Abstract
Objectives
In response to ER stress, endothelial cells initiate corrective pathways such as the unfolded protein response (UPR). Recent studies suggest that reactive oxidant species (ROS) produced on the ER may participate in homeostatic signaling through Ras in response to ER stress. We sought to identify mechanisms responsible for this focal signaling pathway.
Approach and Results
In endothelial cells we found that ER stress induced by tunicamycin activates the NADPH oxidase Nox4 focally on the ER surface but not on the plasma membrane. Ras activation is also restricted to the ER, occurs downstream of Nox4, and is required for activation of the UPR. In contrast, treatment with the growth factor VEGF results in Ras activation and ROS production confined instead to the plasma membrane and not the ER, demonstrating local coupling of ROS-Ras signals. We further identify the calcium-responsive, ER resident guanyl exchange factors RasGRF1 and RasGRF2 as novel upstream mediators linking Nox4 with Ras activation in response to ER stress. Oxidation of the calcium pump SERCA and increases in cytosolic calcium caused by ER stress are blocked by Nox4 knockdown, and reduction in cytosolic free calcium prevents both Ras activation and the UPR.
Conclusions
ER stress triggers a localized signaling module on the ER surface involving Nox4-dependent calcium mobilization, which directs local Ras activation through ER-associated, calcium-responsive RasGRF.
Keywords: Ras, ROS, Nox, Nox4, ER stress, tunicamycin, autophagy, RasGRF1, RasGRF2
INTRODUCTION
The integrity of client protein folding within the ER is exquisitely sensitive to a variety of changes in the environment, including nutrient availability, calcium levels, mechanical stimuli, and redox state. Consequently, a number of cellular stress programs that respond to such perturbations are integrated with ER stress sensing and signaling pathways. Proteins that control cell cycle entry, apoptosis, calcium flux, metabolism, and autophagy decorate the ER endomembrane. These proteins allow the ER to respond to proteotoxic and oxidative stress, loss of anchorage, insufficient or excessive nutrient availability, increases in synthetic demands, and other intrinsic and extrinsic stressors 1–4. The ER surface therefore functions as an important integrative signaling domain to organize stress response signaling.
The Nox family of NADPH oxidases and the Ras GTPases both have broad signaling functions including the response to various cellular stresses. Accurate subcellular routing of both protein families to discrete signaling platforms such as the ER is critical to transducing appropriate responses. Restriction of the single Ras gene product of the yeast Schizosaccharomyces pombe to the plasma membrane, for instance, supports the mating and starvation responses but not cell shape changes, while ER-restricted Ras exclusively controls cell morphology 5. In mammalian cells, ER-resident Ras isoforms are activated by growth factors and differentially activate downstream pathways compared to Ras proteins residing on other membrane domains 6. Nox proteins are likewise targeted to different subcellular structures in order to direct diverse signaling outputs 7. For example, Nox4 localized to focal adhesion sites controls focal adhesion turnover and traction force generation 8. In contrast, nuclear envelope-localized Nox4 promotes export of HDAC4 to activate NFAT, and mitochondrial Nox4 oxidizes aconitase-2 and citrate synthase, signaling mitochondria-dependent death 9, 10.
