Supplemental Digital Content is available in the text.
Abstract
Background:
Autologous engineered skin substitutes comprised of keratinocytes, fibroblasts, and biopolymers can serve as an adjunctive treatment for excised burns. However, engineered skin lacks a vascular plexus at the time of grafting, leading to slower vascularization and reduced rates of engraftment compared with autograft. Hypothetically, vascularization of engineered skin grafts can be improved by treatment with proangiogenic agents at the time of grafting. Epoxyeicosatrienoic acids (EETs) are cytochrome P450 metabolites of arachidonic acid that are inactivated by soluble epoxide hydrolase (sEH). EETs have multiple biological activities and have been shown to promote angiogenesis. Inhibitors of sEH (sEHIs) represent attractive therapeutic agents because they increase endogenous EET levels. We investigated sEHI administration, alone or combined with EET treatment, for improved vascularization of engineered skin after grafting to mice.
Methods:
Engineered skin substitutes, prepared using primary human fibroblasts and keratinocytes, were grafted to full-thickness surgical wounds in immunodeficient mice. Mice were treated with the sEHI 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU), which was administered in drinking water throughout the study period, with or without topical EET treatment, and were compared with vehicle-treated controls. Vascularization was quantified by image analysis of CD31-positive areas in tissue sections.
Results:
At 2 weeks after grafting, significantly increased vascularization was observed in the TPPU and TPPU + EET groups compared with controls, with no evidence of toxicity.
Conclusions:
The results suggest that sEH inhibition can increase vascularization of engineered skin grafts after transplantation, which may contribute to enhanced engraftment and improved treatment of full-thickness wounds.
Tissue-engineered skin replacements have been developed to meet the needs of patients with large burns and insufficient donor sites for skin autografting, and also of patients with chronic nonhealing wounds. In particular, engineered skin substitutes comprised of autologous epidermal keratinocytes, dermal fibroblasts, and biopolymers have been shown to facilitate healing of large excised burn wounds, reducing the harvesting of donor skin for autograft and providing stable skin replacement.1–3 However, because engineered skin contains only 2 cell types, they cannot replace all of the functions of uninjured skin. For example, engineered skin grafts in vitro lack a vascular plexus, which can delay vascularization in vivo compared with split-thickness autograft. In the absence of a preformed vascular network in engineered skin, vascularization is achieved by angiogenesis, the ingrowth of newly formed blood vessels from the wound bed. In contrast, autograft is vascularized more rapidly by a combination of inosculation, the anastomosis of vessels in the graft with vessels in the wound bed, and angiogenesis. Delays in vascularization can compromise engraftment by increasing time for reperfusion, ischemia, and nutrient deprivation of transplanted cells. Previous preclinical studies from our laboratory demonstrated that engineered skin containing cells genetically modified to overexpress vascular endothelial growth factor, an angiogenic cytokine, led to enhanced and accelerated vascularization after grafting to immunodeficient mice.4 Vascular endothelial growth factor overexpression was accompanied by increased graft stability and improved engraftment, suggesting that engraftment could be increased by accelerating early vascularization.5 Approaches that improve vascularization without genetically modified cells should face fewer regulatory hurdles and move more rapidly to clinical application. Hypothetically, treatment with systemic or topical drugs with angiogenic activity may enhance vascularization of engineered skin substitutes without the need to use genetically modified cells.
Epoxyeicosatrienoic acids (EETs) are bioactive lipid signaling molecules that modulate inflammation and stimulate angiogenesis.6–9 EETs are generated from arachidonic acid by cytochrome P450 (CYP) monooxygenase enzymes.8,10 CYPs have been referred to as the “third pathway” of the arachidonic acid cascade because they have received less attention than the cyclooxygenase and lipoxygenase pathways, which generate prostaglandins and leukotrienes, respectively (Fig. 1).8,10 EETs modulate numerous signaling cascades to regulate vascular tone, angiogenesis, and inflammation.11 The EETs are unstable in vivo because of rapid metabolism by the enzyme soluble epoxide hydrolase (sEH), which converts EETs to their corresponding 1,2-diols, the dihydroxyeicosatrienoic acids (DiHETEs).12 Inhibitors of sEH (sEHIs) represent attractive therapeutic agents because they elevate endogenous EET levels by stabilizing the EETs in vivo, thereby increasing their associated benefits. Recently, it was demonstrated that EETs and sEHIs enhance angiogenesis and epithelialization in mouse ear wounds.13,14 In animal studies, sEHIs have low toxicity and few off-target effects. Several potent, metabolically stable sEHIs have been developed for clinical application in treatment of hypertension and inflammatory disorders,6 and at least 3 have been tested in early clinical trials.15–17
Fig. 1.
