Abstract
RECQL1, a key member of the RecQ family of DNA helicases, is required for DNA replication and DNA repair. Two recent studies have shown that germ-line RECQL1 mutations are associated with increased breast cancer susceptibility. Whether altered RECQL1 expression has clinicopathological significance in sporadic breast cancers is unknown. We evaluated RECQL1 at the transcriptomic level [METABRIC cohort, n=1977] and at the protein level [cohort 1, n=897; cohort 2, n= 252; cohort 3 (BRCA-germline deficient), n=74]. In RECQL1-depleted breast cancer cells we investigated anthracycline sensitivity. High RECQL1 mRNA was associated with intClust.3 (p=0.026) which is characterised by low genomic instability. On the other hand, low RECQL1 mRNA was linked to intClust.8 (luminal A ER+ sub-group) (p=0.0455) and intClust.9 (luminal B ER+ sub-group) (p=0.0346) molecular phenotypes. Low RECQL1 expression was associated with shorter breast cancer specific survival (p=0.001). At the protein level, low nuclear RECQL1 level was associated with larger tumour size, lymph node positivity, high tumour grade , high mitotic index, pleomorphism, de-differentiation, ER negativity and HER-2 overexpression (p values<0.05). In ER+ tumours that received endocrine therapy, low RECQL1 was associated with poor survival (p=0.008). However, in ER− negative tumours that received anthracycline based chemotherapy, high RECQL1 was associated with poor survival (p=0.048). In RECQL1-depleted breast cancer cell lines we confirmed doxorubicin sensitivity which was associated with DNA double strand breaks accumulation, S-phase cell cycle arrest and apoptosis. We conclude that RECQL1 has prognostic and predictive significance in breast cancers.
Keywords: RECQL1, helicase, breast cancer, biomarker, prognosis, doxorubicin
INTRODUCTION
DNA helicases unwind DNA, a process essential during replication and DNA repair. Human RecQ family of DNA helicases includes RECQL1, RECQL4, RECQL5, BLM and WRN (1, 2). RECQL1 (also known as RECQL or RECQ1) is localised to chromosome 12p12 and encodes a 649 amino acid protein (3-6). RECQL1 is the smallest and the most abundant of human RecQ helicases. RECQL1 is an integral component of the replication complex and is required for the maintenance of replication fork progression (7-9). RECQL1 is also essential for the maintenance of genomic stability through roles in DNA repair. RECQL1, besides a DNA 3’-5’ helicase activity, can promote branch migration of Holliday junctions and also has strand annealing activity (10). Moreover, to accomplish its various biological functions RECQL1 is known to interact with various proteins involved in DNA repair including PARP1, RPA, RAD51, Top3α, EXO1, MSH2/6, MLH1-PMS2 and Ku70/80 (3-6). The essential role played by RECQL1 in DNA repair is underpinned by the fact that RECQL1 depletion in cells results in increased frequency of spontaneous sister chromatid exchanges, chromosomal instability, DNA damage accumulation and increased sensitivity to cytotoxic chemotherapy (11).
Emerging data suggest a role for RECQL1 in breast cancer pathogenesis. Importantly, two recent studies have shown that germ-line RECQL1 mutations are associated with increased breast cancer susceptibility (12-14). Sun et.al. have identified pathogeneic mutations in RECQL1 gene in 9/448 Chinese patients with BRCA- negative familial breast cancers (12). Similarly, Cybulski et.al. identified deleterious mutations in 7/1013 and 30/13,136 Polish breast cancer patients (13). Although germ-line mutations in RECQL1 are rare, the data provides evidence that RECQL1 is a tumour suppressor. However whether RECQL1 also influences sporadic breast cancer pathogenesis and prognosis is currently unknown.
In the current study we have comprehensively investigated RECQL1 in large cohorts of sporadic breast cancer and have provided the first clinical evidence that altered RECQL1 expression is associated with aggressive breast cancers and poor prognosis. Pre-clinically, RECQL1 depletion in breast cancer cells increased anthracycline chemosensitivity. We conclude that RECQL1 expression has prognostic and predictive significance in sporadic breast cancers.
METHODS
Clinical study
RECQL1 mRNA expression in breast cancer
RECQL1 mRNA expression was investigated in METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) cohort. The METABRIC study protocol, detailing the molecular profiling methodology in a cohort of 1977 breast cancer samples is described by Curtis et al (15). Patient demographics are summarised in supplementary Table S1 of supporting information. ER positive and/or lymph node negative patients did not receive adjuvant chemotherapy. ER negative and/or lymphnode positive patients received adjuvant chemotherapy. For this cohort, the mRNA expression was hybridized to Illumina HT-12 v3 platform (Bead Arrays), and the data were pre-processed and normalised as described previously. RECQL1 expression was evaluated in this data set (RECQL1 probe ID: ILMN_1692705). The probe was a perfect match and quality for its target, having a GC content of 58%, 0 SNPs and it does not possess a polyG tail at the end. Samples were classified into the intrinsic subtypes based on the PAM50 gene list. A description of the normalisation, segmentation, and statistical analyses was previously described (15). Real time RT-qPCR was performed on the ABI Prism 7900HT sequence detection system (Applied Biosystems) using SYBR1 Green reporter. All the samples were analysed as triplicates. The Chi-square test was used for testing association between categorical variables, and a multivariate Cox model was fitted to the data using as endpoint breast cancer specific death. Xtile (Version 3.6.1) was used to identify a cut-off in gene expression values such that the resulting subgroups had significantly different survival courses (16).
RECQL1 protein expression in breast cancer
The study was performed in a consecutive series of 1650 patients with primary invasive breast carcinomas who were diagnosed between 1986 and 1999 and entered into the Nottingham Tenovus Primary Breast Carcinoma series. Patient demographics are summarised in Supplementary Table S2. This is a well-characterised series of patients with long-term follow-up that have been investigated in a wide range of biomarker studies (17-23). All patients were treated in a uniform way in a single institution with standard surgery (mastectomy or wide local excision), followed by Radiotherapy. Prior to 1989, patients did not receive systemic adjuvant treatment (AT). After 1989, AT was scheduled based on prognostic and predictive factor status, including Nottingham Prognostic Index (NPI), oestrogen receptor-α (ER-α) status, and menopausal status. Patients with NPI scores of <3.4 (low risk) did not receive AT. In pre-menopausal patients with NPI scores of ≥3.4 (high risk), classical Cyclophosphamide, Methotrexate, and 5-Flurouracil (CMF) chemotherapy was given; patients with ER-α positive tumours were also offered endocrine therapy. Postmenopausal patients with NPI scores of ≥3.4 and ER-α positivity were offered endocrine therapy, while ER-α negative patients received classical CMF chemotherapy. Median follow up was 111 months (range 1 to 233 months). Survival data, including breast cancer specific survival (BCSS), disease-free survival (DFS), and development of loco-regional and distant metastases (DM), was maintained on a prospective basis. DFS was defined as the number of months from diagnosis to the occurrence of local recurrence, local lymph node (LN) relapse or DM relapse. Breast cancer specific survival (BCSS) was defined as the number of months from diagnosis to the occurrence of BC related-death. Local recurrence free survival (LRS) was defined as the number of months from diagnosis to the occurrence of local recurrence. DM-free survival was defined as the number of months from diagnosis to the occurrence of DM relapse. Survival was censored if the patient was still alive at the time of analysis, lost to follow-up, or died from other causes.
We also evaluated an independent series of 252 ER-α negative invasive BCs diagnosed and managed at the Nottingham University Hospitals between 1999 and 2007. All patients were primarily treated with surgery, followed by radiotherapy and anthracycline chemotherapy. The characteristics of this cohort are summarised in supplementary Table S3. In addition we also explored RECQL1 expression in a cohort of BRCA germ-line deficient tumours. Patient demographics in this cohort is summarised in supplementary Table S4.
Tumor Marker Prognostic Studies (REMARK) criteria, recommended by McShane et al (24), were followed throughout this study. Ethical approval was obtained from the Nottingham Research Ethics Committee (C202313).
