Abstract
Background:
The present study was conducted to find out a tool to enable improved functional recovery with proximal nerve injury. In this experimental study, nerve regeneration was compared between side-to-side (STS), end-to-side (ETS), and end-to-end repairs.
Methods:
The walk track analysis was used as an outcome of functional recovery. Nerve regeneration was studied with morphometry and histology 6 or 26 weeks postoperatively.
Results:
All 3 repair techniques showed regeneration of the nerve. From 12 weeks onward, the functional results of the 3 intervention groups were significantly better compared with the unrepaired control group. End-to-end repair was significantly better when compared with the STS and ETS groups. At 26 weeks, the functional and morphometric results and histologic findings did not differ between the STS and ETS groups. The functional results correlated with the morphometric findings in all groups.
Conclusions:
STS neurorrhaphy showed nerve regeneration, and the end results did not differ from clinically widely used ETS repair. Further studies are warranted to optimize the neurorrhaphy technique and examine possible applications of STS repair in peripheral nerve surgery.
Successful regeneration of the peripheral nerve with proximal injury has remained a challenging situation. Regenerating axons have a limited time to reach the end organs. In these cases, distal end-to-end (ETE) nerve transpositions1,2 and end-to-side (ETS) repairs3–12 have been used to overcome the problem. However, in these repair techniques, the distal end is used for reconstructions. The side-to-side (STS) repair technique leaves both injured nerve ends free and thus offers a tool for further nerve reconstructions. Only a few studies have been made with the STS nerve repair technique. In clinical reports, sensory regeneration13,14 and motor14,15 regeneration were noticed.
The objective of the present experimental study is to compare comprehensively nerve regeneration between the STS, ETS, and ETE repair techniques.
METHODS
Animals
Eighty female young adult Wistar rats (Harlan Laboratories Netherlands B.V., Melderslo, The Netherlands) weighing 300 to 340 g were used. The local laboratory animal care committee approved the experiment, which followed the principles of laboratory animal care.
Operative Procedure
The animals were randomly divided into 10 groups (Table 1). They were anesthetized with an intraperitoneal injection of 5 μg/kg medetomidine hydrochloride (Domitor; Orion Oyj, Espoo, Finland) and 750 μg/kg ketamine hydrochloride (Ketalar; Pfizer Oy, Helsinki, Finland). The same investigator (H.R.) carried out all operations with microsurgical instruments and a surgical microscope (Zeiss, Jena, Germany). The bifurcation of the common peroneal nerve (CPN) and tibial nerve (TN) was exposed (Fig. 1). The CPN was transected 5 mm distally to the bifurcation. In the STS group, a 2-mm long epineural window was created to both the CPN and TN 15 mm distally to the bifurcation, and the neurorrhaphy was performed with four 10-0 sutures (Nylon; S&T AG, Neuhausen, Switzerland). In the ETS group, a 2-mm long epineural window was performed to the lateral surface of the TN similarly to the previous group, and the neurorrhaphy with the distal end of the CPN was performed with four 10-0 sutures. In the ETE group, the CPN transection was repaired with four 10-0 sutures. In the STS repair group, both nerve ends of the CPN and, in the ETS repair group, the proximal end of the CPN were ligated with 8-0 Nylon sutures. The stumps were turned to the opposite directions and sutured to the neighboring muscles with three 10-0 sutures. In the unrepaired group, the CPN was cut, and the nerve ends were ligated, turned to the opposite directions, and sutured to the muscle. In the sham-repaired group, the sciatic nerve trunk was revealed and left intact. The wounds were closed in separate layers with 5-0 sutures (Deknatel Bondek Plus; Teleflex Medical, Durham, N.C.). The analgesic treatment was ensured by a subcutaneous injection of 5 mg/kg carprofen (Rimadyl; Vericode Ltd., Dundee, United Kingdom) 3 days postoperatively.
Table 1.
Experimental Groups
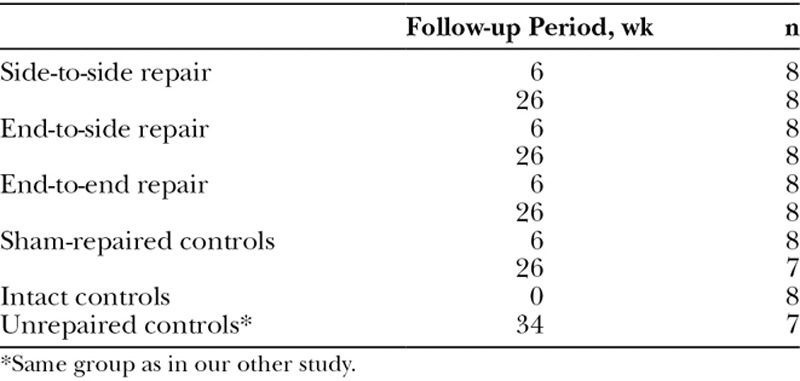
Fig. 1.