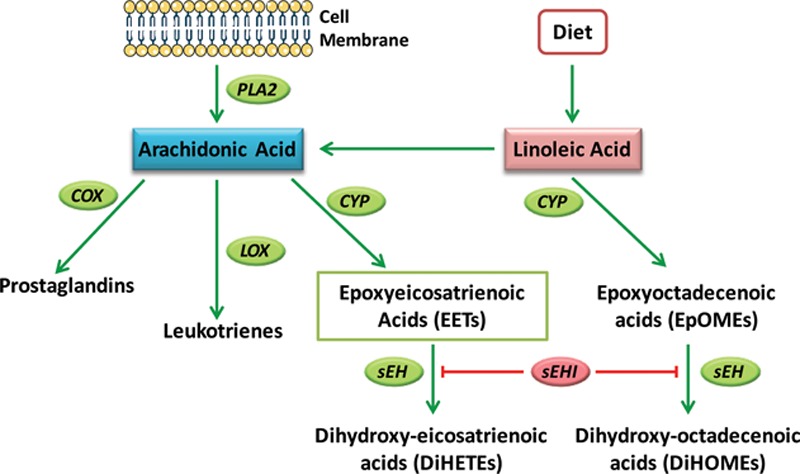
Epoxyeicosatrienoic acid formation and metabolism by soluble epoxide hydrolase. Upon activation of cells by external stimuli, arachidonic acid is released from membrane phospholipids by phospholipase A2 (PLA2). Arachidonic acid is also derived from dietary linoleic acid. Arachidonic acid can be metabolized along one of 3 pathways: the cyclooxygenase (COX) pathway, the lipoxygenase (LOX) pathway, or the CYP pathway. CYP epoxygenases metabolize arachidonic acid to produce EETs. These have been shown to act as autocrine and paracrine mediators with proangiogenic, antihypertensive, and antiinflammatory activities. These properties of EETs are attenuated by their metabolism to DiHETEs by the enzyme sEH. sEHIs prevent the metabolism of EETs to DiHETEs leading to EET accumulation.
This study investigated early vascularization of human tissue–engineered skin substitutes transplanted to immunodeficient mice treated with an orally available sEHI, alone or in combination with topical EET treatment, compared with vehicle-treated controls.
METHODS
Cell Culture and Preparation of Engineered Skin Substitutes
Deidentified human skin was obtained from a 16-year-old African American girl undergoing an elective breast reduction at the University of Cincinnati (UC) Medical Center. The UC Institutional Review Board determined that this activity did not constitute human subjects research and was therefore exempt from requirements for informed consent according to the US Code of Federal Regulations Policy 45CFR46.101(b)(4).18 Primary cultures of dermal fibroblasts and epidermal keratinocytes were initiated and propagated as described.19–22 Collagen-glycosaminoglycan dermal substrates were fabricated as previously described.23 Fibroblasts (5 × 105/cm2) were inoculated onto the dermal substrates. After 24 hours, keratinocytes (1 × 106/cm2) were inoculated. Skin substitutes were cultured at the air–liquid interface for 14 days with daily media changes.21,24
Preparation of EETs for Topical Application
The EET methyl ester regioisomeric mixture was prepared as previously published with modifications.25 Briefly, methyl arachidonate was dissolved in dichloromethane and the mixture was stirred at 0°C. 3-chloroperbenzoic acid was added to the stirring solution, which was warmed to room temperature. The mixture was concentrated using a rotary evaporator, filtered, and the filtrate was washed with saturated potassium bicarbonate. The organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated to dryness, and dissolved in 10% ethyl acetate in hexane. The crude mixture was purified by flash chromatography yielding the final mixture of methyl epoxy docosapentaenoate. The product was characterized by hydrolyzing the esters with LiOH to the corresponding acids,26 which were analyzed by liquid chromatography–tandem mass spectrometry.27 For topical application, a 0.1% mixture of EET methyl ester regioisomers (14,15-EET, 11,12-EET, 8,9-EET, and 5,6-EET methyl esters in a ratio of 2.2:1.6:1.1:1, respectively) was mixed into Aquaphor ointment (Beiersdorf Inc.; Wilton, Conn.). The methyl esters are more stable but are rapidly hydrolyzed to free acids in the cell.
Grafting of Engineered Skin to Mice and Experimental Treatments
All animal procedures were approved by the UC Institutional Animal Care and Use Committee. These studies utilized the potent, stable, sEH inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU), which was prepared in-house (Hammock laboratory).28 TPPU was dissolved in polyethylene glycol 400 and diluted into drinking water at 22.0 μg/ml, resulting in an oral dose of approximately 5 mg/kg/d. For controls, an equal amount of polyethylene glycol 400 was added to drinking water. Mice also received topical treatment consisting of 0.75 g ointment (Aquaphor vehicle control or 0.1% EET in Aquaphor), applied at grafting and 1 week after grafting. Twenty-four mice were divided into 3 groups (8/group): group 1, vehicle-only; group 2, TPPU in drinking water and topical ointment vehicle; group 3, TPPU in drinking water and topical 0.1% EET. TPPU treatments were initiated 48 hours before grafting and were continued throughout the study. Engineered skin substitutes (2 cm × 2 cm) were grafted to full-thickness wounds on the right flanks of female athymic mice (Foxn1nu/nu, Envigo; Indianapolis, Ind.). Grafts were sutured in place, and, using tie-over stent dressings, ointment-coated gauze pads (vehicle control or EET) were applied. The grafts were covered with occlusive dressings (OPSITE, Smith & Nephew; Andover, Mass.), and mice were wrapped using self-adhesive bandages (Coban, 3M; St. Paul, Minn.). After 7 days, dressings were removed, a second topical ointment treatment was administered, and fresh dressings were applied. Mice were euthanized after 14 days. Blood and tissue biopsies of the grafted areas were collected.
