Abstract
Background:
A single-center study assessing the efficacy of Nagor’s Silgel STC-SE silicone gel to reduce the appearance of hypertrophic and keloid scars.
Methods:
A 16-week controlled study of 36 patients with hypertrophic or keloid scars. The subjects were divided between 2 cohorts: one assessing recently healed scars (<6 mo) and other assessing older scars (6 mo to 2 y). The efficacy of Silgel STC-SE on the scar was evaluated by skin hydration, skin moisture evaporation, skin elasticity, basic scar measurements, subjective patient questionnaire data, and image analysis. All subjects had data collected at baseline and weeks 1, 4, 8, 12, and 16. Photographs were taken for image analysis at baseline, week 8, and week 16. Statistical analysis was conducted on all data.
Results:
Twenty-nine patients completed the study (27 presented with hypertrophic scars and 2 with keloid scars), and 90% reported a marked improvement in their scar appearance. Patient questionnaire data showed great satisfaction with the product. Image analysis showed visual improvement with a statistically significant reduction of the “red” color of scars. Overall, scar dimensions were significantly reduced. There was a significant decrease from baseline levels in average scar length. Skin elasticity, skin hydration, and skin moisture evaporation did not change significantly from baseline.
Conclusions:
The results of this study indicate that Silgel STC-SE is an effective treatment in reducing the appearance and red color of hypertrophic scars up to 2 years old. Further study is required to draw significant conclusion in regard to the treatment of keloid scars.
An epidemiologic study by Kantar Health (2009) estimated that there were a total of 39.2 million potential scar-producing procedures performed in the United States during 2007, and this is estimated to increase to 44.4 million potential scar-producing procedures in 2020.1 With this increasing number of procedures, treatment of postsurgical hypertrophic and keloid scars is becoming more critical.
Scars can have a major consequence on a patients’ overall well-being, appearance, and satisfaction with a surgery especially when the scars are in sensitive places on the body or cannot be covered by clothes.2 Treatment of scars can be potentially life changing for a patient and relieve a painful burden, which patients may have been carrying for years.3 The increase in the number of cosmetic surgeries has resulted in the visual appearance of the final result of the surgery, growing in importance to both surgeons and patients.2
A key identifying feature of hypertrophic and keloid scars is that both types of scars are fibro-proliferative disorders, where there is excess healing and wound repair. In hypertrophic and keloid scars, there is an absent or poorly functioning repair process that results in excess scar tissue production.3 Hypertrophic scars are those localized to the original wound parameters. A keloid scar is characterized by excess production of scar tissue, which grows as a tumor of scar tissue, exceeding initial scar boundaries. This type of scar has a genetic predisposition in some individuals with autosomal-dominant features.3
According to Kwon et al, 2014, topical gel is the first-line treatment for hypertrophic and keloid scars as it is noninvasive and has shown to be clinically effective in many randomized, controlled studies.2,4–9
de Giorgi et al2 (2009) and Tandara and Mustoe10 (2008) found that early use of the product can interrupt and influence the healing process. Chan et al5 (2005) also found that intervention of wound healing and epithelization is critical in the early phase of healing. Although the mechanism of silicone gel is unknown, there are many hypotheses that have been investigated throughout the literature. One of these mechanisms, proposed by de Giorgi et al (2015), is that it forms a thin film membrane over the skin because of its lack of absorption and cross-linking properties, which acts as a barrier to protect the scar and enable hydration of the scar. Increased hydration of the scar inhibits the proliferation of fibroblasts, a key mediator in scar formation, and in turn inhibits the fibroblasts’ ability to deposit collagen.2 The study conducted by McCauley et al11 (1990) proved that human skin cultures with fibroblasts that were treated with silicone gel showed a decrease in fibroblast proliferation.
Gallant-Behm and Mustoe, 2010, present a similar hypothesis called occlusion. This hypothesis states that there is increased hydration of the scar resulting in cellular effects.12
This study looks to provide an effective means of scar treatment and satisfy the needs of individual patients, providing the best, personal aesthetic outcome of their scars.
METHODS
A single-center study was conducted to evaluate the effects of Silgel (Nagor, United Kingdom), a polysiloxane silicone gel, on hypertrophic and keloid scars. Silgel will be used throughout this article in place if Silgel STC-SE and refers to the study device; when discussing other brands, they will be referred to as the generic, silicone gel. Investigation was performed over a 16-week period by Alba Science Ltd. to provide efficacy data and support claims for the reduction of keloid and hypertrophic scarring appearance within human subjects.