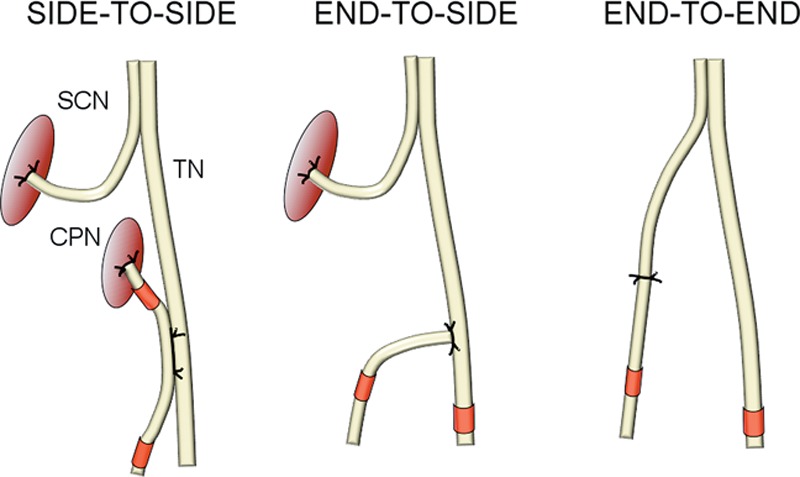
Schematic representation of intervention groups: STS, ETS, and ETE nerve repairs. Red marks show the sites of harvested nerve samples for morphometric study. SCN = sciatic nerve.
Walk Track Analysis
The walk track analysis was performed before the operation and 2, 4, and 6 weeks postoperatively on all animals and, further, 8, 12, 16, 20, and 26 weeks postoperatively on animals with a longer follow-up period. The print length (PL; distance between the heel and third toe) and the toe spread (TS; distance between the first and fifth toe) were measured from the footprints. The results were calculated as a mean value of 3 measurements. The peroneal function index (PFI = 174.9 [(EPL − NPL)/NPL] + 80.3 [(ETS − NTS)/NTS] − 13.4) was calculated. “N” refers to the normal, unoperated side, and “E” refers to the experimental side.16 The investigator had passed the self-education test17 to minimize interobserver differences.
Sample Preparation
The animals were killed at 6 or 26 weeks (Table 1) with an intraperitoneal injection of 60 mg/kg sodium pentobarbital (Mebunat; Orion Oyj). In 7 of 8 animals, the tissues were fixed with intracardiac perfusion of phosphate-buffered formalin. The operated nerves and tissue samples of the long peroneal muscle were removed and fixed in phosphate-buffered formalin. The sites of nerve samples are shown in Figure 1. Nerve and muscle samples were embedded in paraffin. From the paraffin blocks, 4-μm-thick sections were cut both for morphometry with neurofilament immohistochemistry–stained sections and for histology with hematoxylin and eosin–stained sections.
One animal of 8 per group was perfused intracardially with Millonig phosphate buffer and glutaraldehyde. Nerve and muscle samples were removed and postfixed with osmium tetroxide and embedded in epon. One-micrometer-thick sections were cut and stained with toluidine blue for qualitative histologic study.
Neurofilament Protein Immunocytochemistry
Four-micrometer-thick sections were cut from the paraffin blocks. The staining was performed with a biotin-free Poly-HRP-Anti-Mouse kit (BrightVision; Immunologic BV, Duiven, The Netherlands) according to the protocol of the manufacturer. Mouse monoclonal neurofilament (200 and 68 kDa) Ab1 (Clone 2F11) antibody (Thermo Fisher Scientific, Fremont, Calif.) was applied and incubated. Normal antibody diluent (Immunologic BV) was used to dilute and stabilize horseradish peroxidase conjugates. The sections were then incubated with peroxidase-compatible chromogen (Bright-DAB; Immunologic BV) and finally counterstained and cover slipped.
Morphometry
Morphometry was performed with neurofilament-stained sections. The whole-nerve cross-sections of immunohistochemically stained samples were photographed with the AxioVert 200M microscope and AxioCam HRc microscope camera (Carl Zeiss, Göttingen, Germany). The images were stitched as a mosaic image by using AxioVision software (Carl Zeiss, Jena, Germany). The digitalized images of the subperineural areas of the nerve cross-sections were processed with imaging software (Graphics Suite X6/Photo-Paint; Corel Corp., Ottawa, Ontario, Canada). Morphometric measurements were done with BioImageXD.18 The nerve area (μm2), nerve fiber count, and areas of nerve fibers (μm2) were measured. The following outcomes were calculated: total fiber area (sum of fiber areas [μm2]), fiber density (fiber count/nerve area [number/mm2]), the mean fiber area (total fiber area/fiber count [μm2]), and the percentage of the fiber area (total fiber area/nerve area × 100).
Statistical Analysis
The statistical analyses were done with SPSS (version 21; IBM Corp., Armonk, N.Y.) and SAS System for Windows (version 9.4; SAS Institute Inc., Cary, N.C.). The results are expressed as means and SD. P values smaller than 0.05 were considered statistically significant.
The comparisons between the groups of the walk track analysis were done with analysis of covariance for repeated measurements after adjustment for baseline PFI values. The heterogeneous autoregressive covariance structure was used to consider the correlation between observations in these longitudinal data.
In the morphometric analysis, the groups were compared with two-way analysis of variance (ANOVA). Unrepaired groups with only a long follow-up period were compared with other groups with one-way ANOVA. Comparison between the 2 different sites of the CPN of the STS group was performed with the paired t test.
In the comparisons of the fiber area, there was a dependency between the observations because of the thousands of values measured from each animal. It was taken into account with the linear mixed model with the random intercept for animal. The data were normally distributed after log10-transformation.
The effect of multiple comparisons in the analyses mentioned above was considered by using Tukey–Kramer and Dunnett adjustments.
The correlations between the PFI and morphometric outcomes were calculated with Pearson correlation coefficients.
