Abstract
Background:
Provision of optimal postoperative analgesia should facilitate postoperative ambulation and rehabilitation. An optimal multimodal analgesia technique would include the use of nonopioid analgesics, including local/regional analgesic techniques such as surgical site local anesthetic infiltration. This article presents a novel approach to surgical site infiltration techniques for abdominal surgery based upon neuroanatomy.
Methods:
Literature searches were conducted for studies reporting the neuroanatomical sources of pain after abdominal surgery. Also, studies identified by preceding search were reviewed for relevant publications and manually retrieved.
Results:
Based on neuroanatomy, an optimal surgical site infiltration technique would consist of systematic, extensive, meticulous administration of local anesthetic into the peritoneum (or preperitoneum), subfascial, and subdermal tissue planes. The volume of local anesthetic would depend on the size of the incision such that 1 to 1.5 mL is injected every 1 to 2 cm of surgical incision per layer. It is best to infiltrate with a 22-gauge, 1.5-inch needle. The needle is inserted approximately 0.5 to 1 cm into the tissue plane, and local anesthetic solution is injected while slowly withdrawing the needle, which should reduce the risk of intravascular injection.
Conclusions:
Meticulous, systematic, and extensive surgical site local anesthetic infiltration in the various tissue planes including the peritoneal, musculofascial, and subdermal tissues, where pain foci originate, provides excellent postoperative pain relief. This approach should be combined with use of other nonopioid analgesics with opioids reserved for rescue. Further well-designed studies are necessary to assess the analgesic efficacy of the proposed infiltration technique.
Enhanced recovery after surgery, which involves implementation of evidence-based multimodal procedure-specific perioperative care pathways, has been shown to improve postoperative outcome and reduce length of hospital stay.1 One of the major elements of a successful program for enhanced recovery after surgery is the provision of optimal postoperative analgesia to facilitate ambulation and rehabilitation therapy.2 An optimal multimodal analgesia technique would include the use of nonopioid analgesics with different mechanisms of action, with the aim of reducing the need for opioids.2 Reduction in opioid requirements should reduce opioid-related adverse events, which have been shown to increase perioperative morbidity and delay ambulation and rehabilitation therapy.2,3 An ideal multimodal analgesic technique would include local/regional analgesic techniques (i.e., neuraxial blocks [epidural and paravertebral analgesia], field blocks [e.g., transversus abdominis plane blocks and rectus sheath block], and surgical site infiltration) combined with acetaminophen and either a nonsteroidal antiinflammatory drug or a cyclooxygenase-2 selective inhibitor and also analgesic adjuncts such as single intraoperative dose dexamethasone.2
This article presents a novel approach to surgical site infiltration techniques for abdominal surgical procedures based upon neuroanatomy.
ORIGIN OF PAIN IN ABDOMINAL SURGERY
The origin of pain from abdominal surgery is multifactorial, including a parietal (or somatic) component originating from the surgical incision and a visceral component originating from the peritoneum and the manipulation of the intraabdominal structures.4 The somatic innervation of the anterior abdominal wall arises from the thoracolumbar spinal nerves (i.e., T6–L1).5 A recent cadaveric study assessing the course of anterior abdominal wall nerves found that there is extensive branching and communications between the abdominal nerves.5 The communications occur at several sites, including the intercostal plexus, anterolateral large branch communication, the transversus abdominis plane plexus that lies between the internal oblique and the transversus abdominis muscles (T9–L1 segmental nerves adjacent to the deep circumflex iliac artery), and the rectus sheath plexus that comprises all the segmental nerves (i.e., T9–L1) adjacent to the deep inferior epigastric artery.6 In most cases, these nerves also pierce the posterior surface of the rectus abdominis muscle.7 The muscular and cutaneous branches of these segmental nerves enter the muscle and finally terminate in the skin. The skin above the umbilicus is supplied by the cutaneous nerves derived from T6 to T9, the area around the umbilicus is innervated by T10, and the skin below the umbilicus is derived from T11, T12, and L1.
