Abstract
It was recently demonstrated the penfluridol inhibited breast tumor growth and metastasis and this was associated with downregulation of α6- and β4-integrins. In this study, we observed the penfluridol induced reactive oxygen species (ROS) and this was the primary mechanism of action. Penfluridol-mediated growth inhibition, induction of apoptosis, and inhibition breast cancer cell migration was attenuated after cotreatment with glutathione (GSH). Penfluridol also downregulated Sp transcription factors Sp1, Sp3 and Sp4 through epigenetic downregulation of cMyc and cMyc-regulated microRNAs (miR-27a and miR-20a/miR-17) and induction of the miR-regulated Sp transcriptional repressors ZBTB10 and ZBTB4. α6- and β4-Integrins as well as α5- and β1-integrin are Sp-regulated genes that are also coregulated by the orphan nuclear receptor NR4A1 and these integrins can be targeted by agents such as penfluridol that suppress Sp1, Sp3 and Sp4 and also by NR4A1 antagonists.
Keywords: penfluridol, ROS, integrin, Sp proteins, NR4A1
INTRODUCTION
Repositioning clinically-approved drugs for cancer chemotherapy has several advantages including a more rapid drug approval process coupled with potential development of mechanism-based compounds that can be clinically used to target important pro-oncogenic pathways. This approach has been particularly successful with non-steroidal antiinflammatory drugs (NSAIDs) and antidiabetics such as metformin (1,2). Phenothiazine-derived antipsychotic drugs such as thioridazine and chlorpromazine exhibit anticarcinogenic activity in several different cancer cell lines (3–9). More recent studies have demonstrated that penfluridol, another antipsychotic drug, also inhibits breast and pancreatic cancer cell growth (9,10). For example, in pancreatic cancer a series of phenothiazene analogs induced apoptosis and inhibited clonogenic growth and colony formation, and more detailed studies with penfluridol indicated that induction of protein phosphatase 2A (PP2A) was a key effect of this compound (9). Penfluridol exhibited antimetastatic activity in triple negative breast cancer cells and inhibited tumor growth and brain metastasis in three different in vivo models and the key elements were inhibition of α6- and β4-integrin expression (10). However, the mechanisms of the penfluridol-induced responses were not well defined, and this limits potential clinical applications of the compound.
Recent studies in this laboratory showed that β1- and β3-integrin expression in breast cancer cells is regulated by specificity protein 1 (Sp1) transcription factor (TF) in combination with the orphan nuclear receptor 4A1 (NR4A1, Nur77, TR3) which acts as a nuclear cofactor (11). Many of the effects observed in breast and other cancer cell lines treated with penfluridol and other phenothiazine derivatives are similar to that observed after knockdown of Sp transcription factors Sp1, Sp3 or Sp4 or after treatment with agents that target Sp TFs (10–18). For example, knockdown of Sp1, Sp3 or Sp4 individually or combined decreased proliferation and migration/invasion of breast (MDA-MB-231 and SKBR3) and other cancer cell lines (12) and similar results were observed for drugs that repress Sp TF expression (13–18). Moreover, the effects of penfluridol and other phenothazines on inhibition of several genes including cyclin D1, bcl-2, vascular endothelial growth factor (VEGF) receptors, myc and activation/cleavage caspase-3/PARP (3–10) have also been observed after Sp knockdown (10–18). It was recently reported that the antimetastatic activity of penfluridol in triple negative breast cancer cells was related to downregulation of α6- and β4-integrin expression (10); however, since both integrin gene promoters are GC-rich, it is possible that Sp1 and other Sp TFs may regulate expression of α6- and β4-integrins as well as α5-integrin (19–21).
Therefore, we hypothesize that the mechanism of action of penfluridol as an antimetastatic agent for triple negative breast cancer is due to downregulation of Sp TFs. This hypothesis was confirmed in this study which shows that penfluridol induces reactive oxygen species (ROS) in breast cancer cells and ROS-dependent downregulation of Sp1, Sp3 and Sp4 and Sp-dependent genes including α6-, α5-, β1- and β4-integrins which are also coregulated by NR4A1 and decreased by NR4A1 antagonists.
MATERIALS AND METHODS
Cell lines and antibodies
Breast cancer (SKBR3, MDA-MB-231) cell lines were purchased from American Type Culture Collection (Manassas, VA) and were kept frozen until initiation of these studies. The cells were received at low passage (<15) and new frozen stocks were used every 6–8 weeks. The two cell lines were authenticated by Biosynthesis (Lewisville, TX, USA) on February 3, 2015. Cells were maintained 37°C in the presence of 5% CO2 in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium with 10% fetal bovine serum with antibiotic. Dulbecco’s Modified Eagle’s Medium was purchased from GenDepot (Barker, TX). Penfluridol, N-acetylcysteine (NAC), catalase and 36% formaldehyde were purchased from Sigma-Aldrich (St. Louis, MO). Glutathione (GSH) reduced free acid was purchased from Millipore (Temecula, CA). Hematoxylin was purchased from Vector Laboratories (Burlingame, CA). Apoptotic, Necrotic, and Healthy Cells Quantification Kit was purchased from Biotium (Hayward, CA). Antibodies were purchased as outlined in Supplementary Table 1.
Cell proliferation, luciferase and ROS assays and Annexin V staining
Cell proliferation and ROS assays using the cell permeable fluorescent CM-H3DCFD4 probe were carried out as described previously (11–13) (also see Supplemental Methods), and changes in cell number were determined by Coulter Z1 cell counter. Annexin V staining used the Vybrant apoptosis kit according to the manufacturer’s protocol. The GC-rich promoter luciferase pGL3-pGC3-luc construct and transfection/luciferase assays were carried out as previously described (22).
Boyden chamber assay
SKBR3 and MDA-MB-231 cancer cells (3.0 × 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were seeded and subsequently treated with varying concentrations of penfluridol for 24 hr (± GSH, 3 hr prior to treatment) and for subsequent 24 hr of cotreatment. Cells were trypsinized, counted, placed in 12-well 8.0 µm pore ThinCerts from Greiner Bio-one (Monroe, NC), allowed to migrate for 24 hr, fixed with formaldehyde, and then stained with hematoxylin. Equal numbers of cells were used for each assay and cells that migrated through the pores were then counted as described (11–13).
RT-PCR
miRNA was isolated using the mirVana miRNA isolation kit (Ambion, Austin, TX) according to the manufacturer's protocol. Quantification of miRNA (RNU6B and miR-17, miR-20a, and miR-27a) was done using the TaqMan miRNA assay kit (Life Technologies) according to the manufacturer's protocol with real-time PCR. U6 small nuclear RNA was used as a control to determine relative miRNA expression.
Western blot analysis
SKBR3 and MDA-MB-231 cancer cells (3.0 × 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were treated with varying doses of penfluridol for 24 hr. Cells were analyzed by western blot as described previously (11–13) and the catalase inhibition studies were determined as described (17).
Small interfering RNA interference assay
siRNAs used are outlined in Supplementary Table 1. SiRNA experiments were conducted as described previously (11–13).
