Abstract
Nuclear factor kappa B (NF-κB) is a potent transcription factor highly expressed in the central nervous system (CNS) where it has been shown to be required for multiple behavioral paradigms of learning and memory in both mammalian and invertebrate systems. NF-κB dimers are found in neuronal cell bodies, are also present at synapses, and can participate in the activity-dependent regulation of gene expression in response to excitatory neurotransmission. Multiple serine-directed phosphorylation events are critical in the canonical NF-κB activation pathway, including activation of the IκB kinase complex (IKK) and phosphorylation and degradation of the inhibitor of NF-κB (IκB). In this chapter, we describe methods for immunoprecipitation (IP) of the IKK complex from dissociated cultured murine hippocampal neurons, followed by in vitro kinase assay to evaluate excitatory neurotransmission-induced IKK activation by monitoring phosphorylation of a GST-IκBα substrate. These methods can also be successfully implemented in subcellular-reduced brain preparations, such as biochemically isolated synapses.
Keywords: IKK in vitro kinase assay, GST-IκBα, Murine neurons, Excitatory neurotransmission, Synaptic activity
1 Introduction
The nuclear factor kappa B (NF-κB) family of transcription factors was initially characterized as key regulators of genes involved in both innate and adaptive immune responses [1–5]. Dysregulated NF-κB signaling has been linked to cancer and inflammatory and autoimmune disorders [6–9]. Over the past decade, research from multiple laboratories, including our own, has revealed a conserved role for NF-κB in the regulation of synaptic plasticity, learning, and memory [10–21]. While NF-κB is ubiquitously expressed in all tested cell types of the central nervous system (CNS), previous work has demonstrated that NF-κB is present at neuronal synapses and can mediate synaptic activity-dependent regulation of gene expression in response to excitatory neurotransmission [17, 22, 23]. Synaptic input can alter the complement of expressed genes and represents a critical pathway employed by the nervous system to affect the enduring adaptation required in development, differentiation, and plasticity. Neuronal NF-κB is classified as an activity-dependent transcription factor that can be induced by multiple stimuli, including excitatory neurotransmitters [17, 24, 25], cytokines (e.g., tumor necrosis factor alpha (TNFα)) [5, 26], and growth factors (BDNF, NGF) [27, 28]. In addition to these physiological activators, NF-κB has also been shown to become activated during brain injury, ischemia, and oxidative stress.
The mammalian NF-κB family (also known as the Rel family) consists of five members which can hetero- and homo-dimerize: p50 (product of the NF-κB1 gene), p52 (product of the NF-κB2 gene), p65 (also known as Rel A), c-Rel, and RelB [29]. The expression of all NF-κB family members has been reported in brain tissue, although the predominant neuronal dimers under basal conditions appear to be p65:p50 and p50:p50 [17, 24, 30]. All Rel family members contain a conserved Rel homology domain that includes an immunoglobulin-fold DNA-binding domain, a dimerization domain, and a nuclear localization sequence (NLS). RelA, RelB, and c-Rel have a transactivation domain (TAD) in their carboxyl-termini.