Analysis of TPPU and Lipids
TPPU levels in blood and graft biopsies were determined using high-performance liquid chromatography–tandem mass spectrometry.29,30 For analysis of circulating oxylipins, plasma was isolated from whole blood by centrifugation using ethylenediamine-tetraacetic acid–coated tubes (Sarstedt AG & Co.; Nümbrecht, Germany), and 2 μl of antioxidant solution (0.2% triphenylphosphine/0.2% butylated hydroxytoluene/0.1% ethylenediamine-tetraacetic acid) was added per 100 μl plasma. Lipid profiles were determined in plasma and graft biopsies using solid-phase extraction liquid chromatography–electrospray ionization/multistage mass spectrometry.27,29,31 Levels of arachidonic acid metabolites EET and DiHETE and linoleic acid metabolites epoxyoctadecenoic acid (EpOME) and dihydroxyoctadecenoic acid (DiHOME) were evaluated.
Quantitation of Vascularization
Vascularization was quantified by calculating the percent dermal area positive for the endothelial cell marker CD31. Biopsies were embedded in Shandon M1 Embedding Matrix (Invitrogen/Thermo Scientific; Waltham, Mass.). Cryosections were fixed by incubation in methanol followed by acetone at −20°C. Immunohistochemistry was performed using standard techniques with a rat antibody against mouse CD31 (BD Biosciences) followed by an Alexa Flour 594-conjugated goat anti-rat antibody (Invitrogen/Thermo Fisher Scientific). Coverslips were mounted on slides using Vectashield containing 4,6-diamidino-2-phenylindole (Vector Laboratories; Burlingame, Calif.) for counterstaining of nuclei. Sections were viewed with a Nikon Eclipse 90i microscope, photographed at 10× magnification with a DS-Ri1 Digital Camera, and image analysis was performed using NIS-Elements AR3.1 (Nikon Instruments Inc.; Melville, N.Y.). Three nonoverlapping microscopic fields were analyzed per mouse; image analysis was used to calculate percent area CD31-positive. Mean values for each mouse were calculated, and group means were calculated using a single mean value for each mouse.
Statistical Analyses
Statistical analyses were performed using SigmaPlot Version 11.0 (SyStat Software, Inc.; San Jose, Calif.). One-way analysis of variance was used to identify statistically significant differences among groups; post hoc pairwise multiple comparison procedures were performed using the Holm-Sidak method. Differences were considered statistically significant at P < 0.05. One plasma sample from group 2 was too low for accurate lipid analysis, so only 7 plasma samples were analyzed for this group. Thus, all comparisons were n = 8 per group, except for lipid analysis of group 2.
RESULTS
At 2 weeks after transplantation to immunodeficient mice, engineered skin grafts were healed and adhered to wound beds and wound margins, with no gross differences observed among groups (See figure, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A328). TPPU was not detected in blood or graft tissue in vehicle-treated control mice. TPPU was detected in TPPU- and TPPU + EET–treated mice, with significantly elevated levels in blood and graft tissue compared with controls (See figure, Supplemental Digital Content 2, http://links.lww.com/PRSGO/A329). No toxicity was observed.
To investigate the efficacy of sEH inhibition by TPPU, EET levels were measured in plasma and tissue of mice 2 weeks after grafting. Plasma levels of arachidonic acid–derived epoxides 14,15-EET and 11,12-EET were modestly but not significantly increased in both TPPU-treated groups (Fig. 2A). Tissues levels of these EETs were significantly increased in group 3 mice, treated with oral TPPU plus topical EETs, compared with controls (group 1) or TPPU-treated mice (group 2) (Fig. 2B). The significant elevation of 14,15-EET and 11,12-EET in group 3 graft tissue may be due, in part, to topical treatment with exogenous EETs. Levels of the CYP epoxygenase–derived linoleic acid metabolites, 12,13-EpOME and 9,10-EpOME, were also increased in TPPU-treated mice, but statistically significant increases were only observed for 12,13-EpOME in plasma (Fig. 3). No significant differences were observed between TPPU-treated and TPPU + EET–treated groups, but both of these groups had significantly elevated 12,13-EpOME plasma levels compared with controls. In tissue, 14,15-EET and 11,12-EET were significantly elevated in TPPU + EET–treated mice (Fig. 2B); together, these EETs represented nearly all of the total EETs measured in tissue (See figure, Supplemental Digital Content 3, http://links.lww.com/PRSGO/A330). Similarly, 12,13-EpOME, which was significantly elevated in plasma of TPPU and TPPU + EET–treated mice, constituted the majority of total EpOMEs measured in plasma of these mice (See figure, Supplemental Digital Content 3, http://links.lww.com/PRSGO/A330).