The sample size of 8 animals per group was calculated from the expected difference in the walk track analysis. The sample size gives 90% power and a type I error rate of no more than 5% to detect a difference of 15 or more in the mean PFI values between the intact controls and the intervention groups. This expected difference is based on the following assumptions:
A mean (SD) PFI value of −10 (3) among the intact control animals from pilot studies by the investigators.
A mean (SD) PFI value of −25 (13) among animals undergone ETE repair reached values of −28.41 (4.16) at 30 days, −22.92 (3.62) at 60 days, −13.94 (2.68) at 150 days,19 and −14.5 (3.9) at 12 weeks.20 ETS repair reached the PFI value of −77.0 (11.5) at 6 weeks and −37.3 (13.5) at 12 weeks20 and −48.5 (SEM, 2.2) at 28 weeks.21
RESULTS
Two animals did not wake up from anesthesia and were excluded from the study. The sample size of 7 gives 0.86 power to the test. No cases of autotomy or flexion contracture were detected.
Walk Track Analysis
The STS and ETS groups did not differ significantly at any time point. From 6 weeks onward, PFI was better in the ETE group compared with the STS and ETS groups. PFI of the STS and ETS was significantly better when compared with the unrepaired group from 12 weeks onward. The PFI values at 12 weeks were as follows: STS, −40.3 (12.2); ETS, −42.6 (17.3); ETE, −19.1 (5.7); sham repaired, −12.6 (1.4); and unrepaired, −75.8 (12.0; Fig. 2).
Fig. 2.
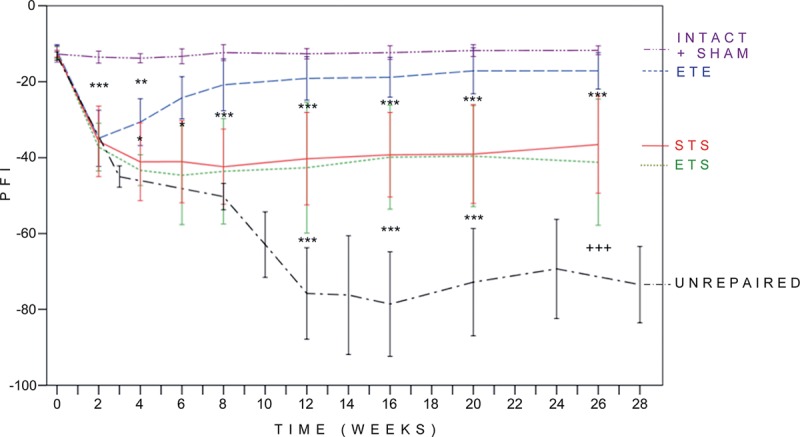
Results of walk track analysis. Normal values of PFI correspond to 0 to −15, and total impairment approaches −100. Differences between the PFI values of the examination groups larger than 15 were considered significant. STS and ETS groups did not differ from each other and reached their maximum regeneration level at 4 weeks. PFI was significantly higher from 12 weeks onward in the STS and ETS groups compared with the unrepaired group. The results of the ETE group were significantly better from 4 weeks onward when compared with the ETS group and from 6 weeks onward when compared to the STS group. There were no significant differences between the ETE group and the sham-repaired group from 6 weeks onward. P < 0.05, **P < 0.01, ***P < 0.001, and +++P < 0.001, when compared other groups with the mean value of 24 and 28-week pooled unrepaired group. Error bar, ± 1 SD.
Morphometry
CPN
All intervention groups showed significantly higher values of the fiber count, total fiber area, fiber density, and percentage of the fiber area when compared with the unrepaired group both at 6 and 26 weeks (Fig. 3). At 26 weeks, there were no significant differences between the STS and ETS groups in any outcome. At 6 and 26 weeks, the fiber count, total fiber area, fiber density, and percentage of the fiber area of the ETE reached significantly higher values than the ETS (all P < 0.02) and STS (all P < 0.001) with the exception of a nonsignificant difference with the total fiber area (P = 0.06) at 6 weeks between the ETE and ETS (Fig. 3).
Fig. 3.