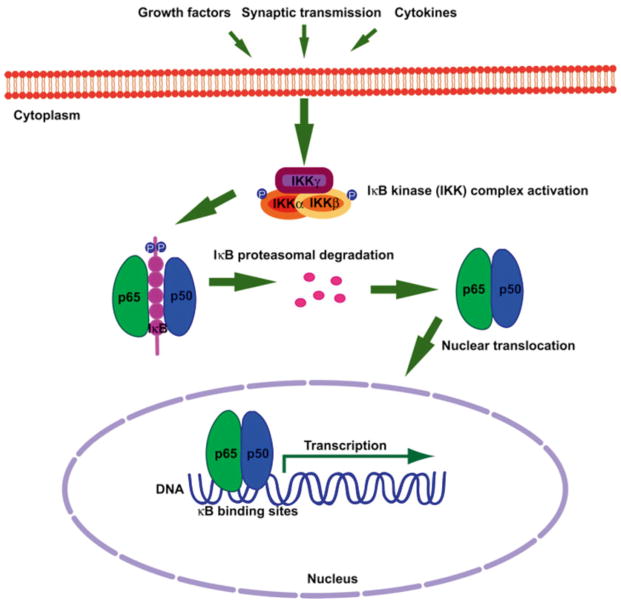
Dimers of NF-κB are held latent in the cytoplasm by non-covalent interactions with a class of inhibitors called inhibitor of NF-κB (IκB) proteins. Canonical pathway activation of NF-κB is mediated by the IκB kinase complex (IKK), which is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ. IKK phosphorylates the inhibitor of kappa B protein (IκB) and leads to its subsequent ubiquitination and degradation by the 26S proteasome [31]. IκB degradation exposes the DNA-binding domain and NLS of NF-κB and permits stable translocation to the nucleus (Fig. 1) followed by binding to consensus κB-binding sites of target genes and the regulation of gene expression. While IKK activation is well characterized as a pathway mediating NF-κB activation, it should be noted that at least one IKK subunit (IKKα) has also been demonstrated to regulate gene expression through a distinct mechanism of chromatin remodeling in multiple tissues, including brain [32]. In some settings, it has been determined that the kinase activity of IKKα is also requisite for this non-NF-κB-related function [33].
Fig. 1.
Canonical pathway of NF-κB activation. Dimers of NF-κB are held latent in the cytoplasm by IκB. Extracellular stimuli (e.g., synaptic transmission, cytokines, and growth factors) induce IKK complex activation leading to site-specific phosphorylation of IκBα on conserved serine residues. Phosphorylated IκBα is targeted for ubiquitination and directed to the 26S proteasome for degradation. NF-κB is then able to stably translocate to the nucleus and regulate transcription
In this chapter, we describe a protocol for the assay of neuronal NF-κB activation produced by excitatory synaptic stimulation and include instructions for the production of dissociated murine neuronal cultures. Synaptic excitation is produced by transient competitive inhibition of GABAA receptors (using bicuculline), which results in enhanced excitatory synaptic responses and the effective production of NF-κB activation in mature neuronal cultures with fully developed inhibitory GABA networks. Activation of the NF-κB pathway can be monitored using multiple approaches. In this protocol, we describe an approach for the measurement of IKK activation in neurons by in vitro kinase assay of IKK. This protocol derives from previous protocols [34–36], but is optimized for use in mouse neuronal tissue. IKK complexes are immunoprecipitated from control or stimulated neurons and presented with an IκBα-based substrate. We also include a protocol for the production and purification of this recombinant in vitro IKK substrate.
2 Materials
2.1 Neuronal Culture Stocks
1 mM AraC (cytosine β-D-arabinofuranoside; Sigma-Aldrich, St. Louis, MO, USA): in sterile nanopure H2O. Sterile filter, make 1 ml aliquots in Eppendorf tubes, and store at −20 °C.
50× B27 supplement for optimal growth of neurons (Life Technologies, Carlsbad, CA, USA): make 1 ml aliquots in Eppendorf tubes and store at −20 °C.
200 mg/ml BSA: BSA fraction V in sterile nanopure H2O. Sterile filter, make 1 ml aliquots in Eppendorf tubes, and store at −20 °C.
25 mg/ml cysteine: L-cysteine in sterile nanopure H2O. Sterile filter, make 200 μl aliquots, and store at −20 °C.
5 mg/ml DNase I (Worthington #LS002004, Code D, 5 mg): in sterile nanopure H2O. Sterile filter, make 400 μl aliquots, and store at −20 °C.
5 ng/ml basic FGF (bFGF, Life Technologies): make 50 μg/ml aliquots in Neurobasal A (Life Technologies #10888-022), and store at −20 °C.
0.2 mg/ml glucose: in nanopure H2O. Sterile filter and store at room temperature.
200× glutamine: 200 mM L-glutamine.
10× HBSS without Ca2+/Mg2+/NaHCO3: 100 ml bottle (Life Technologies).