Chromatin immunoprecipitation
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express magnetic chromatin immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer's protocol. SKBR3 and MDA-MB-231 cells were treated with DMSO, DIM-C-pPhOH (15 or 20 µM), DIM-C-pPhCO2Me (15 or 20 µM) for 24 hr, or penfluridol (5 µM) for 3 or 6 hr. Cells were then fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to the desired chromatin length (~200 to 1,500 bp). The sonicated chromatin was immunoprecipitated with normal IgG, p300, Sp1, Sp3, Sp4, NR4A1, or RNA polymerase II antibodies and protein A-conjugated magnetic beads at 4°C for overnight. After the magnetic beads were extensively washed, protein-DNA cross-links were reversed and eluted. DNA was prepared by proteinase K digestion followed by PCR amplification. Primers used for detecting PCR products are listed in Supplementary Table 1. PCR products were resolved on a 2% agarose gel in the presence of RGB-4103 GelRed Nucleic Acid Stain.
Xenograft studies
Female athymic nude mice 4–6 weeks old were purchased, and MDA-MB-231 cancer cells (2.0 × 106 cells) were suspended in Matrigel (1:1 ratio) and injected into the mammary fat pad of athymic nude mice. When tumors became palpable (150–200 mm3), mice were randomly assigned to control (corn oil vehicle) and penfluridol (10 mg/kg/day), and then treated every day for 19 days. Tumor volumes, tumor weights, and tumor lysates were determined and analyzed as previously described (13,14).
Statistical analysis
Statistical significance of differences between the treatment groups was determined as previously described (11–13).
RESULTS
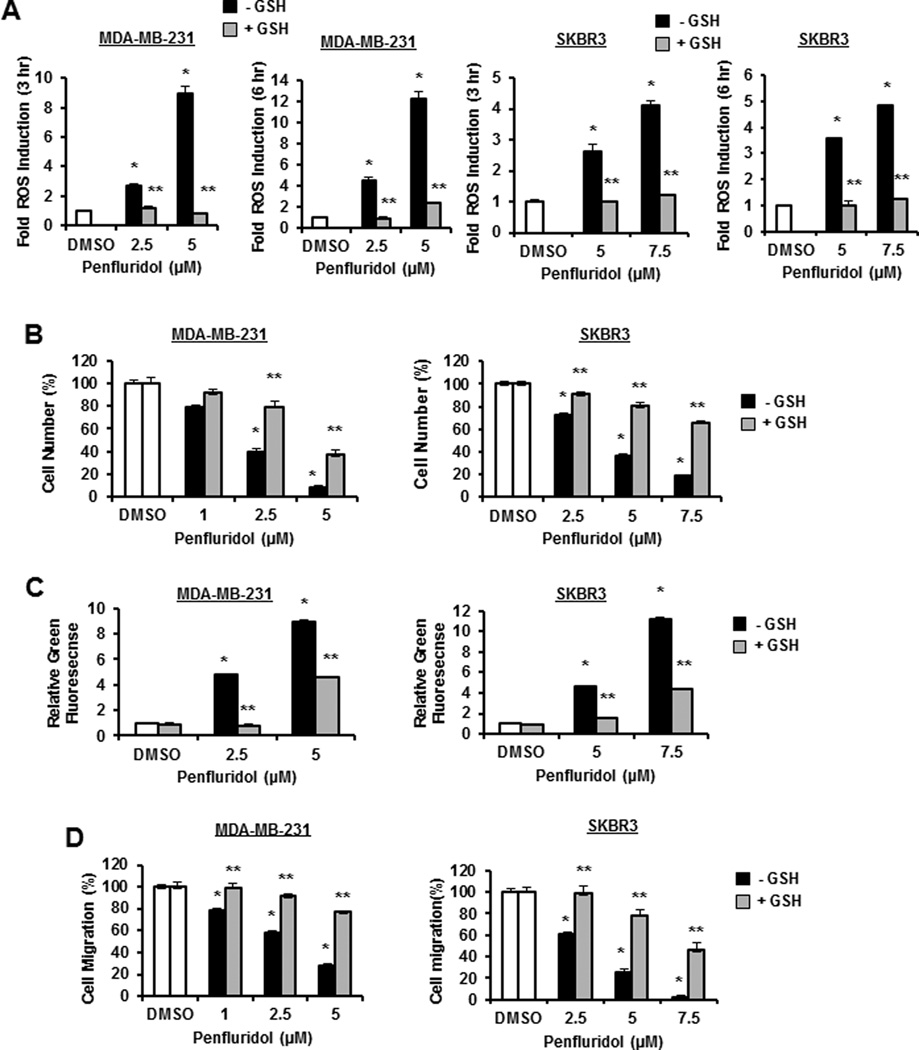
Drugs that downregulate Sp TFs act through ROS-dependent and -independent pathways (18), and results in Figure 1A show that different concentrations of penfluridol induced ROS in MDA-MB-231 and SKBR3 cells after treatment for 3 and 6 hr and similar results were observed after 24 hr (Suppl. Fig. S1A). Moreover, after cotreatment with 5 mM GSH, the penfluridol-mediated induction of ROS as detected using the fluorescent probe CM-H2DCFD4 was significantly attenuated. The role of penfluridol-induced ROS on cell proliferation (Fig. 1B), Annexin V staining (apoptosis) (Fig. 1C), and migration in a Boyden chamber assay (Fig. 1D) was also determined in MDA-MBA-231 and SKBR3 cells treated with penfluridol alone and in combination with GSH. Penfluridol inhibited cell growth and induced Annexin V staining; however, we now show for the first time that all of these responses were ROS-dependent and significantly attenuated after cotreatment with GSH. SKBR3 cells appeared to be more resistant to penfluridol than MDA-MB-231 cells and this may be due to several factors including constitutive ROS levels, GSH and reductant levels and differential expression of drug-metabolizing and transport enzymes.
Figure 1.
Penfluridol induces ROS and ROS-dependent responses in breast cancer cells. (A) MDA-MB-231 and SKBR3 cells were treated with DMSO, 2.5 or 5.0 µM penfluridol alone, or in combination with 5 mM GSH for 3 hr, and ROS activity was determined as outlined in the Materials and Methods. The cells were treated as outlined above for 24 hr and effects on cell proliferation (B), Annexin V staining (C), and migration (D) in a Boyden chamber assay were determined as outlined in the Materials and Methods. Results are expressed as means ± SE for 3 replicates for each data points and significant (p<0.05) modulation by penfluridol (*) and reversal by GSH (**) are indicated.