Tissue Microarrays (TMAs) and immunohistochemistry (IHC)
Tumours were arrayed in tissue microarrays (TMAs) constructed with 0.6mm cores sampled from the periphery of the tumours. The TMAs were immunohistochemically profiled for RECQL1 and other biological antibodies (Supplementary Table S5) as previously described (18, 19, 21, 23). Immunohistochemical staining was performed using the Thermo Scientific Shandon Sequenza chamber system (REF: 72110017), in combination with the Novolink Max Polymer Detection System (RE7280-K: 1250 tests), and the Leica Bond Primary Antibody Diluent (AR9352), each used according to the manufacturer’s instructions (Leica Microsystems). Leica Autostainer XL machine was used to dewax and rehydrate the slides. Pre-treatment antigen retrieval was performed on the TMA sections using sodium citrate buffer (pH 6.0) and heated for 20 minutes at 95°C in a microwave (Whirpool JT359 Jet Chef 1000W). A set of slides were incubated for 60 minutes with the primary anti-RECQL1 antibody (Bethyl Laboratories, catalog no. A300-450A) at a dilution of 1:1000 respectively. Negative and positive (by omission of the primary antibody and IgG-matched serum) controls were included in each run. The negative control ensured that all the staining was produced from the specific interaction between antibody and antigen.
Evaluation of immune staining
Whole field inspection of the core was scored and intensities of nuclear staining were grouped as follows: 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining. The percentage of each category was estimated (0-100%). H-score (range 0-300) was calculated by multiplying intensity of staining and percentage staining. RECQL1 expression was categorised based on the frequency histogram distributions. The tumour cores were evaluated by two scorers (AA and MA) and the concordance between the two scorers was excellent (k = 0.79). Xtile (Version 3.6.1) was used to identify a cut-off in protein expression values such that the resulting subgroups had significantly different survival courses. An H score of ≥215 was taken as the cut-off for high RECQL1 level. Not all cores within the TMA were suitable for IHC assessments as some cores were missing or containing inadequate invasive cancer (<15% tumour).
Statistical analysis
Data analysis was performed using SPSS (SPSS, version 17 Chicago, IL). Where appropriate, Pearson’s Chi-square, Fisher’s exact, Student’s t and ANOVA one way tests were used. Cumulative survival probabilities were estimated using the Kaplan–Meier method, and differences between survival rates were tested for significance using the log-rank test. Multivariate analysis for survival was performed using the Cox proportional hazard model. The proportional hazards assumption was tested using standard log-log plots. Hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated for each variable. All tests were two-sided with a 95% CI and a p value <0.05 considered significant. For multiple comparisons, p values were adjusted according to Benjamini-Hochberg method (25).
Breast cancer cell lines and culture
MCF-7 (ER+/PR+/HER2−, BRCA1 proficient), MDA-MB-231 (ER−/PR−/HER2−, BRCA1 proficient), MDA-MB-468 (ER−/PR−/HER2−, BRCA1 proficient) and MDA-MB-436 (ER−/PR−/HER2−, BRCA1 deficient) were purchased from ATCC and were grown in RPMI (MCF-7) or DMEM (MDA-MB-231, MDA-MB-468 and MDA-MB-436) medium with the addition of 10% foetal bovine serum and 1% penicillin/streptomycin. Cells in culture were routinely checked for mycoplasma contamination by PCR (Sigma, catalog no. MP0035).The cells characterisation were performed by ATCC and passaged in the laboratory for fewer than 6 months.
RECQL1 depletion in breast cancer cells
On-Target plus SMARTpool small interfering RNAs (siRNAs) against RECQL1 (NM_032941), and non-targeting control (CTL) were purchased from Dharmacon (catalog nos. L-013597-00-0005 and D-001810-10-05, respectively). We have previously established the specificity of the siRNA pool (5). All siRNA transfections (in MCF-7, MDA-MB-231 and MDA-MB-468 breast cancer cells) were performed by reverse transfection at a final concentration of 20 nM using Lipofectamine RNAiMAX (Invitrogen, catalog no. 13-778-075) as instructed by the manufacturer. Stable shRNA-mediated knockdown of RECQL1 in MDA-MB-231 cells was achieved using a lentiviral system (26). Briefly, lentivirus particles were produced by cotransfecting 293T cells with the pLKO.1 lentiviral shRNA expression vector containing the RECQL1 targeting sequence (5”-GAGCTTATGTTACCAGTTA-3”) or the gene encoding Luciferase (5”-ACGCTGAGTACTTCGAAATGT-3”) with the packaging plasmids psPAX2 and pM2D.G; and used to transduce MDA-MB-231 cells, followed by selection with puromycin (8 μg/ml). All cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C and routinely checked for mycoplasma contamination (Sigma, catalog no. MP0035). The level of RECQL1 depletion was verified by western blotting.
Western Blot Analysis
Whole-cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail (Sigma, catalog no. 11873580001), and protein was quantified using Bio-Rad DC protein assay kit (Bio-Rad, catalog no. 5000111). Fifty microgram of total protein per lane was used for immunoblotting. The following primary antibodies were used: RECQL1 (1:1000; Bethyl Laboratories, catalog no. A300-450A), γH2AX (1:1000; Cell Signaling, catalog no. 2577), GAPDH (1:1000; Cell Signaling, catalog no. 5174), ERα (1:100, EP1 clone, Dako, catalog no. IS08430-2.), and β actin (1:10000; Abcam, catalog no. ab8226). Following incubation with infrared dye-labelled (Li-Cor) [IRDye 800CW Mouse Anti-Rabbit IgG and IRDye 680CW Rabbit Anti-Mouse IgG; 1:10000] or HRP-conjugated secondary antibodies (Vector Laboratories) for 1 h, membranes were scanned with a Li-Cor Odyssey machine (700 and 800nm) or GeneGnome XRQ Chemidoc System (Syngene) to determine protein expression and signal intensities were quantified using ImageJ.
Quantitative real time PCR
Total RNA was extracted from MCF-7, MDA-MB-231, MDA-MB-468 and MDA-MB-436 cells using RNeasy Mini kit (QIAGEN, UK). The quantification of the extracted RNA was done using a NanoDrop 2000c Spectrophotometer (Thermo Scientific). The cDNA was synthesised from 0.5 μg of total RNA using RT2 first strand kit (QIAGEN). The real-time qPCR was performed using SYBR Green PCR Master mix (Applied Biosystems) with primer set (RECQL1 QuantiTect Primer Assay, catalog no. QT00034503, QIAGEN) targeting RECQL1 gene. The glyceraldehyde-3-phosphate dehydrogenase housekeeper gene was used as an internal control (GAPDH QuantiTect Prier Assay, catalog no. QT00079247, QIAGEN). The real-time PCR for each RNA sample was performed in triplicate. NTC (No Template Control) was used to rule out cross contamination of reagents and surfaces. NTC included all the RT-PCR reagents except the RNA template. Minus reverse transcriptase (- RT) control was used to rule out genomic DNA contamination.
Cytotoxicity and cell cycle analysis
Cells, stably transduced or 48 h after siRNA transfection, plated in quadruplicates into a 96-well plate (5×103 cells/well) were treated with increasing concentrations of doxorubicin and cell viability was measured after 5 days by the WST-8 based colorimetric assay using Cell Counting Kit-8 (Dojindo Laboratories) according to the manufacturer’s instructions. For cell cycle analysis, cells were fixed in cold ethanol before being stained with propidium iodide (Sigma, 0.45 mg/mL). Resuspended cells were analysed for DNA content by flow cytometry performed on a BD Accuri C6 flow cytometer equipped with BD Accuri C6 software (BD Biosciences). Means from two independent experiments were plotted with their respective standard errors of the means (SEM). Statistically significant differences between cell populations was confirmed using a 2-tailed t-test, assuming equal variances and are presented on figures as *= p ≤ 0.05, ** = p ≤ 0.005.
