Abstract
Eukaryotic cells have evolved a complex mechanism for sensing DNA damage during genome replication. Activation of this pathway prevents entry into mitosis to allow for either DNA repair or, in the event of irreparable damage, commitment to apoptosis. Under conditions of replication stress, the damage signal is initiated by the ataxia-telangiectasia-mutated and Rad3-related kinase ATR. We recently demonstrated that the human immunodeficiency virus type 1 (HIV-1) gene product viral protein R (Vpr) arrests infected cells in the G2 phase via the activation of ATR. In the present study, we show that the activation of ATR by Vpr is analogous to activation by certain genotoxic agents, both mechanistically and in its downstream consequences. Specifically, we show a requirement for Rad17 and Hus1 to induce G2 arrest as well as Vpr-induced phosphorylation of histone 2A variant X (H2AX) and formation of nuclear foci containing H2AX and breast cancer susceptibility protein 1. These results demonstrate that G2 arrest mediated by the HIV-1 gene product Vpr utilizes the cellular signaling pathway whose physiological function is to recognize replication stress. These findings should contribute to a greater understanding of how HIV-1 manipulates the CD4+-lymphocyte cell cycle and apoptosis induction in the progressive CD4+-lymphocyte depletion characteristic of HIV-1 pathogenesis.
Human immunodeficiency virus type 1 (HIV-1) has four genes, vif, vpr, vpu, and nef, termed accessory genes, that are dispensable for viral replication in vitro (14). Many important functions related to HIV-1 pathogenesis have been ascribed to these accessory genes. Specifically, Vpr has been implicated in long terminal repeat transactivation, nuclear import of the preintegration complex, induction of G2 cell cycle arrest, and apoptosis. Recent studies identifying single amino acid changes in Vpr in a cohort of HIV-1-infected long-term nonprogressors substantiated the role of Vpr in HIV-1 pathogenesis in vivo (29, 43). Vpr induces G2 arrest and apoptosis in infected CD4+ lymphocytes (18, 21, 35, 38). G2 arrest by Vpr is effected in HeLa cells through activation of the ATR-dependent DNA damage checkpoint pathway (40). These data support previous work demonstrating the inhibition of cyclin B1-p34cdc2 complexes by Vpr (18) and establish the identity of the upstream regulators of Cdc2. ATR-dependent activation of Chk1 kinase leads to the inhibition of Cdc25C phosphatase, which is normally required to dephosphorylate and activate Cdc2 (40).
The signaling pathway downstream of ATR activation was recently characterized in detail (reviewed in references 1 and 33). Because Vpr activates the ATR-specific checkpoint, we wished to examine whether other molecules in the ATR pathway are required. In addition to initiating G2 arrest signaling through Chk1, activated ATR also phosphorylates cellular proteins in separate branches of the DNA damage response (Table 1). In light of the fact that ATR is known to have at least eight known cellular targets for phosphorylation (1, 51), we hypothesized that additional pathways controlled by ATR are affected by its activation by Vpr. Therefore, in this work, we analyzed the activation status of other known ATR targets.
TABLE 1.
Phosphorylation targets of ATR and reported roles in activation of the G2 checkpoint
| ATR target (reference) | Status in the presence of Vpr (reference or source)a |
|---|---|
| Chk1 (25) | P, A (40) |
| Rad17 (54) | N (this work) |
| H2AX (9, 10, 37, 46) | P, F (this work) |
| BRCA1 (45) | F (this work), P (Andersen and Planelles, submitted) |
| Plk1 (13) | ? |
| p53 (24) | D (41) |
| 53BP1 (46, 48) | ? |
| E2F (11, 36) | ? |
P, phosphorylated; A, activated; N, necessary for G2 checkpoint activation; F, focus formation; ?, unknown; D, dispensable for G2 checkpoint activation.
