Abstract
The importance of maintaining contractile function in aortic smooth muscle cells (SMCs) is evident by the fact that heterozygous mutations in the major structural proteins or kinases controlling contraction lead to the formation of aneurysms of the ascending thoracic aorta that predispose to life-threatening aortic dissections. Force generation by SMC requires ATP-dependent cyclic interactions between filaments composed of SMC specific isoforms of α-actin (encoded by ACTA2) and myosin heavy chain (MYH11). ACTA2 and MYH11 mutations shown to disrupt this cyclic interaction predispose to thoracic aortic disease. Movement of the myosin motor domain is controlled by phosphorylation of the regulatory light chain (RLC) on the myosin filament, and loss-of-function mutations in the dedicated kinase for this phosphorylation, myosin light chain kinase (MYLK) also predispose to thoracic aortic disease. Finally, a mutation in the cGMP-activated protein kinase (PRKG1) results in constitutive activation of the kinase in the absence of cGMP, thus driving SMC relaxation in part through increased de-phosphorylation of the RLC, and predisposes to thoracic aortic disease. Furthermore, SMCs cannot generate force without connections to the extracellular matrix (ECM) through focal adhesions, and mutations in the major protein in the ECM, fibrillin-1, linking SMCs to the matrix also cause thoracic aortic disease in individuals with Marfan syndrome. Thus, disruption of the ability of the aortic SMC to generate force through the elastin-contractile units in response to pulsatile blood flow may be a primary driver for thoracic aortic aneurysms and dissections.
Keywords: aortic aneurysm, thoracic, vascular smooth muscle cell, elastin-contractile unit
Subject Codes: Contractile Function, Vascular Biology, Genetics, Aneurysm, Aortic Dissection
The natural history of thoracic aortic aneurysms is to enlarge over time, which progressively increases the risk for an acute ascending aortic dissection 1. Ascending aortic dissections (Stanford classification type A dissections) cause sudden death in approximately half of afflicted individuals, and there is further mortality and high morbidity in survivors after hospitalization and emergency surgical repair 2. Approximately one quarter of patients with thoracic aortic disease have a single gene mutation predisposing to the disease, as illustrated by Marfan syndrome due to mutations in FBN1 3. There has been rapid progress over the past 12 years in identifying additional genes for autosomal dominant inheritance of a predisposition for thoracic aortic disease for both syndromic and nonsyndromic disease, collectively termed heritable thoracic aortic disease, and 13 genes have been identified to date. These genes are inherited in an autosomal dominant manner and encode proteins with specific functions or components of signaling pathways in aortic smooth muscle cells (SMCs). The causative genes can be grouped into the following categories: structural and regulatory proteins involved in SMC contractile function (ACTA2, MYH11, MYLK, PRKG1)4–7; proteins involved in the maintenance of or SMC adhesion to the extracellular matrix (FBN1, MFAP5, LOX) 8, 9; proteins involved in canonical TGF-β signaling (TGFBR2, TGFBR1, SMAD3, TGFB2, TGFB3)10–15; and proteins involved in SMC metabolism (MAT2A) or survival (FOXE3)16, 17.
The identification of alterations in genes encoding proteins critical for SMC contraction and adhesions as a cause of heritable thoracic aortic disease has suggested a role for mechanotransduction in SMCs in maintaining the structure of the ascending aorta throughout a lifetime 18–20. Further evidence that mechanosensing is important in the pathogenesis of this disorder is provided by the fact that the major risk factors for thoracic aortic disease, including poorly controlled hypertension and bodybuilding weight lifting, increased the forces on the ascending aorta21, 22. Here, we review the current understanding of SMC contraction and provide an overview of the mutations in the genes predisposing to thoracic aortic disease that have been shown to or are predicted to disrupt SMC contractile function.
SMC Contraction and the Aortic Elastin-Contractile Unit
Arteries respond to the dynamic pressures introduced by pulsatile blood flow through the active contraction of SMCs in the arterial wall. Vascular SMCs are arranged circumferentially into multiple layers, either embedded between layers of elastin lamellae in large elastic arteries or in a matrix of connective tissue in smaller muscular arteries. For contractile function, SMCs express the smooth muscle-specific isoform of α-actin (SM α-actin; encoded by ACTA2) that oligomerizes to form thin filaments (Figure 1) 23. The thick filaments are composed of a smooth muscle-specific isoform of myosin heavy chain dimer (SM MHC; encoded by MYH11), and four light chains, two regulatory light chains (RLC) and two essential light chains. The thick and thin filaments are assembled into contractile units anchored to focal adhesions on the cell membranes and dense bodies in the cytoplasm, but do not form sarcomeres (the structural unit of myofibrils in skeletal and cardiac muscle). Actomyosin cross-bridge cycling is the mechanism for tension development and shortening in SMCs. The myosin heavy chain has a motor domain containing conserved elements that bind ATP, as well as a more variable region involved in actin binding. Phosphorylation of the RLC on the myosin thick filament is necessary to activate the force generating cycle of smooth muscle myosin motor heads with the actin filaments. The lever arm of the myosin heavy chain is followed by a coiled coil domain that allows dimerization of two myosin heavy chains to form the dimeric, two-headed smooth muscle myosin molecule. The last segment of the coiled coil region initiates assembly of individual smooth muscle myosin heavy chains into filaments.
Figure 1.