Fig. 2.
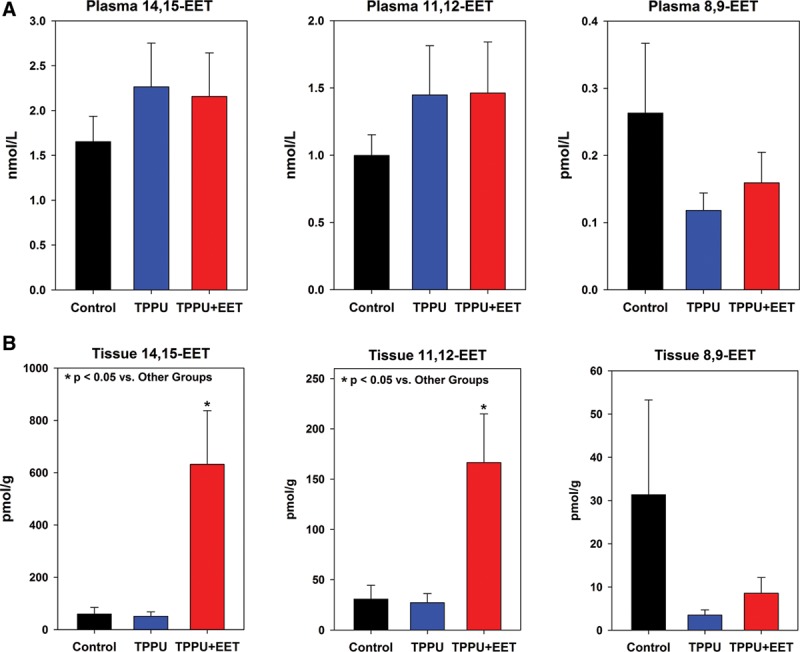
Levels of arachidonic acid–derived EETs measured in plasma (A) and tissue (B) at 2 weeks after transplantation. Shown are values (mean + SEM) for individual regioisomers 14,15-EET (left plots), 11,12-EET (center plots), and 8,9-EET (right plots). Note that 14,15-EET and 11,12-EET are significantly elevated in the TPPU + EET group (*P < 0.05); this may be due, in part, to topical application of EETs.
Fig. 3.
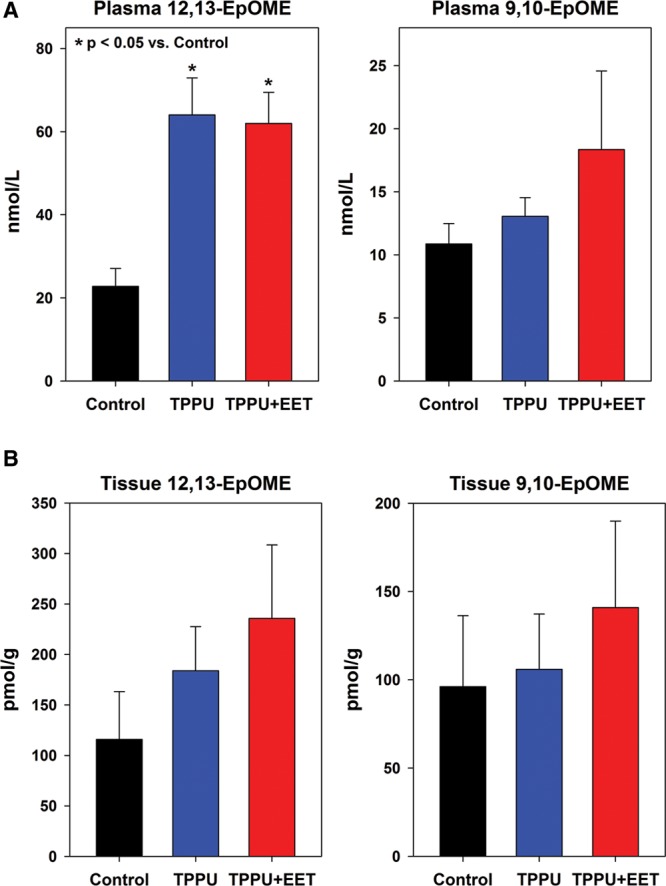
Levels of linoleic acid–derived EpOMEs measured in plasma and tissue at 2 weeks after transplantation. Shown are values (mean + SEM) measured in plasma (A) and tissue biopsies (B) for individual regioisomers 12,-13-EpOME (left plot) and 9,10-EpOME (right plot). Statistically significant differences are marked with asterisks (*P < 0.05).