It is now evident that the peritoneum is a metabolically active organ and responds to surgical insult by manifesting a local and systemic immunologic and inflammatory response.8,9 The peritoneum consists of “silent nociceptors” that are activated by surgical injury and intraperitoneal inflammation and contribute to visceral pain.8,10 The neuro-immuno-humoral pain pathways involved in abdominal surgery include somatic and autonomic nerves such as the afferent fibers of the abdominal vagus nerve.11 Parasympathetic activation has been shown to influence perioperative outcome, as reduced vagal tone augments inflammation and increases gastrointestinal dysfunction.12,13
RATIONALE FOR RECOMMENDED SURGICAL SITE INFILTRATION TECHNIQUE
Based upon the neuroanatomy described above, an optimal surgical site infiltration technique for the abdominal wall would consist of the administration of local anesthetic into the peritoneal, musculofascial, and subdermal tissue planes. Thus, upon completion of the surgical procedure but before closure of the surgical wound, the first tissue plane that should be infiltrated is the peritoneum (Fig. 1). The importance of the peritoneal component to abdominal pain can be explained by the evidence that intraperitoneal local anesthetic nebulization, which distributes local anesthetic throughout the peritoneal cavity, provides excellent pain relief.14 Similarly, intraperitoneal instillation of local anesthetic provides excellent pain relief.15,16 Furthermore, preperitoneal local anesthetic infusion has been shown to provide excellent pain relief after abdominal surgery.17,18 Studies have reported that peritoneal closure increases postoperative pain, suggesting that the trauma from peritoneal closure induces pain,19 probably similar to that caused by peritoneal incision. The next tissue plane that should be infiltrated, after the closure of the peritoneum, is the musculofascial plane (Fig. 2), as the abdominal nerves run through it. Infiltration of the fascial plane with or without local anesthetic infusion through catheters has been reported to improve pain relief, reduce opioid requirements, and improve postoperative outcome.4,20,21 Finally, the subdermal tissue should be infiltrated (Fig. 3) so as to block the peripheral nerve endings.22,23 Of note, infiltration of the subdermal tissue alone has produced variable results.14,24,25
Fig. 1.
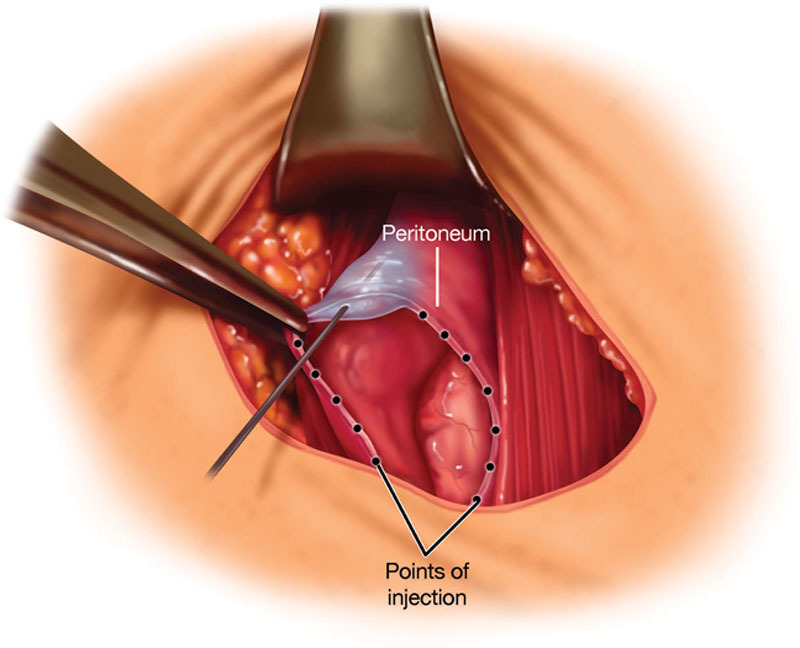
Peritoneal infiltration with local anesthetic solution.
Fig. 2.

Musculofascial infiltration with local anesthetic solution.
Fig. 3.

Subdermal infiltration with local anesthetic solution.