Silgel is a clear, nonsticky, silicone gel that is applied to a closed wound. Silgel is a Class I, CE-marked medical device. The product is 100% polysiloxane and is supplied in a 20-ml tube.
Patients were divided into 2 groups: Those with recent scars, less than 6 months old, and those with older scars, 6 months to 2 years old. A basic outline of the protocol is illustrated in Figure 1. All patients were examined by the same physician to assess the suitability of the scar to be treated. Classification (hypertrophic or keloid) was completed by visual inspection to assess the level of scar growth beyond the original wound site. Patients were prescribed Silgel and instructed to apply a very small amount of the silicone gel to their scar twice daily for 16 weeks. Patients were requested not to use any other cosmetic products on the scarred area or to expose the scarred area to excess ultraviolet light (tanning or sunbathing). Patients were informed to only use the product on clean, intact skin and not to apply the product on the days that they had follow-up visits at the research center. To assess the efficacy of Silgel, various quantifiable parameters were utilized including scar size/visual assessment, skin elasticity, skin hydration, skin moisture evaporation, image analysis/scar color, and subject perception data. All patients visited the research center (Edinburgh, United Kingdom) for their baseline visit and subsequent visits at weeks 1, 4, 8, 12, and 16. At baseline, week 8, and week 16 visits, macro photographs were taken by a trained expert. At each follow-up visit, the same trained nurse collected 3 sets of Corneometer readings, Dermalab TEWL measurements, Dermalab elasticity meter measurements, basic scar measurements (length, width, and height), and subjective patient questionnaire data.
Fig. 1.
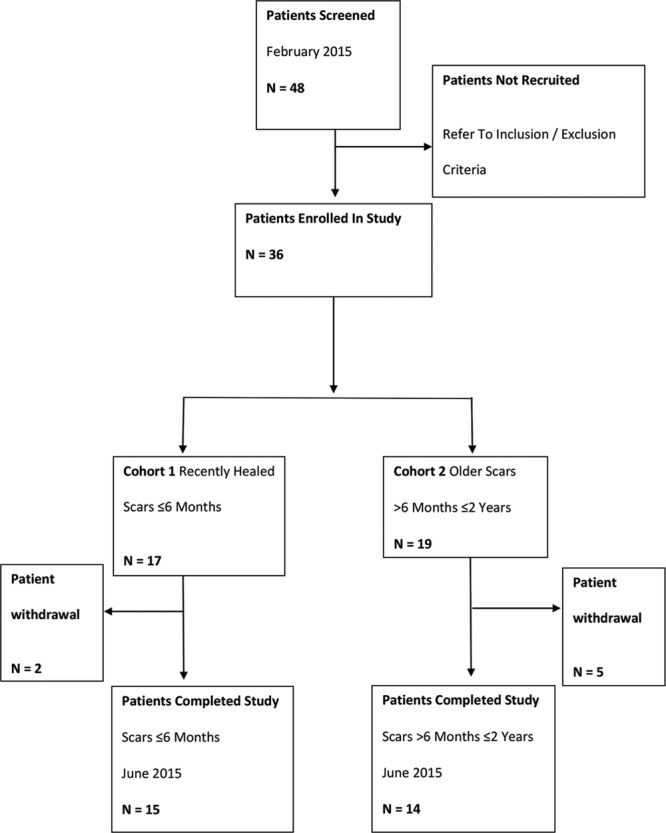
Flow chart illustrating the study protocol.
The patient inclusion criteria were patients in good general health, age 16 to 60 years old with the presence of a keloid or hypertrophic scar no more than 2 years old, no open wounds, and no unhealed tissue or infections. Included scar types were those resulting from plastic surgery procedures including breast surgery; general surgical procedures including C-section, trauma wounds, and burns; trivial injuries such as insect bites, ear piercing, or vaccinations.
The patient exclusion criteria were patients with known allergies or sensitivities to silicone and those with active skin conditions or other skin disorders, such as eczema or psoriasis. Patients were also excluded if they were taking regular medication that may influence the response of the skin, such as antiinflammatory medications or antihistamines. Patients were screened to ensure that they had not participated in a similar study within the last month before recruitment. Female patients were excluded if they were pregnant or at risk of being pregnant.