Signaling Pathways Controlling Regulatory Light Chain (RLC) Phosphorylation and Activation of Vascular Smooth Muscle Contraction and Relaxation. Contraction pathways shown on the left lead to calcium-dependent phosphorylation of myosin RLC by myosin light chain kinase (MLCK) and actin-myosin-dependent force development. Relaxation pathways on the right lead to calcium reduction and myosin dephosphorylation. Mutated genes causing heritable thoracic aortic disease are indicated in red font; these genes disrupt critical proteins involved in SMC contraction. Abbreviations: BK (large-conductance calcium and voltage sensitive potassium channel), CaM (calmodulin), GP (Guanine nucleotide-binding Protein, e.g. Gq/11), GPCR (GP-Coupled Receptor, e.g. α1 adrenergic receptor), IP3R (Inositol triphosphate Receptor), IRAG (Inositol triphosphate Receptor-Associated PKG substrate), LIC (Ligand-gated Ion Channel, e.g. ATP-gated P2X1), RLC (Regulatory Light Chain), -P (phosphorylation), MADP (Membrane-Associated Dense Plaque), PKs (Protein Kinases, e.g. Rho kinase), SR (sarcoplasmic reticulum), VDCC (Voltage-Dependent Calcium Channel, e.g. CaV1.2). Red lightning bolt represents membrane depolarization.
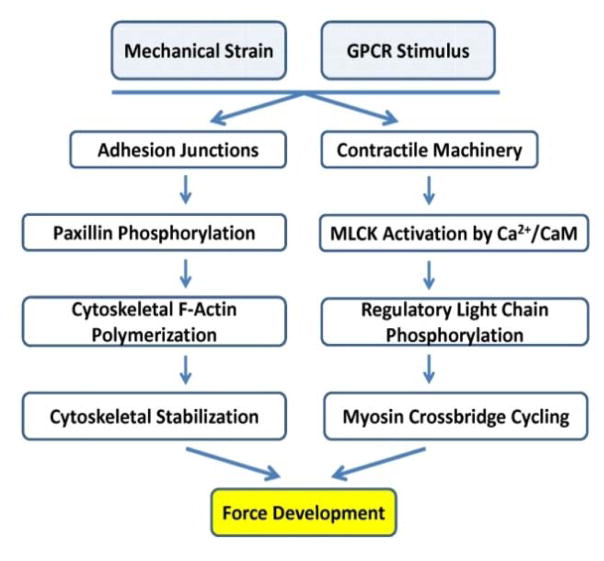
In SMCs, the majority of actin is polymerized into filaments (termed F-actin), leaving a small pool of monomeric actin (G-actin). With contractile stimuli, the pool of G-actin in SMCs decreases further 24–26. Although phosphorylation of RLC is necessary to initiate force, studies have identified other cytoskeletal events required for SMC force generation (Figure 2). In this model, the thick and thin filaments in the actomyosin contractile units are relatively stable and largely responsible for the “stable” pool of F-actin in the cells. Contractile stimuli initiate the assembly of the extracellular matrix and cytoskeleton adhesion complexes through focal adhesions at the cell membrane, which orchestrates the organization of the submembranous network of actin filaments, and subsequently engages the contractile units and actomyosin cross bridging 25–27. A tightly regulated polymerization of G-actin to F-actin is initiated at cell adhesion junctions and provides stabilization of the submembranous cytoskeleton. This dynamic organization of the actin cytoskeleton in the submembranous area may be important for maintaining tone and vascular plasticity by providing membrane rigidity and adaptation to local forces acting on the cell-matrix adhesion junctions 24, 26, 28. This model may also allow for strengthening of the cell membrane for transmission of force generated by the contractile units.
Figure 2.
Vascular smooth muscle force development is dependent on submembranous cytoskeletal actin polymerization initiated at focal adhesions and actomyosin cross-bridge cycling driven by phosphorylation of the myosin regulatory light chain.
Actin is highly conserved and six isoforms are present in humans (α-cardiac, α-skeletal, α-vascular smooth muscle, γ-enteric smooth muscle, and β- and γ-cytoplasmic), which differ primarily in their N-terminal sequences. The major actin isoforms in vascular smooth muscle cells (tonic SMCs) are smooth muscle α-actin (SM α-actin; ~65% of total actin), β-actin (~20%) and SM γ-actin (~15%)29. Studies regarding specific distributions of different actin isoforms into actin filaments in vascular SMCs are controversial 24, 30, 31. Published reports show different actin isoforms polymerizing in response to stimuli that initiate contraction, with SM α-actin being a component of the actomyosin contractile units 32, 33, and β-actin and γ-actin involved in the pool of submembranous actin that polymerizes with contractile stimuli. Additionally, current evidence suggests that tropomyosin may serve to spatially segregate stable actin filaments associated with myosin in the contractile unit from the dynamic submembranous actin 26, 34–36.
An increase in intracellular calcium concentration ([Ca2+]i) in vascular smooth muscle triggers the contractile response37, 38 (Figure 1). Calcium binds to calmodulin (CaM), which then binds and activates the short form of myosin light chain kinase (MLCK; encoded by MYLK) expressed in aortic SMCs in vivo 6. The extent of RLC phosphorylation is determined by the activities of two enzymes: Ca2+/calmodulin-dependent MLCK and myosin light chain phosphatase (MLCP) 38, 39. The activated MLCK phosphorylates Ser19 on the regulatory light chain (RLC) of myosin, which activates the heavy chain myosin motor domain that drives contraction in smooth muscle. RLC phosphorylation is sufficient to activate myosin motors and SMC contraction. MLCK appears to be the only known physiological kinase for this function and the only known physiological substrate for MLCK is myosin RLC; thus, it is a dedicated protein kinase 39–41. One RLC wraps around each alpha-helical neck region of myosin heavy chain. In the unphosphorylated state, intramolecular interactions position the actin-binding interface of one myosin motor head onto the converter domain of the second head, thus inhibiting the activity of both heads 42, 43. When RLC is phosphorylated, the two heads are displaced from each other, allowing cyclic binding to actin filaments. This ATP-dependent chemomechanical transduction leads to SMC shortening and force development.