10× HBSS with Ca2+/Mg2+, EDTA, NaHCO3: 100 ml bottle as above, add 0.1 ml of 0.5 M EDTA (0.5 mM final), and add 9.6 ml of 524 mM NaHCO3 (50 mM final).
1 M HEPES, pH 7.5.
0.2 units/μl papain (Worthington Biochemical Corporation, Lakewood, NJ, USA): resuspend 100 mg vial in sterile nanopure H2O at 4 °C overnight, sterile filter into a 15 ml conical tubes, and store at 4 °C.
100× pen/strep/glutamine: 1:1 mix of penicillin (10,000 units), streptomycin (10 mg), and 200 mM L-glutamine. Sterile filter, make 1 ml aliquots in Eppendorf tubes, and store at −20 °C.
40× pen/strep/pyruvate/glucose: 200 μl pen/strep, 200 μl 200 mg/ml glucose, 400 μl 100 mM sodium pyruvate, 200 μl sterile nanopure H2O. Sterile filter, make 1 ml aliquots in Eppendorf tubes, and store at −20 °C.
100 mM sodium pyruvate: store at 4 °C.
50 ml of plating medium: 48.5 ml of Neurobasal A, 1 ml of 50× B-27 supplement, 0.5 ml of 100× pen/strep/L-glutamine, 5 μl of 5 ng/ml bFGF.
50 ml of growth medium: 48.75 ml of Neurobasal A, 1 ml of 50× B-27 supplement, 0.25 ml of L-glutamine, 5 μl of 5 ng/ml bFGF (see Note 1).
Trituration medium: 20 ml of DMEM, 200 μl of 200 mg/ml BSA (2 mg/ml final), 0.5 ml of 40× pen/strep/glucose/pyruvate, 200 μl of 5 mg/ml DNase (0.05 mg/ml final).
Centrifugation medium: 5 ml of trituration medium, 400 μl of 200 mg/ml BSA (16 mg/ml final); add three drops of sterile 0.1 N NaOH or until medium is slightly pink.
Papain solution: 1.5 ml of 6.67× HBSS/NaHCO3/EDTA, 128 μl of 25 mg/ml cysteine (2.13 mg/ml final), 0.25 ml of 40× pen/strep/glucose/pyruvate, 8 ml of H2O. Store on Ice. Immediately prior to use, warm to room temperature, add 1/2 to flask, and gently aerate with 95 % O2/5 % CO2. Add 0.2 units/μl papain later.
Dissection solution: 2 ml of 10× HBSS without Ca2+/Mg2+/NaHCO3, 200 μl of 1 M HEPES (1 mM final), 0.5 ml of 40× pen/strep/glucose/pyruvate, 17.3 ml of H2O.
0.1 M sodium borate buffer: add 38.14 g of sodium borate (Na3BrO3) to 1 L of H2O. Adjust pH to 8.4 with HCl. Sterile filter or autoclave.
Poly-L-lysine (PLL: 0.5 mg/ml) solution: add 10 mg of PLL (Sigma) to 100 ml of 0.1 M sodium borate buffer (pH 8.4). Sterile filter and make 5 ml aliquots in 50 ml conical tubes (concentration 333 nM and final concentration 33 nM after adding 45 ml of sodium borate buffer). Freeze at −20 °C. Add 45 ml of sodium borate buffer to the 5 ml of PLL aliquots. Add the PLL solution to the wells (500 μl/well of 24-well plate). Incubate for 4 h at 37 °C; overnight is better. Wash 1× in a large volume with 1× PBS or sterile water. Vacuum dry and plate immediately or store in the tissue culture incubator until plating.
100× stop solution for bicuculline stimulation for neurons: 500 mM sodium HEPES, use 0.3 M NaOH to pH to 7.4, 100 mM kynurenic acid, anhydrous (Sigma K-3375), 1 M MgCl2, sterilized. The kynurenate solution must be filtered with a prewashed 0.2 μm nylon filter to remove any undissolved material and bacteria (see Note 2), followed by aliquoting (1 ml per 1.5 ml tube) and storage at −20 °C.