A significant fraction of Nox4 resides on the ER endomembrane, where it participates in ER signaling. ER-localized Nox4 terminates EGF signaling through oxidative inactivation of the ER resident phosphatase PTP-1B, and glucose deprivation activates autophagy through ROS produced by ER-associated Nox4 11, 12. Previously, we demonstrated that a Nox4-Ras pathway acts locally to activate the unfolded protein response (UPR) and initiate autophagy as a protective response against specific ER stressors 13. However, the molecular basis for the coupling of Nox4 and Ras in response to ER stress is not known, nor is the significance of their shared residence on the ER surface. Here, we find that ER stress initiates a Nox4-dependent calcium signal which in turn restricts Ras activation to the ER and not plasma membrane through the calcium-responsive, ER-localized guanyl exchange factor RasGRF. This novel signaling pathway therefore integrates ROS, calcium, and Ras signaling in response to ER stress through regional interaction of ER-based proteins.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Nox4 mediates ER-localized ROS production
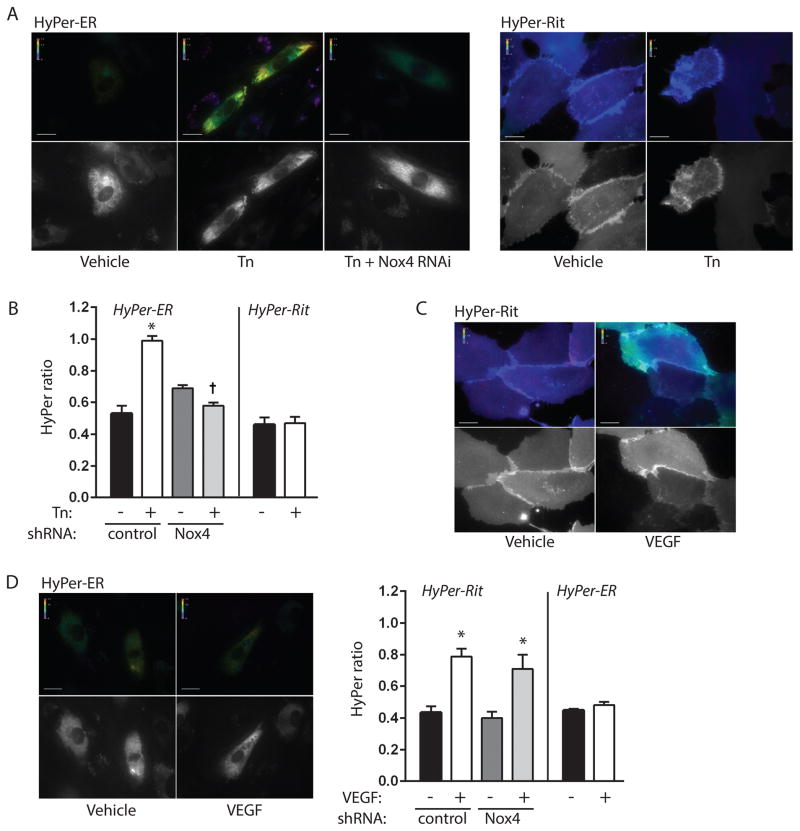
We first examined sites of ROS production during ER stress following treatment of HUVEC with the well-known ER stressor tunicamycin. Both Nox4 and Ras are anchored to the plasma membrane as well as endomembranes such as the ER, thus we focused our attention on focal production of ROS at these two subcellular membrane domains. To spatially distinguish sites of oxidant production we employed HyPer, a H2O2-specific ratiometric sensor composed of cpYFP inserted into the regulatory domain of OxyR 14. We have previously shown that HyPer-ER, which contains an ER leader sequence and C-terminal retention peptide, accurately targets the endothelial ER and reports ER H2O2 levels 13. Consistent with prior observations 13, we found increases in H2O2 levels within the ER by 16 h of treatment with tunicamycin, and further, that RNAi-dependent knockdown of Nox4 abrogated tunicamycin-dependent increases in ER H2O2 production (Figure 1A–B, Figure I A). To interrogate the plasma membrane for focal ROS production, we fused HyPer to the C-terminal membrane targeting domain of Rit (HyPer-Rit). This domain localizes exclusively to the plasma and not ER membrane, and unlike CAAX-containing proteins it does not require posttranslational modifications and does not direct trafficking through ER endomembranes 5; it is therefore insensitive to ER integrity. ER stress induced by tunicamycin had no effect on HyPer-Rit ratiometric signals, indicating a lack of increase in H2O2 production at the plasma membrane (Figure 1A–B). To verify our ability to spatially discriminate sites of ROS production, we stimulated cells with VEGF, which has also been linked with Nox4 signaling 15. Like nearly all plasma membrane receptor-triggered events, VEGF membrane signaling proceeds over minutes rather than hours; thus, VEGF-induced ROS production was found within 5 min, on a different time course than tunicamycin. Notwithstanding, addition of VEGF resulted in the opposite spatial activation pattern in that H2O2 levels increased at the plasma membrane but not at the ER (Figure 1C–D). Thus, tunicamycin-induced ER stress initiates ROS production specifically at the ER and not plasma membrane through Nox4.
Figure 1. Focal production of ROS by tunicamycin and VEGF.
A. HUVEC expressing control or Nox4 shRNA were transduced with HyPer-ER and exposed to vehicle (DMSO) or tunicamycin (Tn, 10 μg/ml) for 16 h. Pseudocolored ratiometric images for excitation wavelengths 492/405 nm are shown in top panels. Grayscale images at bottom show 405 nm excitation images to document HyPer-ER expression. B. Quantification of HyPer intensity ratios for indicated conditions. *P<0.001 compared to vehicle with control shRNA, † P<0.001 compared to Tn with control shRNA, mean ± SEM of 18–39 determinations. C, D. Ratiometric images of HUVEC expressing HyPer-Rit (C) or HyPer-ER (D) and stimulated with saline or VEGF (50 U/ml) for 3–10 min. Bar graphs in D show quantification of HyPer intensity ratios for indicated conditions. *P<0.01 compared to vehicle with corresponding control or Nox4 shRNA, mean ± SEM of 11–20 determinations. All scale bars are 20 μm.