Immunofluorescence staining analysis
For γH2AX staining, control and RECQL1 knockdown cells were grown on coverslips in the medium containing 0.1 μM doxorubicin for 4 h and allowed to recover in drug free medium for indicated time periods. Cells were fixed in 3.75% paraformaldehyde for 10 min at room temperature, permeabilized in 0.5% Triton-X100 in PBS for 10 min and blocked with 1% BSA in PBS for 1 h at room temperature followed by incubation with mouse monoclonal anti-γH2AX (1:200; Upstate, JBW301) antibody for 1 h at 37°C. After three washes in PBS for 5 min each, the cells were incubated with Alexa Fluor 488 goat anti-mouse IgG (1:400; Invitrogen) secondary antibody for 1 h at 37°C, washed thrice with PBS and mounted in Prolong Gold containing DAPI (Invitrogen). Immunostained cells were imaged with a Nikon fluorescence microscope (Eclipse Ti) equipped with imaging capabilities and Elements imaging software. Scoring for each individual condition (siRNA or shRNA, cell line, drug treatment etc.) within an experiment was carried out on at least 10 separate fields of view and about 50-100 cells in total. Means from two independent experiments were plotted with their respective standard errors of the means (SEM). Statistically significant differences between cell populations was confirmed using a 2-tailed t-test, assuming equal variances and are presented on figures as *= p ≤ 0.05, ** = p ≤ 0.005.
RESULTS
RECQL1 mRNA expression in human breast cancer
We then evaluated RECQL1 mRNA expression in the METABRIC cohort. 31.7% (626/1971) of breast tumours had low RECQL1 mRNA expression and 68.3% (1345/1971) of breast tumours had high RECQL1 mRNA expression. Clinicopathological associations are summarized in Supplementary Table S6. The METABRIC study by joint clustering of copy number and gene expression data identified 10 novel biological subgroups [labelled integrative clusters (intClust) 1-10] (15). We investigated whether RECQL1 mRNA expression would associate with these distinct biological subgroups (Supplementary Table S7). High RECQL1 mRNA was associated with intClust.3 (p=0.026) which is characterised by low genomic instability (15). On the other hand low RECQL1 mRNA was linked to intClust.8 (p=0.0455) and intClust.9 (p=0.0346) phenotypes. Of note, intClust.8 belongs to Luminal A ER+ sub-group where as intClust.9 belongs to luminal B ER+ sub-group (15).
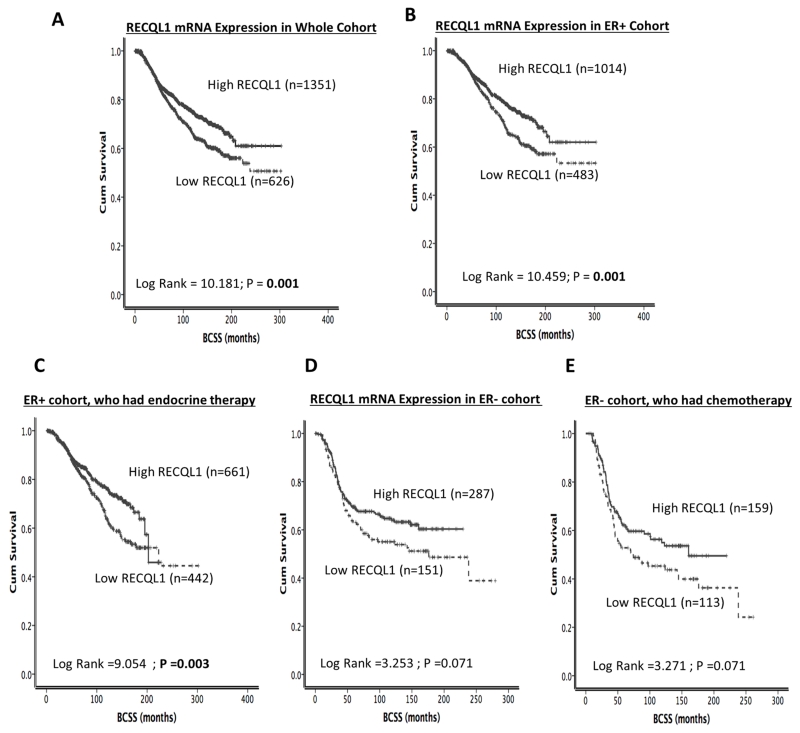
We then proceeded to survival analysis in the METABRIC cohort. Low RECQL1 mRNA expression was associated with poor BCSS (p=0.001) in the whole cohort [Figure 1B]. In ER+ tumours, low RECQL1 mRNA expression remained associated with poor BCSS (p=0.001) [Figure 1C], including in patients who received adjuvant endocrine therapy (p=0.003) [Figure 1D]. However, in ER− tumours, RECQL1 mRNA expression, although borderline, did not significantly influence outcome in the ER− cohort, including in patients who received adjuvant chemotherapy (p=0.071 and p=0.071 respectively) [Figure 2E, 2F].
Figure 1. RECQL1 mRNA expression and breast cancer survival.
A. RECQL1 mRNA expression and survival in the whole cohort. B. RECQL1 mRNA expression and survival in ER+ tumours. C. RECQL1 mRNA expression and survival in patients with ER+ tumours who received endocrine therapy. D. RECQL1 mRNA expression and survival in ER− tumours. E. RECQL1 mRNA expression and survival in patients with ER− tumours who received chemotherapy.
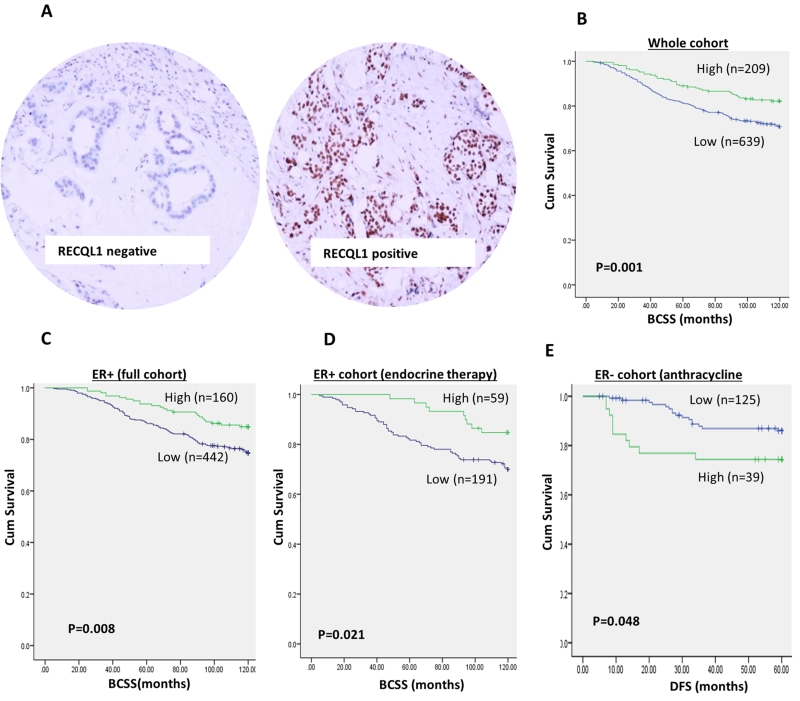
Figure 2. RECQL1 protein level and breast cancer survival.
A. Photomicrographs of RECQL1 protein expression in breast cancers. B. RECQL1 protein level and survival in the whole cohort. C. RECQL1 protein level and survival in ER+ tumours. D. RECQL1 protein level and survival in patients with ER+ tumours who received endocrine therapy. E. RECQL1 protein level and survival in patients with ER− tumours who received anthracycline chemotherapy. [BCSS= breast cancer specific survival, DFS = Disease free survival].
Together the data provides evidence that RECQL1 mRNA level has clinicopathological and prognostic significance in various sub-types of breast cancers. We then proceeded to evaluate RECQL1 protein level in breast cancers.
RECQL1 protein level in human breast cancer
A total of 897 early breast cancers were suitable for RECQL1 expression analysis. We observed only nuclear expression where 677/897 (75.5%) of tumours had low RECQL1 level and 220/897 (25.5%) had high RECQL1 level (Figure 2B). We also evaluated 15 normal breast tissues for RECQL1 expression where high nuclear staining in the terminal duct lobular units in the sections was observed (mean H-score =226) suggesting differential expression of RECQL1 in breast cancer tissues compared to normal breast tissue. No cytoplasmic staining was observed in any normal breast or tumour tissue.