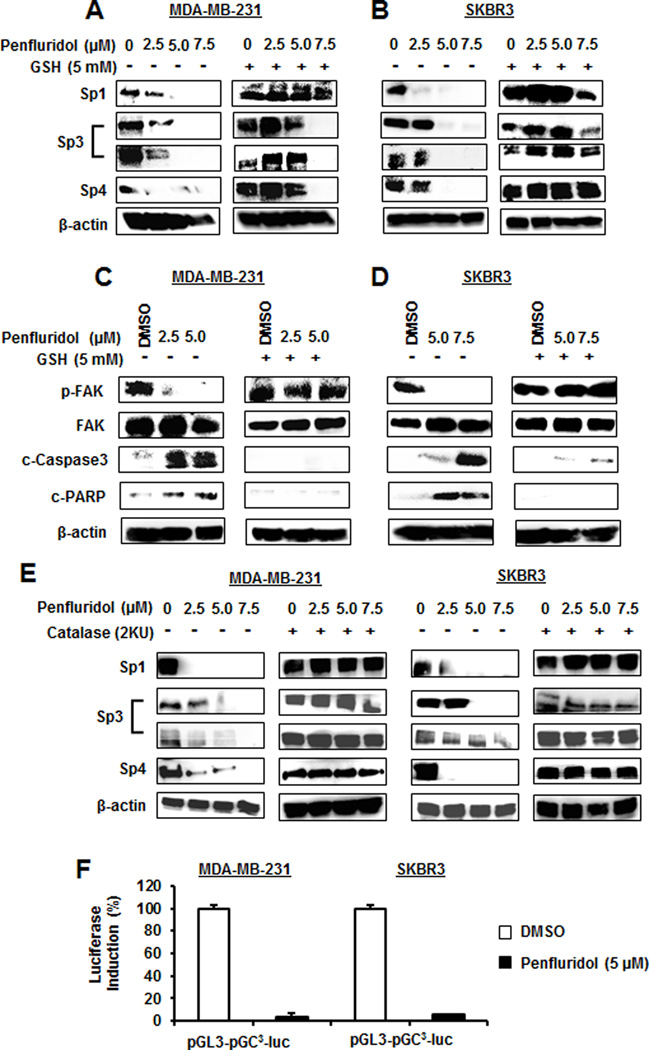
Results illustrated in Figures 2A and 2B show that penfluridol decreased expression of Sp1, Sp3 (both high and low molecular weight forms), and Sp4 in MDA-MB-231 and SKBR3 cells and cotreatment with GSH attenuated these effects, and similar results were observed for NAC (Suppl. Fig. S1B). For some responses (e.g. Sp1), addition of GSH or NAC enhanced endogenous expression of these proteins. Penfluridol decreased focal adhesion kinase (FAK) phosphorylation and activated (cleaved) caspase 3 in breast cancer cells (Figs. 2C and 2D) as previously reported (10) and the responses were attenuated by cotreatment with GSH. Penfluridol also induced ROS-dependent caspase 3/PARP cleavage (apoptosis) in both cell lines (Figs. 2C and 2D). In addition, penfluridol-mediated downregulation of Sp proteins was also inhibited after addition of catalase (Fig. 2E) and penfluridol also decreased luciferase activity in these cells transfected with a construct containing 3 consensus (Sp) binding sites (Fig. 2F), confirming that induction of apoptosis and the growth and migration inhibitory effects of penfluridol in breast cancer cells were ROS-dependent.
Figure 2.
Penfluridol induces ROS-dependent modulation of Sp1, Sp3, Sp4 and other responses in breast cancer cells. MDA-MB-231 and SKBR3 cells were treated with different concentrations of penfluridol alone or in combination with GSH for 24 hr, and whole cell lysates were analyzed for Sp1, Sp3 and Sp4 (A and B, respectively) and other responses (C and D, respectively) as outlined in the Materials and Methods. (E) Penfluridol-mediated downregulation of Sp proteins was also determined in the presence or absence of catalase by western blots of whole cell lysates. (F) MDA-MB-231 and SKBR3 cells were transfected with pGL3-GC3-luc and luciferase was determined as described (22).
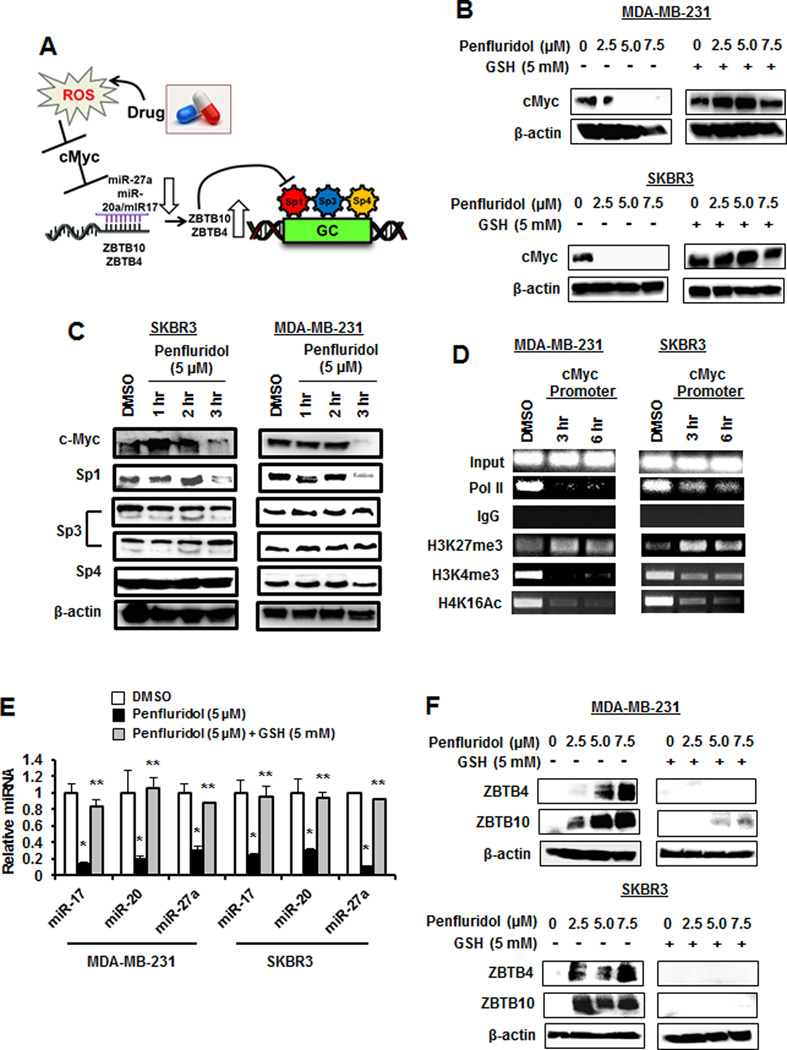
Penfluridol activates the ROS ⊣ Myc ⊣ miR-27a/miR-20/17a → ZBTB pathway
Figure 3 illustrates the cascade of events leading to ROS-dependent downregulation of Sp TFs and this is initiated by cMyc downregulation due to rapid shifts of chromatin-modifying complexes from non-GC-rich to GC-rich (e.g. cMyc) gene promoters (13,14,23). Results in Figure 3B show that penfluridol also decreased cMyc expression in MDA-MB-231 and SKBR3 cells and this response was totally reversed after cotreatment with GSH, and Figure 3C shows that cMyc and Sp1 were rapidly decreased in MDA-MB-231 and SKBR3 cells after treatment with penfluridol. ChIP analysis showed that pol II association with the GC-rich cMyc promoter was decreased after treatment of MDA-MB-231 and SKBR3 cells with penfluridol for 3 and 6 hr, and this was accompanied by an increase in the inhibitory H3K27me3 and a decrease in the activating H3K4me4 and H4K16Ac histone marks (Fig. 3D). The ROS-dependent decrease in cMyc was accompanied by a decrease in the cMyc-regulated miR-27a and miR-20a/miR-17 after treatment penfluridol and this decrease was also inhibited after cotreatment with GSH (Fig. 3E). Penfluridol-mediated repression of miR-27a and miR-20/miR-17 (part of the miR-17–92 cluster) resulted in induction of the miR-regulated Sp repressors ZBTB10 and ZBTB4, respectively, and this induction response was also attenuated in cells cotreated with GSH (Fig. 3F). These results demonstrate that the mechanism of penfluridol-induced ROS is initiated by ROS-mediated epigenetic repression of cMyc (Fig. 3A) and this has previously been observed for phenethylisothiocyanate (PEITC) and HDAC inhibitors in pancreatic cancer and rhabdomyosarcoma (RMS) cells, respectively (13,14).