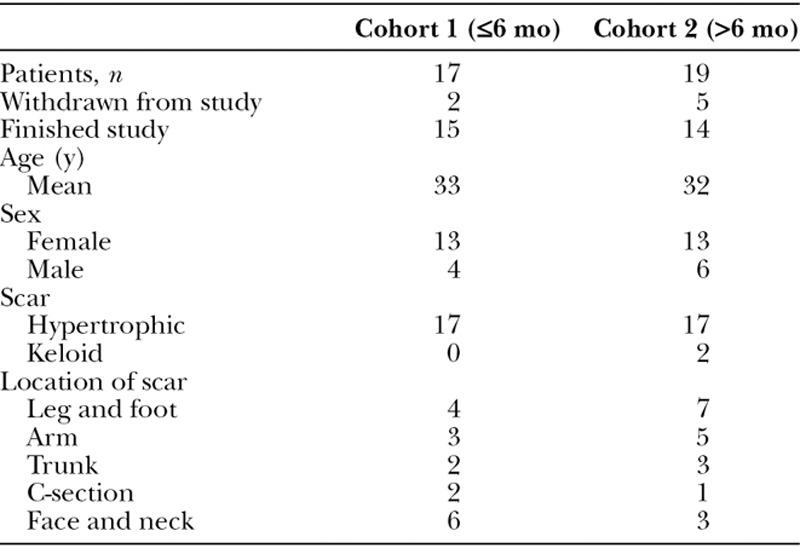
Forty-eight potential patients were screened with 12 subjects failing the initial assessment as they did not meet the inclusion criteria. A description of patient demographics is presented in Table 1. Seven subjects were withdrawn during the course of the study. Three subjects were withdrawn because of not being able to attend test visits, 3 were withdrawn for missing scheduled visits, and 1 was withdrawn because the patient developed active eczema.
Table 1.
Subject and Scar Characteristics
The study physician assessed each scar at baseline and visually identified the scar as hypertrophic or keloid. Scar measurements were taken to determine any change in the size of the scar after treatment. Corneometer readings were used to measure skin hydration. A cortex Dermalab TEWL was used to measure changes in water content of skin. A Dermalab Elasticity meter was used to measure skin elasticity. Skin elasticity measurements consist of 3 values, the necessary force needed to lift the skin a certain distance using negative pressure (e), the retraction time or stiffness (r), and the viscoelasticity over time, which is calculated from both e and r. Macro photography using a Nikon D7100 camera and standardized lighting and positioning was performed. Color assessments (L*a*b) were performed on the resulting photographs (L*a*b analysis is as follows: L, white; a, red; and b, yellow/brown spectrum).
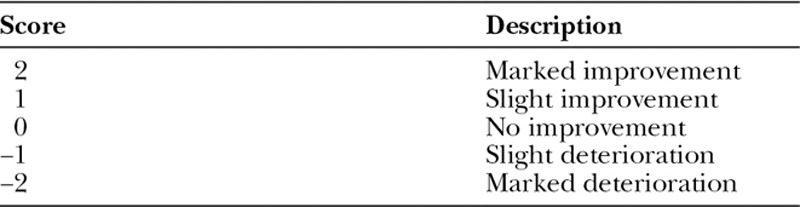
Patient opinion for the overall change in appearance of scars was gathered using a Patient Scoring Scale shown in Table 2. It was used subjectively to grade their scar changes from baseline and to provide opinions of the product and changes observed during treatment.
Table 2.
Visual Analog Scale for Changes in Overall Appearance from the Baseline
Adverse events, as outlined by the study protocol, were directed immediately to the study sponsor. Any compliance issues with patients were recorded throughout the study period.
The study protocol, including informed consent, was reviewed and approved by an independent ethics committee. The study protocol is consistent with the Principles of Good Clinical Practice and was conducted in accordance with the Declaration of Helsinki.
Data were recorded onto an electronic case report form throughout the study. Statistical significance was determined using a 5% type 1 error rate (2-tailed tests) along with the associated 95% confidence intervals.
RESULTS
Scars were analyzed to assess changes in scar size, color and skin conditions, and patients satisfaction after using Silgel over a relatively short time. Patients were categorized into 3 groups for analysis: group 1 included all subjects in the study, which allows examination of all treated scars up to 2 years old; group 2 included subjects who had scars 6 months or younger, to identify trends specific to early use of the test product; and group 3 included subjects with scars over 6 months and up to 2 years old, to identify trends specific to older scars. Mean values for each of the measurements were evaluated for all 3 groups.