As shown in Table 1, in the whole cohort, low nuclear RECQL1 levels were significantly associated with larger tumour size, lymph node positivity, higher tumour stage, high tumour grade, high mitotic index, pleomorphism, de-differentiation and tumour type (ps<0.05). ER−, PR− and HER-2 overexpression was more common in tumours with low nuclear RECQL1 protein level (p<0.05). High risk Nottingham Prognostic Index (NPI) >3.4 was also more common in tumours with low RECQL1 level (p=0.0006). Low PARP1, BRCA1 negative, low RAD51, low ATM, low nuclear pChk1, low nuclear Chk2, low XRCC1, low FEN1, low SMUG1, low DNA-PKcs were significantly more likely in tumours with low nuclear RECQL1 protein level (p<0.05). Moreover, low RECQL1 tumours were also significantly associated with low levels of other RecQ helicases including RECQL4, BLM and WRN (ps<0.05). We then proceeded to analysis separately in ER+ and ER− cohort.
Table 1.
RECQL1 protein levels and breast cancer.
| VARIABLE | RECQL1 protein level | P values | ||
|---|---|---|---|---|
|
| ||||
| Low N (%) |
High N (%) |
Unadjusted | adjusted | |
|
| ||||
| A) Pathological Parameters | ||||
|
| ||||
| Tumour Size | ||||
| <1cm | 53 (7.8) | 31 (14.1) | 0.031 | 0.0472 |
| >1-2cm | 338 (49.9) | 108 (49.1) | ||
| >2-5cm | 268 (39.6) | 78 (35.5) | ||
| >5cm | 18 (2.7) | 3 (1.4) | ||
|
| ||||
| Lymph Node Status | ||||
| Negative | 351 (58.5) | 143 (68.8) | 0.029 | 0.0483 |
| Positive (1-3) | 199 (33.2) | 54 (26.0) | ||
| Positive (>3) | 50 (8.3) | 11 (5.3) | ||
|
| ||||
| Tumour Stage | ||||
| 1 | 410 (60.5) | 157 (70.7) | 0.017 | 0.0313 |
| 2 | 210 (29.6) | 52 (23.4) | ||
| 3 | 67(9.9) | 13 (5.9) | ||
|
| ||||
| Tumour Grade | ||||
| G1 | 88 (13.0) | 46 (20.9) | 0.0001 | 0.0018 |
| G2 | 230 (33.9) | 89 (40.5) | ||
| G3 | 360 (53.1) | 85 (38.6) | ||
|
| ||||
| Mitotic Index | ||||
| M1 (low; mitoses < 10) | 212 (32.2) | 105 (48.2) | 0.0001 | 0.0012 |
| M2 (medium; mitoses 10-18) | 131 (19.9) | 34 (15.6) | ||
| M3 (high; mitosis >18) | 315 (47.9) | 79 (36.2) | ||
|
| ||||
| Tubule Formation | ||||
| 1 (>75% of definite tubule) | 25 (3.8) | 19 (8.7) | 0.006 | 0.0162 |
| 2 (10%-75% definite tubule) | 213 (32.4) | 78 (35.8) | ||
| 3 (<10% definite tubule) | 420 (63.8) | 121 (55.5) | ||
|
| ||||
| Pleomorphism | ||||
| 1 (small-regular uniform) | 13 (2.0) | 7 (3.2) | 0.001 | 0.0044 |
| 2 (Moderate variation) | 218 (33.2) | 101 (46.5) | ||
| 3 (Marked variation) | 425 (64.8) | 109 (50.2) | ||
|
| ||||
| Tumour Type | ||||
| IDC-NST | 421 (63.3) | 107 (49.5) | 0.017 | 0.0298 |
| Tubular Carcinoma | 122 (18.3) | 59 (27.3) | ||
| Medullary Carcinoma | 17 (2.6) | 6 (2.8) | ||
| ILC | 63 (9.5) | 27 (12.5) | ||
| Others | 8 (1.2) | 4 (1.9) | ||
| Mixed NST/lobular/Special Type | 34 (5.1) | 13 (6.0) | ||
|
| ||||
| ER | ||||
| Negative | 187 (28.1) | 42 (19.7) | 0.015 | 0.0309 |
| Positive | 478 (71.9) | 171 (80.3) | ||
|
| ||||
| PgR | ||||
| Negative | 310 (48.0) | 67 (31.5) | 0.0001 | 0.0007 |
| Positive | 336 (52.0) | 146 (68.5) | ||
|
| ||||
| Her2 overexpression | ||||
| No | 559 (84.1) | 197 (90.0) | 0.032 | 0.0431 |
| Yes | 106 (15.9) | 22 (10.0) | ||
|
| ||||
| Triple Negative Phenotype | ||||
| No | 570 (83.8) | 183 (81.7) | 0.459 | 0.4725 |
| Yes | 110 (16.2) | 41 (18.3) | ||
|
| ||||
| NPI | ||||
| ≤ 3.4 | 117 (27.7) | 88 (41.5) | 0.0001 | 0.0006 |
| >3.4 | 462 (72.3) | 124 (58.5) | ||
|
| ||||
| B) DNA Repair | ||||
|
| ||||
| XRCC1 (Nuclear) | ||||
| Low | 98 (18.6) | 9 (5.6) | 0.0001 | 0.0005 |
| High | 430 (81.4) | 151 (94.4) | ||
|
| ||||
| BRCA1 (Nuclear) | ||||
| Low | 104 (18.9) | 18 (10.7) | 0.014 | 0.0306 |
| High | 447 (81.1) | 150 (89.3) | ||
|
| ||||
| SMUG1 (Nuclear) | ||||
| Low | 288 (58.2) | 64 (45.1) | 0.002 | 0.007 |
| High | 207 (41.8) | 78 (54.9) | ||
|
| ||||
| FEN1 (Nuclear) | ||||
| Low | 389 (75.4) | 103 (65.2) | 0.012 | 0.0280 |
| High | 127 (24.6) | 55 (34.8) | ||
|
| ||||
| FEN1 (Cytoplasmic) | ||||
| Low | 285 (55.2) | 70 (44.3) | 0.016 | 0.0311 |
| High | 231 (44.8) | 88 (55.7) | ||
|
| ||||
| PARP1 | ||||
| Low | 270 (49.0) | 67 (39.6) | 0.033 | 0.0481 |
| High | 281 (51.0) | 102 (60.4) | ||
|
| ||||
| TOPO2 | ||||
| Low | 237 (47.0) | 64 (38.6) | 0.057 | 0.0739 |
| High | 267 (53.0) | 102 (61.4) | ||
|
| ||||
| KU 70/80 | ||||
| Low | 60 (13.0) | 22 (17.7) | 0.181 | 0.2112 |
| High | 400 (87.0) | 102 (82.3) | ||
|
| ||||
| DNA-PKcs | ||||
| Low | 87 (18.0) | 7 (5.6) | 0.001 | 0.0044 |
| High | 397 (82.0) | 117 (94.4) | ||
|
| ||||
| ATR | ||||
| Low | 110 (35.4) | 38 (37.6) | 0.682 | 23.87 |
| High | 201 (64.6) | 63 (62.4) | ||
|
| ||||
| Chk1 (Nuclear) | ||||
| Low | 605 (89.1) | 164 (73.2) | 0.0001 | 0.0005 |
| High | 74 (10.9) | 60 (26.8) | ||
|
| ||||
| Chk1 (Cytoplasmic) | ||||
| Low | 211 (31.1) | 75 (33.5) | 0.451 | 0.478 |
| High | 468 (68.9) | 149 (66.5) | ||
|
| ||||
| ATM | ||||
| Low | 238 (56.9) | 54 (41.5) | 0.002 | 0.007 |
| High | 180 (43.1) | 76 (58.5) | ||
|
| ||||
| CHK2 | ||||
| Low | 112 (25.2) | 19 (15.7) | 0.029 | 0.327 |
| High | 333 (74.8) | 102 (84.3) | ||
|
| ||||
| RAD51 (Nuclear) | ||||
| Low | 234 (55.1) | 43 (39.1) | 0.003 | 0.0095 |
| High | 191 (44.9) | 67 (60.9) | ||
|
| ||||
| C) Other RecQ helicases | ||||
|
| ||||
| RECQL5 (Nuclear) | ||||
| Low | 273 (46.5) | 68 (40.0) | 0.133 | 0.1605 |
| High | 314 (53.5) | 102 (60.0) | ||
|
| ||||
| RECQL4 (Nuclear) | ||||
| Low | 245 (65.9) | 173 (50.6) | 0.00004 | 0.0014 |
| High | 127 (34.1) | 169 (49.4) | ||
|
| ||||
| RECQL4 (Cytoplasmic) | ||||
| Low | 297 (53.6) | 73 (46.2) | 0.100 | 0.125 |
| High | 257 (46.4) | 85 (53.8) | ||
|
| ||||
| BLM (Nuclear) | ||||
| Low | 163 (27.7) | 28 (16.9) | 0.005 | 0.0146 |
| High | 426 (72.3) | 138 (83.1) | ||
|
| ||||
| BLM (Cytoplasmic) | ||||
| Low | 438 (74.5) | 114 (70.3) | 0.292 | 0.3194 |
| High | 150 (25.5) | 48 (29.6) | ||
|
| ||||
| WRN (Nuclear) | ||||
| Low | 197 (48.8) | 53 (42.1) | 0.188 | 0.214 |
| High | 207 (51.2) | 73 (57.9) | ||
|
| ||||
| WRN (Cytoplasmic) | ||||
| Low | 221 (54.7) | 52 (41.3) | 0.008 | 0.02 |
| High | 183 (45.3) | 74 (58.7) | ||
Bold = Statistically significant;HER2: human epidermal growth factor receptor 2; ER: oestrogen receptor; PgR: progesterone receptor;Triple negative: ER−/PgR−/HER2− .