Figure 3.
Mechanism of penfluridol-induced Sp downregulation. (A) Molecular mechanism of drug-induced ROS and ROS effects on miR-ZBTB interactions and Sp downregulation (14). MDA-MB-231 and SKBR3 cells were treated with penfluridol alone or in combination with GSH for 24 hr (B) or penfluridol alone for different times (C), and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. (D) Cells were treated with 5 µM penfluridol, and effects on interaction with the proximal GC-rich cMyc promoter were by ChIP assays as outlined in the Materials and Methods. (E) Cells were treated as outlined in (B) and expression of microRNAs (miRs) was determined by real time PCR. Penfluridol significantly (p<0.05) decreased all miRs and GSH significantly reversed these responses. (F) Cells were treated as outlined in (B) and expression of ZBTB10 and ZBTB4 were determined by western blot analysis as outlined in the Materials and Methods.
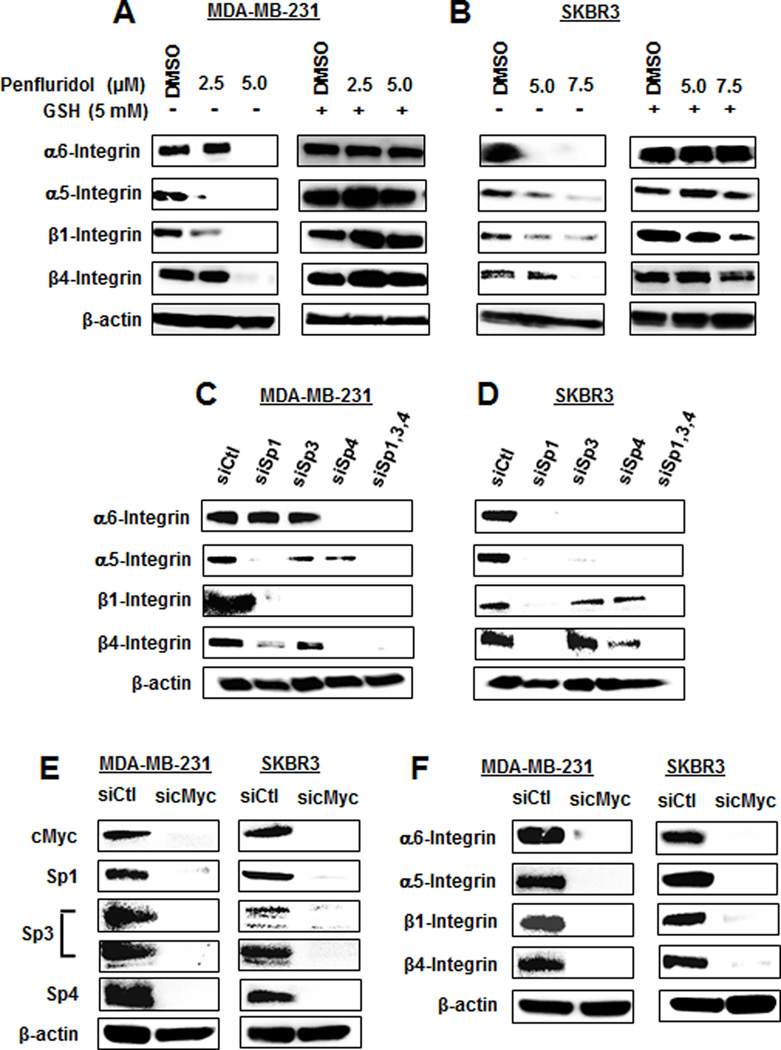
Penfluridol induces ROS-dependent repression of integrins
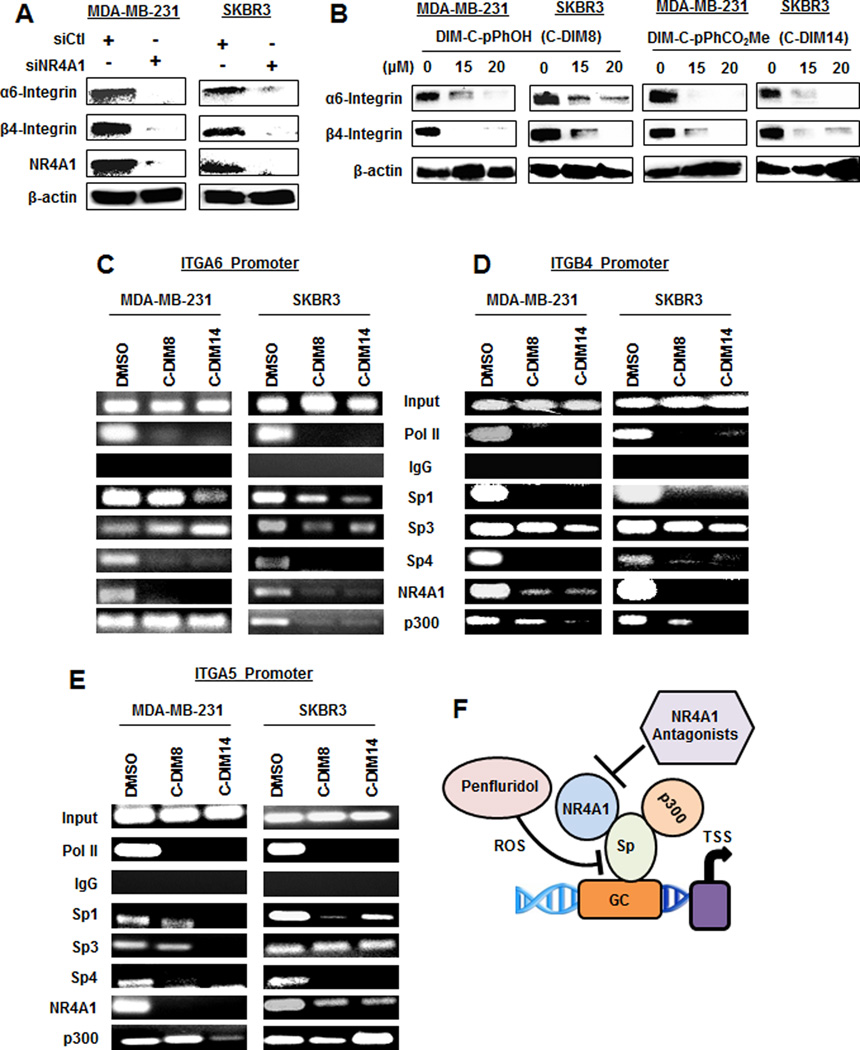
Penfluridol was reported to decrease α6- and β4-integrin in breast cancer cells (10). Our results confirm this same response in MDA-MB-231 and SKBR3 cells, and cotreatment with GSH which attenuated this effect (Fig. 4A) indicating that this response was also ROS-dependent. We also observed that α5- and β1-integrin expression were decreased by penfluridol and rescued after cotreatment with GSH (Figs. 4A and 4B) and this was consistent with our recent studies showing that β1-integrin is an Sp-regulated gene and α5-integrin appears to be coregulated with β1-integrin (11). Confirmation that α6, α5-, β4- and β1-integrin are Sp-regulated genes was determined by RNA interference where knockdown of one or more of Sp1 (siSp1), Sp3 (siSp3), Sp4 (siSp4) or all three Sps (siSp1,3,4) decreased expression of the integrins in MDA-MB-231 (Fig. 4C) and SKBR3 (Fig. 4D) cells. Lysates for this western blot were obtained from a previous study which showed efficient knockdown of Sp1, Sp3 and Sp4 (12). Results of Sp knockdown show that α5- and β4-integrins were coregulated by two or all three of the Sp TFs; however, α6-integrin and β1-integrin were regulated only by Sp4 (MDA-MB-231) and Sp1 (SKBR3), respectively. Knockdown of cMyc by RNA decreased