Scar metrics were measured weekly for height, length, and width. Changes in the scar metrics can be seen in Figure 2. There is an observable decrease in average scar width across the 3 groups; this was not validated statistically. Scar height showed a slight decrease in size; however, this also was not validated by statistical significance. The average length was observed to decrease from around week 4 after treatment with Silgel. A statistically significant change in mean scar length was found from baseline at weeks 4 to 16 for group 1 (P = 0.0455, P = 0.0016, P = 0.0009, P = 0.0005, weeks 4–16, respectively), week 8 for group 2 only (P = 0.0429), and weeks 8 to 16 for group 3 (P = 0.0131, P = 0.0035, P = 0.0016, weeks 8–16, respectively). This statistical validation indicates a significant decrease in mean scar length, which corresponds to the observational indications.
Fig. 2.
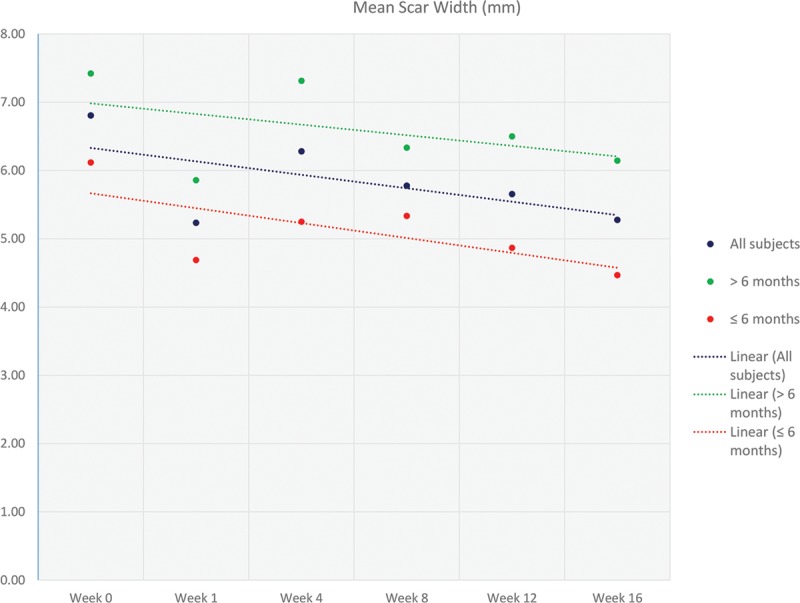
Graph illustrating the mean change in scar width (mm) at the various time points (0, 1, 4, 8, 12, and 16 wk). Linear trend lines were included to more clearly illustrate the data. The data are represented as 3 groups: all subjects, <6 months, and >6 months.
There was no observable difference in skin hydration for the patient groups; slight variations were observed between younger and older scars; however, they were not statistically significant. There were statistically significant increases in hydration at various time points: all groups at week 1 (P = 0.0002, P = 0.0435, P = 0.0007, respectively) and for groups 1 and 3 at week 8 (P = 0.0102 and P = 0.0077, respectively); however, it did not increase with time but rather was observed at random time points.
Skin moisture evaporation showed very little observable change; it fluctuated between all 3 groups. A statistically significant increase in moisture evaporation was found from baseline at week 1 for groups 1 and 3 (P = 0.0494 and P = 0.0261, respectively) indicating a slight deterioration in skin barrier condition after immediate use, which was not detected at any other time point. There was also a statistically significant decrease in moisture evaporation detected from baseline at week 8 for groups 1 and 2 (P = 0.0225 and P = 0.0398, respectively) indicating an improvement in skin barrier, also not detected at any other time point. The initial decrease at week 1 followed by increase at week 8 are likely just fluctuations and are not indicative of a significant overall increase or decrease.
There is an observable although small decrease in skin viscoelasticity. No statistically significant different changes in elasticity were detected at any time point.
High-resolution photographs were taken at baseline, week 8, and week 16 and showed great visual improvement as shown in Figure 3. Analysis of the change in scar color indicates an average increase in the white color (L) of the scar and an average decrease in the red color (a) of the scar over the 16 weeks for all groups and a general overall color change (ΔE). A graph representing the change in redness is shown in Figure 4.
Fig. 3.