Levels of the diol metabolites of EETs and EpOMEs, the DiHETEs and DiHOMEs, respectively, were measured in plasma and tissue 2 weeks after grafting. Efficient inhibition of sEH is predicted to reduce diol levels and increase the epoxide/diol ratio, although relative levels may vary in different tissues.32 No significant differences were observed in plasma DiHETEs; however, significantly higher levels were detected in tissue biopsies of mice treated with TPPU + EET compared with TPPU-treated mice and controls (See figure, Supplemental Digital Content 4, http://links.lww.com/PRSGO/A331). This can likely be attributed to breakdown of topically applied exogenous EETs in this group. This significant increase in total DiHETE levels in tissue resulted in a significant decrease, rather than increase, in the EET/DiHETE ratio (Fig. 4A). EET/DiHETE ratios in plasma and tissue in TPPU- treated mice were increased, although increases were not statistically significant (Fig. 4A). Previously, reductions in plasma DiHOME levels and increased plasma EpOME/DiHOME ratios were observed after oral TPPU treatment of rodents, which demonstrated target engagement and sEH inhibition.32 In this study, plasma levels of total DiHOMEs were reduced, and the reduction was statistically significant versus controls in the TPPU + EET group (Fig. 4B). Furthermore, plasma EpOME/DiHOME ratios were significantly increased in TPPU and TPPU + EET groups compared with controls (Fig. 4B). This finding, coupled with the significant increase in plasma 12,13- EpOME levels in TPPU and TPPU + EET groups (Fig. 3A), indicates that 12,13-EpOME may serve as a biomarker for sEHI activity in this mouse model.
Fig. 4.
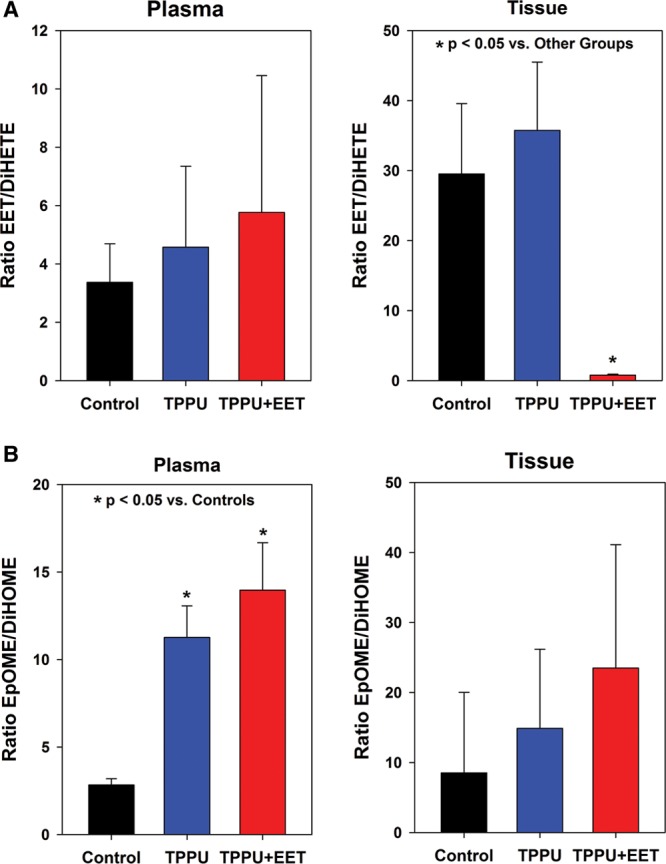
Epoxide:diol ratios in plasma and tissue at 2 weeks after grafting. The ratios of total EET/DiHETE levels in plasma (upper left) and tissue (upper right) are shown in (A). The ratios of total EpOME/DiHOME levels in plasma (lower left) and tissue (lower right) are shown in (B). Values plotted represent mean + SEM. Significant differences are indicated by asterisks (*P < 0.05).
At the histological level, grafts in all groups displayed a differentiated epidermis with a well-cornified surface (See figure, Supplemental Digital Content 5, http://links.lww.com/PRSGO/A332). In engineered skin grafts from control, untreated mice, CD31-positive blood vessels were observed in the lower dermal regions near the centers of the grafts, although higher densities of vessels were observed at graft edges, adjacent to the wound margins (Figs. 5A, B). In TPPU- and TPPU + EET–treated groups, blood vessels were more evenly distributed throughout the dermis (Figs. 5C, D). Quantitative analysis showed no significant difference between TPPU- and TPPU + EET–treated groups, but both treatments resulted in significantly higher graft vascularization levels compared with control, untreated mice (Fig. 6).
Fig. 5.