Sp1, Sp3 and Sp4 expression in MDA-MB-231 and SKBR3 cells (Fig. 4E), and the same treatment also decreased expression of α6-, α5-, β1- and β4-integrin in the same cell lines (Fig. 4F). Similar results were observed with a second oligonucleotide targeting cMyc (Suppl. Fig. S1C). Thus, penfluridol activation of ROS results in cMyc downregulation, leading to decreased expression of Sp1, Sp3, Sp4 and Sp-regulated genes α6-, α5-, β1- and β4-integrin. The critical role of cMyc was confirmed in studies showing that penfluridol-induced downregulation of Sp proteins, Sp-regulated genes and caspase 3/PARP cleavage was rescued by Myc overexpression (Suppl. Fig. S2). Moreover, similar results were observed after knockdown of ZBTB10 (Suppl. Fig. S3).
Figure 4.
Penfluridol targets integrin expression via an ROS-cMyc (downregulation) pathway. MDA-MB-231 (A) and SKBR3 (B) cells were treated with penfluridol alone or in combination with GSH, and whole cell lysates were analyzed by western blots. Cells were transfected with oligonucleotides specifically targeted to Sp transcription factors [MDA-MB-231(C) and SKBR3 (D)] or cMyc (E and F), and whole cell lysates were analyzed by western blots. Whole cell lysates obtained after Sp knockdown in MDA-MB-231 and SKBR3 cells were generated in a previous study which also reports effects of these oligonucleotides on Sp1, Sp3 and Sp4 expression (C and D) (12).
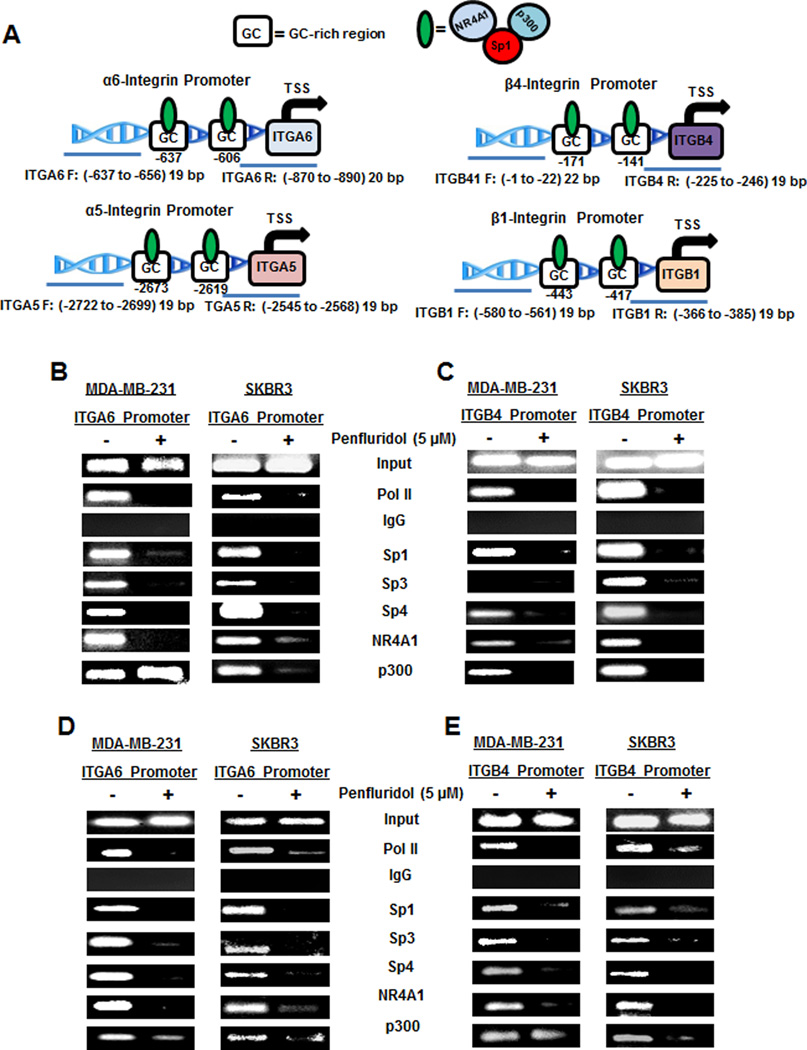
Since the α6-, α5-, β1- and β4-integrins are Sp-regulated genes, we also investigated the effects of penfluridol on association of pol II and Sp1, Sp3, Sp4 with the GC-rich regions of the integrin gene promoters (Fig. 5A) using a ChIP assay. In addition, since we previously observed that NR4A1 and p300 act as cofactors for Sp-dependent activation of β1-integrin (11), the effects of penfluridol on their association with the integrin promoters was also determined. Figures 5B–5E show that pol II, Sp1, Sp3, Sp4, p300 and NR4A1 were all associated with the α6-, β4-, α5- and β1-integrin gene promoters as determined in a ChIP assay. With the exception of p300, treatment of MDA-MB-231 and SKBR3 cells with penfluridol decreased association of Sp1, Sp3, Sp4 and NR4A1 with the four integrin promoters, whereas the loss of p300 was promoter- and cell context-dependent.