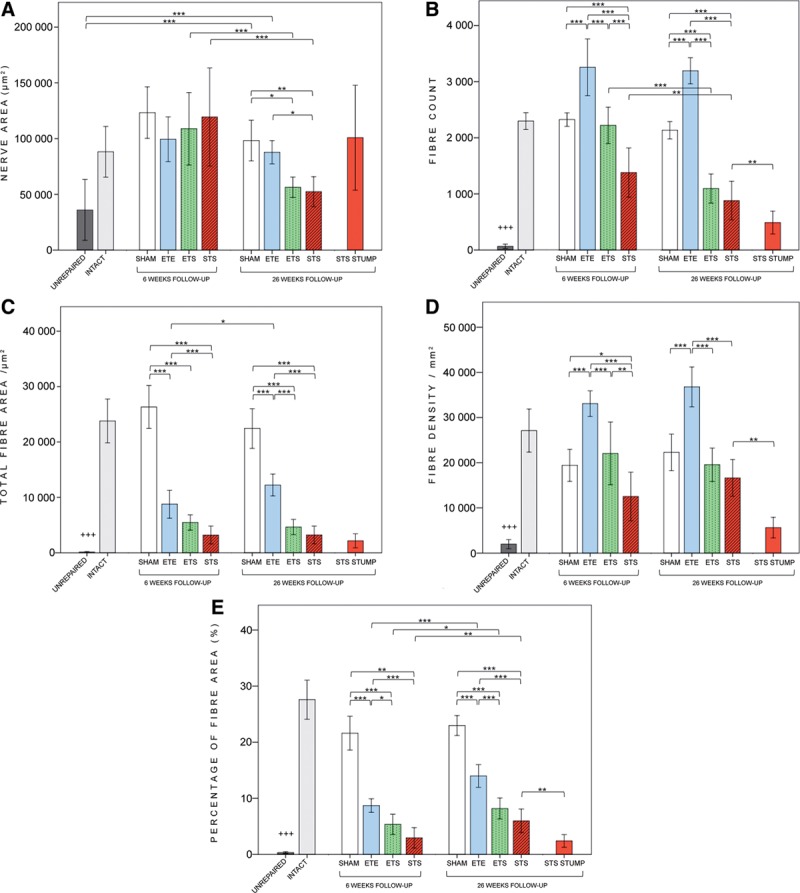
Results of the morphometric analyses of the common peroneal nerve. The fiber count (B), total fiber area (C), fiber density (D), and percentage of the fiber area (E): values of all intervention groups at 26 weeks were significantly higher when compared with the unrepaired group (P < 0.001). There were no significant differences between the STS and ETS groups in any outcome at 26 weeks. Both at 6 and 26 weeks, the ETE group showed significantly higher values than the STS and ETS groups in the fiber count, total fiber area, fiber density, and percentage of the fiber area values, with the exception of the nonsignificant difference in the total fiber between the ETE and ETS at 6 weeks. On the distal side of STS neurorrhaphy, the fiber count, fiber density, and percentage of the fiber area were significantly higher compared with the nerve stump. *P < 0.05, **P < 0.01, ***P < 0.001, and +++ P < 0.001, comparison of experimental groups to the unrepaired group at 26 weeks. Error bar, ± 1 SD.
The mean nerve area of the STS, ETS, and ETE did not differ at 6 weeks, but at 26 weeks, the STS was significantly smaller than the ETE. There were no differences between the ETS and STS groups (Fig. 3).
In the STS group, the morphometric analysis of the CPN was performed on both sides of the neurorrhaphy (Fig. 1). The fiber count, fiber density, and percentage of the fiber area were significantly higher on the distal side compared with the stump (Fig. 3).
The mean nerve fiber areas did not differ between the STS, ETS, and ETE groups (Fig. 4). In all 3 groups, the values of the mean fiber area and percentage of the fiber area were significantly higher at 26 weeks compared with 6 weeks (group by time interaction effect, P = 0.01).
Fig. 4.
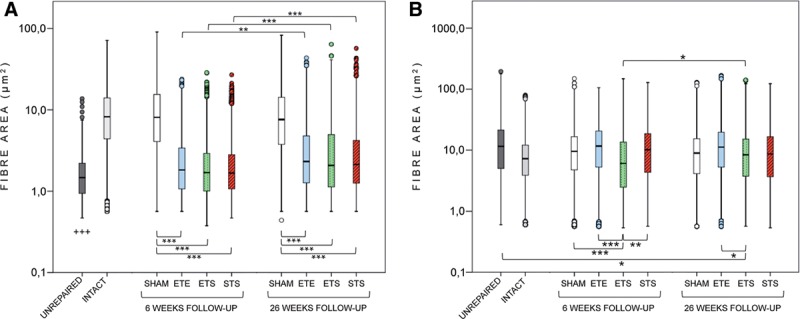
Fiber area values of common peroneal nerve (A) and tibial nerve (B). In peroneal nerve (A), the mean fiber area did not differ between the STS, ETS, and ETE groups at 6 or 26 weeks. The mean fiber areas of the STS, ETS, and ETE groups were significantly larger at 26 weeks compared with 6 weeks. The mean fiber areas of the 3 groups were significantly smaller when compared with the sham-repaired groups and larger when compared with the unrepaired group. In tibial nerve (B), the mean fiber area of the ETS group was significantly smaller when compared with the STS, ETE, and sham-repaired groups at 6 weeks. At 26 weeks, the mean fiber area of the ETE group was larger compared with the ETS group. In y-axis, the values are logarithmic. The box plot diagram shows the median and the upper and lower quartiles; whiskers indicate variability outside the quartiles, and outliers are plotted as individual points. *P < 0.05, **P < 0.01, ***P < 0.001, and +++ P < 0.001, comparison of experimental groups to the unrepaired group at 26 weeks.
All morphometric parameters of the distal CPN at 26 weeks (Table 2) correlated with PFI: nerve area (Pearson correlation, 0.73; P < 0.001), fiber count (0.82; P = 0.000), mean fiber area (0.68; P < 0.001), total fiber area (0.77; P < 0.001), fiber density (0.77; P < 0.001), and percentage of the fiber area (0.80; P < 0.001).
Table 2.
Results of Morphometric Analyses of Common Peroneal Nerve
TN
The mean fiber area of the ETS was smaller at 6 weeks when compared with the STS, ETE, and sham repaired (all P < 0.002) and at 26 weeks when compared with the ETE group (P = 0.03; Fig. 4).
The values of the mean nerve area, fiber count, fiber density, and percentage of the fiber area (Fig. 5) did not differ significantly between the STS, ETS, and ETE groups both at 6 and 26 weeks. The mean fiber count values of the TN at 26 weeks were as follows: STS, 5,064 (542); ETS, 5,026 (384); ETE, 5,272 (411); intact, 5,158 (232); sham repaired, 5,138 (284); and unrepaired, 5,301 (295).