Tunicamycin initiates Nox4-dependent Ras activation on the ER
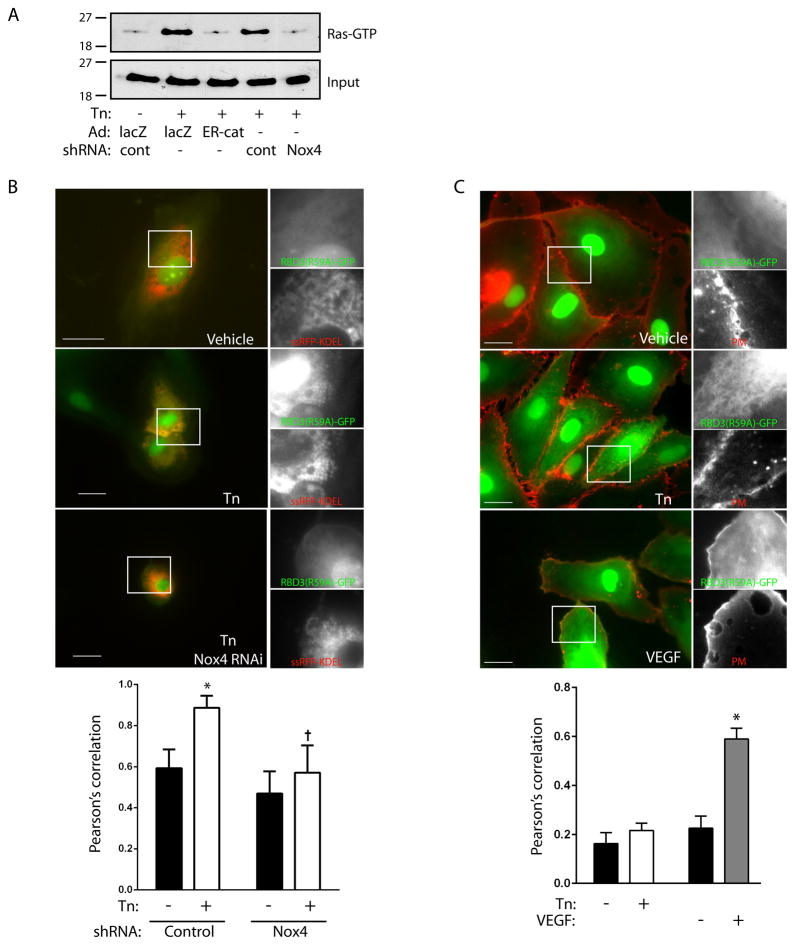
Using a Ras-GTP pulldown assay, we found that activation of Ras accompanied tunicamycin-induced ER stress (Figure 2A). Ras activation was blocked by expression of ER-targeted catalase or by Nox4 knockdown, both consistent with activation of Ras by H2O2 on the ER endomembrane (Figure 2A, Figure I B). To provide further support for ER-restricted Ras activation, we expressed a triconcatenated Ras binding domain of Raf fused to GFP (RBD3(R59A)-GFP), which reports sites of activation of endogenous Ras 16. Coexpression of this probe with ssRFP-KDEL, which marks the ER, revealed that tunicamycin treatment caused translocation of RBD3(R59A)-GFP to the ER (Figure 2B). Quantification confirmed colocalization of this Ras-GTP-avid probe with the ER which was diminished by Nox4 knockdown. Further, knockdown of K-Ras abrogated translocation of RBD3(R59A)-GFP to the ER, supporting its ability to report sites of Ras activation (Figures II A–D). In contrast, using a viable membrane stain to mark plasma membranes, we found that VEGF stimulation but not tunicamycin induced RBD3(R59A)-GFP translocation to the plasma membrane, indicating Ras activation on plasma membranes by VEGF but not ER stress (Figure 2C). Thus spatial compartmentalization of Ras activation parallels that of ROS generation in an agonist-specific fashion.
Figure 2. Ras activation by tunicamycin is restricted to the ER.
A. HUVEC expressing control or Nox4-targeted shRNA were transduced with lacZ or ER-targeted catalase (ER-cat) and treated with tunicamycin (10 μg/ml) for 16 h. Ras activity was assessed by pulldown, with input shown in lower panel. Representative of two separate experiments. B. Representative photomicrographs of cells cotransfected with RBD3(R59A)-GFP and the ER marker ssRFP-KDEL, under the conditions as labeled. Inset shows magnification of green and red channels separately. Quantification of colocalization for the red and green channels for whole-cell regions of interest are shown in bar graph below. *P<0.001 compared to vehicle with control shRNA, † P<0.001 compared to Tn with control shRNA, mean ± SEM of 9 fields. C. Cells were transduced with RBD3(R59A)-GFP (green channel), stimulated with Tn (10 μg/ml, 16 h) or VEGF (50 U/ml, 5 min), and plasma membranes stained (PM, red channel). Bar graph shows quantification of red/green colocalization for membrane regions of interest. *P<0.001 compared to vehicle. Mean ± SEM of 15–20 determinations. All scale bars are 20 μm.