A recent study in women undergoing open abdominal hysterectomy through a horizontal incision found that surgical site infiltration that included peritoneal, musculofascial, and subdermal planes provides superior pain relief compared to bilateral transversus abdominis plane blocks.26 An infiltration technique for hernia surgery would involve local anesthetic infiltration around the neck of the hernia sac (i.e., peritoneal tissue), musculofascial, and subdermal layers.27 Thus, a systematic surgical site infiltration with overlapping areas of coverage may be more effective because of anatomic variation of nerve branching that makes field blocks (e.g., transversus abdominis plane blocks) less reliable in interrupting the pain pathway.
LOCAL ANESTHETICS
The local anesthetic solutions that can be used for postoperative pain control include bupivacaine (maximum dose ~150 mg, varies based on body weight), ropivacaine (maximum dose ~300 mg, varies based on body weight), and liposomal bupivacaine (maximum dose 266 mg). The duration of analgesia achieved with bupivacaine HCl and ropivacaine is typically 6 to 8 hours. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.; San Diego, Calif.) is a sustained-release local anesthetic, approved for administration into the surgical site.28–30 The maximum recommended dose of liposomal bupivacaine is 266 mg (20 mL).28 It can be diluted with preservative-free normal (0.9%) sterile saline up to 300 mL.28 Liposomal bupivacaine should not be admixed with lidocaine, as it displaces bupivacaine from the liposomal formulation, which can lead to bupivacaine toxicity.28,30 Liposomal bupivacaine 20 mL (266 mg) is combined with 30 mL, 0.25% bupivacaine HCl (75 mg) with epinephrine, and saline to achieve the total volume. Although liposomal bupivacaine has 3% bupivacaine HCl, dilution with saline would reduce this amount, and therefore, the addition of bupivacaine HCl to liposomal bupivacaine allows for a faster onset and improved pain relief in the immediate postoperative period (i.e., postanesthesia care unit).
The concerns with local or regional analgesia include the potential for local anesthetic systemic toxicity, wound infection and delayed healing, and myotoxicity.31,32 The likelihood and intensity of potential systemic local analgesic toxicity depend on the cumulative local analgesic dosages administered, the vascularity of the injection site, and the use of additives such as epinephrine.31 Local anesthetics generally have favorable safety profiles, particularly when infiltrated at the surgical site.31 A recent study reported that liposome bupivacaine has a favorable safety profile compared with bupivacaine HCl when administered to dogs via intravascular route.33 Also, liposome bupivacaine given locally at the surgical site infiltration does not have clinically evident impact on wound healing at doses up to 532 mg across different surgical models.34
INFILTRATION TECHNIQUE
It is important to ensure that all layers of the surgical incision are infiltrated under direct visualization in a controlled and meticulous manner. It is best to infiltrate with a 22-gauge, 1.5-inch needle. The needle is inserted approximately 0.5 to 1 cm into the tissue plane (e.g., peritoneal, musculofascial, or subdermal planes), and local anesthetic solution is injected while slowly withdrawing the needle, which should reduce the risk of intravascular injection. Proper infiltration technique involves using a continuous motion fanning technique (commonly referred to as a “moving needle technique”; Fig. 4).
Fig. 4.

Infiltration with moving needle technique to optimize distribution of the local anesthetic solution.
The volume of local anesthetic would depend on the size of the incision. The typical volume for surgical site infiltration would be 1 to 1.5 mL every 1 to 2 cm of surgical incision per layer. Thus, for a transverse (e.g., Pfannenstiel) incision for open abdominal hysterectomy, which is typically 12 to 15 cm long, the total volume could be 60 mL, with 20 mL injected into the peritoneal plane, 20 mL injected into the musculofascial plane, and 20 mL injected into the subdermal plane. For abdominal wall reconstruction using the transversus abdominis release approach, the total volume of the injection is 100 to 150 mL because of larger areas of dissection.