Rad17 is a replication factor C-related protein that, in a complex with Rfc2, Rfc3, Rfc4, and Rfc5, loads the heterotrimeric sliding clamp consisting of Rad9, Rad1, and Hus1 (9-1-1 complex) at sites of DNA damage (54). ATR, Rad17, and the 9-1-1 complex colocalize and activate one another to signal G2 checkpoint activation (54). Rad17 and the 9-1-1 complex are necessary for downstream signaling of G2 arrest through ATR activation (54). In the present study, we investigated the potential requirement for Rad17 and Hus1 in Vpr-induced G2 arrest.
Activated ATR can also phosphorylate proteins other than those required for G2 arrest (Table 1). One of these substrates is histone 2A variant X (H2AX). H2AX is deposited randomly throughout chromatin, comprising approximately 10% of total nucleosomal histone H2A (34). H2AX has a highly conserved serine residue at position 139 that is phosphorylated by ATR and/or ATM in response to DNA damage (10, 37, 46). It is estimated that hundreds to thousands of H2AX molecules are phosphorylated per double-stranded break (37). ATM-dependent H2AX phosphorylation occurs in response to double-stranded DNA breaks (10, 46, 47). In contrast, ATR phosphorylates H2AX under circumstances of replication stress, such as stalled replication forks (9). In the presence of DNA damage or replication stress, H2AX molecules that are located in the vicinity of the DNA lesion become phosphorylated in a highly specific localized manner (34). Thus, immunofluorescence staining for phosphorylated H2AX (also referred to as γ-H2AX) following DNA damage produces a staining pattern of distinct nuclear foci (34). γ-H2AX is thought to amplify the DNA damage signal by enhancing and stabilizing the recruitment of DNA damage sensor proteins, such as ATR, ATM, Rad17, and the 9-1-1 complex, and DNA repair proteins, such as breast cancer susceptibility protein 1 (BRCA1), Nbs1, Mre11, and Rad50, to sites of DNA damage (15). This action may effectively “mark” the site of DNA damage, maintaining checkpoint signaling at the damaged region until DNA repair is completed.
Another substrate of activated ATR is BRCA1. BRCA1 is important for both checkpoint activation and DNA repair. BRCA1 colocalizes with DNA repair factors, such as Rad51, PCNA, and Mre11-Rad50-Nbs1 (15). It has been proposed that BRCA1 may represent an essential link in coordinating cell cycle arrest with genomic repair efforts (reviewed in reference 27) and with the induction of apoptosis.
In the present study, we show that both Rad17 and Hus1 are required for Vpr-mediated G2 arrest. In addition, HIV-1 Vpr expression leads to the formation of intense γ-H2AX and BRCA1 nuclear foci, characteristic markers of DNA damage. These results confirm and extend previous observations suggesting a role of Vpr in activating the ATR-dependent G2 checkpoint and may help to explain other aspects of HIV-1 pathogenesis, such as the induction of apoptosis.
MATERIALS AND METHODS
Cell culture.
Exponentially growing HeLa cells were cultured in Dulbecco minimal essential medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin-l-glutamate (PSG) (Invitrogen). Hus1−/− p21−/− and Hus1+/+ p21−/− mouse embryonic fibroblasts were cultured on glycerin-coated plates with RPMI medium (Invitrogen) supplemented with 10% FBS, 1% PSG, and 10 mM nonessential amino acids (Invitrogen). Primary human thymic cultures were prepared as previously described (44).
RNA interference.
SmartPool small interfering RNA (siRNA) duplexes (Dharmacon, Lafayette, Colo.) were transfected at a concentration of 100 nM into approximately 3 × 105 exponentially dividing HeLa cells in serum-free OPTI-MEM (Invitrogen) by using 5 μl of Lipofectamine 2000 (Invitrogen).
Immunofluorescence staining.