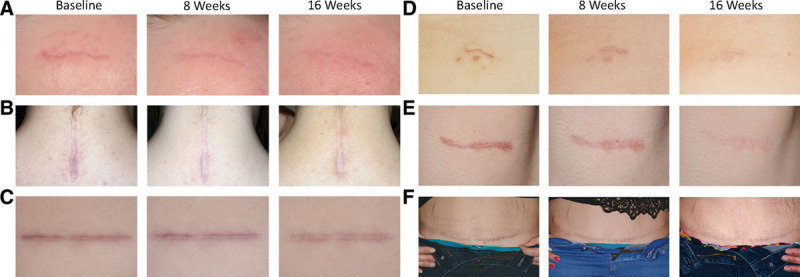
High-resolution photographs of 6 patient’s scars at 0, 8, and 16 weeks with Silgel treatment. A, Hypertrophic scar on the right forehead, (B) hypertrophic scar on posterior neck, (C) hypertrophic scar on right upper abdomen, (D) hypertrophic scar on midabdomen, (E) hypertrophic scar on right neck, (F) hypertrophic scar on lower abdomen (C-section).
Fig. 4.
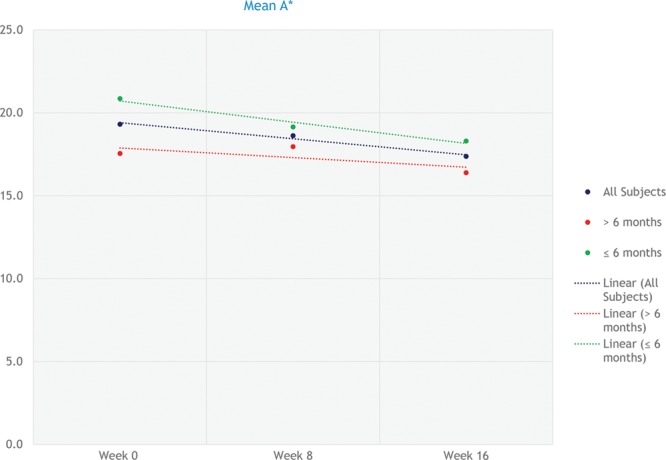
Graph illustrating the mean change in a* at the various time points (0, 1, 4, 8, 12, and 16 wk). Linear trend lines were included to more clearly illustrate the data. The data are represented as 3 groups: all subjects, <6 months, and >6 months.
Statistical analysis showed that there was a statistically significant difference for group 1 for L* for weeks 8 and 16 (P = 0.0000 for both), a* for weeks 8 and 16 (P = 0.0409 and P = 0.0004, respectively), and ΔE for weeks 8 and 16 (P = 0.0000 for both). There was a statistically significant difference for group 2 for L* for weeks 8 and 16 (P = 0.0008 and P = 0.0000, respectively), a* for weeks 8 and 16 (P = 0.0376 and P = 0.0069, respectively), and ΔE for weeks 8 and 16 (P = 0.0000 for both). There was a statistically significant difference for group 3 for L* for weeks 8 and 16 (P = 0.0068 and P = 0.0000, respectively), a* for week 16 (P = 0.0254), and ΔE for weeks 8 and 16 (P = 0.0002 and P = 0.0000, respectively). The statistical analysis confirms the observed improvement in the scars and lends credence to the scars becoming more white or lighter over time.
The subjective assessment of the patient’s scars at weeks 1, 8, and 16 is listed in Table 3. Feedback showed improvement over all categories, many of which were significant. The surveys demonstrated that by week 16, 93% of patients reported to like Silgel and 86% of patients reported that the product reduced the redness of their scar. Ninety-three percent of patients would recommend Silgel, and 86% of patients agreed that Silgel helped fade the appearance of their scar.
Table 3.
Visual Analog Scale for Change in Overall Appearance of Scar from Baseline

Subject opinion of scar improvement showed that there was a gradual improvement in the overall scar appearance over 16 weeks for all groups. Analysis showed that there was a statistically significant difference at each visit from week 4 to 16 for all 3 groups, indicating that there was no deterioration in scar appearance. A sampling of the survey is shown in Figure 5 and illustrates the marked improvement from week 1 to week 16.
Fig. 5.