Figure 5.
Penfluridol decreases interactions of Sp proteins and other nuclear factors with integrin gene promoters. (A) Outline of GC-rich regions for α6-integrin (ITGA6), β4-integrin (ITGB4), α5-integrin (ITGA5) and β1-integrin (ITGB1) gene promoters and primers targeting these regions. Breast cancer cells were treated with DMSO and 5 µM penfluridol, and interactions of Sp1, Sp3, Sp4 and other nuclear cofactors with the ITGA6 (B), ITGB4 (C), ITGA5 (D) and ITGB1 (E) gene promoters were determined in a ChIP assay as outlined in the Materials and Methods.
NR4A1/Sp regulation of integrins
NR4A1/Sp regulate expression of β1- and α5-integrin in breast cancer cells, and knockdown of Sp or NR4A1 or treatment with an NR4A1 antagonist inhibits their expression (11). Therefore, we investigated the activity of penfluridol as an NR4A1 ligand in Panc1 cells transfected with a GAL4-NR4A1 chimera and a reporter gene containing a GAL4 response element; this assay is used for screening NR4A1 antagonists (24,25), and penfluridol did not inhibit or activate this system (Suppl. Fig. S4). Knockdown of NR4A1 (siNR4A1) or treatment with the NR4A1 antagonists 1,1-bis(3'-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) or 1,1-bis(3'-indolyl)-1-1(p-carbomethoxyphenyl)methane (DIM-C-pPhCO2Me) decreased expression of α6- and β4-integrin in MDA-MB-231 and SKBR3 cells (Fig. 6A) and similar results were previously observed for β1- and α5-integrin (11). Treatment of MDA-MB-231 and SKBR3 cells with DIM-C-pPhOH (C-DIM8) or DIM-C-pPhCO2Me (C-DIM14) showed that these compounds induced loss of pol II from the α6- (Fig. 6C), β4- (Fig. 6D) and α5- (Fig. 6E) integrin promoters in a ChIP assay and this was previously observed for the β1-integrin promoter (11). Unlike penfluridol which decreased expression of Sp1, Sp3 and Sp4, resulting in loss of these TFs from the integrin promoters (Fig. 5), NR4A1 antagonists or NR4A1 knockdown do not affect Sp expression (11) and retention or loss of Sp1, Sp3 or Sp4 from the integrin promoters in ChIP assays is highly variable and gene promoter- and cell context-dependent (11). This variability was observed for p300 (Figs. 6C–6E) and also in a previous study (11). Nevertheless, the results show that both penfluridol and NR4A1 antagonists downregulate expression of α5, α6-, β1- and β-4 integrin by selectively targeting the Sp and NR4A1 transcription factors, respectively, which are required for expression of the integrin genes (Fig. 6F).
Figure 6.
Integrin genes targeted by penfluridol are coregulated by Sp transcription factors and NR4A1. Breast cancer cells were transfected with siNR4A1 (A) or treated with the NR4A1 antagonists DIM-C-pPhOH (C-DIM8) and DIM-C-pPhCO2Me (C-DIM14) (B), and whole cell lysates were analyzed by western blots. Breast cancer cells were treated with DMSO and the two NR4A1 antagonists, and interactions of Sp1, Sp3, Sp4 and other nuclear cofactors with the ITGA6 (C), ITGB4 (D) and ITGA5 (E) gene promoters were determined in a ChIP assay. The same interactions with the ITGB1 promoter were previously reported (11). (F) Model of NR4A1/Sp interactions with GC-rich integrin gene promoters and the differential targeting by penfluridol and NR4A1 antagonists.
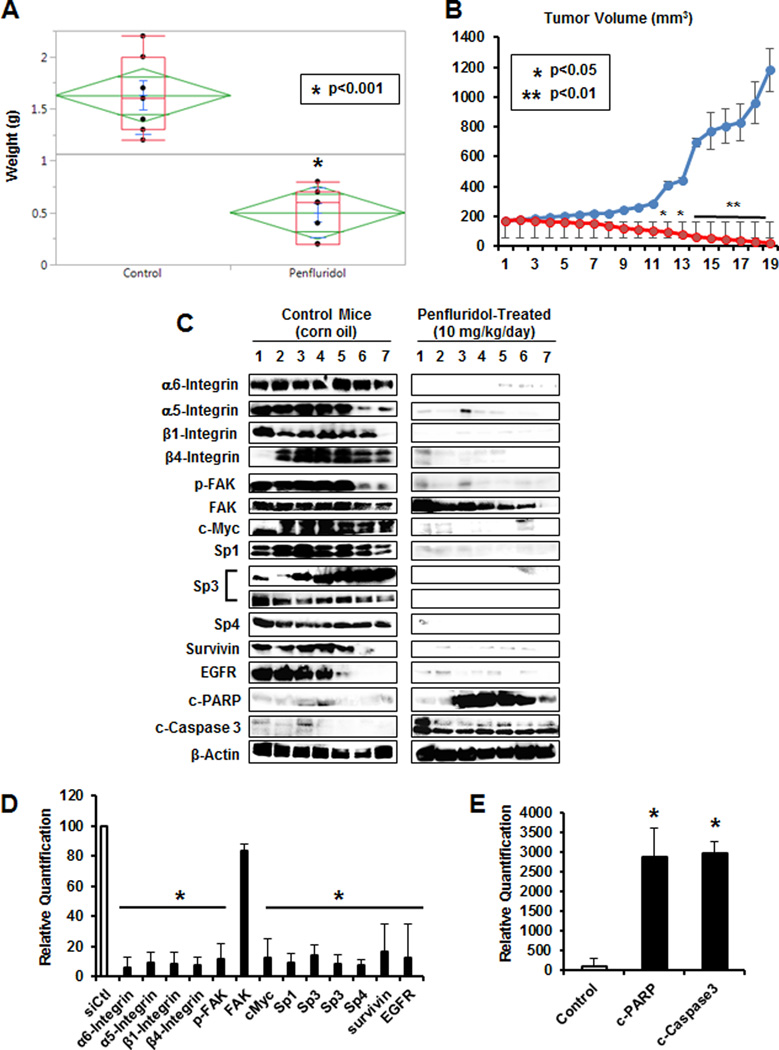
Treatment of athymic nude mice bearing MDA-MB-231 cells in an orthotopic model with penfluridol (10 mg/kg/day) decreased tumor volume over the treatment period (Fig. 7A) and inhibited tumor growth (Fig. 7B), and toxicity (organ damage or weight loss) was not observed. Western blot analysis of lysates from control (solvent) and penfluridol-treated mice showed that penfluridol decreased expression of Sp1, Sp3 and Sp4, prototypical Sp-regulated genes (survivin and EGFR) including α6-, α5-, β1- and β4-integrin and induced apoptosis (Fig. 7C). These effects were also quantitated (Fig. 7D) and penfluridol significantly decreased expression of Sp proteins and Sp-regulated genes or increased caspase 3/PARP cleavage compared to control protein set at 100% and normalized to β-actin. Thus, the in vivo results complement the in vitro studies demonstrating that the anticancer activities of penfluridol are ROS-dependent and this includes NR4A1/Sp-mediated downregulation of integrins.