30 mM bicuculline (Sigma): dissolve in water and store in 50 μl aliquots at −20 °C.
2.2 GST-IκBα and In Vitro Kinase Assay Stocks
LB: 20 g of LB in 1 L of nanopure water, autoclaved.
1× phosphate buffered solution (PBS), pH 7.3: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.4 mM KH2PO4, autoclaved and stored at room temperature. For washing neurons at harvesting, add 0.9 mM MgCl2 to PBS.
Glutathione buffer: 50 mM Tris, pH 7.5, 10 mM glutathione (Sigma).
1 mM IPTG (Isopropyl β-D-1-thiogalactopyranoside).
Mini EDTA-free protease inhibitor cocktail (Roche, Madison, WI, USA): add fresh, prior to use, 1 tablet/10 ml lysis buffer.
Lysozyme.
Glutathione Sepharose beads (GE Healthcare, Mickleton, NJ, USA).
Poly-Prep chromatography elution columns (Bio-Rad, Hercules, CA, USA).
Bradford protein assay kit (Bio-Rad).
Kinase reaction buffer: 10 mM ATP (“cold”), 5 mCi of [γ-P32]-ATP (10 mCi/ml), 1 mg of GST-IκBα(1–62). Make fresh prior to use in radioactive designated area.
1× kinase buffer: 10 mM HEPES, pH 7.9, 5 mM MgCl2, 1 mM MnCl2. Store at room temperature. Add fresh, prior to use, 50 mM DTT and phosphatase inhibitors: 12.5 mM beta-glycerophosphate, 2 mM NaF, 50 mM Na3VO4. You can make 50× mixed stock of phosphatase inhibitors stored in small aliquots at −20 °C.
2.3 Mini SDS-PAGE Gel, Immunoblotting, and Immunoprecipitation (IP)
Stacking gel: 1.67 ml of 0.5 M Tris, pH 6.8, 0.83 ml of 30 % acrylamide/bis solution (Bio-Rad), 0.067 ml of 10 % SDS, 0.333 ml of 1.5 % ammonium persulfate, 3.77 ml of nanopure water, 0.005 ml of TEMED (N,N,N′,N′-tetramethyl-ethylenediamine).
12 % resolving gel: 1.25 ml of 3 M Tris, pH 8.8, 4 ml acryl-amide–bisacrylamide (30 %:0.8 %), 0.1 ml of 10 % SDS, 0.5 ml of 1.5 % ammonium persulfate, 4.13 ml of nanopure water, 0.005 ml of TEMED.
1× TBST: 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1 % Tween 20. Make a 10× stock and store at room temperature.
6× SDS sample loading buffer: 1 M Tris, pH 6.8, 10 % SDS, 30 % glycerol, 0.012 % bromophenol blue. Store in 10 ml aliquots in 15 ml conical tubes at room temperature. Prior to use, heat gently to bring into solution, and add 20 μl of 3 M DTT to 100 μl of 6× sample buffer.
1× running buffer: 25 mM Tris–HCl, 200 mM glycine, 0.1 % SDS. Make 5× stock and store at room temperature.
1× transfer buffer: 25 mM Tris, 192 mM glycine, 10 % methanol. Make 10× stock, without methanol, and store at room temperature. Add the methanol prior to use, when you make the 1× working solution.
IP lysis buffer: 20 mM Tris, pH 7.5, 150 mM NaCl, 1.0 % Triton X-100, 1.0 mM EDTA. Store at room temperature. Add fresh phosphatase inhibitors: 20 mM beta-glycerophosphate, 10 mM NaF, 100 μM Na3VO4. Add fresh mini EDTA-free pro-tease inhibitor cocktail prior to use.
Primary antibody: mouse anti-IKKα monoclonal (Novus Biologicals, Littleton, CO, USA).
Secondary antibody anti-mouse HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA): 1:5,000 dilution.
Enhanced chemiluminescence reagents such as the Pierce ECL Western Blotting Substrate (Thermo Scientific, Waltham, MA, USA).