Of note, levels of Nox4 protein did not appreciably change in response to tunicamycin treatment for up to 24 h, suggesting activation of the oxidase (Figure III A). Currently, mechanisms of Nox4 activation are incompletely understood. The only clear modifier of Nox4 activity is polymerase-δ-interacting protein 2 (Poldip2), which associates strongly with focal adhesions and stress fibers 8, 17 but is not known to be present in the ER. Accordingly, knockdown of Poldip2 had no effect on Ras activation (Figures III B–C), suggesting Nox4 activation by an alternate pathway.
RasGRF controls ER stress-induced Ras activation upstream of the UPR
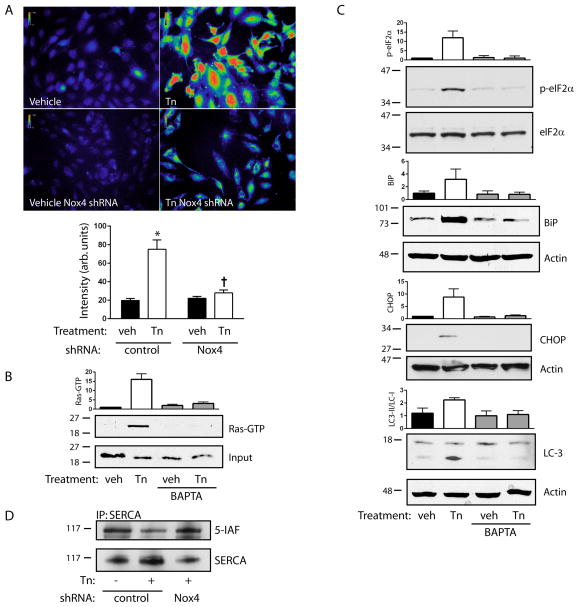
Activation of the UPR has been linked to pathways downstream of Ras 13, 18, 19. Indeed, knockdown of K-Ras blocked induction of BiP and CHOP, as well as phosphorylation of eIF2α, suggesting that the Nox4/Ras signaling pathway controls the UPR in response to tunicamycin-induced ER stress (Figure 3A–C). As an independent marker of sustained ER stress, we noted the conversion of LC3-I to LC3-II, a marker of autophagy which is known to be triggered following prolonged ER stress states 13, 20, 21 (Figure 3D). LC3-II formation was also reversed by K-Ras knockdown, indicating that ER Ras coordinates multiple responses to ER stress. To further support the link between ER ROS, Ras, and the UPR, we found that ER-targeted catalase also effectively blocked BiP and CHOP induction, eIF2α phosphorylation, and LC3-I to LC3-II conversion (Figure IV A–C).
Figure 3. K-Ras mediates the UPR.
A. Cells expressing control or K-Ras shRNA were stimulated with tunicamycin (Tn, 10 μg/ml for 16 h). Ras and BiP protein expression are shown. K-Ras knockdown was 85% efficient. Bar graph shows BiP levels, mean ± SEM of 3 determinations. B–D. Cells expressing control or K-Ras shRNAs were stimulated with Tn and eIF2α phosphorylation (B), CHOP (C), and LC3-I (upper band, D) to LC3-II (lower band, D) conversion were determined. Bar graphs show mean ± SEM of 3 determinations.