In ER+ tumour (supplementary Table S7), low nuclear RECQL1 level was significantly associated with higher mitotic index (p= 0.033). PR− and High risk Nottingham Prognostic Index (NPI) >3.4 was also more common in tumours with low RECQL1 level (p values<0.05). Low XRCC1, low TOPO2A, were also more likely in tumours with low nuclear RECQL1 protein level (p<0.05). However, in ER− tumours (supplementary Table S8), no significant clinicopathological associations were observed.
We then proceeded to survival analyses. In the whole cohort, patients whose tumours had low RECQL1 level were significantly more likely to have shorter BCSS compared to those with high RECQL1 level (p=0.001) (Figure 2C). In ER+ tumours, similarly, low RECQL1 was associated with poor BCSS (p = 0.008) (Figure 2D) including in patients who received adjuvant endocrine therapy (p= 0.021) (Figure 2E). However, in patients who received no endocrine therapy, RECQL1 level did not influence survival (p=0.485) (Supplementary Figure S1A). In ER− tumours, RECQL1 did not influence survival including in patients who received CMF (cyclophosphamide+methotrexate+5-FU) chemotherapy (Supplementary Figure S1B, C and D). However, in this historical cohort, patients received CMF chemotherapy which is currently not the standard adjuvant treatment in breast cancer. We therefore investigated RECQL1 level and survival in a further cohort of 252 ER− tumours that received more modern anthracycline based adjuvant chemotherapies. The characteristics of this cohort are summarised in supplementary Table S3. As the long term follow-up data has not yet matured, we investigated the impact of RECQL1 expression on disease free survival at 5 years in patients who received adjuvant doxorubicin chemotherapy. At 5 years, 176/252 were alive, 73/252 were dead from breast cancer recurrence and 3/252 died from other causes. Patients with high RECQL1 expression were more likely to suffer disease recurrence compared to patients with low RECQL1 expression (p=0.048) (Figure 2F).
We also investigated RECQL1 expression in 49 BRCA1 germ-line deficient and 25 BRCA2 germ-line deficient breast tumours. No significant clinicopathological associations were observed (data not shown). RECQL1 expression also did not influence survival outcomes BRCA1/2 germ-line deficient tumours (data not shown).
Taken together, the data suggests that RECQL1 overexpression may predict resistance to doxorubicin chemotherapy in sporadic ER− breast cancers. To investigate this possibility further we proceeded to pre-clinical studies in breast cancer cell lines.
RECQL1 depletion and doxorubicin chemosensitivity in breast cancer cell lines
RECQL1 deficiency leads to genomic instability and sensitivity to a range of genotoxins (3-6). However, the impact of RECQL1 depletion in breast cancer cells and anthracycline sensitivity has not been investigated.
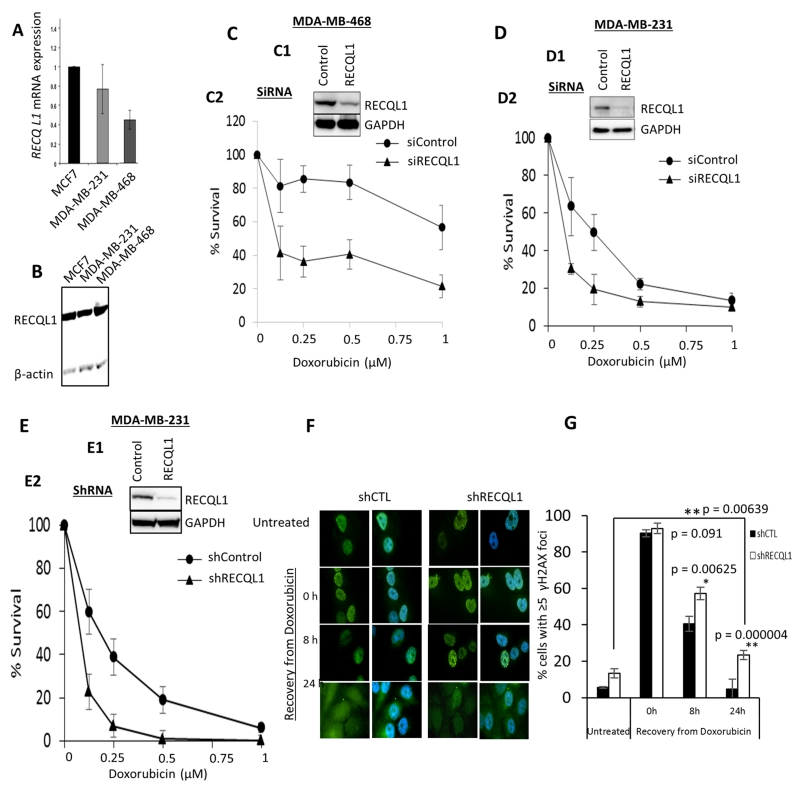
We initially profiled MCF-7, MDA-MB-468 and MDA-MB 231 breast cancer cell lines. At the mRNA level, MCF-7 and MDA-MB-231 cells have high RECQL1 mRNA expression compared to MDA-MB-468 cells (Figure 3A). At the protein level, all three cell lines have robust RECQL1 protein expression (Figure 3B). We then utilised siRNA to transiently deplete RECQL1 in MDA-MB-468, MDA-MB-231 and MCF-7 cells. We transfected cells with a control siRNA (siControl) or a pool of 4 siRNAs (smart pool, 20 nM) targeting RECQL1 (siRECQL1) (Figures 3C1, 3D1 & supplementary Figure S2A1). As compared to control cells, RECQL1 depleted cells displayed significantly reduced survival to doxorubicin treatment in MDA-MB-468, MDA-MB 231 (Figure 3C2, 3D2) and MCF-7 cells (supplementary Figure S2A2) (p<0.05 at all drug concentration tested). To determine if increased sensitivity to doxorubicin was also sustained in cells depleted of RECQL1 over a longer period of time, we transduced MDA-MB-231 cells with a RECQL1-specific shRNA (Figure 3E1). As compared to control shRNA (shCTL) transduced MDA-MB-231 cells, the RECQL1 shRNA (shRECQL1) transduced cells displayed significantly reduced survival to doxorubicin treatment (Figure 3E2).
Figure 3. RECQL1 depletion and doxorubicin sensitivity in breast cancer cell lines.