2.4 Labware and Instruments
Pipetters and pipette tips.
Eppendorf 1.5 and 15 ml conical tubes.
24-well culture plates and culture flasks (75 cm2).
Eppendorf 1.5 and 15 ml tube centrifuges with temperature control.
Tube rotator at 4 °C.
Gel casting apparatus and accessories, gel running and transfer apparatus accessories, and power source for gel running and transfer. We use the Mini-Protean gel system (Bio-Rad).
Hot block for Eppendorf tubes.
Radiation badge, Geiger counter, radioactivity designated area, and instruments.
CO2 and 95 % O2/5 % CO2 gas tanks.
Tissue culture room, tissue culture hood, and tissue culture incubator set at 37 °C and 5 % CO2.
Ethanol-based flame lamp.
Dissection scope, instruments, and dissection designated area (see Note 3).
UV sterilizer.
PhosphorImager instrument.
3 Methods
3.1 Murine Hippocampal Neuron Dissociation and Culture
3.1.1 Dissection and Digestion of Murine Hippocampi
For additional details and visual aid, see refs. 37 and 38:
Prepare fresh trituration, centrifugation, papain, and dissection solutions.
Make a hole (using soldering iron) in the top of a sterile 75 cm2 culture flask, and connect the hole by tubing to oxygen/CO2 (95/5 %).
Place 3 × 35 mm culture dishes containing dissection medium on ice: one for the whole brains, another for dissected hippocampi, and a third for the final hippocampi with meningeal membranes removed.
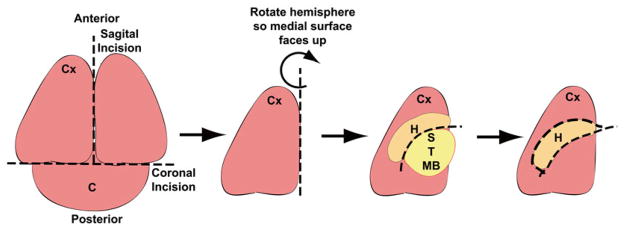
Dissect the hippocampi from postnatal P0 mouse pups using a dissecting scope (Fig. 2) (see Note 4).
While dissecting, keep the hippocampi on ice in dissection solution.
Remove the meninges using fine tweezers under the dissecting microscope.
When the dissection is finished, transfer the tissue to the flask connected by tubing to oxygen/CO2 (95/5 %), taking care to transfer as little dissection solution as possible, and add 100 U papain.
Digest for ~10–15 min at room temperature, depending upon papain age and temperature.
Add 80 U of papain and 100 μl of 5 mg/ml DNase to the remaining papain solution and add to the digestion mix in the flask.
Digest for another 10–15 min, and monitor by eye watching for feathering of the tissue edges.
Fig. 2.
Diagram depicting hippocampal dissection from neonatal mouse pups. Place the whole brain with the caudal surface toward the bottom of the dissection dish. Make an initial coronal incision to remove the cerebellum (C). Cut the remaining of the brain sagitally on the midline to separate the two brain hemispheres. Rotate the hemisphere placing the medial surface facing up. Remove the striatal (S), thalamic (T), and midbrain (MB) structures to expose the hippocampus (H). Dissect out the hippocampus
3.1.2 Trituration and Plating
Label three 15 ml conical tubes, #1–#3.
Once the digestion is over, move the flask to a tissue culture (TC) hood. Allow the tissue to settle in one corner, and then remove the tissue to #1 conical tube using a 5 ml plastic pipette.
Allow the tissue to settle and remove as much of the digestion solution as possible from over the tissue. Add 4 ml of trituration solution with mixing, allow the tissue to settle, and again remove as much solution as possible from over the tissue.
Triturate the tissue gently up and down four times with a sterile Pasteur pipette, and then add 3 ml of trituration solution and allow chunks to settle.
Remove solution from the top, which is free of chunks, and transfer to #2 conical tube.