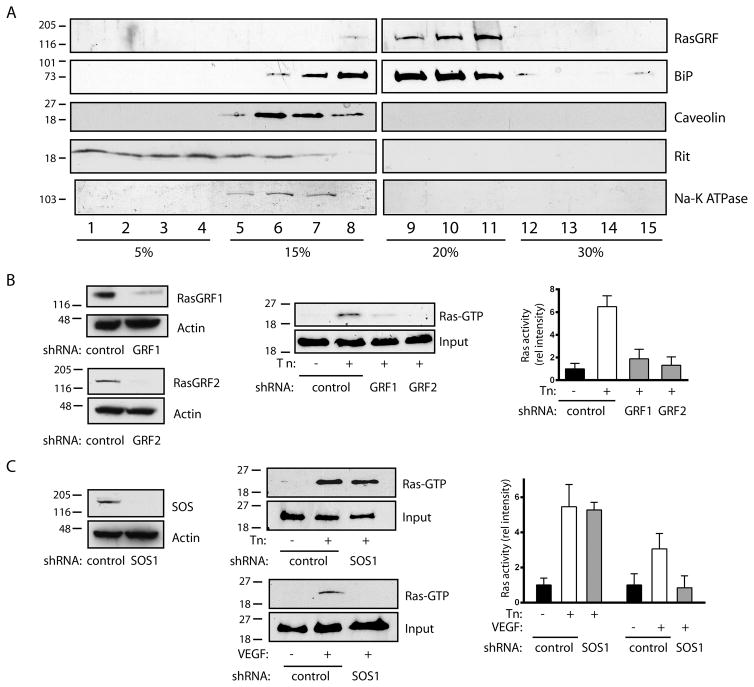
We hypothesized that an ER-resident Ras guanyl exchange factor (GEF) was specifically activated during ER stress, leading to Ras activation. Of the known Ras GEFs, RasGRF1 and RasGRF2 have been shown to associate with both plasma membrane and ER endomembranes, although their function at ER sites is unknown 22. In HUVECs, iodixanol density gradients demonstrated that RasGRF cosedimented quantitatively with ER fractions, marked by BiP, with a lesser amount cosedimenting with plasma membrane fractions, marked by caveolin, Rit, and Na-K ATPase (Figure 4A). Further, knockdown of either RasGRF1 or RasGRF2 alone markedly reduced Ras activation by tunicamycin (Figure 4B). The RasGRF isoforms are known to require homo or heterodimerization for full activation 23, suggesting a possible explanation for the pronounced effect of single isoform knockdowns. In contrast, knockdown of SOS1, known to associate with the plasma membrane, had no effect on ER stress-induced Ras (Figure 4C). Further, a time course study indicated that Ras becomes activated by 8 h of tunicamycin treatment but not at early time points, precluding the involvement of SOS1 at earlier time points (Figure V A). Accordingly, SOS1 knockdown did not significantly affect Ras activation at any time point (Figure V B). Consistent with the activation of plasma membrane Ras by VEGF, SOS1 knockdown completely blocked Ras activation by VEGF (Figure 4C).
Figure 4. RasGRF is required for tunicamycin-induced Ras activation.
A. HUVEC were subjected to discontinuous iodixanol gradient fractionation. RasGRF cosedimented with ER fractions (9–11, marked by BiP), and not plasma membrane fractions (5–7, marked by caveolin, Rit, and Na-K ATPase). B. Effect of shRNAs against RasGRF1 and RasGRF2 on protein expression is shown in left panels. Cells expressing control or RasGRF shRNAs were stimulated with Tn (10 μg/ml for 16 h) and assessed for Ras activity. Representative pulldowns in center panel, graph at right shows Ras activity, mean ± SEM of 3 determinations. Knockdowns were 77% (RasGRF1) and 93% (RasGRF2) efficient. C. Effect of shRNA against SOS1 on protein expression shown in left panel. Cells expressing control or SOS1 shRNA were stimulated with Tn (10 μg/ml for 16 h) or VEGF (50 U/ml, 5 min) and assessed for Ras activity. Representative pulldowns in center panel, graph at right shows Ras activity mean ± SEM of 3 determinations. SOS1 knockdown was 98% efficient.
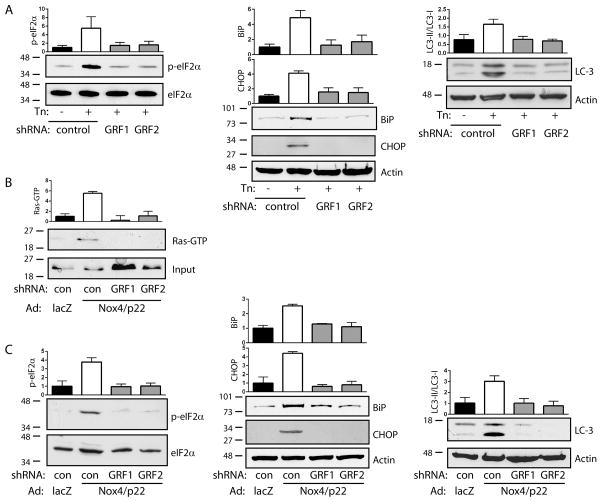
As further confirmation that ER-associated Ras GRFs act upstream of the UPR, we found that knockdown of RasGRF1 or RasGRF2 also prevented tunicamycin-induced eIF2α phosphorylation, BiP and CHOP induction, and LC3-I to LC3-II conversion (Figure 5A). In addition, overexpression of Nox4 and its requisite partner p22phox caused Ras activation (Figures 5B and VI A), eIF2α phosphorylation, BiP and CHOP induction, and LC3-I to LC3-II conversion (Figure 5C). Nox4-induced Ras activation, as well as induction of the UPR and autophagy, were blocked by knockdown of RasGRF1 or RasGRF2 (Figure 5B–C), confirming the transduction of Nox4-dependent ER stress signals by RasGRF. In contrast, knockdown of RasGRF1 or RasGRF2 had no effect on VEGF-induced Ras activation (Figure VI B). Together, these data indicate that tunicamycin-induced ER stress triggers focal activation of Ras through Nox4 and ER-associated RasGRF, leading to downstream initiation of the UPR.