HeLa cells were harvested 48 h posttransduction by trypsinization. Single-cell suspensions of minced thymic tissue were prepared for immunostaining 20 h after HIV-1NL4-3 infection. Cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 35 min at 4°C and then washed three times for 5 min each time in PBS. All subsequent steps were carried out at room temperature. Samples were blocked and permeabilized for 20 min in blocking buffer (3% bovine serum albumin [BSA], 0.2% Triton X-100, and 0.01% skim milk in PBS). Primary antibody (rabbit anti-γ-H2AX [48] or rabbit anti-BRCA1 [Bethyl Laboratories, Montgomery, Tex.]) was diluted 1:400 in incubation buffer (1% BSA and 0.02% Triton X-100 in PBS) and incubated with cells for 45 min. Cells were washed with PBS, after which secondary antibody (goat anti-rabbit immunoglobulin G [IgG]-AlexaFluor568 conjugate [Cell Signaling, Eugene, Oreg.]) diluted 1:500 in incubation buffer was applied for 35 min. Cells were washed with PBS as before and mounted on glass slides by using FluorSave reagent (CalBiochem, San Diego, Calif.). Cells were visualized for γ-H2AX or BRCA1 immunostaining and green fluorescent protein (GFP) expression by scanning fluorescence confocal microscopy (FluoView FV300; Olympus, Melville, N.Y.).
Cell cycle analysis.
At 48 h after infection, cells were detached by trypsinization, washed with fluorescence-activated cell sorting (FACS) buffer (2% FBS and 0.02% sodium azide in PBS), fixed with 2% paraformaldehyde in PBS, and permeabilized with 0.01% Triton X-100 in PBS for 15 min. Cells were washed again with FACS buffer, incubated in DNA staining buffer (10 μg of propidium iodide/ml and 11.25 kU of RNase A/ml in FACS buffer) for 15 min, and analyzed by FACScan flow cytometry for GFP expression or DNA content (Beckton Dickinson, Franklin Lakes, N.J.). In experiments involving transduction with lentivirus vectors, only experiments with 90% transduction efficiency or higher were analyzed. Cell cycle profiles were modeled by using ModFit software (Verity Software, Topsham, Maine).
Lentivirus vectors.
Lentivirus vectors were produced by transient transfection of HEK293T cells. For defective lentivirus vector production, plasmids pHR-GFP and pHR-Vpr were cotransfected with pCMVΔR8.2ΔVpr (4) and pHCMV-VSVG (3) by calcium phosphate-mediated transfection (53). Virus supernatants were collected at 48, 72, and 96 h posttransfection. Harvested supernatants were cleared by centrifugation at 2,000 rpm (828 × g). Cleared supernatants were concentrated by ultracentrifugation at 25,000 rpm (115,889 × g) for 1.5 h at 4°C. Concentrated virus was allowed to resuspend overnight at 4°C, and the suspension was frozen at −80°C for storage. Vector titers were measured by infection of HeLa cells as described below, followed by flow cytometric analysis of cells that were positive for the reporter molecule, GFP. Vector titers were calculated with the equation [(F × C0)/V] × D, where F is the frequency of GFP-positive cells found by flow cytometry, C0 is the total number of target cells at the time of infection, V is the volume of inoculum, and D is the virus dilution factor. The virus dilution factor used for titrations was 10. The total number of target cells at the time of infection was 106. Infections were performed at a multiplicity of infection (MOI) of 2.5 with 10 μg of Polybrene/ml for 3 h. Infections of siRNA-treated cells were performed 48 h after siRNA transfection.
HIV-1 infection.
HIV-1NL4-3 stocks were prepared as previously described (20). Virus was diluted in Iscove's medium supplemented with 2% FBS and used to infect primary thymocytes at an MOI of 1.0.
Drug treatment.
Cells were incubated with 10 mM hydroxyurea (HU) for 2 h before immunostaining was done.
Western blotting.