Figure 7.
Penfluridol inhibits tumor growth in vivo. Penfluridol (10 mg/kg/day) inhibit tumor volume (A) and weight (B) in athymic nude mice bearing MDA-MB-231 cells in an orthotopic model. Western blot analysis of tumor lysates (C) and quantitation of various proteins (D and E) where control proteins normalized to β-actin were set at 100% and penfluridol-mediated changes (normalized to β-actin) compared to controls were determined as outlined in the Materials and Methods. Both high and low molecular weight forms of Sp3 were detected.
DISCUSSION
Integrins are cell surface receptors that function as α/β heterodimers which are formed from the 18 α and 8 β subunits, and the functions of the known 24 α/β-integrin heterodimers are highly tissue-/cell type-specific. In cancer cells, integrins are important for migration and invasion, and overexpression of some integrins in cancer patients is a negative prognostic factor (26,27). Integrins play a key role in breast cancer stem cells and mammary tumors, and there is extensive evidence for the role of β1-, β4-, α5- and α6-integrins and their α/β heterodimers in breast cancer cell migration, invasion and metastasis (28–34). Although integrin inhibitors have been developed, their applications for cancer chemotherapy have been limited (26) and this is due, in part, to β1–β3-integrin switching in which drugs targeting β1-integrin induce β3-integrin (35,36). We recently showed that the orphan nuclear receptor NR4A1 regulates expression of β1-integrin and treatment of breast cancer cells with bis-indole-derived (C-DIMs) NR4A1 antagonists such as DIM-C-pPhOH and DIM-C-pPhCO2Me decrease both β1- and β3-integrin expression and eliminate the switching pathway (11). Both integrins are regulated by an NR4A1-Sp complex which binds to GC-rich regions of their gene promoters (Fig. 6F), suggesting that β1- and β3-integrin can be targeted by NR4A1 antagonists or drugs that target Sp transcription factors.
It was previously reported that penfluridol decreased expression of α6- and β4-integrin in breast cancer cells (10). Since these cell lines highly express Sp1, Sp3 and Sp4 (37) and since there is evidence for Sp-regulation of these integrins (19,20), we hypothesized that the mechanism of action of penfluridol may be due to repression of Sp proteins. We also investigated α5-integrin expression which also has a GC-rich gene promoter (21). Using SKBR3 and MDA-MB-231 cells as a model, it was clear that penfluridol induced ROS and the resulting inhibition of cell growth and migration and induction of apoptosis were all inhibited after cotreatment with GSH (Fig. 1). Subsequent experiments show that induction of ROS was the key factor in mediating downregulating Sp1, Sp3 and Sp4 through ROS-dependent epigenetic repression of cMyc, decreased expression of cMyc-regulated miR-27a and miR-20a/miR-17, and induction of the Sp repressors ZBTB10 and ZBTB4. This ROS-dependent pathway has previously been observed for other ROS inducers such as PEITC and HDAC inhibitors (13,14). The ROS-miR-ZBTB-Sp pathway (Fig. 3A) has also been observed in cancer cells treated with a nitro-aspirin derivative, celastrol, betulinic acid and curcumin; other ROS inducers including arsenic trioxide, ascorbate, hydrogen peroxide and t-butyl hydroperoxide also downregulate Sp1, Sp3 and Sp4 in cancer cell lines (15–17,38–41). We also observed that penfluridol targeted Sp TFs and pro-oncogenic Sp-regulated genes in vivo and this was consistent with potent inhibition of tumor growth in an orthotopic model (Fig. 7) and complemented results of previous studies on the anticancer activities of penfluridol (9,10).
This paper also demonstrates that penfluridol-mediated downregulation of α5-, α6-, β1 and β4-integrins is also ROS-dependent and Sp TFs regulate expression of these genes in breast cancer cell lines. Moreover, like β1- and β3-integrin (11), the α5-, α6- and β4-integrins are coregulated by NR4A1 and expression of this family of integrins is due to interactions of the NR4A1-Sp complex with their corresponding GC-rich gene promoter elements (Fig. 6G). Penfluridol does not exhibit NR4A1 antagonist activity but targets the integrins through repression of Sp TFs, whereas the C-DIM/NR4A1 antagonists inactivate the coactivator-like activity of NR4A1 (Fig. 6F). Nuclear receptor-mediated activation of genes through interaction with DNA bound Sp TFs has been observed for several other receptors (42) and like the integrins in this study can be targeted through inactivation of the receptor or downregulation of Sp TFs. The identification of the mechanism of action of penfluridol coupled with the reported effectiveness of this compound as an inhibitor of breast cancer metastasis will facilitate the design of future clinical applications of this ROS inducer for breast cancer therapy. This would also include applications in treating tumors such as rhabdomyosarcomas which exhibit high endogenous ROS levels (9,43,44).
Supplementary Material
Acknowledgments
Funding: The financial assistance of the National Institutes of Health (P30-ES023512, S. Safe), Texas AgriLife Research and Sid Kyle endowment, is gratefully acknowledged.
Abbreviations
- C-DIM8
1,1-bis(3'-indolyl)-1-(p-hydroxyphenyl)methane
- C-DIM14
1,1-bis(3'-indolyl)-1-(p-carboxymethylphenyl)methane
- DIM-C-pPhCO2Me
1,1-bis(3'-indolyl)-1-(p-carboxymethylphenyl)methane
- DIM-C-pPhOH
1,1-bis(3'-indolyl)-1-(p-hydroxyphenyl)methane
- FAK
focal adhesion kinase
- GSH
glutathione
- NAC
N-acetylcysteine
- NR4A1
nuclear receptor 4A1
- NSAIDs
non-steroidal anti-inflammatory drugs
- PEITC
phenethylisothiocyanate
- PP2A
protein phosphatase 2A
- RMS
rhabdomyosarcoma
- ROS
reactive oxygen species
- Sp1
specificity protein 1
- TFs
transcription factors
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest: There are no conflicts of interest to declare.