Fig. 5.
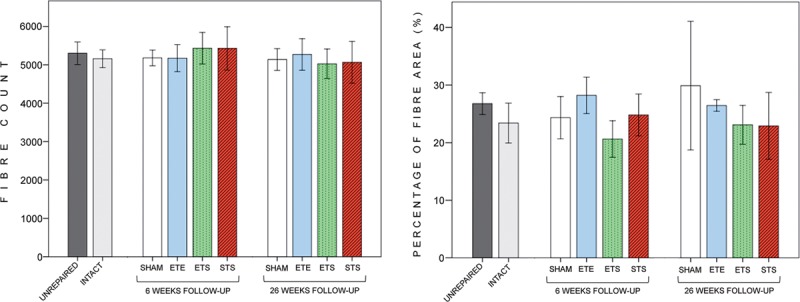
Results of the morphometric analysis of the donor tibial nerve. The biopsy sites are seen in Figure 1. Fiber count (A) and percentage of the fiber area (B) values of different groups did not differ significantly from each other at 6 and 26 weeks. *P < 0.05, **P < 0.01, and ***P < 0.001. Error bar, ± 1 SD.
Qualitative Light Microscopy
In the TN proximal to the neurorrhaphy, axon density seemed normal in all groups. In the STS and ETS groups, some axon sprouts were observed outside the perineurium at 6 weeks but not at 26 weeks.
At the site of the neurorrhaphy at 6 and 26 weeks, a number of axon sprouts were observed similarly in the STS and ETS groups in the lateral areas of the TN inside the perineurium and the regenerative segment continued to the subperineurial areas of the CPN.
Distal to the neurorrhaphy in the CPN, the epineurium seemed normal, and no misdirected axons were seen outside the perineurium in the STS and ETS groups. Axon density looked similar in both groups (Fig. 6). Mild changes of fibrosis could be seen in the STS group at 26 weeks. In the ETE group, axon density seemed to be high and myelin sheaths thicker compared with the STS and ETS groups.
Fig. 6.
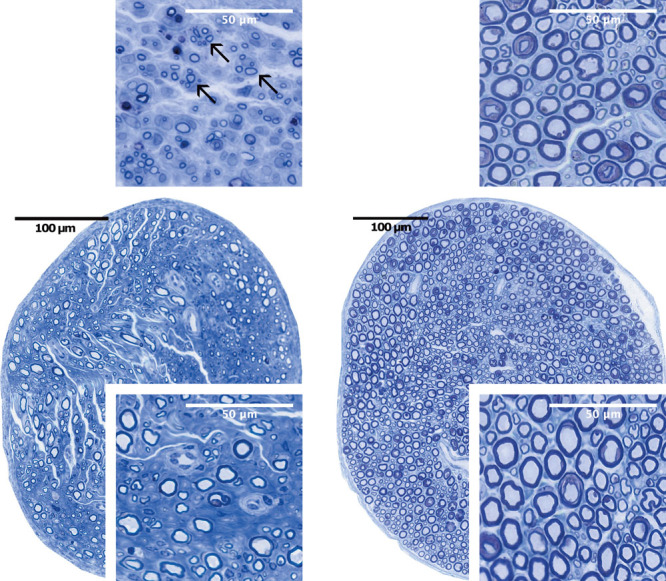
Nerve cross-sections of side-to-side repair (left) and sham repair (right) at 6 (above) and 26 weeks (below) postoperatively. Clusters of regenerative axon sprouts (arrows) can be seen 5 mm distal to the site of operation at 6 weeks (above left). At 26 weeks, axons are myelinated and larger in size (below left). Well-preserved nerve fibers in the sham-repaired group at 6 (above right) and at 26 weeks (below right): toluidine blue staining.
Distal to the neurorrhaphy in the TN in the STS and ETS groups, small amounts of axon sprouts were seen in the peripheral areas inside the perineurium; otherwise, the view looked normal at 6 and 26 weeks.
In the long peroneal muscle, there were focal signs of atrophy in both the STS and ETS groups (Fig. 7) at 6 and 26 weeks. In the ETE group, only some atrophic muscle fibers could be observed. In the unrepaired group, there were changes in advanced muscle denervation.
Fig. 7.
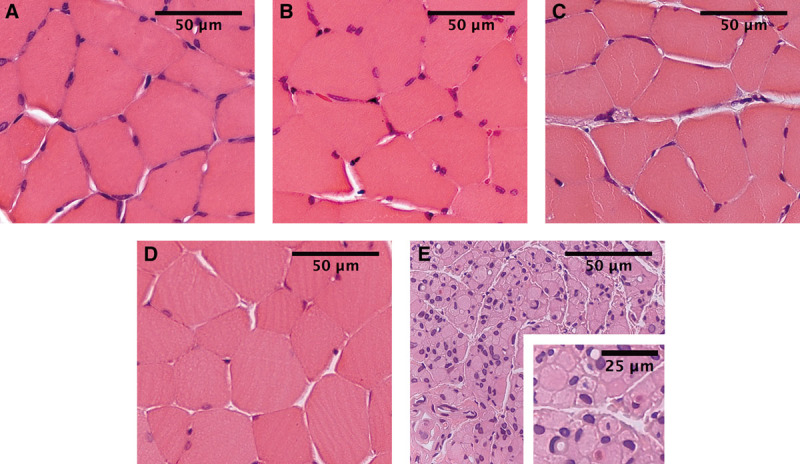
Muscle samples from the long peroneal muscle at 26 weeks. STS (A), ETS (B), ETE (C), intact control (D), and unrepaired groups (E). In the STS and ETS groups, focal signs of muscle atrophy were more clearly seen than in the ETE group. In the unrepaired group, muscle cells were small and atrophied, and their shape was angular. Pyknotic nuclear clumps and signs of group atrophy with some areas of preserved muscle fibers were noticed: HE staining.