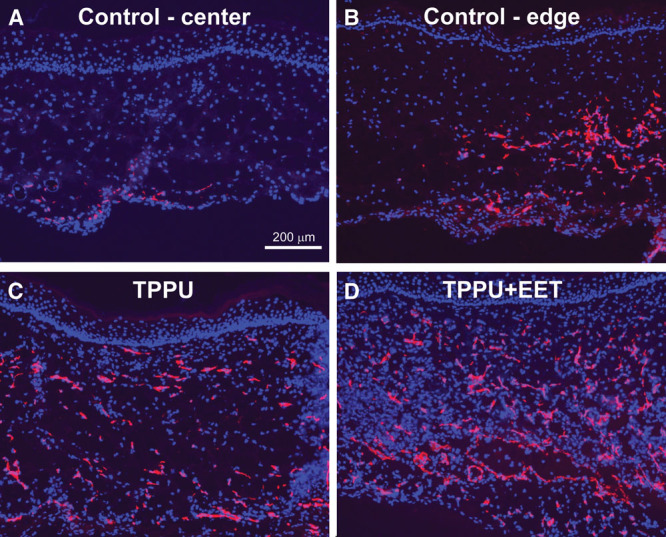
Localization of endothelial cells in engineered skin grafts. Immunohistochemistry was used to localize CD31-positive endothelial cells (red) in blood vessels; nuclei were counterstained blue with 4,6-diamidino-2-phenylindole. Shown are representative sections from control grafts (group 1) in upper panels; images are from center of graft (A) and from edge of graft near wound margin (B; wound margin is toward right of panel). Bottom panels show representative sections from TPPU-treated mice (group 2; C) and TPPU + EET–treated mice (group 3; D). Scale bar (A; 200 μm) is the same for all panels.
Fig. 6.
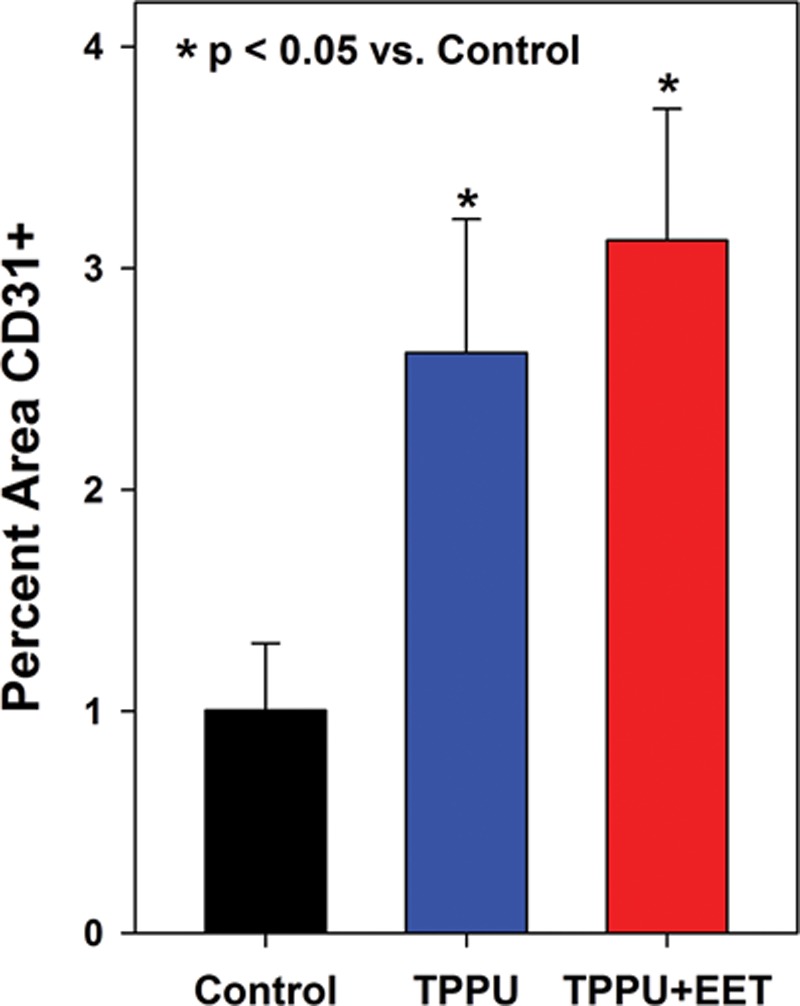
Vascularization of engineered skin grafts is increased in TPPU-treated mice at 2 weeks after transplantation. Plotted are values (mean + SEM) for the relative area (percent of total dermal area) staining positive for the endothelial cell–specific marker CD31. Mice treated with TPPU or TPPU + EET had significantly greater values for percent area CD31-positive compared to controls (*P < 0.05).
DISCUSSION
The results of this study suggest that TPPU treatment contributed to elevated levels of bioactive epoxy-fatty acids and was associated with enhanced vascularization of engineered skin grafts 2 weeks after transplantation. Previous studies demonstrated that enhanced early vascularization of engineered skin substitutes was accompanied by increased engraftment.5 Therefore, treatment with TPPU or other sEHIs at the time of transplantation may contribute to improved engraftment of engineered skin. Additional studies investigating extended time points after grafting will be required to confirm this prediction. In addition to their proangiogenic activities, EETs and sEHIs have other beneficial effects that may increase their therapeutic value in burn-injured patients. Previous studies demonstrated analgesic effects of EETs and sEH inhibition in animal models of inflammatory and neuropathic pain.33,34 Hypothetically, these agents may serve to reduce burn-induced hyperalgesia while enhancing skin-graft healing.