OPTIMAL INFILTRATION TECHNIQUE FOR OPEN ABDOMINAL WALL RECONSTRUCTION SURGERY
Infiltration of local anesthetics for abdominal wall reconstruction using the transversus abdominis release approach also includes infiltration of the peritoneal, musculofascial, and subdermal tissue planes. The first injection plane occurs after the retrorectus dissection reveals the neurovascular bundles at the lateral border of the posterior rectus sheath as they course through the transversus abdominis anteriorly toward the anterior rectus fascia. The injection through the posterior rectus sheath and into the transversus abdominis musculofascial plane and preperitoneal space creates a hydrodissection plane, which often makes separation of the transversus abdominis from the peritoneum much easier. Optionally, after the mesh has been fixated transfascially, another musculofascial plane infiltration can be performed into the area of mesh fixation. At the time of incision closure, a subdermal infiltration is performed before skin closure is completed. Because of the large surface area required to perform infiltration for abdominal wall reconstruction, dilution of the chosen anesthetic may be required.
OPTIMAL INFILTRATION TECHNIQUE FOR LAPAROSCOPIC VENTRAL HERNIA REPAIR SURGERY
The goal of local anesthetic infiltration during laparoscopic ventral hernia repair is to minimize pain at both the laparoscopic port sites and the area of mesh fixation, with or without primary closure of the facial defect. Port-site infiltration may be done before insertion, after insertion, or after port removal. Infiltration after port removal may be technically easier because there is no port to work around; the anesthetic is simply infiltrated under direct vision into the tissues under direct vision. The targets for infiltration include the peritoneum, fascial plane between the internal oblique and transversus abdominis muscle layers, and the subdermal tissue. If the ports are infiltrated during initial entry, the tissue planes are infiltrated for the first port. All secondary port sites are then infiltrated under direct laparoscopic visualization. One to 2 mL of anesthetic is injected per centimeter of skin incision. Also, 5 to 10 mL is infiltrated between the internal oblique and transversus abdominis layers, depending on the port size.
After completion of the laparoscopic ventral hernia repair and placement/fixation of the mesh underlay, the abdominal wall is infiltrated around the entire circumference of the mesh. This typically requires 150 to 300 mL of anesthetic, depending on the area to be infiltrated. A 22-gauge 1½-inch needle is generally used, although obese patients may require spinal needle length. The needle is inserted into the abdominal wall and, under laparoscopic visualization, positioned so the tip is between the transversus abdominis and internal oblique muscle layers. To confirm proper placement, a 1- to 2-mL test dose is infiltrated. If the tip is in the proper layer, a prominent “bulge” should be evident in the transversus abdominis muscle without elevation of the peritoneum. Once tip placement is confirmed, aliquots of 10 to 20 mL are injected. This technique is then repeated, working at 3- to 5-cm intervals around the mesh perimeter of the mesh edge. Care is taken to ensure overlap of the infiltration areas and to minimize needle sticks to the mesh itself.
If the fascia was closed primarily before mesh placement and fixation, the same infiltration technique described above may be used. Infiltration of anesthetic around the perimeter of the mesh effectively creates a field block to the entire anterior abdominal wall, which covers both the fascia closure and mesh fixation sites.
DISCUSSION
The importance of optimal pain management is well known. Nevertheless, treatment of postoperative pain continues to pose challenges, and studies report that inadequate postoperative pain relief remains common.35 Suboptimal postoperative pain management may be related to inadequate or improper use of available analgesic therapies.2 An optimal analgesic technique would block all noxious stimuli that result from surgical insult, including parietal and visceral components.
Previous studies evaluating analgesic efficacy of surgical site infiltration analgesia have reported variable degrees of success,24,25 most likely because of inadequate or improper technique (i.e., indiscriminate subcutaneous injection after the closure of the wound). Infiltration techniques vary from procedure to procedure, requiring knowledge of each surgical site and its anatomy to produce optimal results. Attention to proper infiltration technique is essential to attain the maximum benefits. Systematic and extensive placement of local analgesic into peritoneal, musculofascial, and subdermal tissues, where pain foci originate, is critical. Of note, such a meticulous surgical site infiltration in the various tissue planes requires the use of larger volumes of local anesthetic solution.
In summary, meticulous, systematic, and extensive surgical site local anesthetic infiltration in the various tissue planes including the peritoneal, musculofascial, and subdermal tissues, where pain foci originate, provides excellent postoperative pain relief. This approach should be combined with use of other nonopioid analgesics with opioids reserved for rescue. Further, well-designed studies are necessary to assess the analgesic efficacy of the proposed infiltration technique.