DISCUSSION
The objective of the present study was to compare the STS, ETS, and ETE repair techniques comprehensively. Previously, Yüksel et al22 studied STS neurorrhaphy experimentally. However, in their study, CPN was transected only 3 weeks after the primary operation. Furthermore, Ladak et al23 used nerve grafts between the donor and the recipient nerve in their distal neurorrhaphy. To our knowledge, this study is the first experimental examination with a similar model in STS and ETS repairs. In the present study, the donor-side window was identical in the STS and ETS repairs, and on the recipient side, the nerve end was used similarly in the ETS and ETE repairs.
All 3 repair groups showed functional recovery. From 12 weeks onward, the functional results of the 3 intervention groups were significantly better compared with the unrepaired control group. The functional results did not differ between the STS and ETS groups. In the present study, the results of the walk track analysis at 26 weeks had a significant correlation with the morphometric parameters. It is clear that although there is robust axonal regeneration in the distal nerve stump, but appropriate connections to the muscles are not reached, functional regeneration remains poor, and there is no correlation between the outcomes.24–26
The present results of the walk track analysis are in accordance with those of previous studies. In our study, the PFI of the STS at 26 weeks (−36.6) was in line with the study by Yüksel et al at 28 weeks (−30.4). The PFI of the present ETS group (−41.2) at 26 weeks is also comparable with the results of the previous ETS studies: Eren et al,21 −48.5 after 28 weeks; Liu et al,20 −37.3, and Ozmen et al,27 −54.8 after 12 weeks.
In the morphometric analysis, we analyzed the whole cross-sections of nerves, which ensures unbiased analysis of different nerve regions. Immunohistochemical staining was conducted with neurofilament antibody, which allows the calculation of even small and unmyelinated axon sprouts. The protocol allowed to add axons to the mask and to remove nonaxonal particles from the mask.
The morphometric parameters, histological findings, and functional results were superior in the ETE repair compared with the STS and ETS repairs. It can be explained by a better axonal flow from the transsectional donor nerve end compared to the epineural window. On the recipient side, the transectional nerve end (ETS) showed no advantage compared with the window (STS), as in the long-term, there were no significant differences between the STS and ETS groups in the morphometric parameters, histologic degenerative signs of the muscle, and functional results. Considering these 2 results, it seems that the donor side of the neurorrhaphy is decisive to the end result when all 3 repair techniques are compared. After successful end-organ connection, axons will maturate and the myelin layer will thicken. Our morphometric and histological findings showed that during regeneration from 6 to 26 weeks, the axons grew in size in the STS, ETS, and ETE groups (Fig. 4) and the myelin layer seemed thickened (Fig. 6).
To clarify the mechanism of regeneration in the STS repair, nerve samples were taken from both sides of the neurorrhaphy of the CPN. The values of the fiber count, fiber density, and percentage of the fiber areas were significantly higher on the distal side compared with the stump. Thus, the results of regenerating axons in the STS group cannot be explained with contamination.
According to the previous reports, donor muscle denervation has been reported to be negligible from 3 to 12 months after ETS repair.27–31 However, signs of acute donor muscle denervation28 and decrease in the number of myelinated nerve fibers distal to ETS neurorrhaphy compared with the proximal values31,32 have been reported. In our study, a histologically small amount of axon sprouts was noticed in the TNs of both STS and ETS groups as slight signs of donor nerve injury both at 6 and 26 weeks. Although deliberate injury to the donor nerve was avoided at operations, it is obvious that axonal injury cannot be completely excluded when epineural windows are created.
Despite encouraging results, we are aware that nerve regeneration is faster with rats compared with humans. Despite the known “blow through effect,”33 we considered it important to get long-term results as well to ensure the stability of the regeneration results. Form the methodological point of view, quantitative morphometry was performed with neurofilament staining, which cannot distinguish the myelin sheath. Further studies are warranted to analyze the development of myelin thickness and differentiation of sensory and motor axon sprouting.
The present results are in accordance with previous promising clinical results.13,14 According to the literature with ETS repair34–36 and our findings with STS neurorrhaphy, the noted number of axons may be limited to ensure sufficient regeneration. The purpose of STS and ETS repairs is to serve axon sprouts into the severed nerve and end organ rapidly enough after nerve injury. These so-called “baby-sitting” procedures aim to maintain the growth-supporting atmosphere in the distal nerve stump and to reduce muscle atrophy.23,37–41 When compared with the ETS technique, the advantage of the STS technique is that it leaves both nerve stumps available for further reconstructions. Further studies are needed to optimize the size of epi- or perineural windows to enhance regeneration and to combine the STS technique to proximal ETE repair.
CONCLUSIONS
Nerve regeneration was compared between STS, ETS, and ETE techniques. The present results with the walk track analysis and the morphometric and histological findings showed that nerve regeneration occurs in all 3 groups. STS repair showed similar regeneration when compared with ETS repair.