A. RECQL1 mRNA expression in MCF-7, MDA-MB-231 and MDA-MB-468 breast cancer cell lines. B. RECQL1 protein level in MCF-7, MDA-MB-231 and MDA-MB-468 breast cancer cell lines. C. Transient RECQL1 depletion by siRNA (C1) and doxorubicin sensitivity in MDA-MB-468 (C2) [The graph shows the cellular surviving fractions measured at different doses of doxorubicin treatment in control and RECQL1-depleted cells. Surviving fraction values are the mean ± SEM from three independent experiments]. D. Transient RECQL1 depletion by siRNA (D1) and doxorubicin sensitivity in MDA-MB-231 (D2) [The graph shows the cellular surviving fractions measured at different doses of doxorubicin treatment in control and RECQL1-depleted cells. Surviving fraction values are the mean ± SEM from three independent experiments]. E. Stable RECQL1 depletion by shRNA (E1) and doxorubicin sensitivity in MDA-MB-231 (E2) [The graph shows the cellular surviving fractions measured at different doses of doxorubicin treatment in control and RECQL1-depleted cells. Surviving fraction values are the mean ± SEM from three independent experiments]. F. Representative immunofluorescence staining of γH2AX foci (green) and its merge with nuclear DNA stain DAPI (blue) in control and RECQL1-depleted MDA-MB-231 cells is shown here. G. Analysis of γH2AX foci in MDA-MB-231 cells stably transduced with control or RECQL1 shRNA. The percentage of cells exhibiting ≥5 γH2AX foci at indicated time points following recovery from doxorubicin treatment (0.1 μM for 4 h) was determined by immunofluorescence. Quantitative data shown represent the average from two independent experiments with associated SEMs.
To determine whether the cellular level of RECQL1 protein modulates overall DNA damage in breast cancer cell lines, we examined γH2AX as a surrogate of DNA double-strand breaks in control and RECQL1 knockdown cells exposed to doxorubicin. MDA-MB-231 cells stably transduced with control or RECQL1 shRNA were treated with 0.1 μM doxorubicin for 4 h and the percentage of cells exhibiting ≥5 γH2AX foci at various time points following recovery from drug treatment was determined by immunofluorescence (Figure 3F). Consistent with constitutively elevated DNA damage upon RECQL1 knockdown reported in other cell types (8, 27), RECQL1-depleted MDA-MB-231 cells displayed spontaneous γH2AX foci under untreated condition. Doxorubicin treatment induced comparable level of DNA double strand breaks in both control and RECQL1 knockdown MDA-MB-231 cells. However, following 8 h recovery from the doxorubicin treatment, significantly greater fraction of RECQL1-depleted cells was scored positive for γH2AX foci. After 24 h in drug-free medium, γH2AX foci were persistent in about 25% RECQL1-depleted cells as compared to 5% control MDA-MB-231 cells (Figure 3G). We note that the initial numbers of γH2AX positive induced spontaneously in control versus RECQL1-depleted cells is different, however, the difference between control and RECQL1 knockdown cells for the percentage of γH2AX positive cells during recovery (8 and 24 h) from doxorubicin treatment is statistically significant (p≤0.05). These results suggest that RECQL1 promotes repair of doxorubicin induced DNA damage. In MCF-7 cells, similarly, RECQL1-depleted cells retain statistically significant proportion of γH2AX positive cells at 8 and 24 h following recovery in drug-free medium (Supplementary Figure S2B).
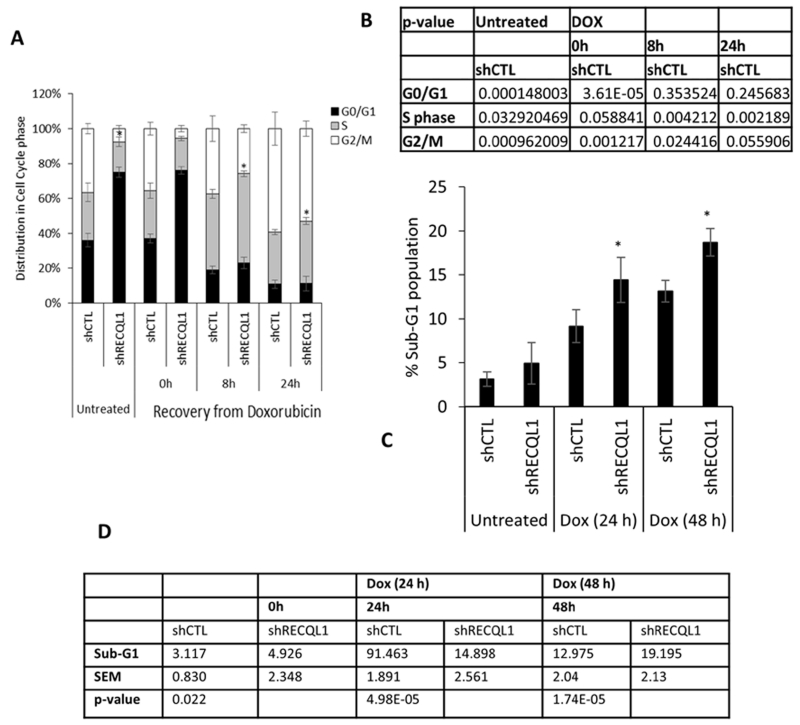
We next analysed cell cycle progression in these cells using FACS analysis (Figure 4). Stable knockdown of RECQL1 in MDA-MB-231 cells resulted in predominant accumulation in G0/G1 phase of the cell cycle. Cell cycle distribution of control and RECQL1 knockdown MDA-MB-231 cells was largely unaltered after doxorubicin treatment (0.1 μM, 4 h). MDA-MB-231 cells, with or without knockdown of RECQL1 expression, accumulated in S-phase following 8 h after recovery from treatment, and in G2/M following 24 h recovery in drug-free medium; however, a significantly greater proportion of RECQL1-depleted cells remained in S-phase at 8 h (p≤0.05) and 24 h (p≤0.05) of recovery (Figure 4A & 4B). Doxorubicin induced S-phase arrest is consistent with the formation of DNA adducts that prevent replication fork progression and formation of double strand breaks downstream of Topoisomerase II inhibition. Relative accumulation in the S-phase together with the increased double strand breaks and sensitivity to doxorubicin observed in RECQL1 knockdown MDA-MB-231 cells is consistent with a role of RECQL1 in resolving stalled or broken replication forks and DNA repair. Doxorubicin induced DNA strand breaks can ultimately result in apoptosis. To determine the extent of doxorubicin induced cell death in RECQL1 knockdown cells, we analysed the percentage of MDA-MB-231 cells stably transduced with control or RECQL1 shRNA having sub-G1 DNA content (Figure 4C& 4D). Following treatment with doxorubicin (0.1 μM) for 24 h, 9±1.73% control and 15±2.61% RECQL1 knockdown cells were in the sub-G1 population; treatment for 48 h resulted in 13±2.04% control and 19.2±2.01% RECQL1 knockdown cells in sub-G1 population(Figure 4C& 4D). In RECQL1-depleted MCF-7 cells, although S- phase accumulation was not evident Supplementary Figure S2C, there was significant accumulation of sub-G1 cells upon 24 h and 48 h of doxorubicin treatment (Supplementary Figure S2D).
Figure 4. RECQL1 depletion and cell cycle progression.
A. Cell cycle distributions of MDA-MB-231 cells stably transduced with either control or RECQL1 shRNA at the indicated times following recovery from doxorubicin treatment (0.1 μM for 4 h). B. Data shown represent the average from two independent experiments with associated SEMs. Individual p values are summarized as a table here. C. Sub-G1 population in control and RECQL1-depleted MDA-MB-231 cells after doxorubicin treatment (0.1 μM) for indicated time. D. Data shown represent the average from two independent experiments with associated SEMs. Individual p values are summarized as a table here.
RECQL1 depletion and estrogen receptor- α (ER α) levels
Given the recent evidence that RECQL1 may modulate gene expression (26, 28), we conducted preliminary studies to explore if RECQL1 may impact upon ERα expression in breast cancer cell lines. In control cells, as expected, ERα expression was not detectable in MDA-MB-468 cells and MCF-7 cells have proficient ERα expression. We detected ERα expression in MDA-MB-231. Although unexpected, previous studies have reported ERα expression in MDA-MB-231 cells (29, 30). As shown in supplementary Figure S3, 48 h after RECQL1 siRNA transfection, we observed significant depletion of ERα levels in MCF-7 and MDA-MB-231 cells. The data suggest that either RECQL1 depletion impairs ERα expression or promotes ERα degradation. Detailed mechanistic studies are currently underway to explore these possibilities.
Taken together, preclinical and clinical data provide evidence that RECQL1 has prognostic and predictive significance in breast cancers.