In this study, some mice were treated topically with a mixture of EETs, in addition to TPPU treatment, to determine whether the local delivery of these lipids would synergistically increase vascularization in mice treated with TPPU. Differences in plasma EET levels were not observed in mice treated with TPPU with or without topical EETs, suggesting that topical EETs did not reach systemic circulation. Although local EET levels were significantly elevated in TPPU + EET–treated mice compared with TPPU treatment alone, there was no significant difference in vascularization between these groups. This suggests that topical EET treatment, when used in conjunction with systemic TPPU treatment, does not have a significant effect on vascularization of engineered skin grafts. It may be that TPPU is sufficiently effective on its own, making it difficult to demonstrate a synergistic or additive effect when combined with exogenous EETs. This was previously observed for TPPU in a mouse model of angiotensin-II–dependent hypertension.35 Because of the potency of TPPU and the rapid metabolism of EETs, it is often difficult to show synergy in vivo.35 The relatively high level of DiHETEs and the decreased EET/DiHETE ratio in tissue suggest that much of the topically applied EETs were metabolized during the period between application and analysis. Future studies will investigate higher levels of EETs, applied as described here and in slow-release formulations.
Although topical EET did not seem to potentiate the effects of systemic TPPU on engineered skin graft vascularization, we cannot rule out the possibility that topical EETs may enhance vascularization of native skin autograft, which possesses a vascular plexus at the time of transplantation. Topical EET treatment was previously shown to increase neovascularization in mouse ear wounds, similar to results observed with sEHI treatment.14 In this study, systemic sEHI treatment enhanced neovascularization of engineered skin grafts, which required ingrowth of vessels from the wound bed and wound margins, whereas treatment of the surface of initially avascular grafts with EET provided no additional benefit. EETs have been shown to act on endothelial cells to increase proliferation, migration, and tube formation.36–38 Furthermore, endothelial-derived EETs were shown to promote neovascularization and tissue regeneration in vivo.39 It is reasonable to speculate, therefore, that topical EETs, EET mimics, or sEHI treatment may improve vascularization of transplanted native skin autograft, which contains endothelial cells and vascularizes via a combination of inosculation and angiogenesis.
For this study, we chose not to investigate topical EET treatment alone because it represents a less attractive therapeutic approach, because of their relatively low stability and high cost. However, newer synthetic methods are expected to dramatically lower their production costs, reducing one barrier to clinical application. From a translational perspective, sEHIs currently represent a superior approach because they increase endogenous EET levels. Multiple stable, highly potent sEHIs have been developed. Vascularization is a critical step required for viability and function of not just engineered skin, but essentially any tissue-engineered organ.40 Therefore, as a drug that inhibits sEH systemically with low toxicity, TPPU may be an attractive adjunct to improve the engraftment of tissue-engineered constructs. However, systemic sEHI treatment must be investigated with caution because of the potential risk that elevated EETs may contribute to increased tumor angiogenesis. Long-term systemic treatment may not be required to achieve local increases necessary to improve vascularization of engineered tissues. Future studies are needed to investigate the long-term effects of systemic sEHI treatment and the effects of local sEH inhibition on engraftment of engineered skin.
ACKNOWLEDGMENTS
The authors thank Mark Kleiner and Chris Lloyd for preparation of culture media and biopolymer substrates and Mary Rolfes in the Shriners Hospitals for Children – Cincinnati Histology Core Facility for preparation of histological sections.
Supplementary Material
Footnotes
Supported by Developmental Grant #85220 from the Shriners Hospitals for Children. Partial support was received from NIEHS grant R01 ES002710, NIEHS Superfund Research Program Grant P42 ES004699, NIH/NIDDK U24 DK097154 Metabolomics Resources Core, and NIH CounterACT U54 NS079202. Kin Sing Stephen Lee is partially supported by the NIH Pathway to Independence Award from NIH/NIEHS (K99 ES024806).
Some of the results described in this article were presented at the joint Symposium on Advanced Wound Care and the Wound Healing Society annual meeting (SAWC/WHS), Atlanta, Ga., 2016.