Myosin light chain phosphatase dephosphorylates RLC, and induces relaxation after a decrease in [Ca2+]i returns MLCK to an inactive state (Figure 1). MLCP is a holoenzyme composed of distinct subunits: a small catalytic subunit (PP1cδ), and a large regulatory subunit (MYPT1) 38. Biochemically, MYPT1 binds PP1cδ and myosin, and thus functions as a scaffolding protein. Signaling pathways converge to inhibit MLCP activity by distinct mechanisms, thereby increasing RLC phosphorylation without changing elevated Ca2+ sensitization ([Ca2+]i). Inhibition of MLCP activity occurs, in part, due to phosphorylation of MYPT1 (Figure 1). Thus, the extent of RLC phosphorylation is determined by the relative activities of MLCP and Ca2+/calmodulin-dependent MLCK, each of which are modulated by different SMC signaling pathways.
To contract productively, the contractile units in SMCs need to be linked to the extracellular matrix (ECM). The human ascending aorta is composed of over 50 alternating layers of elastic lamellae and SMCs, and the contractile units in the SMCs attach to the ECM through integrin-containing focal adhesions (also called dense plaques) on the cell surface. The integrin receptors in the focal adhesions bind to microfibrils that surround the elastin fibers. The continuous connection of the elastin fibers to the contractile units in the SMCs has been termed the “elastin-contractile unit” 44. Interestingly, despite the fact that the ascending aorta is composed of over 50 layers of elastic lamellae and SMCs organized into “contractile-elastin units”, the elasticity of the aorta is due to the elastin fibers and not the contraction of the SMCs 45. The aortic pathology associated with thoracic aortic disease, termed medial degeneration, is characterized by loss of elastin lamellae and SMCs, and accumulation of proteoglycans, and is present with disease triggered by either genetic mutations or hypertension.
ACTA2 Mutations
ACTA2 is a highly conserved gene and relatively invariant in the general population. Forty-one heterozygous ACTA2 mutations located throughout the actin monomer have been identified that predispose to thoracic aortic aneurysms and aortic dissections (Figure 3) 4, 46–50. Forty of these variants are missense mutations predicted to produce a mutant SM α-actin monomer; one mutation is predicted to lead to aberrant splicing of exon 8 and produce a protein truncated at glycine 270 with an additional 35 aberrant amino acids added. One in frame deletion and a splice site mutation deleting the second to last exon (exon 8) of the gene have been identified in thoracic aortic disease patients but have not been shown to segregate with thoracic aortic disease. Thus, there is currently no definitive evidence that ACTA2 variants leading to haploinsufficiency predispose to thoracic aortic disease.
Figure 3.
Missense mutations in ACTA2 identified in individuals and families with heritable thoracic aortic disease. Residues highlighted in boxes have the following correlations between genotype and phenotype: R179 mutations cause Multisystemic Smooth Muscle Dysfunction Syndrome; R258 and R39 mutations predispose to a moyamoya-like cerebrovascular disease; R149 and R118 predispose to early onset coronary artery disease; and G160 and W88 disruption predispose to decreased penetrance and later onset thoracic aortic disease. Side chains of other residues known to be mutated are shown as sticks (cyan). G48V and M49V are in a disordered region (D-loop) and not seen in this crystal structure. Actin structure is PDB code 1NWK.
ACTA2 mutations disrupt amino acids located in all 4 subdomains of actin and are predicted to produce structurally-altered actin monomers (Figure 3). The 3-dimensional structure of an actin monomer (G-actin) reveals 4 subdomains. A nucleotide and divalent cation site reside in the center of the molecule. ATP is rapidly hydrolyzed once the monomer assembles into the filament, but phosphate release occurs more slowly, thus marking the age of the filament. The N- and C-termini are located in subdomain 1, which is the primary site for myosin interaction. A hinge region connects the small (subdomains 1 and 2) and large (subdomains 3 and 4) domains of actin. Subdomains 2 and 4 (at the pointed end), interact with subdomains 1 and 3 (barbed end) of another monomer in the actin filament; the barbed end is the fast growing end of the filament. The “hydrophobic plug” has longitudinal contacts with the other actin strand in the filament. Subdomains 1 and 2 are on the outside of the filaments, and 3 and 4 are toward the inside.
The effect of an ACTA2 missense mutation, p.Arg258Cys (denoted R258C), on actin function was investigated at the molecular level by in vitro assays, using expressed human SM α-actin 51. The mutation is located in subdomain 4, and is at the interface between the two strands that form the actin filament. The mutant actin showed many functional defects compared to its wildtype counterpart. The R258C filaments were less stable than filaments composed of wildtype actin, and more readily severed by cofilin, even in the presence of tropomyosin, which is usually protective. Surprisingly, R258C actin bound profilin ~20-fold more tightly than wildtype actin, which implies that the monomeric pool of actin will be higher in SMCs expressing mutant actin. Phosphorylated human smooth muscle myosin moves R258C actin more slowly than wildtype actin in an in vitro motility assay, even when mixed 50:50 with wild type actin, which mimics the condition found in a heterozygous human. Notably, many of the observed changes must happen allosterically, as the mutation is not located in either the myosin or the profilin binding site. Thus, the observed defects align with the hypothesis that ACTA2 mutations disrupt force generation by SMCs and this defective contractile function contributes to the pathogenesis of TAAD.