DISCUSSION
RECQL1 is a key member of the RecQ family of DNA helicases. RECQL1 has important roles in the maintenance of replication fork progression, DNA repair and gene expression mechanisms (3-6). Recently, RECQL1 germ-line mutations were discovered in non-BRCA hereditary breast cancer patients (12, 13) implying a critical tumour suppressor function for RECQL1. However, the role of RECQL1 in cancer pathogenesis appears to be complex. In normal cells, RECQL1 may function as a ‘caretaker of the genome’ (3-6). On the other hand, established tumours may be dependent on RECQL1 to tolerate replication induced DNA damage, a feature seen in proliferating cancer cells. In fact RECQL1 has been shown to be overexpressed in glioblastoma (31), hepatocellular carcinoma (32), ovarian cancers (33), melanoma (34) and head & neck cancer models (35). Whether RECQL1 also impacts sporadic breast cancer pathogenesis is currently unknown. We have conducted comprehensive analysis and demonstrated prognostic and predictive significance of RECQL1 in sporadic breast cancers.
Genomic analyses have revealed that breast cancer represents a heterogeneous group of diseases with distinct prognostic outcomes (15). In addition to ER, PR and HER-2 expression status, markers of proliferation and genomic stability appear to influence biological and clinical behaviour of breast cancers (15, 36, 37). Given the role of RECQL1 in DNA replication and repair, we anticipated differential roles of RECQL1 in various molecular sub-types of breast cancers. As expected, high RECQL1 mRNA was associated with intClust.3 which is characterised by low genomic instability (15). On the other hand, low RECQL1 mRNA was linked to intClust.8 luminal A ER+ sub-group (low proliferating) phenotype (15). Interestingly, low RECQL1 mRNA was also observed in intClust.9 phenotype which belongs to luminal B sub-group implying a more complex role for RECQL1 in this sub-group. In the METABRIC cohort, low RECQL1 mRNA was associated with poor survival. At the protein level, similarly, low RECQL1 was associated with aggressive phenotypes and poor survival including in ER+ tumours. However, a limitation to the current study is that mRNA expression and protein expression studies were conducted in two independent cohort. Although, low levels of RECQL1 appear to be prevalent in the breast cancers, the mechanism for such down-regulation is currently unknown. As epigenetic silencing of the BRCA1 promoter has been reported in up to 11%-14% of breast tumours (37), it is likely that similar mechanisms may be operating for RECQL1 in sporadic breast cancers. An interesting observation was that we did not observe any cytoplasmic staining for RECQL1. This is in contrast to the cytoplasmic staining observed for BLM, RECQL4 and WRN in breast cancers (16, 38, 39). The data suggests differential regulation of localisation for different RecQ helicases. In pre-clinical studies, RECQL1 deficiency has been shown to promote genomic instability resulting in increased frequency of spontaneous sister chromatid exchanges, chromosomal instability, DNA damage accumulation and mutagenesis (3-6). A ‘mutator phenotype’ (38) due to RECQL1 deficiency may therefore promote an aggressive phenotypes in ER+ breast cancers. In the current study we observed that low PARP1, BRCA1 negative, low RAD51, low ATM, low nuclear pChk1, low nuclear Chk2, low XRCC1, low FEN1, low SMUG1 and low DNA-PKcs were significantly more likely in tumours with low nuclear RECQL1 protein level. The data suggests that RECQL1 loss may increase genomic instability which may in turn lead to dysregulation of other DNA repair factors thereby promoting a ‘mutator phenotype’. A novel observation in the current study is that low RECQL1 also influenced survival in ER+ cohorts that received endocrine therapy implying that RECQL1 could also have predictive significance. Given the recent evidence that RECQL1 may modulate gene expression (26, 28), we speculate that ER and/or ER mediated gene expression could be influenced by low RECQL1 in tumours. To explore this hypothesis we investigated ERα protein levels in control and RECQL1-depleted breast cancer cells. We observed significant depletion of ERα levels in RECQL1-depleted MCF-7 and MDA-MB-231 cells. The preliminary data would suggest that either RECQL1 depletion impairs ERα expression or promotes ERα degradation. Therefore, detailed mechanistic studies are required to explore these possibilities in detail.
In ER− sub-group, RECQL1 did not appear to influence survival either in patient who received no chemotherapy or who received historical CMF chemotherapy. Interestingly, in ER− negative tumours that received the more modern anthracycline chemotherapy, we observed that overexpression of RECQL1 was associated with poor disease free survival. The data suggest that ER− tumours may be dependent on RECQL1 to tolerate replication induced DNA damage, such as those induced by doxorubicin chemotherapy. To support this hypothesis we depleted RECQL1 in breast cancer cells. We not only demonstrated doxorubicin sensitivity in RECQL1-depleted cells but also showed that the observed sensitivity was associated with DNA double strand breaks accumulation, S-phase cell cycle arrest and apoptosis.
RecQ family of DNA helicases include RECQL1, RECQL4, RECQL5, BLM and WRN (1, 2). We have recently investigated the expression of RECQL4, RECQL5, BLM and WRN in breast cancers (17, 39-41). Whereas high RECQL4, high RECQL5 and high BLM expression were associated with aggressive breast cancers (17, 39, 40), low WRN expression was linked to poor outcomes (41). Interestingly, RecQ helicase mRNA levels are linked to biologically distinct integrative clusters reported in the METABRIC study (15). For example, intClust 3 subgroup that is characterised by low genomic instability was consistent seen with tumours with low BLM, low RECQL4 and low RECQL5 mRNA levels. On the other hand, high RECQL1 or high WRN mRNA levels correlated to intClust 3 sub-group. Similarly, intClust 9 (8q cis-acting/20qamplified mixed subgroup with aggressive phenotype) was more common in tumours with high BLM, high RECQL4, high RECQL5, low RECQL1 or low WRN mRNA levels. Taken together, the mRNA and protein expression data would suggest that differential helicase expressions lead to distinct molecular phenotypes. We speculate that proliferative functions (of BLM, RECQL4 and RECQL5 helicases) and genomic stability functions (of RECQL1 and WRN) may influence breast cancer pathogenesis. Moreover, the data presented here would also suggest that RecQ helicase deficient sporadic tumours may be suitable for a synthetic lethality approach, an exciting new personalised treatment strategy recently demonstrated for PARP inhibitors in BRCA deficient cancers (42). Moreover, given the recent development of helicase inhibitors (1) such as those targeting BLM (43, 44), our data would indicate potential application for these new helicase inhibitors for personalization of breast cancer therapy.
In conclusion we have shown that RECQL1 has prognostic and predictive significance in breast cancer.
Supplementary Material
Acknowledgments
Financial information: This work was funded by the NIGMS/NIH grant 5SC1GM093999-06 to S. Sharma.