Cells were detached at the time of cell cycle analysis and lysed in Laemmli sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris-HCl, 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 14.4 mM 2-mercaptoethanol in double-distilled H2O) at a concentration of 5 × 105 cells/100 μl of buffer. Lysates were boiled for 5 min prior to being loaded on SDS-10% polyacrylamide gels for electrophoretic separation. Proteins were transferred to polyvinylidene difluoride membranes by a semidry transfer method (Bio-Rad, Hercules, Calif.) and then blocked for 45 min at room temperature in blocking solution (5% skim milk and 0.1% Tween 20 in PBS). Rabbit primary antibodies against Rad17 (Santa Cruz Biotechnology, Santa Cruz, Calif.), actin (Santa Cruz), ATM (Novus Biologicals, Littleton, Colo.), or ATR (32) were applied for 90 min at room temperature. Blots were washed three times in TPBS (0.1% Tween 20 in PBS) for 10 min each time at room temperature. Secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies were applied for 45 min at room temperature. Blots were washed again three times in TPBS before protein detection with enhanced chemiluminescence reagent (Amersham).
RESULTS
Rad17 is necessary for Vpr-mediated G2 arrest.
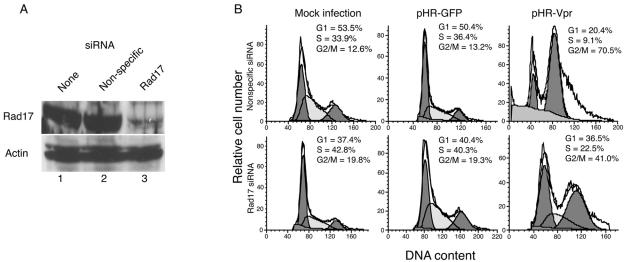
In an effort to examine the role of Rad17 in Vpr-mediated G2 arrest, we used RNA interference to reduce endogenous Rad17 levels. We transfected siRNA duplex oligonucleotides targeted at Rad17 mRNA (54) and used, in parallel, siRNA with a nonspecific target sequence and mock transfection as controls. In these experiments, endogenous Rad17 protein levels were reduced by approximately 85% relative to those of mock-transfected or nonspecific siRNA-transfected cells (Fig. 1A). Following transfection, cells were transduced with lentivirus vectors expressing either Vpr and GFP cDNAs separated by an internal ribosome entry site (pHR-Vpr) or GFP alone (pHR-GFP) (40). At 48 h after transduction, cells were analyzed by flow cytometry for infection efficiency and DNA content, as reported by GFP expression and propidium iodide staining, respectively. We then analyzed the cell cycle distributions of the various experimental cell populations after electronic gating of GFP-positive (transduced) and GFP-negative (untransduced) cells. Infection with pHR-GFP did not affect the cell cycle profile of any of the transfected populations. Infection with pHR-Vpr induced a marked accumulation of cells in G2 phase, as expected (Fig. 1B). When cells were pretreated by transfection with a Rad17-specific siRNA (but not with nonspecific siRNA), Vpr-induced accumulation in G2 was dramatically relieved (Fig. 1B). Therefore, Rad17 is required for activation of the G2 checkpoint by Vpr.
FIG. 1.
Rad17 inhibition by RNA interference relieves Vpr-mediated G2 arrest. (A) Immunoblot of total Rad17 (upper panel) or actin (lower panel). Cells were mock transfected (lane 1), transfected with nonspecific siRNA (lane 2), or transfected with Rad17-specific siRNA (lane 3). (B) Cell cycle analysis of HeLa cells transfected as indicated and infected with lentivirus vector pHR-Vpr or pHR-GFP. Cell cycle distributions were analyzed 48 h after infection. Left peaks represent diploid cells in G1. Right peaks represent tetraploid cells in G2/M.
Hus1 is necessary for Vpr-mediated G2 arrest.