MYH11 Mutations
Rare variants in MYH11 are found throughout the gene but only a subset of these rare variants predispose to heritable thoracic aortic disease. In-frame deletions in the coiled-coil domain of the SMC-specific isoform of myosin heavy chain have been demonstrated to predispose to thoracic aortic disease associated with patent ductus arteriosus (PDA) through segregation in large families with thoracic aortic disease 5 (Figure 4). Additionally, two MYH11 missense variants that altered the coiled-coil were identified on one allele domain (p.Leu1264Pro and p.Arg1275Leu) and segregated with thoracic aortic disease and PDA in a three generation family 52. The Leu1264 substitution is predicted to disrupt coiled-coil formation and interfere with protein interactions, while the Arg1275 substitution is less likely to be damaging. Another MYH11 missense variant, p.Arg712Gln, also segregated with disease in a family with TAAD and PDA and is located in the ATPase motor head region. However, other rare MYH11 missense variants that are predicted to have damaging effects on the protein do not segregate with TAAD or PDA in families 53. Interestingly, duplications of a region of 16p13.1 that contains 9 genes, including MYH11, increase the risk for thoracic aortic dissections greater than 10-fold in the general population 54. Corresponding deletions of the same region of 16p13.1 also occur in the general population but there is no evidence that these deletions predispose to thoracic aortic disease. These data indicate that haploinsufficiency for MYH11 does not appear to cause disease, whereas increased expression of MYH11, as would occur with duplications leading to three copies of the gene, does predispose to acute aortic dissections. Studies exploring how increased expression of MYH11 leads to thoracic aortic disease identified increased autophagy and degradation of SM-MHC and endoplasmic reticulum stress in SMCs with overexpression of the myosin heavy chain55.
Figure 4.
Schematic of a SMC myosin filament with the location of the regulatory light chain (RLC) and essential light chain (ELC) and protein domains of the myosin heavy chain indicated. In frame deletions in the coiled coil domain predispose to heritable thoracic aortic disease.
It is not entirely clear why some of the MYH11 genetic variants cause heritable thoracic aortic disease and others do not. Interestingly, MYH11 rare variants disrupting amino acids in the motor head of SM-MHC, such as the p.Arg247Cys mutation, have been identified in the general population but do not cause familial thoracic aortic disease. We found that MYH11 p.Arg247Cys disrupted the myosin motor leading to decreased contractile force generation in aortic rings in response to agonists and does not cause aortic disease in a mouse model 56. At the same time, we recently found that a subset of the Myh11R247C mice die of acute aortic dissection when hypertension in induced but the wildtype mice do not (unpublished data). It may be that in the absence of other disease modifiers, such a mutation is tolerated because the SMCs can simply increase the average level of myosin RLC phosphorylation to compensate for the lower force per myosin head. On the other hand, MYH11 missense mutations that are possibly disease-causing, such as the p.Arg712Gln described above, may have unique dominant effects, such as those described for zebrafish myh11 mutations 57, 58. These effects include loss of regulation, leading to constitutive ATPase activity in the mutant myosin filaments and energetic overload on the SMCs, in addition to impairment of myosin motor function and decreased contraction.
MYLK Mutations
Heterozygous loss-of-function mutations in MYLK are a cause of heritable thoracic aortic disease. The corresponding protein, myosin light chain kinase (MLCK) is the kinase that controls phosphorylation of the RLC and subsequent actomyosin cross-bridge cycling for SMC contraction 6. Heterozygous MYLK mutations cause either haploinsufficiency or alter the calmodulin binding domain such that calmodulin does not properly bind to activate the kinase. Interestingly, no mutations in the catalytic domain of MLCK have been reported in familial thoracic aortic disease.
MYLK encodes three gene products expressed from separate promotors; two of these isoforms contain the catalytic and calmodulin domain required for activation and kinase activity, the 220 kDa long form (amino acids 1 through 1914) and the 130 kDa short form (amino acids 923 through 1914) (Figure 5). It is important to note that only the short form of MLCK is expressed in human aorta 6, 59. Based on this fact, it is questionable whether mutations disrupting amino acids 1 through 922 of MLCK cause thoracic aortic disease.
Figure 5.
Schematic of the protein domains in the three isoforms of myosin light chain kinase (MLCK). Three distinct promoters control the expression of these isoforms of MLCK. The short form is expression in the aorta. Pathogenic mutations that cause heritable thoracic aortic disease are shown and lead to haploinsufficiency or disrupt calmodulin binding, which is required to activate the kinase.
Recent studies have sought to determine why haploinsufficiency of MYLK causes thoracic aortic disease and have found that aortic SMCs have less physiological reserve relative to signaling of RLC phosphorylation when compared to SMCs in the bladder. Activation of a small fraction of MLCK, achieved by limiting amounts of free Ca2+/calmodulin and inhibition of myosin light chain phosphatase, is sufficient for robust RLC phosphorylation and contractile responses in bladder SMCs 60. However, similarly limiting MLCK activity combined with modest Ca2+ sensitization responses in aortic SMCs diminishes contractile reserve. Thus, haploinsufficiency of MLCK should diminish aortic SMC contraction.