Fire-polish a sterile Pasteur pipette to slightly decreased diameter and repeat the trituration. Again add 3 ml of trituration solution, allow chunks to settle, and remove the top solution to #2 conical tube.
Repeat the above trituration procedure fire-polishing pipettes to progressively smaller diameters.
Transfer the solution from conical tube #2 to conical tube #3 using a small-bore Pasteur pipette. Retriturate bits at the bottom of #2 if necessary.
Layer 5 ml of centrifugation solution underneath the triturated cells in conical tube #3.
Centrifuge to pellet cells for 10 min (150 × g, at 20 °C).
Remove the supernatant and resuspend the cell pellet in plating medium (with pen/strep), and count cells with hemocytometer to plate at ~235,000 cells/cm2.
Put plated cells in an incubator.
Approximately 5–7 h later suck off about half of the medium and replace (see Note 5).
The next morning (no more than 16 h post-plating), gently swirl the dishes, remove by suction 1/2–1/3 of the medium, and replace with fresh growth medium (without pen/strep) (see Notes 6 and 7).
3.2 IKK Substrate (GST-IκBα) Preparation
Transform 100 ng plasmid expressing recombinant-tagged GST-IκBα (1–62) [39] into 100 μl bacteria (BL21) (see Note 8).
Starter culture: inoculate 10 ml of LB plus ampicillin (final concentration 0.1 mg/ml) with transformed GST-IκBα (1–62) in BL21 bacteria by shaking at 30 °C overnight.
Add the starter culture into 250 ml of LB plus ampicillin and shake at 37 °C.
When the bacterial culture reaches OD600 = 0.6–0.8 (optical density at 600 nm using UV/visible light spectrophotometer), induce GST-IκBα(1–62) expression in the cultured bacteria with a final concentration of 1 mM IPTG by shaking at room temperature for 2 h.
Spin down the bacteria for 20 min at 4,000 × g at 4 °C.
Resuspend the bacterial pellet with 12.5 ml of cold PBS with protease inhibitors.
Add lysozyme to a final concentration of 0.5 mg/ml and incubate at room temperature for 15 min.
Sonicate to reduce viscosity.
Add Triton X-100 to a final concentration of 1 %.
Spin at 10,000 × g for 10 min at 4 °C.
To the supernatant, add 400 μl of a 50 % slurry of Glutathione Sepharose beads that have been pre-equilibrated in PBS; incubate with rotation at 4 °C for 3 h.
Spin down the tube from step 11 at 1,000 × g at room temperature for 1 min to collect the beads from the lid. Collect all beads and supernatant and load onto an elution column.
Wash the column with 8 ml of PBS.
Elute in 200 μl fractions with glutathione buffer. After adding each 200 μl aliquot of elution buffer, plug the bottom of the column, and let it sit for 10 min at room temperature. Collect 6–10 elution fractions.
Determine the protein concentration of each fraction by Bradford protein assay.
Aliquot each fraction separately and store at −80 °C; make small aliquots to avoid freeze-thaw degradation.
Run a sample of each fraction on a 12 % polyacrylamide mini SDS-PAGE gel, followed by Coomassie blue staining to confirm the size of the purified substrate that should migrate at 37 kDa.
3.3 IKK Complex Immunoprecipitation
Use days in vitro (DIV) 21 hippocampal dissociated neuronal culture plated in 24-well plates.
Stimulate the neurons with a GABAAR blocker, bicuculline, at a final concentration of 50 μM for 30 s (see Notes 9 and 10).
Stop the stimulation with 1× stop solution (using 100× kynurenate magnesium stock solution) by incubation at 37 °C for 10 min.
The stop solution is washed by removal of 50 % of the medium and replacement with fresh growth medium, and then incubate the neurons at 37 °C 3 h before harvesting.
Put the plates on ice and wash once with ice-cold PBS with added 0.9 mM MgCl2 (see Note 11).
Lyse the cells with 100 μl of cold lysis buffer per well from a 24-well plate.
Incubate the cells on ice for 10 min, scrape the cells into a 1.5 ml tube, and leave for 10 more minutes on ice.