ACKNOWLEDGMENT
The authors thank Deborah Keller, MD, of Colorectal Surgical Associates for her contributions to the development of this consensus recommendation.
Footnotes
Technical editorial assistance was provided, under the direction of the authors, by Synchrony Medical Communications, LLC, West Chester, Pa. Funding for this support was provided by Pacira Pharmaceuticals, Inc.
Disclosures: Dr. Joshi has received honoraria from Pacira Pharmaceuticals, Inc., Mallinckrodt Pharmaceuticals, and Baxter, Inc. Dr. Haas has received unrestricted educational grants and has been a consultant and speaker for Pacira Pharmaceuticals. Dr. Janis is a consultant for LifeCell, receives royalties from Quality Medical Publishing/CRC Press, and has received honoraria from Pacira Pharmaceuticals, KCI, and Bard. Dr. Ramshaw has received honoraria and been a consultant and speaker for Pacira Pharmaceuticals, Surgiquest, Covidien, Ethicon, WL Gore, BG Medical, B Braun, and Novus Scientific. Dr. Nihira has received honoraria from Pacira Pharmaceuticals and the Postsurgical Pain Congress. Dr. Dunkin’s institution has received payment for courses sponsored by Pacira. The Article Processing Charge was paid through an unrestricted grant from Pacira Pharmaceuticals.
REFERENCES
- 1.Gustafsson UO, Scott MJ, Schwenk W, et al. Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 2.Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol. 2014;28:191–201. doi: 10.1016/j.bpa.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Oderda GM, Gan TJ, Johnson BH, et al. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70. doi: 10.3109/15360288.2012.751956. [DOI] [PubMed] [Google Scholar]
- 4.Rackelboom T, Le Strat S, Silvera S, et al. Improving continuous wound infusion effectiveness for postoperative analgesia after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:893–900. doi: 10.1097/AOG.0b013e3181f38ac6. [DOI] [PubMed] [Google Scholar]
- 5.Rozen WM, Tran TM, Ashton MW, et al. Refining the course of the thoracolumbar nerves: a new understanding of the innervation of the anterior abdominal wall. Clin Anat. 2008;21:325–333. doi: 10.1002/ca.20621. [DOI] [PubMed] [Google Scholar]
- 6.McLoughlin TM. Advances in Anesthesia. vol 29. Philadelphia, PA: Elsevier Health Sciences; 2011. [Google Scholar]
- 7.Tank PW. Grant’s Dissector. 15th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2012. [Google Scholar]
- 8.Sammour T, Kahokehr A, Soop M, et al. Peritoneal damage: the inflammatory response and clinical implications of the neuro-immuno-humoral axis. World J Surg. 2010;34:704–720. doi: 10.1007/s00268-009-0382-y. [DOI] [PubMed] [Google Scholar]
- 9.Paddison JS, Booth RJ, Fuchs D, et al. Peritoneal inflammation and fatigue experiences following colorectal surgery: a pilot study. Psychoneuroendocrinology. 2008;33:446–454. doi: 10.1016/j.psyneuen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 11.Mirakaj V, Dalli J, Granja T, et al. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211:1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubbers T, Buurman W, Luyer M. Controlling postoperative ileus by vagal activation. World J Gastroenterol. 2010;16:1683–1687. doi: 10.3748/wjg.v16.i14.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingelmo PM, Bucciero M, Somaini M, et al. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: a double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2013;110:800–806. doi: 10.1093/bja/aes495. [DOI] [PubMed] [Google Scholar]
- 15.Perniola A, Fant F, Magnuson A, et al. Postoperative pain after abdominal hysterectomy: a randomized, double-blind, controlled trial comparing continuous infusion vs patient-controlled intraperitoneal injection of local anaesthetic. Br J Anaesth. 2014;112:328–336. doi: 10.1093/bja/aet345. [DOI] [PubMed] [Google Scholar]