ACKNOWLEDGMENT
We thank Pasi Kankaanpää, PhD, for his technical assistance in the morphometric analysis. We are grateful to the laboratory staff of the University of Turku, especially to Mrs. Sinikka Collanus and Mrs. Leena Järvi.
Footnotes
Part of the results of this study has been presented at the following meetings: XIXth Federation of European Societies for Surgery of the Hand Meeting, Paris, France, June 18 to 21, 2014 (abstract and oral presentation): Side-to-Side Repair in Peripheral Nerve Surgery-Histomorphometric Results and World Society for Reconstructive Microsurgery, World Congress Chicago, Ill., July 11 to 14, 2013 (abstract and oral presentation): Side-to-Side Repair in Peripheral Nerve Regeneration. An Experimental Study with Rats.
Disclosure: Dr. Rönkkö has received financial support for this study from the following foundations: Research Foundation of Instrumentarium, Finnish Research Foundation for Orthopaedics and Traumatology, Finnish Society for Surgery of Hand, Medical Research Fund of Tampere University Hospital, and Medical Research Fund of Turku University Hospital. None of the other authors has any financial disclosures. The Article Processing Charge was paid for by Medical Research Fund of Turku University Hospital.
REFERENCES
- 1.Tung TH, Mackinnon SE. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010;35:332–341. doi: 10.1016/j.jhsa.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Oberlin C, Béal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232–237. doi: 10.1016/0363-5023(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.Ballance CA, Ballance HA, Stewart P. Remarks on the operative treatment of chronic facial palsy of peripheral origin. Br Med J. 1903;1:1009–1013. doi: 10.1136/bmj.1.2209.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beris A, Lykissas M, Korompilias A, et al. End-to-side nerve repair in peripheral nerve injury. J Neurotrauma. 2007;24:909–916. doi: 10.1089/neu.2006.0165. [DOI] [PubMed] [Google Scholar]
- 5.Dvali LT, Myckatyn TM. End-to-side nerve repair: review of the literature and clinical indications. Hand Clin. 2008;24:455–460, vii. doi: 10.1016/j.hcl.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Geuna S, Papalia I, Tos P. End-to-side (terminolateral) nerve regeneration: a challenge for neuroscientists coming from an intriguing nerve repair concept. Brain Res Rev. 2006;52:381–388. doi: 10.1016/j.brainresrev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Pannucci C, Myckatyn TM, Mackinnon SE, et al. End-to-side nerve repair: review of the literature. Restor Neurol Neurosci. 2007;25:45–63. [PubMed] [Google Scholar]
- 8.Viterbo F, Amr AH, Stipp EJ, et al. End-to-side neurorrhaphy: past, present, and future. Plast Reconstr Surg. 2009;124(6 Suppl):e351–e358. doi: 10.1097/PRS.0b013e3181bf8471. [DOI] [PubMed] [Google Scholar]
- 9.Artiaco S, Tos P, Conforti LG, et al. Termino-lateral nerve suture in lesions of the digital nerves: clinical experience and literature review. J Hand Surg Eur Vol. 2010;35:109–114. doi: 10.1177/1753193409337959. [DOI] [PubMed] [Google Scholar]
- 10.Battiston B, Artiaco S, Conforti LG, et al. End-to-side nerve suture in traumatic injuries of brachial plexus: review of the literature and personal case series. J Hand Surg Eur Vol. 2009;34:656–659. doi: 10.1177/1753193409104673. [DOI] [PubMed] [Google Scholar]
- 11.Mennen U. End-to-side nerve suture in clinical practice. Hand Surg. 2003;8:33–42. doi: 10.1142/s0218810403001352. [DOI] [PubMed] [Google Scholar]
- 12.Mouilhade F, Barbary S, Apard T, et al. End-to-side neurorrhaphy for median nerve repair after elbow tumor resection: case report. J Hand Surg Am. 2009;34:83–86. doi: 10.1016/j.jhsa.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Yüksel F, Peker F, Celiköz B. Two applications of end-to-side nerve neurorrhaphy in severe upper-extremity nerve injuries. Microsurgery. 2004;24:363–368. doi: 10.1002/micr.20058. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Ji F, Tong D, et al. Side-to-side neurorrhaphy for high-level peripheral nerve injuries. Acta Neurochir (Wien) 2012;154:527–532. doi: 10.1007/s00701-011-1264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cage TA, Simon NG, Bourque S, et al. Dual reinnervation of biceps muscle after side-to-side anastomosis of an intact median nerve and a damaged musculocutaneous nerve. J Neurosurg. 2013;119:929–933. doi: 10.3171/2013.5.JNS122359. [DOI] [PubMed] [Google Scholar]
- 16.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Brown CJ, Mackinnon SE, Evans PJ, et al. Self-evaluation of walking-track measurement using a Sciatic Function Index. Microsurgery. 1989;10:226–235. doi: 10.1002/micr.1920100317. [DOI] [PubMed] [Google Scholar]
- 18.Kankaanpää P, Paavolainen L, Tiitta S, et al. BioImageXD: an open, general-purpose and high-throughput image-processing platform. Nat Methods. 2012;9:683–689. doi: 10.1038/nmeth.2047. [DOI] [PubMed] [Google Scholar]