Spin the lysate at 15,600 × g for 10 min at 4 °C and transfer the supernatant to a new 1.5 ml tube.
Add 2 μg of anti-IKKα antibody to at least 1,000 μg of lysate and rotate for 3 h at 4 °C (see Note 12).
Add 20 μl of prewashed protein A/G Sepharose (50 % slurry, protein A/G Sepharose should be prewashed three times in PBS with added protease inhibitors), and bind for 2 h rotating at 4 °C (see Note 13).
Wash three times with 1 ml of cold lysis buffer (rotate each wash at 4 °C for 5 min, then briefly spin down at 15,600 × g in a 4 °C microfuge, and remove the supernatant before the next wash) and one time with 1 ml of cold kinase buffer. Use a 23 G bent needle attached to a vacuum line to remove the supernatant, being careful not to remove the beads.
Remove the kinase buffer fully with a needle (gauge 27) attached to a vacuum line after the last wash.
3.4 In Vitro Kinase Assay Followed by Immunoblot and PhosphorImaging
Use all standard precautions in conformity with radiation safety regulations. Use a lab coat designated for radiation use only. Always wear a radiation badge when handling radiolabeled phosphate. Monitor frequently for radioactive spillage:
To each tube from Subheading 3.3, add 30 ml of kinase reaction buffer.
Incubate for 30 min at 30 °C with gentle agitation.
Terminate the reaction with 10 ml of 5× SDS-PAGE sample buffer, and boil the samples for 2–3 min.
Run the samples and pre-stained molecular weight marker on 12 % polyacrylamide mini SDS-PAGE gel, and transfer to nitrocellulose or PVDF (run the gel at a constant 90 V and transfer overnight at 35 constant voltage, 4 °C).
Wrap the blot in Saran wrap and expose in a PhosphorImager cassette overnight or as required. Quantitate using a PhosphorImager.
Unwrap the blot (rehydrate if necessary), and block for 1 h in TBST with 5 % nonfat milk at room temperature.
Incubate the blot for 1 h in TBST with 5 % milk and 1:1,000 dilution of anti-IKKα antibody at room temperature.
Wash five times for 5 min each with excess of TBST.
Incubate the blot with the secondary anti-mouse HRP antibody in 10 ml of TBST with 5 % milk for 1 h at room temperature.
Wash five times for 5 minutes each with excess of TBST.
Place the blot on Saran wrap, develop by ECL, and expose the developed blot to film.
Footnotes
Basic fibroblast growth factor (bFGF) is optimally in the growth medium only at the time of plating and no longer than the first 3 days of culture, to avoid excessive glia proliferation.
Kynurenate may be difficult to dissolve. The solution is typically left in the refrigerator for several days, shaking it once or twice a day, or just warm at room temperature starting in the morning. Very small residual particles may be tolerated.
Dissection can take place on the lab bench provided the area is cleansed with 70 % ethanol, sterile bench techniques are practiced, and dissecting instruments are sterilized with UV.
The use of pups on the day of birth is optimal to ensure the survival of the greatest numbers of neurons. It is better to dissect the minimum number of hippocampi that yield the size of the culture required (generally from dissecting up to 15 pups), since a shorter dissection duration allows better survival of the dissociated neurons. If required, for the inhibition of glial proliferation, AraC (10 μM) may be included in the culture medium at DIV 3 for 48 h.
During changing culture medium, sufficient volume to cover the surface of the cells must always be maintained.
Plating in medium containing pen/strep may be used to reduce the possibility of bacterial infection, but cultures must be switched to WITHOUT pen/strep as soon as possible and no later than 18 h after plating to reduce the toxicity to neurons. To avoid contamination, always use sterile techniques when handling neuronal cultures, including keeping a separate set of pipette tips that is opened only in the TC hood.
The use of glial conditioned medium can be useful to improve neuronal viability, especially when the B27 lot is less than optimal or when it is desired to maintain a low-density culture (e.g., for immunohistochemistry).