Disclosure: Bora Inceoglu, Kin Sing Stephen Lee, and Bruce D. Hammock are coinventors on patents related to soluble epoxide hydrolase by the University of California. Bruce D. Hammock and Bora Inceoglu are cofounders of EicOsis LLC. Steven T. Boyce has patents and intellectual property that are assigned to the University of Cincinnati and Shriners Hospitals for Children and are licensed for commercial development. The Article Processing Charge was paid for by the Shriners Hospitals for Children.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Boyce ST, Kagan RJ, Yakuboff KP, et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269–279. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce ST, Kagan RJ, Greenhalgh DG, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60:821–829. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 3.Boyce ST, Simpson PS, Rieman MT, et al. Randomized, paired-site comparison of autologous engineered skin substitutes and split-thickness skin graft for closure of extensive, full-thickness burns. J Burn Care Res. 2016 doi: 10.1097/BCR.0000000000000401. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supp DM, Supp AP, Bell SM, et al. Enhanced vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J Invest Dermatol. 2000;114:5–13. doi: 10.1046/j.1523-1747.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 5.Supp DM, Boyce ST. Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J Burn Care Rehabil. 2002;23:10–20. doi: 10.1097/00004630-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem. 2012;55:1789–1808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82:60–67. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Inceoglu B, Schmelzer KR, Morisseau C, et al. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 11.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS, Liu JY, Wagner KM, et al. Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J Med Chem. 2014;57:7016–7030. doi: 10.1021/jm500694p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sander AL, Sommer K, Neumayer T, et al. Soluble epoxide hydrolase disruption as therapeutic target for wound healing. J Surg Res. 2013;182:362–367. doi: 10.1016/j.jss.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Sander AL, Jakob H, Sommer K, et al. Cytochrome P450-derived epoxyeicosatrienoic acids accelerate wound epithelialization and neovascularization in the hairless mouse ear wound model. Langenbecks Arch Surg. 2011;396:1245–1253. doi: 10.1007/s00423-011-0838-z. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Whitcomb R, MacIntyre E, et al. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. 2012;52:319–328. doi: 10.1177/0091270010397049. [DOI] [PubMed] [Google Scholar]
- 16.Tran L, Kompa AR, Wang BH, et al. Evaluation of the effects of urotensin II and soluble epoxide hydrolase inhibitor on skin microvessel tone in healthy controls and heart failure patients. Cardiovasc Ther. 2012;30:295–300. doi: 10.1111/j.1755-5922.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- 17.Lazaar AL, Yang L, Boardley RL, et al. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol. 2016;81:971–979. doi: 10.1111/bcp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services, Office for Human Research Protections: Basic HHS Policy for Protection of Human Research Subjects. Code of Federal Regulations Title 45. Rockville, MD: Public Welfare[Part 46, Protection of Human Subjects]; 2009. Jan 15, [PubMed] [Google Scholar]
- 19.Boyce ST, Ham RG. Cultivation, frozen storage, and clonal growth of normal human epidermal keratinocytes in serum-free media. J Tiss Cult Meth. 1985;9:83–93. [Google Scholar]
- 20.Boyce ST. Methods for the serum-free culture of keratinocytes and transplantation of collagen-GAG-based skin substitutes. Methods Mol Med. 1999;18:365–389. doi: 10.1385/0-89603-516-6:365. [DOI] [PubMed] [Google Scholar]
- 21.Boyce ST, Supp AP, Swope VB, et al. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002;118:565–572. doi: 10.1046/j.1523-1747.2002.01717.x. [DOI] [PubMed] [Google Scholar]
- 22.McFarland KL, Glaser K, Hahn JM, et al. Culture medium and cell density impact gene expression in normal skin and abnormal scar-derived fibroblasts. J Burn Care Res. 2011;32:498–508. doi: 10.1097/BCR.0b013e3182223cb1. [DOI] [PubMed] [Google Scholar]
- 23.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988;22:939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 24.Smiley AK, Klingenberg JM, Aronow BJ, et al. Microarray analysis of gene expression in cultured skin substitutes compared with native human skin. J Invest Dermatol. 2005;125:1286–1301. doi: 10.1111/j.0022-202X.2005.23971.x. [DOI] [PubMed] [Google Scholar]
- 25.Morisseau C, Inceoglu B, Schmelzer K, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falck JR, Kodela R, Manne R, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Schmelzer K, Georgi K, et al. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose TE, Morisseau C, Liu JY, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulu A, Appt S, Morisseau C, et al. Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. Br J Pharmacol. 2012;165:1401–1412. doi: 10.1111/j.1476-5381.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Sun GY, Liu T, et al. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell Tissue Res. 2016;363:399–409. doi: 10.1007/s00441-015-2262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JY, Lin YP, Qiu H, et al. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48:619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostermann AI, Herbers J, Willenberg I, et al. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-(1-propanoylpiperidin-4-yl)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU): Resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inceoglu B, Jinks SL, Ulu A, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inceoglu B, Bettaieb A, Trindade da Silva CA, et al. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulu A, Stephen Lee KS, Miyabe C, et al. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2014;64:87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Wei S, Pozzi A, et al. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch Biochem Biophys. 2009;489:82–91. doi: 10.1016/j.abb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Wei X, Xiao X, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 38.Pozzi A, Macias-Perez I, Abair T, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 39.Panigrahy D, Kalish BT, Huang S, et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci U S A. 2013;110:13528–13533. doi: 10.1073/pnas.1311565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laschke MW, Menger MD. Vascularization in tissue engineering: angiogenesis versus inosculation. Eur Surg Res. 2012;48:85–92. doi: 10.1159/000336876. [DOI] [PubMed] [Google Scholar]