- 19.Lykissas MG, Korompilias AV, Batistatou AK, et al. Can end-to-side neurorrhaphy bridge large defects? An experimental study in rats. Muscle Nerve. 2007;36:664–671. doi: 10.1002/mus.20861. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Chen LE, Seaber AV, et al. Motor functional and morphological findings following end-to-side neurorrhaphy in the rat model. J Orthop Res. 1999;17:293–300. doi: 10.1002/jor.1100170220. [DOI] [PubMed] [Google Scholar]
- 21.Eren F, Yuksel F, Ulkur E, et al. Nerve regeneration through a healthy nerve trunk: a new and hopeful conduit for bridging nerve defects. Plast Reconstr Surg. 2005;116:1697–1705. doi: 10.1097/01.prs.0000186538.04622.7a. [DOI] [PubMed] [Google Scholar]
- 22.Yüksel F, Karacaoğlu E, Güler MM. Nerve regeneration through side-to-side neurorrhaphy sites in a rat model: a new concept in peripheral nerve surgery. Plast Reconstr Surg. 1999;104:2092–2099. doi: 10.1097/00006534-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Ladak A, Schembri P, Olson J, et al. Side-to-side nerve grafts sustain chronically denervated peripheral nerve pathways during axon regeneration and result in improved functional reinnervation. Neurosurgery. 2011;68:1654–1665. doi: 10.1227/NEU.0b013e31821246a8. [DOI] [PubMed] [Google Scholar]
- 24.Wolthers M, Moldovan M, Binderup T, et al. Comparative electrophysiological, functional, and histological studies of nerve lesions in rats. Microsurgery. 2005;25:508–519. doi: 10.1002/micr.20156. [DOI] [PubMed] [Google Scholar]
- 25.Munro CA, Szalai JP, Mackinnon SE, et al. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21:1095–1097. doi: 10.1002/(sici)1097-4598(199808)21:8<1095::aid-mus20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Kanaya F, Firrell JC, Breidenbach WC. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast Reconstr Surg. 1996;98:1264–1271, discussion 1272. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Ozmen S, Latifoglu O, Ayhan S, et al. Impact of epineurial excision of the distal recipient nerve in terminolateral neurorrhaphy. J Reconstr Microsurg. 2004;20:385–397. doi: 10.1055/s-2004-830002. [DOI] [PubMed] [Google Scholar]
- 28.Cederna PS, Kalliainen LK, Urbanchek MG, et al. “Donor” muscle structure and function after end-to-side neurorrhaphy. Plast Reconstr Surg. 2001;107:789–796. doi: 10.1097/00006534-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Johnson EO, Vekris MD, et al. Long-term evaluation of rabbit peripheral nerve repair with end-to-side neurorrhaphy in rabbits. Microsurgery. 2006;26:262–267. doi: 10.1002/micr.20237. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Soucacos PN, Beris AE, et al. Long-term evaluation of rat peripheral nerve repair with end-to-side neurorrhaphy. J Reconstr Microsurg. 2000;16:303–311. doi: 10.1055/s-2000-7338. [DOI] [PubMed] [Google Scholar]
- 31.Giovanoli P, Koller R, Meuli-Simmen C, et al. Functional and morphometric evaluation of end-to-side neurorrhaphy for muscle reinnervation. Plast Reconstr Surg. 2000;106:383–392. doi: 10.1097/00006534-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Papalia I, Geuna S, Tos PL, et al. Morphologic and functional study of rat median nerve repair by terminolateral neurorrhaphy of the ulnar nerve. J Reconstr Microsurg. 2003;19:257–264. doi: 10.1055/s-2003-40582. [DOI] [PubMed] [Google Scholar]
- 33.Brenner MJ, Moradzadeh A, Myckatyn TM, et al. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28:265–272. doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarasidis G, Watanabe O, Mackinnon SE, et al. End-to-side neurorraphy: a long-term study of neural regeneration in a rat model. Otolaryngol Head Neck Surg. 1998;119:337–341. doi: 10.1016/S0194-5998(98)70074-9. [DOI] [PubMed] [Google Scholar]
- 35.Tarasidis G, Watanabe O, Mackinnon SE, et al. End-to-side neurorrhaphy resulting in limited sensory axonal regeneration in a rat model. Ann Otol Rhinol Laryngol. 1997;106:506–512. doi: 10.1177/000348949710600612. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi A, Pannucci C, Moradzadeh A, et al. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp Neurol. 2008;211:539–550. doi: 10.1016/j.expneurol.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidge KM, Yee A, Moore AM, et al. The supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer for restoring intrinsic function: clinical experience. Plast Reconstr Surg. 2015;136:344e–352e. doi: 10.1097/PRS.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 38.Farber SJ, Glaus SW, Moore AM, et al. Supercharge nerve transfer to enhance motor recovery: a laboratory study. J Hand Surg Am. 2013;38:466–477. doi: 10.1016/j.jhsa.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mersa B, Tiangco DA, Terzis JK. Efficacy of the “baby-sitter” procedure after prolonged denervation. J Reconstr Microsurg. 2000;16:27–35. doi: 10.1055/s-2000-7538. [DOI] [PubMed] [Google Scholar]
- 40.Terzis JK, Tzafetta K. The “babysitter” procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg. 2009;123:865–876. doi: 10.1097/PRS.0b013e31819ba4bb. [DOI] [PubMed] [Google Scholar]
- 41.Placheta E, Wood MD, Lafontaine C, et al. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg. 2015;135:460–471. doi: 10.1097/PRS.0000000000000893. [DOI] [PubMed] [Google Scholar]