Figure 5. RasGRF mediates Nox4-dependent ER stress signaling.
A. Cells expressing control, RasGRF1, or RasGRF2 shRNAs were stimulated with Tn (10 μg/ml for 16 h) and eIF2α phosphorylation (left), BiP and CHOP expression (center), and LC3-I to LC3-II conversion (right) were determined. Bar graphs are mean ± SEM of 3 determinations. B. Cells expressing control, RasGRF1, or RasGRF2 shRNAs were transduced with lacZ or Nox4/p22phox and Ras activity was assessed by pulldown. Bar graph is mean ± SEM of 3 determinations. C. Cells were treated as in (B) and eIF2α phosphorylation (left), BiP and CHOP expression (center), and LC3-I to LC3II conversion (right) were determined. Bar graphs are mean ± SEM of 3–4 determinations.
Nox4 triggers Ca2+-induced ER signaling
RasGRF is known to respond to calcium through the binding of calcium-calmodulin to the RasGRF IQ domain, consistent with its calcium-sensitive activation in neuronal cells 24. We therefore hypothesized that calcium may link Nox4 with Ras activation through RasGRF. Tunicamycin increased cytosolic free calcium in a perinuclear space consistent with an ER distribution (Figure VII A). Increases in cytosolic calcium following treatment with tunicamycin were markedly reduced by Nox4 knockdown as well as ER-targeted catalase (Figure 6A, Figure VII B), confirming that Nox4-dependent H2O2 acts upstream of calcium signals and possibly RasGRF activation. Consistent with this scenario, reduction of cytosolic calcium levels using BAPTA-AM blocked tunicamycin-induced activation of Ras (Figure 6B) as well as eIF2α phosphorylation, BiP and CHOP induction, and LC3-I to LC3-II conversion (Figure 6C). Oxidants are known to induce increases in cytosolic free calcium, in large part through cysteine oxidation of ER-associated calcium transporters. Of known oxidant-sensitive calcium transport modulators, very low levels of the ryanodine and IP3 receptors were found. However, significant amounts of sarcoendoplasmic reticulum calcium ATPase (SERCA) were detected. Using 5-IAF to label reactive (reduced) cysteine residues, tunicamycin was found to markedly increase cysteine oxidation of SERCA (corresponding to reduced 5-IAF labeling) (Figure 6D). Knockdown of Nox4 restored levels of reduced cysteine, indicating that SERCA is a direct oxidation target of Nox4. Taken together, our data demonstrate that ER stress triggers a Nox4-Ca2+-RasGRF-Ras signaling module on the ER surface which is required to activate an ER stress response through the UPR.
Figure 6. Calcium mediates Ras-dependent ER signaling.
A. Fluo-3-AM fluorescence intensities were measured following treatment of control or Nox4 shRNA-expressing cells with Tn (10 μg/ml, 16 h). Top panels show representative pseudocolored Fluo-3-AM images, bar graph below shows mean ± SEM of 50 determinations; *P<0.001 compared to vehicle with control shRNA, † P<0.001 compared to Tn with control shRNA. B. Cells were preloaded with BAPTA-AM and treated with Tn. Ras activity was assessed by pulldown. Bar graph is mean ± SEM of 3 determinations. C. Cells were treated as in (B) and eIF2α phosphorylation, BiP and CHOP expression, and LC3-I to LC3-II conversion were determined. Bar graphs are mean ± SEM of 3 determinations. D. Cells expressing control or Nox4 shRNA were treated with Tn and reactive cysteines were alkylated with 5-iodoacetamidofluorescein (5-IAF). Lysates were immunoprecipitated for SERCA and immunoblotted for either SERCA or fluorescein.
DISCUSSION
In this study, we found a novel basis for the functional coupling of Nox4 and Ras using tunicamycin to study the specific example of ER stress in endothelial cells. We found first that despite the co-occupancy of Nox4 and Ras on different subcellular membrane domains, ROS production and Ras GTP loading colocalize specifically on the ER surface in response to tunicamycin. In support of the general importance for local ROS-Ras coupling, we found that VEGF, known to initiate signaling on the plasma membrane, caused ROS production and Ras activation restricted instead to the plasma and not ER membrane, demonstrating agonist-specific spatial coactivation. Following ER stress, the mechanism of Nox4 activation remains unclear. While expression of this oxidase is clearly inducible, Nox4-dependent increases in ROS production have been shown to occur within minutes in response to angiotensin II, insulin, VEGF, arachidonic acid, and mechanical stress 25–28. Thus activation of a latent pool of ER-associated Nox4 is likely to occur, although the precise mechanism for activation remains unknown.
