Cho EJ, et al. Optimal administration routes for adipose-derived stem cells therapy in ischaemic flaps. J Tissue Eng Regen Med. 2012;8:596–603. doi: 10.1002/term.1552. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Mirabella T, Hartinger J, Lorandi C, et al. Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells Dev. 2012;21:2179–2188. [Google Scholar]
- 21.Oh M, Chang H, Minn KW. The effects of tadalafil on axial- pattern skin flap survival in rats. Dermatol Surg. 2008;34:626–630. doi: 10.1111/j.1524-4725.2007.34118.x. [DOI] [PubMed] [Google Scholar]
- 22.Shafighi M, Olariu R, Fathi AR, et al. Dimethyloxalylglycine stabilizes HIF-1α in cultured human endothelial cells and increases random-pattern skin flap survival in vivo. Plast Reconstr Surg. 2011;128:415–422. doi: 10.1097/PRS.0b013e31821e6e69. [DOI] [PubMed] [Google Scholar]
- 23.Sheng L, Yang M, Li H, et al. Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med. 2011;224:229–234. doi: 10.1620/tjem.224.229. [DOI] [PubMed] [Google Scholar]
- 24.Simman R, Craft C, McKinney B. Improved survival of ischemic random skin flaps through the use of bone marrow nonhematopoietic stem cells and angiogenic growth factors. Ann Plast Surg. 2005;54:546–552. doi: 10.1097/01.sap.0000158068.86576.73. [DOI] [PubMed] [Google Scholar]
- 25.Ulusoy MG, Uysal A, Koçer U, et al. Improved flap viability with site-specific delivery of sildenafil citrate using fibrin glue. Ann Plast Surg. 2005;55:292–296. doi: 10.1097/01.sap.0000175483.35073.ea. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Yi C, Xia W, et al. Mesenchymal stem cells transduced by vascular endothelial growth factor gene for ischemic random skin flaps. Plast Reconstr Surg. 2008;121:59–69. doi: 10.1097/01.prs.0000293877.84531.5a. [DOI] [PubMed] [Google Scholar]
- 27.Harder Y, Amon M, Laschke MW, et al. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg. 2008;61:503–511. doi: 10.1016/j.bjps.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Laschke MW, Kleer S, Scheuer C, et al. Vascularisation of porous scaffolds is improved by incorporation of adipose tissue-derived microvascular fragments. Eur Cell Mater. 2012;24:266–277. doi: 10.22203/ecm.v024a19. [DOI] [PubMed] [Google Scholar]
- 29.Gao W, Qiao X, Ma S, et al. Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1α. J Cell Mol Med. 2011;15:2575–2585. doi: 10.1111/j.1582-4934.2011.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichioka S, Kudo S, Shibata M, et al. Bone marrow cell implantation improves flap viability after ischemia-reperfusion injury. Ann Plast Surg. 2004;52:414–418. doi: 10.1097/01.sap.0000099709.95938.59. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Zan T, Li Y, et al. Transplantation of adipose-derived stem cells promotes formation of prefabricated flap in a rat model. Tohoku J Exp Med. 2010;222:131–140. doi: 10.1620/tjem.222.131. [DOI] [PubMed] [Google Scholar]
- 32.Lu F, Mizuno H, Uysal CA, et al. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008;121:50–58. doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Tepper OM, Galiano RD, et al. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg. 2004;113:284–293. doi: 10.1097/01.PRS.0000091169.51035.A5. [DOI] [PubMed] [Google Scholar]
- 34.Uysal AC, Mizuno H, Tobita M, et al. The effect of adipose-derived stem cells on ischemia-reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg. 2009;124:804–815. doi: 10.1097/PRS.0b013e3181b17bb4. [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Sheng L, Li H, et al. Improvement of the skin flap survival with the bone marrow-derived mononuclear cells transplantation in a rat model. Microsurgery. 2010;30:275–281. doi: 10.1002/micr.20779. [DOI] [PubMed] [Google Scholar]
- 36.Yi C, Xia W, Zheng Y, et al. Transplantation of endothelial progenitor cells transferred by vascular endothelial growth factor gene for vascular regeneration of ischemic flaps. J Surg Res. 2006;135:100–106. doi: 10.1016/j.jss.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Yue Y, Zhang P, Liu D, et al. Hypoxia preconditioning enhances the viability of ADSCs to increase the survival rate of ischemic skin flaps in rats. Aesthetic Plast Surg. 2013;37:159–170. doi: 10.1007/s00266-012-9993-z. [DOI] [PubMed] [Google Scholar]
- 38.McDaniel JS, Pilia M, Ward CL, et al. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J Surg Res. 2014;192:214–222. doi: 10.1016/j.jss.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Ergün S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15:981–995. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- 40.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Chen CW, Corselli M, Péault B, et al. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Nakano M, Nakajima Y, Kudo S, et al. Effect of autotransplantation of microvessel fragments on experimental random-pattern flaps in the rat. Eur Surg Res. 1998;30:149–160. doi: 10.1159/000008571. [DOI] [PubMed] [Google Scholar]