PRKG1 Mutations
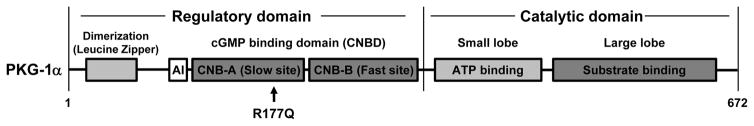
A single heterozygous PRKG1 mutation, p.Arg177Gln (designated R177Q), causes heritable thoracic aortic disease 7. Other PRKG1 rare variants are found in thoracic aortic disease patients and are present in the general population, but the only variant confirmed to cause thoracic aortic disease is PRKG1 R177Q. PRKG1 encodes a type I cGMP-dependent protein kinase (PKG-1), which is activated upon binding of cGMP and controls SMC relaxation (Figure 6). The kinase contains N-terminal dimerization/docking and auto-inhibitory domains, two regulatory cGMP binding sites, and a catalytic domain. Upon binding of cGMP to the two cGMP binding sites, the regulatory domain undergoes a major conformational change, releasing and activating the catalytic domain. PKG-1 R177Q is located in the high-affinity cGMP binding site, and introduction of R177Q into this domain abolished binding of cGMP. Paradoxically, introduction of R177Q into the entire kinase led to the enzyme being constitutively active even in the absence of cGMP, both in vitro and in intact cells 7.
Figure 6.
Schematic of type I cGMP-dependent protein kinase (PKG-1), which is activated upon binding of cGMP to the CNB-A and CNB-B domains. With cGMP binding, the autoinhibitory domain (AI) is released from the catalytic domain to activate the kinase. A single mutation, R177Q, has been identified to cause heritable thoracic aortic disease and is located in the CNB-A cGMP binding domain. The mutation leads to constitutive activation of the kinase such that it is no longer regulated by cGMP.
Although two PKG-1 splice variants (PKG-1α and PKG-1β) exist in mammalian tissues, PKG-1α is the major isoform present in vascular SMCs and is highly expressed in SMCs61. PKG-1 induces SMC relaxation through phosphorylation of multiple target proteins, including inositol 1,4,5-trisphosphate receptor I-associated protein (IRAG) and large-conductance calcium-activated potassium channels (BK) channels to lower [Ca2+]i, as well as MYPT1, and RhoA to activate MLCP 62, 63. The PKG-1 R177Q variant is constitutively activated and increases the rate of dephosphorylation of RLC in response to cGMP in aortic SMCs. Thus, the overall effect of the PRKG1 R177Q alteration on RLC phosphorylation is similar to the MYLK loss-of-function mutations, specifically decreased RLC phosphorylation and diminished SMC force generation.
PKG1 regulates vascular tone in vivo, and both Prkg1−/− mice and mice harboring a PKG1α mutation that disrupts kinase dimerization and substrate targeting are hypertensive 64, 65. Patients with the constitutively active mutation, PRKG1 p.Arg177Gln, would be expected to be hypotensive, but paradoxically, only a few mutation carriers are hypotensive and others are hypertensive 7.
Other genes for heritable thoracic aortic disease and SMC contraction
Although the heritable thoracic aortic disease genes reviewed here encode proteins directly involved in SMC contraction and relaxation, additional genes for this condition either have the potential or have been shown to decrease aortic SMC contraction. SMCs need to adhere to the extracellular matrix to productively contract, and in the aorta, the contractile elements link to microfibrils surrounding the elastin fibers through focal adhesions on the cell surface of SMCs. The major protein in microfibrils is the large glycoprotein, fibrillin-1, and genetic alterations in the gene coding for this protein, FBN1, predispose to thoracic aortic disease in patients with Marfan syndrome 3. Additionally, common variants in FBN1 have been shown to predispose to thoracic aortic disease in the general population through genome wide association studies 21. Fibrillin-1 is secreted by cells and polymerizes to form microfibrils, and FBN1 mutations disrupt the structure and deposition of microfibrils into the matrix 66–68. In fact, the connections between the SMCs and elastin fibers are completely lost in a hypomorphic mouse model of Marfan syndrome 69. Studies of mouse models of Marfan syndrome (Fbn1C1039G/+) found decreased contraction of isolated aortic rings in response to agonists, consistent with diminished force transduction by SMCs predicted to occur with loss of SMC connections to elastin fibers 70. Thus, decreased force generation by aortic SMCs may contribute to aortic disease in individuals with Marfan syndrome. Additional genes encoding other microfibril proteins (MFAP5) and enzymes important for the structural integrity of the elastin fibers (LOX) may also disrupt the adherence of SMCs to the elastin fibers 8, 9
Genetic alteration of the genes that encode proteins in the canonical TGF-β signaling pathway can also predispose to heritable thoracic aortic disease. Although initial studies suggested increased TGF-β signaling was the primary driver for thoracic aortic disease, the mutations disrupting TGF-β pathway are predicted or have been shown to decrease TGF-β signaling 13, 14, 71. TGF-β signaling is critical for the differentiation of neural crest cells into SMCs populating the ascending thoracic aorta during development 72, 73; thus, lower TGF-β signaling may disrupt proper differentiation of neural crest-derived SMCs that populate the ascending aorta. Consistent with this hypothesis, SMCs explanted from individuals with TGFBR2 mutations are relatively de-differentiated in culture when compared to control SMCs and fail to differentiate to the same extent as control SMCs when exposed to TGF-β1 71. Differentiation of SMCs is defined by the expression of SMC contractile proteins, including SM α-actin and SM myosin heavy chain. Thus, a lack of differentiation due to loss-of-function mutations in genes involved in TGF-β signaling may also limit the number of contractile units in aortic SMCs and decrease SMC force generation.