Secondly, our data indicate that the Ras-selective GEFs RasGRF1 and RasGRF2 activate ER signaling upstream of Ras. These GEFs are predominantly associated with the ER and nuclear envelope, with some plasma membrane association, and are excluded from the Golgi 22. RasGRF1/2 are best known as regulators of neuronal differentiation and synaptic plasticity during memory consolidation, and are activated in association with calcium transients following synaptic receptor activation 24, 29–31. More recently, participation of RasGRF1/2 in T-cell activation has also been described 32, 33. However, despite their strong ER localization, these RasGRFs have not previously been demonstrated to participate in ER signaling. Our data suggest that their location on the ER endomembrane and their sensitivity to calcium signals confer unique properties to rapidly and specifically respond to ER stress, a function not previously assigned to the RasGRFs.
The activation of specific GEFs targeted to different subcellular domains is an important cellular strategy to compartmentalize Ras activation, which in turn discriminates downstream signaling events. Differential targeting of the Ras GEFs Ste6 and Efc25, for instance, distinguishes Ras involvement in yeast mating versus morphogenic signaling 34, and Golgi-restricted RasGRP1 directs Ras signaling to the Golgi apparatus of T-cells as opposed to the plasma membrane 35. Our data suggest that the initiation of ER stress signaling requires a similar arrangement whereby ER-localized RasGRF confines Ras activation to the ER surface and assures an appropriate ER-based response.
Finally, we found that the role of Nox4 in this cascade appears to be the triggering of calcium signals that are required for RasGRF activation. The initiation of calcium signals and ROS production are now known to be tightly linked, as all major calcium-handling molecular complexes are redox-sensitive 36. Our studies indicate that Nox4 targets SERCA in response to ER stress. Cysteine oxidation of SERCA, which constitutively pumps calcium from the cytosol into the ER, inhibits its activity and raises cytosolic calcium. Besides SERCA, it is possible that other proteins which regulate transport of calcium across the ER membrane are targeted by Nox4-derived H2O2.
From an evolutionary standpoint, genes encoding Nox and Ras arose together in simple eukaryotes to control overlapping signaling pathways, particularly those which respond to environmental stresses. Following nutrient deprivation, for instance, both filamentous fungi and slime molds form spore-bearing fruiting bodies, a process requiring both Nox and Ras genes 37–41. Likewise, penetration of rice plants by the filamentous fungus Magnaporthe grisea requires coactivation of both Ras and Nox proteins within a specialized appendage, the appressorium 42, 43. Recently, an ancient Nox protein, Yno1p, was found in yeast cells to be quantitatively associated with the ER and to relay mitochondrial stress signals in collaboration with Ras 44. Therefore, the cooperation we observe between Ras and Nox4 in ER stress signaling may exemplify a conserved interaction between these two spatiotemporally co-regulated protein families in stress response pathways.
Supplementary Material
HIGHLIGHTS.
ER stress caused by tunicamycin initiates a calcium signal downstream from Nox4.
RasGRF1/2 is an ER resident, calcium-dependent Ras GEF which is activated by ER stress.
RasGRF1/2 activates Ras on the ER surface, causing local coupling of ROS and Ras on the ER surface.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the American Heart Association, the Cancer Prevention Research Institute of Texas, and the NIH, grants T32-HL098040 to CDO and R01-CA208620 to LST.
ABBREVIATIONS
- Nox
NADPH oxidase
- GRF
Guanine nucleotide releasing factors
- HUVEC
Human umbilical vein endothelial cells
- ROS
Reactive oxidant species
- SERCA
Sarcoendoplasmic reticulum calcium ATPase
- UPR
Unfolded protein response
Footnotes
DISCLOSURES
None.