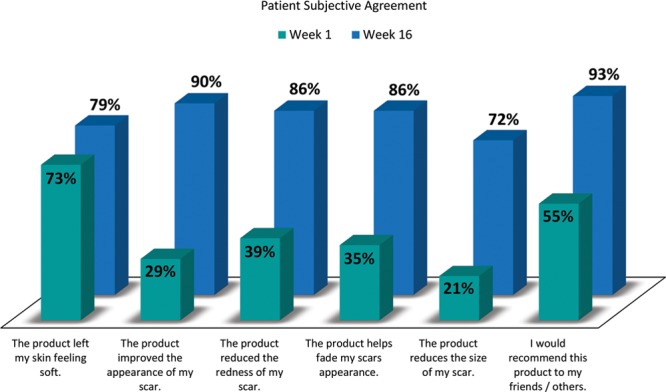
Patients (as a percentage) agreed with the listed statements in the subjective questionnaire. Note: Remaining percentage of patients were “neither agreed or disagreed.”
Patients also reported that Silgel increased the softness of their skin, was easy to apply, dried easily on the skin, reduced the intensity of color of the scar, and reduced the height of the scar.
DISCUSSION
Scar treatment is an important field of study in which there is not a significant number of proven effective treatments.13 The active ingredient in Silgel, polysiloxane, has been proven effective by de Giorgi et al in 2009, Kwon et al in 2014, and many other reputable studies.2,4–10,14 Although the mechanism of action of silicone gel is not fully understood, it has been indicated that it acts as a barrier on the skin.10 Skin hydration, skin moisture evaporation, and skin elasticity were monitored to support this claim, and image analysis was conducted using high-resolution photographs to determine any aesthetic improvement. As scars can have significant psychological impact on a patient’s well-being; patient satisfaction with the product was monitored throughout the study.15
This study was initially implemented to assess the treatment of both hypertrophic and keloid scars using Silgel. However, only 2 patients presented with keloid scars, thus making any significant conclusions about the treatment of this type of scar difficult to substantiate.
In addition, keloid scars can take 3 months to develop after wound healing and can continue to grow for up to 1 year. Therefore, for the purposes of this study and the assessment of the effectiveness of Silgel to treat scars, conclusions can be drawn regarding the treatment of hypertrophic scars only.
The overall size of scars showed reduction after treatment with Silgel; the length of the scars showed a statistically significant decrease. The observed time until a statistically significant decrease varied between younger and older scars; younger scars showed reduction in length after 4 weeks, whereas older scars showed a reduction after 8 weeks. This corresponds with other studies (Chan et al 2005) that demonstrate the effect of silicone gel on reducing the size of scars.5
Skin hydration, skin moisture evaporation, and skin elasticity were assessed to determine whether a “barrier” effect was observed at the scar location after application of Silgel. The semiocclusive properties of silicone gel are thought to allow water to evaporate but still act to regenerate the protective barrier function of the epidermis.10 Initial increases in skin moisture evaporation from baseline levels were observed; however, after week 8, there was a significant decrease in water evaporation. Further study is required to support the hypothesis proposed by Tandara and Mustoe10, 2008, that silicone gel treatment impedes water loss. There was no statistical change in skin hydration during the study that does not prove the occlusive barrier hypothesis proposed by Beckenstein et al16 2004 and Gallant-Behm and Mustoe12 2010. Elasticity fluctuated between increasing and decreasing values, and no statistical significance was found, thus indicating that there was no change in the overall elasticity of the scar site after treatment with Silgel. Fluctuations are likely due to the inaccessibility of some of the scars for accurate measuring. The results for these 3 parameters did not show a vast improvement or decline indicating that it maintained the skin barrier, and neither caused an increase or decrease in skin conditions.
There was a statistically significant improvement for image analysis in all 3 categories across all 3 groups, indicating that the treatment of hypertrophic scars will decrease the red color and increase the white color of scars over a 16-week period.
Subjects indicated an improvement in overall scar appearance from week 1, and statistical significance was observed for weeks 4 to 16 across all 3 groups. This percentage improved significantly from week 1 to 16 for questions regarding the appearance, color, and fading of their scars. An advantage of silicone gel treatment, also demonstrated by previous studies, was the easy application and compliance with the product.6 Overall acceptance of Silgel increased from 74% at week 1 to 93% at week 16, indicating a high margin of acceptance for Silgel as an effective scar treatment.