Recombinant GST-IκBα(1–62) can be cloned into a bacterial expression vector: pGEX (GE Healthcare) by in-frame insertion of an amino-terminal fusion with GST to the first 62 amino acid residues of human IκBα).
High basal synaptic activity may dampen the apparent activation of NF-κB, particularly if the cultures are of high density. If necessary, basal synaptic activity may be inhibited prior to stimulation using several possible approaches: (a) reduction of B27 (to 50 %) in growth medium, up to 8 h prior to stimulation, with stimulation in normal growth medium, or (b) blocking NMDA receptors using 150 μM APV and/or voltage-gated L-type sodium channels using 10 μM nimodipine, up to 8 h prior to stimulation, and then wash the neurons and stimulate in normal growth medium.
Inhibitory GABA networks may not be fully formed in cultures at DIV ≤ 18 and may not allow sufficient induction of endogenous excitation using a GABAAR blocker. In this case, other stimuli are more suitable, including glutamate or KCl.
MgCl2 is used to reduce neuronal activation during harvesting.
Anti-IKKγ (NEMO) can be used instead.
Use a pipette tip with the end cut to pipette the 50 % slurry. Mark the level of the 50 % slurry on the tube exterior with a marker, and wash beads in a large volume of cold PBS by inverting the tubes repeatedly. Spin down the tubes at 850 × g at 4 °C, and then remove the supernatant to the marked level.
References
- 1.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 5.Verhelst K, Carpentier I, Beyaert R. Regulation of TNF-induced NF-kappaB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor Rev. 2011;22:277–286. doi: 10.1016/j.cytogfr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 10.Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 12.Christoffel DJ, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freudenthal R, Romano A. Participation of Rel/NF-kappaB transcription factors in long-term memory in the crab Chasmagnathus. Brain Res. 2000;855:274–281. doi: 10.1016/s0006-8993(99)02358-6. [DOI] [PubMed] [Google Scholar]
- 14.Freudenthal R, Romano A, Routtenberg A. Transcription factor NF-kappaB activation after in vivo perforant path LTP in mouse hippocampus. Hippocampus. 2004;14:677–683. doi: 10.1002/hipo.20020. [DOI] [PubMed] [Google Scholar]
- 15.Kaltschmidt B, et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Meffert MK, et al. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 18.O’Mahony A, et al. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Riordan KJ, et al. Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors. J Neurosci. 2006;26:4870–4879. doi: 10.1523/JNEUROSCI.4527-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmeisser MJ, et al. IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci. 2012;32:5688–5703. doi: 10.1523/JNEUROSCI.0111-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boersma MC, et al. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31:5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech Dev. 1993;43:135–147. doi: 10.1016/0925-4773(93)90031-r. [DOI] [PubMed] [Google Scholar]
- 24.Guerrini L, Blasi F, Denis-Donini S. Synaptic activation of NF-kappa B by glutamate in cerebellar granule neurons in vitro. Proc Natl Acad Sci U S A. 1995;92:9077–9081. doi: 10.1073/pnas.92.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc Natl Acad Sci U S A. 1995;92:9618–9622. doi: 10.1073/pnas.92.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-kappaB pathway. FEBS J. 2011;278:862–876. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- 27.Carter BD, et al. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 28.Yeiser EC, et al. Neurotrophin signaling through the p75 receptor is deficient in traf6−/− mice. J Neurosci. 2004;24:10521–10529. doi: 10.1523/JNEUROSCI.1390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 30.Kaltschmidt C, et al. Constitutive NF-kappa B activity in neurons. Mol Cell Biol. 1994;14:3981–3992. doi: 10.1128/mcb.14.6.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev. 2012;246:77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 32.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, et al. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 34.DiDonato JA, et al. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. EMBO J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zandi E, et al. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 37.Beaudoin GM, 3rd, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7:1741–1754. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 38.Nunez J. Primary culture of hippocampal neurons from p0 newborn rats. J Vis Exp. 2008;19:e895. doi: 10.3791/895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geleziunas R, et al. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]