References
- 1.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–61. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 5.Onken B, Wiener H, Philips MR, Chang EC. Compartmentalized signaling of Ras in fission yeast. Proc Natl Acad Sci USA. 2006;103:9045–50. doi: 10.1073/pnas.0603318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, 2nd, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4:343–50. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 7.Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174:615–23. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307:H945–57. doi: 10.1152/ajpheart.00918.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–64. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–63. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res. 2013;113:1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30:3553–68. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–6. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 15.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–24. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 16.Augsten M, Pusch R, Biskup C, Rennert K, Wittig U, Beyer K, Blume A, Wetzker R, Friedrich K, Rubio I. Live-cell imaging of endogenous Ras-GTP illustrates predominant Ras activation at the plasma membrane. EMBO Rep. 2006;7:46–51. doi: 10.1038/sj.embor.7400560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo YS, Sun Z, Ma J, Cui W, Gao B, Zhang HY, Han YH, Hu HM, Wang L, Fan J, Yang L, Tang J, Luo ZJ. 17beta-Estradiol inhibits ER stress-induced apoptosis through promotion of TFII-I-dependent Grp78 induction in osteoblasts. Lab Invest. 2014;94:906–16. doi: 10.1038/labinvest.2014.63. [DOI] [PubMed] [Google Scholar]
- 19.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, Kaufman RJ, Bastian BC, Soengas MS. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–63. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 20.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arozarena I, Matallanas D, Berciano MT, Sanz-Moreno V, Calvo F, Munoz MT, Egea G, Lafarga M, Crespo P. Activation of H-Ras in the endoplasmic reticulum by the RasGRF family guanine nucleotide exchange factors. Mol Cell Biol. 2004;24:1516–30. doi: 10.1128/MCB.24.4.1516-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anborgh PH, Qian X, Papageorge AG, Vass WC, DeClue JE, Lowy DR. Ras-specific exchange factor GRF: oligomerization through its Dbl homology domain and calcium-dependent activation of Raf. Mol Cell Biol. 1999;19:4611–22. doi: 10.1128/mcb.19.7.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Medarde A, Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim Biophys Acta. 2011;1815:170–88. doi: 10.1016/j.bbcan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol. 2003;285:F219–29. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 27.Lin CS, Lee SH, Huang HS, Chen YS, Ma MC. H2O2 generated by NADPH oxidase 4 contributes to transient receptor potential vanilloid 1 channel-mediated mechanosensation in the rat kidney. Am J Physiol Renal Physiol. 2015;309:F369–76. doi: 10.1152/ajprenal.00462.2014. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Yang Z, Jiang Y, Hartnett ME. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Molecular vision. 2014;20:231–41. [PMC free article] [PubMed] [Google Scholar]
- 29.Barman A, Assmann A, Richter S, Soch J, Schutze H, Wustenberg T, Deibele A, Klein M, Richter A, Behnisch G, Duzel E, Zenker M, Seidenbecher CI, Schott BH. Genetic variation of the RASGRF1 regulatory region affects human hippocampus-dependent memory. Frontiers in human neuroscience. 2014;8:260. doi: 10.3389/fnhum.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–84. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 31.Schwechter B, Rosenmund C, Tolias KF. RasGRF2 Rac-GEF activity couples NMDA receptor calcium flux to enhanced synaptic transmission. Proc Natl Acad Sci USA. 2013;110:14462–7. doi: 10.1073/pnas.1304340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, Hsu YM, Yang L, Guan JH, Xu GT, Lin X. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med. 2014;211:2307–21. doi: 10.1084/jem.20132349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz S, Santos E, Bustelo XR. RasGRF2, a guanosine nucleotide exchange factor for Ras GTPases, participates in T-cell signaling responses. Mol Cell Biol. 2007;27:8127–42. doi: 10.1128/MCB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadaki P, Pizon V, Onken B, Chang EC. Two ras pathways in fission yeast are differentially regulated by two ras guanine nucleotide exchange factors. Mol Cell Biol. 2002;22:4598–606. doi: 10.1128/MCB.22.13.4598-4606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bivona TG, Perez De Castro I, Ahearn IM, Grana TM, Chiu VK, Lockyer PJ, Cullen PJ, Pellicer A, Cox AD, Philips MR. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–8. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 36.Nunes P, Demaurex N. Redox regulation of store-operated Ca2+ entry. Antioxid Redox Signal. 2014;21:915–32. doi: 10.1089/ars.2013.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano-Dominguez N, Alvarez-Delfin K, Hansberg W, Aguirre J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell. 2008;7:1352–61. doi: 10.1128/EC.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kana-uchi A, Yamashiro CT, Tanabe S, Murayama T. A ras homologue of Neurospora crassa regulates morphology. Mol Gen Genet. 1997;254:427–32. doi: 10.1007/s004380050435. [DOI] [PubMed] [Google Scholar]
- 39.Malagnac F, Lalucque H, Lepere G, Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol. 2004;41:982–97. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Lardy B, Bof M, Aubry L, Paclet MH, Morel F, Satre M, Klein G. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in Dictyostelium discoideum. Biochim Biophys Acta. 2005;1744:199–212. doi: 10.1016/j.bbamcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Reymond CD, Gomer RH, Nellen W, Theibert A, Devreotes P, Firtel RA. Phenotypic changes induced by a mutated ras gene during the development of Dictyostelium transformants. Nature. 1986;323:340–3. doi: 10.1038/323340a0. [DOI] [PubMed] [Google Scholar]
- 42.Park G, Xue C, Zhao X, Kim Y, Orbach M, Xu JR. Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea. Plant Cell. 2006;18:2822–35. doi: 10.1105/tpc.105.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci USA. 2007;104:11772–7. doi: 10.1073/pnas.0700574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leadsham JE, Sanders G, Giannaki S, Bastow EL, Hutton R, Naeimi WR, Breitenbach M, Gourlay CW. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell metabolism. 2013;18:279–86. doi: 10.1016/j.cmet.2013.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.