The combination of different parameters allowed for a thorough clinical observation of the efficacy of Silgel as a scar treatment for hypertrophic scars. A noticeable decrease in the length of the scars, improvement in the color, and a high level of patient satisfaction indicate that Silgel is an effective product and can work to improve the overall appearance of scars. Further study would be required to determine whether there is an effective “barrier” effect as a result of treatment with Silgel. The timing of the scar was also shown to be statistically important. Newer scars (<6 mo), as opposed to older scars (6 mo to 2 y), were more sensitive to Silgel treatment, making the product more effective. Silgel has proved to be an effective method to improve the appearance of hypertrophic scars.
Footnotes
Supported by Global Consolidated Aesthetics (registered in Ireland with number 450181), which trades as GC Aesthetics. Global Consolidated Aesthetics is the parent company of Nagor Limited, the manufacturer of the study device. The authors of this article are all paid employees of Global Consolidated Aesthetics or its subsidiaries.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by GC Aesthetics.
REFERENCES
- 1.Kantar Health. Epidemiology of the U.S. Scar Market: A Report Prepared for Scarguard. 2009.
- 2.de Giorgi V, Sestini S, Mannone F, et al. The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clin Exp Dermatol. 2009;34:688–693. doi: 10.1111/j.1365-2230.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz P, Barri AS. Plastic surgery: principles. In: Peter CN, editor. In: Scar Prevention Treatment and Revision. Elsevier Health Sciences; 2012. [Google Scholar]
- 4.Kwon SY, Park SD, Park K. Comparative effect of topical silicone gel and topical tretinoin cream for the prevention of hypertrophic scar and keloid formation and the improvement of scars. J Eur Acad Dermatol Venereol. 2014;28:1025–1033. doi: 10.1111/jdv.12242. [DOI] [PubMed] [Google Scholar]
- 5.Chan KY, Lau CL, Adeeb SM, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005;116:1013–1020. doi: 10.1097/01.prs.0000178397.05852.ce. discussion 1021. [DOI] [PubMed] [Google Scholar]
- 6.Chernoff WG, Cramer H, Su-Huang S. The efficacy of topical silicone gel elastomers in the treatment of hypertrophic scars, keloid scars, and post-laser exfoliation erythema. Aesthetic Plast Surg. 2007;31:495–500. doi: 10.1007/s00266-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 7.Hom DB, Hom KA. Do topical products reduce postincision scars? Laryngoscope. 2015;125:282–283. doi: 10.1002/lary.24768. [DOI] [PubMed] [Google Scholar]
- 8.Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Surg. 2009;2:104–106. doi: 10.4103/0974-2077.58527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scuderi N, Dessy LA, Buccheri EM, et al. Phase 2 cross-over multicenter trial on the efficacy and safety of topical cyanoacrylates compared with topical silicone gel in the prevention of pathologic scars. Aesthetic Plast Surg. 2011;35:373–381. doi: 10.1007/s00266-010-9621-8. [DOI] [PubMed] [Google Scholar]
- 10.Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61:1219–1225. doi: 10.1016/j.bjps.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 11.McCauley RL, Riley WB, Jr, Juliano RA, et al. In vitro alterations in human fibroblast behavior secondary to silicone polymers. J Surg Res. 1990;49:103–109. doi: 10.1016/0022-4804(90)90118-l. [DOI] [PubMed] [Google Scholar]
- 12.Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen. 2010;18:235–244. doi: 10.1111/j.1524-475x.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih R, Waltzman J, Evans GR Plastic Surgery Educational Foundation Technology Assessment Committee. Review of over-the-counter topical scar treatment products. Plast Reconstr Surg. 2007;119:1091–1095. doi: 10.1097/01.prs.0000255814.75012.35. [DOI] [PubMed] [Google Scholar]
- 14.Pantlen P, Tilkorn H, Schwipper V. Clinical experience with Silgel™ topical cream and Silgel™ topical gel, containing polysiloxane in the treatment of hypertrophic scars and their prophylaxis. FRG: Department of Plastic and Maxillofacial Surgery, Fachklinik Hornheide, University of Muenster; 2000. [Google Scholar]
- 15.Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245–272. doi: 10.2165/00128071-200304040-00004. [DOI] [PubMed] [Google Scholar]
- 16.Beckenstein MS, Kuniaki T, Matarasso A. The effect of Scarguard on collagenase levels using a full-thickness epidermal model. Aesthet Surg J. 2004;24:542–546. doi: 10.1016/j.asj.2004.08.005. [DOI] [PubMed] [Google Scholar]