Footnotes
Conflict of interest: The authors disclose no potential conflicts of interest
REFERENCES
- 1.Brosh RM., Jr. DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–58. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croteau DL, Popuri V, Opresko PL, Bohr VA. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 2014;83:519–52. doi: 10.1146/annurev-biochem-060713-035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S. An appraisal of RECQ1 expression in cancer progression. Front Genet. 2014;5:426. doi: 10.3389/fgene.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sami F, Sharma S. Probing Genome Maintenance Functions of human RECQ1. Comput Struct Biotechnol J. 2013;6:e201303014. doi: 10.5936/csbj.201303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S, Phatak P, Stortchevoi A, Jasin M, Larocque JR. RECQ1 plays a distinct role in cellular response to oxidative DNA damage. DNA Repair (Amst) 2012;11:537–49. doi: 10.1016/j.dnarep.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Brosh RM., Jr. Distinct roles of RECQ1 in the maintenance of genomic stability. DNA Repair (Amst) 2010;9:315–24. doi: 10.1016/j.dnarep.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thangavel S, Mendoza-Maldonado R, Tissino E, Sidorova JM, Yin J, Wang W, et al. Human RECQ1 and RECQ4 helicases play distinct roles in DNA replication initiation. Mol Cell Biol. 2010;30:1382–96. doi: 10.1128/MCB.01290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popuri V, Croteau DL, Brosh RM, Jr., Bohr VA. RECQ1 is required for cellular resistance to replication stress and catalyzes strand exchange on stalled replication fork structures. Cell Cycle. 2012;11:4252–65. doi: 10.4161/cc.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Parvathaneni S, Hara T, Lal A, Sharma S. Replication stress induces specific enrichment of RECQ1 at common fragile sites FRA3B and FRA16D. Mol Cancer. 2013;12:29. doi: 10.1186/1476-4598-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Sommers JA, Choudhary S, Faulkner JK, Cui S, Andreoli L, et al. Biochemical analysis of the DNA unwinding and strand annealing activities catalyzed by human RECQ1. J Biol Chem. 2005;280:28072–84. doi: 10.1074/jbc.M500264200. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Stumpo DJ, Balajee AS, Bock CB, Lansdorp PM, Brosh RM, Jr., et al. RECQL, a member of the RecQ family of DNA helicases, suppresses chromosomal instability. Mol Cell Biol. 2007;27:1784–94. doi: 10.1128/MCB.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Wang Y, Xia Y, Xu Y, Ouyang T, Li J, et al. Mutations in RECQL Gene Are Associated with Predisposition to Breast Cancer. PLoS Genet. 2015;11:e1005228. doi: 10.1371/journal.pgen.1005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cybulski C, Carrot-Zhang J, Kluzniak W, Rivera B, Kashyap A, Wokolorczyk D, et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet. 2015;47:643–6. doi: 10.1038/ng.3284. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee T, Brosh RM., Jr. RECQL: a new breast cancer susceptibility gene. Cell Cycle. 2015 doi: 10.1080/15384101.2015.1066539. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 17.Arora A, Abdel-Fatah TM, Agarwal D, Doherty R, Moseley PM, Aleskandarany MA, et al. Transcriptomic and Protein Expression Analysis Reveals Clinicopathological Significance of Bloom Syndrome Helicase (BLM) in Breast Cancer. Mol Cancer Ther. 2015;14:1057–65. doi: 10.1158/1535-7163.MCT-14-0939. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Fatah TM, Middleton FK, Arora A, Agarwal D, Chen T, Moseley PM, et al. Untangling the ATR-CHEK1 network for prognostication, prediction and therapeutic target validation in breast cancer. Mol Oncol. 2015;9:569–85. doi: 10.1016/j.molonc.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Fatah TM, Arora A, Alsubhi N, Agarwal D, Moseley PM, Perry C, et al. Clinicopathological significance of ATM-Chk2 expression in sporadic breast cancers: a comprehensive analysis in large cohorts. Neoplasia. 2014;16:982–91. doi: 10.1016/j.neo.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albarakati N, Abdel-Fatah TM, Doherty R, Russell R, Agarwal D, Moseley P, et al. Targeting BRCA1-BER deficient breast cancer by ATM or DNA-PKcs blockade either alone or in combination with cisplatin for personalized therapy. Mol Oncol. 2015;9:204–17. doi: 10.1016/j.molonc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Fatah TM, Perry C, Arora A, Thompson N, Doherty R, Moseley PM, et al. Is there a role for base excision repair in estrogen/estrogen receptor-driven breast cancers? Antioxid Redox Signal. 2014;21:2262–8. doi: 10.1089/ars.2014.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel-Fatah TM, Russell R, Albarakati N, Maloney DJ, Dorjsuren D, Rueda OM, et al. Genomic and protein expression analysis reveals flap endonuclease 1 (FEN1) as a key biomarker in breast and ovarian cancer. Mol Oncol. 2014;8:1326–38. doi: 10.1016/j.molonc.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel-Fatah TM, Russell R, Agarwal D, Moseley P, Abayomi MA, Perry C, et al. DNA polymerase beta deficiency is linked to aggressive breast cancer: a comprehensive analysis of gene copy number, mRNA and protein expression in multiple cohorts. Mol Oncol. 2014;8:520–32. doi: 10.1016/j.molonc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. 1979. [Google Scholar]
- 26.Li XL, Lu X, Parvathaneni S, Bilke S, Zhang H, Thangavel S, et al. Identification of RECQ1-regulated transcriptome uncovers a role of RECQ1 in regulation of cancer cell migration and invasion. Cell Cycle. 2014;13:2431–45. doi: 10.4161/cc.29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Brosh RM., Jr. Human RECQ1 is a DNA damage responsive protein required for genotoxic stress resistance and suppression of sister chromatid exchanges. PLoS One. 2007;2:e1297. doi: 10.1371/journal.pone.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Parvathaneni S, Li XL, Lal A, Sharma S. Transcriptome guided identification of novel functions of RECQ1 helicase. Methods. 2016;108:111–7. doi: 10.1016/j.ymeth.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang HJ, Lee MH, Kang HL, Kim SH, Ahn JR, Na H, et al. Differential regulation of estrogen receptor alpha expression in breast cancer cells by metastasis-associated protein 1. Cancer Res. 2014;74:1484–94. doi: 10.1158/0008-5472.CAN-13-2020. [DOI] [PubMed] [Google Scholar]
- 30.Ford CH, Al-Bader M, Al-Ayadhi B, Francis I. Reassessment of estrogen receptor expression in human breast cancer cell lines. Anticancer Res. 2011;31:521–7. [PubMed] [Google Scholar]
- 31.Mendoza-Maldonado R, Faoro V, Bajpai S, Berti M, Odreman F, Vindigni M, et al. The human RECQ1 helicase is highly expressed in glioblastoma and plays an important role in tumor cell proliferation. Mol Cancer. 2011;10:83. doi: 10.1186/1476-4598-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futami K, Ogasawara S, Goto H, Yano H, Furuichi Y. RecQL1 DNA repair helicase: A potential tumor marker and therapeutic target against hepatocellular carcinoma. Int J Mol Med. 2010;25:537–45. doi: 10.3892/ijmm_00000375. [DOI] [PubMed] [Google Scholar]
- 33.Sanada S, Futami K, Terada A, Yonemoto K, Ogasawara S, Akiba J, et al. RECQL1 DNA repair helicase: a potential therapeutic target and a proliferative marker against ovarian cancer. PLoS One. 2013;8:e72820. doi: 10.1371/journal.pone.0072820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jewell R, Conway C, Mitra A, Randerson-Moor J, Lobo S, Nsengimana J, et al. Patterns of expression of DNA repair genes and relapse from melanoma. Clin Cancer Res. 2010;16:5211–21. doi: 10.1158/1078-0432.CCR-10-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Zhang Z, Zhou X, Qiu W, Chen F, Chen W. Identification of genes associated with cisplatin resistance in human oral squamous cell carcinoma cell line. BMC Cancer. 2006;6:224. doi: 10.1186/1471-2407-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joh JE, Esposito NN, Kiluk JV, Laronga C, Lee MC, Loftus L, et al. The effect of Oncotype DX recurrence score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. Oncologist. 2011;16:1520–6. doi: 10.1634/theoncologist.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 38.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–9. [PubMed] [Google Scholar]
- 39.Arora A, Abdel-Fatah TM, Agarwal D, Doherty R, Croteau DL, Moseley PM, et al. Clinicopathological and prognostic significance of RECQL5 helicase expression in breast cancers. Carcinogenesis. 2016;37:63–71. doi: 10.1093/carcin/bgv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora A, Agarwal D, Abdel-Fatah TM, Lu H, Croteau DL, Moseley P, et al. RECQL4 helicase has oncogenic potential in sporadic breast cancers. J Pathol. 2016;238:495–501. doi: 10.1002/path.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shamanna RA, Lu H, Croteau DL, Arora A, Agarwal D, Ball G, et al. Camptothecin targets WRN protein: mechanism and relevance in clinical breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewari KS, Eskander RN, Monk BJ. Development of Olaparib for BRCA-Deficient Recurrent Epithelial Ovarian Cancer. Clin Cancer Res. 2015;21:3829–35. doi: 10.1158/1078-0432.CCR-15-0088. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee T, Aggarwal M, Brosh RM., Jr. A new development in DNA repair modulation: discovery of a BLM helicase inhibitor. Cell Cycle. 2013;12:713–4. doi: 10.4161/cc.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen GH, Dexheimer TS, Rosenthal AS, Chu WK, Singh DK, Mosedale G, et al. A small molecule inhibitor of the BLM helicase modulates chromosome stability in human cells. Chem Biol. 2013;20:55–62. doi: 10.1016/j.chembiol.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.