- 16.Perniola A, Magnuson A, Axelsson K, et al. Intraperitoneal local anesthetics have predominant local analgesic effect: a randomized, double-blind study. Anesthesiology. 2014;121:352–361. doi: 10.1097/ALN.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 17.Beaussier M, El’Ayoubi H, Schiffer E, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double-blind, placebo-controlled study. Anesthesiology. 2007;107:461–468. doi: 10.1097/01.anes.0000278903.91986.19. [DOI] [PubMed] [Google Scholar]
- 18.Bertoglio S, Fabiani F, Negri PD, et al. The postoperative analgesic efficacy of preperitoneal continuous wound infusion compared to epidural continuous infusion with local anesthetics after colorectal cancer surgery: a randomized controlled multicenter study. Anesth Analg. 2012;115:1442–1450. doi: 10.1213/ANE.0b013e31826b4694. [DOI] [PubMed] [Google Scholar]
- 19.Basarkod S, Ambi US, Ganeshnavar A, et al. Post-operative analgesic requirement in non-closure and closure of peritoneum during open appendectomy: a randomized controlled study. J Clin Diagn Res. 2012;6:264–266. [Google Scholar]
- 20.Liu SS, Richman JM, Thirlby RC, et al. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–932. doi: 10.1016/j.jamcollsurg.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Ventham NT, Hughes M, O’Neill S, et al. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg. 2013;100:1280–1289. doi: 10.1002/bjs.9204. [DOI] [PubMed] [Google Scholar]
- 22.Dahl JB, Møiniche S. Relief of postoperative pain by local anaesthetic infiltration: efficacy for major abdominal and orthopedic surgery. Pain. 2009;143:7–11. doi: 10.1016/j.pain.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Loizides S, Gurusamy KS, Nagendran M, et al. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;3:CD007049. doi: 10.1002/14651858.CD007049.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skjelsager A, Ruhnau B, Kistorp TK, et al. Transversus abdominis plane block or subcutaneous wound infiltration after open radical prostatectomy: a randomized study. Acta Anaesthesiol Scand. 2013;57:502–508. doi: 10.1111/aas.12080. [DOI] [PubMed] [Google Scholar]
- 25.Petersen PL, Mathiesen O, Stjernholm P, et al. The effect of transversus abdominis plane block or local anaesthetic infiltration in inguinal hernia repair: a randomised clinical trial. Eur J Anaesthesiol. 2013;30:415–421. doi: 10.1097/EJA.0b013e32835fc86f. [DOI] [PubMed] [Google Scholar]
- 26.Gasanova I, Alexander J, Ogunnaike B, et al. Transversus abdominis plane block versus surgical site infiltration for pain management after open total abdominal hysterectomy. Anesth Analg. 2015;121:1383–1388. doi: 10.1213/ANE.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 27.Joshi GP, Rawal N, Kehlet H, et al. PROSPECT collaboration. Evidence-based management of postoperative pain in adults undergoing open inguinal hernia surgery. Br J Surg. 2012;99:168–185. doi: 10.1002/bjs.7660. [DOI] [PubMed] [Google Scholar]
- 28.Exparel (bupivacaine liposome injectable suspension) [package insert] San Diego, CA: Pacira Pharmaceuticals, Inc.; 2014. [Google Scholar]
- 29.Hu D, Onel E, Singla N, et al. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33:109–115. doi: 10.1007/s40261-012-0043-z. [DOI] [PubMed] [Google Scholar]
- 30.Uskova A, O’Connor JE. Liposomal bupivacaine for regional anesthesia. Curr Opin Anaesthesiol. 2015;28:593–597. doi: 10.1097/ACO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 31.Neal JM, Bernards CM, Butterworth JF, IV, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–161. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- 32.Brower MC, Johnson ME. Adverse effects of local anesthetic infiltration on wound healing. Reg Anesth Pain Med. 2003;28:233–240. doi: 10.1053/rapm.2003.50050. [DOI] [PubMed] [Google Scholar]
- 33.Joshi GP, Patou G, Kharitonov V. The safety of liposome bupivacaine following various routes of administration in animals. J Pain Res. 2015;8:781–789. doi: 10.2147/JPR.S85424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxter R, Bramlett K, Onel E, et al. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35:312–320.e5. doi: 10.1016/j.clinthera.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]