Summary
To conclude, disruption of the ability of aortic SMCs to generate force in response to pulsatile blood flow through the elastin-contractile unit may be the primary driver for thoracic aortic aneurysms and dissections. Mutated genes that predispose to the disease alter SMC force generation by disruption of the major structural components of the SMC contractile unit, i.e., the kinases regulating contraction through phosphorylation of the RLC or the extracellular matrix to which the contractile units are linked. These data suggest that disruption of the elastin-contractile unit will lead to altered mechanosensing by the SMCs and altered cell signaling pathways to cause disease, which could include increase canonical and noncanonical TGF-β signaling and angiotensin II signaling through the angiotensin type I receptor. The hypothesis that altered mechanosensing through the elastin-contractile unit is the major driver of thoracic aortic disease provides an explanation for other triggers of the disease, such as hypertension. In a hypertensive individual, the elastin-contractile unit may not be altered due to genetic alterations but the forces across the unit will be increased due to the hypertension, thus potentially altering similar cellular pathways downstream of the elastin-contractile unit. It is also relevant to note that studies have pursued the genetic variants that increase the risk for thoracic aortic disease in individuals who do not have heritable thoracic aortic disease, including genome wide association studies and copy number variant analyses. These studies have identified low risk genetic variants in the same genes encoding proteins in the elastin contractile unit, including FBN1 and MYH11 21, 54, 74. Finally, the hypothesis that disruption of SMC force generation is the primary driver of the disease informs the selection of drugs to prevent thoracic aortic disease. The longstanding therapeutic recommendation to decrease the growth rate of thoracic aortic aneurysms and the risk for acute aortic dissections is treatment with β-adrenergic blocking agent (β-blockers). β-blockers decrease both the force and number of beats of the heart and thus decrease the hemodynamic forces across the elastin-contractile unit in the thoracic aorta. In contrast, antihypertensive drugs that act through decreasing SMC force generation may increase the risk of thoracic aortic disease or increase the severity of disease in heritable thoracic aortic disease. Supporting this hypothesis is recent data that calcium channel blockers accelerated thoracic aortic disease in a Marfan mouse model and increased the risk for aortic dissection and need for surgery in patients with Marfan syndrome 75. Similarly, treatment with the antihypertensive hydralazine, also a SMC relaxant, increased the risk for aortic dissection induced by feeding turkeys β-aminopropionitrile 76. Thus, accumulating data suggests that altered mechanosensing of aortic SMCs through the elastin-contractile unit is a primary driver of thoracic aortic disease.
Supplementary Material
Highlights.
Force generation by aortic smooth muscle cells (SMCs) requires ATP-dependent cyclic interactions between filaments composed of SMC specific isoforms of α-actin (encoded by ACTA2) and myosin heavy chain (MYH11).
ACTA2 and MYH11 mutations shown to disrupt this cyclic interaction predispose to thoracic aortic disease.
Movement of the myosin motor domain is controlled by phosphorylation of the regulatory light chain on the myosin filament, and loss-of-function mutations in the dedicated kinase for this phosphorylation, myosin light chain kinase (MYLK) also predispose to thoracic aortic disease.
A mutation in the cGMP-activated protein kinase (PRKG1) results in constitutive activation of the kinase in the absence of cGMP, thus driving SMC relaxation in part through increased de-phosphorylation of the regulatory light chain, and predisposes to thoracic aortic disease.
Disruption of the ability of the aortic SMC to generate force through the elastin-contractile units in response to pulsatile blood flow may be a primary driver for thoracic aortic aneurysms and dissections.
Acknowledgments
We are extremely grateful to the individuals and families who participated in this study and the physicians and genetic counselors who aided in the collection of clinical data from the families.
Sources of Funding
The following sources provided funding for these studies: RO1 HL62594, P01HL110869-01, N01-HV-68199, HHSN268200648199C, John Ritter Foundation, Vivian L. Smith Foundation, and Richard T. Pisani Funds (D.M. Milewicz).
Footnotes
Disclosers: None
References
- 1.LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011 Feb;8:103–13. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 2.Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013 May 21;127:2031–7. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai LY, Keene DR, Renard M, De BJ. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene. 2016 Oct 10;591:279–91. doi: 10.1016/j.gene.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo DC, Pannu H, Papke CL, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007 Dec;39:1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Vranckx R, Khau Van KP, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006 Mar;38:343–9. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Guo DC, Cao J, Gong L, Kamm KE, Regalado E, Li L, Shete S, He WQ, Zhu MS, Offermanns S, Gilchrist D, Elefteriades J, Stull JT, Milewicz DM. Mutations in Myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010 Nov 12;87:701–7. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo DC, Regalado E, Casteel DE, et al. Recurrent Gain-of-Function Mutation in PRKG1 Causes Thoracic Aortic Aneurysms and Acute Aortic Dissections. Am J Hum Genet. 2013 Aug 8;93:398–404. doi: 10.1016/j.ajhg.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbier M, Gross MS, Aubart M, et al. MFAP5 Loss-of-Function Mutations Underscore the Involvement of Matrix Alteration in the Pathogenesis of Familial Thoracic Aortic Aneurysms and Dissections. Am J Hum Genet. 2014 Dec 4;95:736–43. doi: 10.1016/j.ajhg.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo DC, Regalado ES, Gong L, et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016 Mar 18;118:928–34. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuguchi T, Collod–Beroud G, Akiyama T, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004 Aug;36:855–60. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med. 2006 Aug 24;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 12.Pannu H, Fadulu V, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, Shete S, Willing MC, Raman CS, Milewicz DM. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005 Jul 26;112:513–20. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 13.Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos-Cortez RL, Leal SM, Bertoli-Avella AM, Shendure J, Rieder MJ, Nickerson DA, Milewicz DM. Exome Sequencing Identifies SMAD3 Mutations as a Cause of Familial Thoracic Aortic Aneurysm and Dissection With Intracranial and Other Arterial Aneurysms. Circ Res. 2011 Sep 2;109:680–6. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–21. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoli-Avella AM, Gillis E, Morisaki H, et al. Mutations in a TGF-beta ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015 Apr 7;65:1324–36. doi: 10.1016/j.jacc.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo DC, Gong L, Regalado ES, et al. MAT2A Mutations Predispose Individuals to Thoracic Aortic Aneurysms. Am J Hum Genet. 2015 Jan 8;96:170–7. doi: 10.1016/j.ajhg.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuang SQ, Medina-Martinez O, Guo DC, et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest. 2016 Mar 1;126:948–61. doi: 10.1172/JCI83778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milewicz DM, Guo D, Tran-Fadulu V, Lafont A, Papke C, Inamoto S, Pannu H. Genetic Basis of Thoracic Aortic Aneurysms and Dissections: Focus on Smooth Muscle Cell Contractile Dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology. Dysfunctional mechanosensing in aneurysms. Science. 2014 May 2;344:477–9. doi: 10.1126/science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of Mechanotransduction in Vascular Biology: Focus on Thoracic Aortic Aneurysms and Dissections. Circ Res. 2015 Apr 10;116:1448–61. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeMaire SA, McDonald ML, Guo DC, et al. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat Genet. 2011 Oct;43:996–1000. doi: 10.1038/ng.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: more evidence for a connection. Cardiology. 2007;107:103–6. doi: 10.1159/000094530. [DOI] [PubMed] [Google Scholar]
- 23.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010 Nov 15;42A:169–87. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008 Sep;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002 Jan;16:72–6. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 26.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008 Sep;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor E, Kempster L, Wait R, Gosling M, Dunn MJ, Powell JT. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol Cell Proteomics. 2004 Feb;3:115–24. doi: 10.1074/mcp.M300046-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.van den Akker J, Schoorl MJ, Bakker EN, Vanbavel E. Small artery remodeling: current concepts and questions. J Vasc Res. 2010;47:183–202. doi: 10.1159/000255962. [DOI] [PubMed] [Google Scholar]
- 29.Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984 Dec 10;259:14383–8. [PubMed] [Google Scholar]
- 30.Drew JS, Murphy RA. Actin isoform expression, cellular heterogeneity, and contractile function in smooth muscle. Can J Physiol Pharmacol. 1997 Jul;75:869–77. [PubMed] [Google Scholar]
- 31.Stromer MH, Mayes MS, Bellin RM. Use of actin isoform-specific antibodies to probe the domain structure in three smooth muscles. Histochem Cell Biol. 2002 Oct;118:291–9. doi: 10.1007/s00418-002-0453-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol. 2005 May;288:C1145–C1160. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Pavlish K, Benoit JN. Myosin phosphorylation triggers actin polymerization in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2008 Nov;295:H2172–H2177. doi: 10.1152/ajpheart.91437.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2008 Jun;13:130–40. doi: 10.1177/1074248407313737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem. 2008 Dec 26;283:36522–31. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HR, Leavis PC, Graceffa P, Gallant C, Morgan KG. A new method for direct detection of the sites of actin polymerization in intact cells and its application to differentiated vascular smooth muscle. Am J Physiol Cell Physiol. 2010 Nov;299:C988–C993. doi: 10.1152/ajpcell.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 38.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003 Oct;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 39.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001 Feb 16;276:4527–30. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 40.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008 Aug;135:610–20. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang WC, Peng YJ, Zhang GS, et al. Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J Biol Chem. 2010 Feb 19;285:5522–31. doi: 10.1074/jbc.M109.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendt T, Taylor D, Messier T, Trybus KM, Taylor KA. Visualization of head-head interactions in the inhibited state of smooth muscle myosin. J Cell Biol. 1999 Dec 27;147:1385–90. doi: 10.1083/jcb.147.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowey S, Trybus KM. Common structural motifs for the regulation of divergent class II myosins. J Biol Chem. 2010 May 28;285:16403–7. doi: 10.1074/jbc.R109.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest. 1993 Jan;68:89–99. [PubMed] [Google Scholar]
- 45.Berry CL, Greenwald SE, Rivett JF. Static mechanical properties of the developing and mature rat aorta. Cardiovasc Res. 1975 Sep;9:669–78. doi: 10.1093/cvr/9.5.669. [DOI] [PubMed] [Google Scholar]
- 46.Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009 May;84:617–27. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morisaki H, Akutsu K, Ogino H, Kondo N, Yamanaka I, Tsutsumi Y, Yoshimuta T, Okajima T, Matsuda H, Minatoya K, Sasaki H, Tanaka H, Ishibashi-Ueda H, Morisaki T. Mutation of ACTA2 gene as an important cause of familial and nonfamilial nonsyndromatic thoracic aortic aneurysm and/or dissection (TAAD) Hum Mutat. 2009 Oct;30:1406–11. doi: 10.1002/humu.21081. [DOI] [PubMed] [Google Scholar]