LITERATURE CITED
- 1.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature Reviews: Endocrinology. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 3.Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5:4929–4934. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang S, Dong SM, Kim BR, Park MS, Trink B, Byun HJ, et al. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17:989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin T, He S, Shen G, Ye T, Guo F, Wang Y. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol Med Rep. 2015;12:4103–4108. doi: 10.3892/mmr.2015.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antherieu S, Bachour-El Azzi P, Dumont J, Abdel-Razzak Z, Guguen-Guillouzo C, Fromenty B, et al. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology. 2013;57:1518–1529. doi: 10.1002/hep.26160. [DOI] [PubMed] [Google Scholar]
- 7.Min KJ, Seo BR, Bae YC, Yoo YH, Kwon TK. Antipsychotic agent thioridazine sensitizes renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated inhibition of Akt signaling and downregulation of Mcl-1 and c-FLIP(L) Cell Death & Disease. 2014;5:e1063. doi: 10.1038/cddis.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjan A, Srivastava SK. Penfluridol suppresses pancreatic tumor growth by autophagy-mediated apoptosis. Scientific Reports. 2016;6:26165. doi: 10.1038/srep26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien W, Sun QY, Lee KL, Ding LW, Wuensche P, Torres-Fernandez LA, et al. Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer. Molecular Oncology. 2015;9:889–905. doi: 10.1016/j.molonc.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan A, Gupta P, Srivastava SK. Penfluridol: an antipsychotic agent suppresses metastatic tumor growth in triple-negative breast cancer by inhibiting integrin signaling axis. Cancer Research. 2016;76:877–890. doi: 10.1158/0008-5472.CAN-15-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Molecular and Cellular Biology. 2016;36:1383–1394. doi: 10.1128/MCB.00912-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedrick E, Cheng Y, Jin UH, Kim K, Safe S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget. 2016 doi: 10.18632/oncotarget.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick E, Crose L, Linardic CM, Safe S. Histone deacetylase inhibitors inhibit rhabdomyosarcoma by reactive oxygen species-dependent targeting of specificity protein transcription factors. Molecular Cancer Therapeutics. 2015;14:2143–2153. doi: 10.1158/1535-7163.MCT-15-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jutooru I, Guthrie AS, Chadalapaka G, Pathi S, Kim K, Burghardt R, et al. Mechanism of action of phenethylisothiocyanate and other reactive oxygen species-inducing anticancer agents. Molecular and Cellular Biology. 2014;34:2382–2395. doi: 10.1128/MCB.01602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadalapaka G, Jutooru I, Safe S. Celastrol decreases specificity proteins (Sp) and fibroblast growth factor receptor-3 (FGFR3) in bladder cancer cells. Carcinogenesis. 2012;33:886–894. doi: 10.1093/carcin/bgs102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jutooru I, Chadalapaka G, Abdelrahim M, Basha MR, Samudio I, Konopleva M, et al. Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Molecular Pharmacology. 2010;78:226–236. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jutooru I, Chadalapaka G, Sreevalsan S, Lei P, Barhoumi R, Burghardt R, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Experimental Cell Research. 2010;316:2174–2188. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safe S, Imanirad P, Sreevalsan S, Nair V, Jutooru I. Transcription factor Sp1, also known as specificity protein 1 as a therapeutic target. Expert Opinion on Therapeutic Targets. 2014;18:759–769. doi: 10.1517/14728222.2014.914173. [DOI] [PubMed] [Google Scholar]
- 19.Lin CS, Chen Y, Huynh T, Kramer R. Identification of the human alpha6 integrin gene promoter. DNA and Cell Biology. 1997;16:929–937. doi: 10.1089/dna.1997.16.929. [DOI] [PubMed] [Google Scholar]
- 20.Takaoka AS, Yamada T, Gotoh M, Kanai Y, Imai K, Hirohashi S. Cloning and characterization of the human beta4-integrin gene promoter and enhancers. Journal of Biological Chemistry. 1998;273:33848–33855. doi: 10.1074/jbc.273.50.33848. [DOI] [PubMed] [Google Scholar]
- 21.Nam EH, Lee Y, Park YK, Lee JW, Kim S. ZEB2 upregulates integrin alpha5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis. 2012;33:563–571. doi: 10.1093/carcin/bgs005. [DOI] [PubMed] [Google Scholar]
- 22.Wu F, Khan S, Wu Q, Barhoumi R, Burghardt R, Safe S. Ligand structure-dependent activation of estrogen receptor alpha/Sp by estrogens and xenoestrogens. Journal of Steroid Biochemistry and Molecular Biology. 2008;110:104–115. doi: 10.1016/j.jsbmb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Research. 2010;70:6824–6836. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Lee SO, Safe S. Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3'-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochemical Pharmacology. 2012;83:1445–1455. doi: 10.1016/j.bcp.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman SL, Picard M. Integrins as therapeutic targets. Trends in Pharmacological Sciences. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews: Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, et al. Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 2014;7:747–761. doi: 10.1016/j.celrep.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kacsinta AD, Rubenstein CS, Sroka IC, Pawar S, Gard JM, Nagle RB, et al. Intracellular modifiers of integrin alpha 6p production in aggressive prostate and breast cancer cell lines. Biochemical and Biophysical Research Communications. 2014;454:335–340. doi: 10.1016/j.bbrc.2014.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieira AF, Ribeiro AS, Dionisio MR, Sousa B, Nobre AR, Albergaria A, et al. P-cadherin signals through the laminin receptor alpha6beta4 integrin to induce stem cell and invasive properties in basal-like breast cancer cells. Oncotarget. 2014;5:679–692. doi: 10.18632/oncotarget.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. International Journal of Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 32.Zhou B, Gibson-Corley KN, Herndon ME, Sun Y, Gustafson-Wagner E, Teoh-Fitzgerald M, et al. Integrin alpha3beta1 can function to promote spontaneous metastasis and lung colonization of invasive breast carcinoma. Molecular Cancer Research. 2014;12:143–154. doi: 10.1158/1541-7786.MCR-13-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin alpha5beta1 facilitates cancer cell invasion through enhanced contractile forces. Journal of Cell Science. 2011;124:369–383. doi: 10.1242/jcs.071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Shenouda S, Baranwal S, Rathinam R, Jain P, Bao L, et al. Integrin subunits alpha5 and alpha6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Molecular Cancer. 2011;10:84. doi: 10.1186/1476-4598-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parvani JG, Galliher-Beckley AJ, Schiemann BJ, Schiemann WP. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Molecular Biology of the Cell. 2013;24:3449–3459. doi: 10.1091/mbc.E12-10-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truong HH, Xiong J, Ghotra VP, Nirmala E, Haazen L, Le Devedec SE, et al. β1 Integrin inhibition elicits a prometastatic switch through the TGFβ-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal. 2014;7:ra15. doi: 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- 37.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Research. 2007;67:11001–1111. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 38.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFκB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. Journal of Biological Chemistry. 2010;285:25332–25344. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathi SS, Jutooru I, Chadalapaka G, Sreevalsan S, Anand S, Thatcher GR, et al. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Molecular Cancer Research. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pathi SS, Lei P, Sreevalsan S, Chadalapaka G, Jutooru I, Safe S. Pharmacologic doses of ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated genes in colon cancer cells. Nutrition and Cancer. 2011;63:1133–1142. doi: 10.1080/01635581.2011.605984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. Journal of Molecular Endocrinology. 2008;41:263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham J, Nunez-Alvarez Y, Hettmer S, Carrio E, Chen HI, Nishijo K, et al. Lineage of origin in rhabdomyosarcoma informs pharmacological response. Genes and Development. 2014;28:1578–1591. doi: 10.1101/gad.238733.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Stewart E, Shelat AA, Qu C, Bahrami A, Hatley M, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24:710–724. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.