- 48.Milewicz DM, Kwartler CS, Papke CL, Regalado ES, Cao J, Reid AJ. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet Med. 2010 Apr;12:196–203. doi: 10.1097/GIM.0b013e3181cdd687. [DOI] [PubMed] [Google Scholar]
- 49.Milewicz DM, Carlson AA, Regalado ES. Genetic testing in aortic aneurysm disease: PRO. Cardiol Clin. 2010 May;28:191–7. doi: 10.1016/j.ccl.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regalado ES, Guo DC, Prakash S, et al. Aortic Disease Presentation and Outcome Associated With ACTA2 Mutations. Circ Cardiovasc Genet. 2015 Jun;8:457–64. doi: 10.1161/CIRCGENETICS.114.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu H, Fagnant PM, Bookwalter CS, Joel P, Trybus KM. Vascular disease-causing mutation R258C in ACTA2 disrupts actin dynamics and interaction with myosin. Proc Natl Acad Sci U S A. 2015 Aug 4;112:E4168–E4177. doi: 10.1073/pnas.1507587112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007 Oct 15;16:3453–62. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harakalova M, van der SJ, de Kovel CG, et al. Incomplete segregation of MYH11 variants with thoracic aortic aneurysms and dissections and patent ductus arteriosus. Eur J Hum Genet. 2013 May;21:487–93. doi: 10.1038/ejhg.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuang SQ, Guo DC, Prakash SK, et al. Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genet. 2011 Jun;7:e1002118. doi: 10.1371/journal.pgen.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwartler CS, Chen J, Thakur D, Li S, Baskin K, Wang S, Wang ZV, Walker L, Hill JA, Epstein HF, Taegtmeyer H, Milewicz DM. Overexpression of smooth muscle myosin heavy chain leads to activation of the unfolded protein response and autophagic turnover of thick filament associated proteins in vascular smooth muscle cells. J Biol Chem. 2014 Apr 7; doi: 10.1074/jbc.M113.499277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuang SQ, Kwartler CS, Byanova KL, Pham J, Gong L, Prakash SK, Huang J, Kamm KE, Stull JT, Sweeney HL, Milewicz DM. Rare, Nonsynonymous Variant in the Smooth Muscle-Specific Isoform of Myosin Heavy Chain, MYH11, R247C, Alters Force Generation in the Aorta and Phenotype of Smooth Muscle Cells. Circ Res. 2012 May;110:1411–22. doi: 10.1161/CIRCRESAHA.111.261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallace KN, Dolan AC, Seiler C, Smith EM, Yusuff S, Chaille-Arnold L, Judson B, Sierk R, Yengo C, Sweeney HL, Pack M. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell. 2005 May;8:717–26. doi: 10.1016/j.devcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Abrams J, Einhorn Z, Seiler C, Zong AB, Sweeney HL, Pack M. Graded effects of unregulated smooth muscle myosin on intestinal architecture, intestinal motility and vascular function in zebrafish. Dis Model Mech. 2016 May 1;9:529–40. doi: 10.1242/dmm.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herring BP, El-Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol. 2006 Nov;291:C817–C827. doi: 10.1152/ajpcell.00198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao N, Huang J, He W, Zhu M, Kamm KE, Stull JT. Signaling through myosin light chain kinase in smooth muscles. J Biol Chem. 2013 Mar 15;288:7596–605. doi: 10.1074/jbc.M112.427112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010 Sep;62:525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surks HK. cGMP-dependent protein kinase I and smooth muscle relaxation: a tale of two isoforms. Circ Res. 2007 Nov 26;101:1078–80. doi: 10.1161/CIRCRESAHA.107.165779. [DOI] [PubMed] [Google Scholar]
- 63.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (cGK) Handb Exp Pharmacol. 2009:137–62. doi: 10.1007/978-3-540-68964-5_8. [DOI] [PubMed] [Google Scholar]
- 64.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998 Jun;17:3045–51. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanton RM, Takimoto E, Aronovitz M, Thoonen R, Kass DA, Karas RH, Mendelsohn ME. Mutation of the protein kinase I alpha leucine zipper domain produces hypertension and progressive left ventricular hypertrophy: a novel mouse model of age-dependent hypertensive heart disease. J Gerontol A Biol Sci Med Sci. 2013 Nov;68:1351–5. doi: 10.1093/gerona/glt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollister DW, Godfrey M, Sakai LY, Pyeritz RE. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990 Jul;323:152–9. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- 67.Milewicz DM, Pyeritz RE, Crawford ES, Byers PH. Marfan syndrome: defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992 Jan;89:79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Handford PA, Downing AK, Reinhardt DP, Sakai LY. Fibrillin: from domain structure to supramolecular assembly. Matrix Biol. 2000 Nov;19:457–70. doi: 10.1016/s0945-053x(00)00100-1. [DOI] [PubMed] [Google Scholar]
- 69.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of marfan syndrome. Circ Res. 2001 Jan;88:37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- 70.Yang HH, van BC, Chung AW. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vascul Pharmacol. 2010 Jan;52(1–2):37–45. doi: 10.1016/j.vph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Inamoto S, Kwartler CS, Lafont AL, et al. TGFBR2 Mutations Alter Smooth Muscle Cell Phenotype and Predispose to Thoracic Aortic Aneurysms and Dissections. Cardiovasc Res. 2010 Jul;88:520–9. doi: 10.1093/cvr/cvq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997 Jul;124:2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004 May;94:1195–202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- 74.Prakash SK, LeMaire SA, Guo DC, Russell L, Regalado ES, Golabbakhsh H, Johnson RJ, Safi HJ, Estrera AL, Coselli JS, Bray MS, Leal SM, Milewicz DM, Belmont JW. Rare copy number variants disrupt genes regulating vascular smooth muscle cell adhesion and contractility in sporadic thoracic aortic aneurysms and dissections. Am J Hum Genet. 2010 Dec;87:743–56. doi: 10.1016/j.ajhg.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doyle JJ, Doyle AJ, Wilson NK, et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife. 2015 Oct;4:e08648. doi: 10.7554/eLife.08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simpson CF, Taylor WJ. Effect of hydralazine on aortic rupture induced by B-aminopropionitrile in turkeys. Circulation. 1982 Apr;65:704–8. doi: 10.1161/01.cir.65.4.704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.