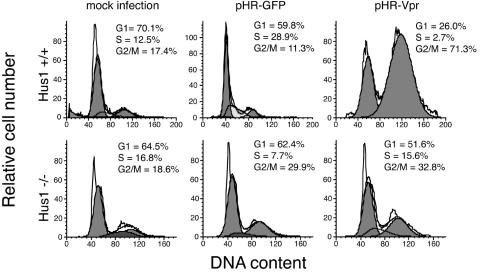
Our finding that Rad17 is necessary for Vpr-induced G2 arrest prompted us to examine another constituent of the ATR signaling pathway, Hus1. To examine the role of Hus1 in Vpr-induced G2 arrest, we used Hus1−/− p21−/− mouse embryonic fibroblasts (49). Both Hus1−/− p21−/− cells and Hus1+/+ p21−/− cells exhibited normal cell cycle distributions when mock infected or infected with pHR-GFP (Fig. 2). Hus1−/− p21−/− cells, however, failed to arrest in G2 after infection with pHR-Vpr, whereas their Hus1+/+ counterparts exhibited robust G2 arrest (Fig. 2). These experiments illustrate a requirement for Hus1 in Vpr-induced G2 arrest.
FIG. 2.
Hus1 is required for Vpr-mediated G2 arrest. Hus1−/− p21−/− or Hus1+/+ p21−/− mouse embryonic fibroblasts were infected with lentivirus vector pHR-Vpr or pHR-GFP. Cell cycle distributions were analyzed at 48 h after infection.
Taken together, the above observations indicate that HIV-1 Vpr activates the G2 checkpoint in a manner that is indistinguishable, mechanistically, from that of certain genotoxic agents (specifically, HU) that cause replication inhibition. Because recognition of DNA damage via ATR can lead to dramatic cellular changes other than checkpoint activation, we undertook studies to investigate the consequences of ATR activation. We first focused on γ-H2AX and BRCA1 because they have been reported to recruit DNA repair factors and induce apoptosis.
Vpr expression induces γ-H2AX and BRCA1 focus formation.
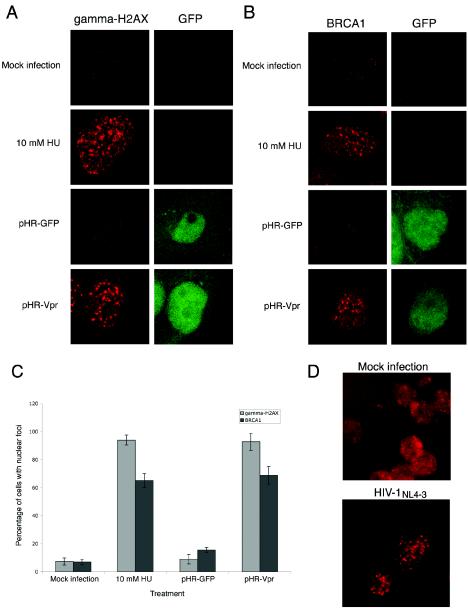
Based on previous findings about ATR (9, 45), we hypothesized that Vpr, through ATR activation, would induce γ-H2AX and BRCA1 focus formation. To test the previous hypothesis, we infected HeLa cells with pHR-Vpr or pHR-GFP and, 48 h later, immunostained cells with γ-H2AX- or BRCA1-specific antibodies. As a positive control, we treated nontransduced cells for 1 h with 10 mM hydroxyurea (HU) 10 min prior to immunostaining (46). Samples then were visualized by fluorescence scanning confocal microscopy (Fig. 3A and B). Cells with multiple (10 or more), intense nuclear foci were visually counted. These quantitations are presented in Fig. 3C. We found that approximately 93 or 69% of Vpr-expressing cells exhibited γ-H2AX or BRCA1 focal staining, respectively, whereas only 9 or 16% of pHR-GFP-infected cells exhibited any γ-H2AX or BRCA1 foci, respectively. Less than 8% of mock-infected cells exhibited γ-H2AX or BRCA1 foci. Approximately 94 or 65% of HU-treated cells exhibited γ-H2AX or BRCA1 foci, respectively. We conclude that Vpr expression leads to γ-H2AX and BRCA1 focus formation. The phosphorylation status of BRCA1 was not formally proven by the above experiments, because recognition by the BRCA1-specific antibody was not dependent on BRCA1 phosphorylation. However, in a separate study, J. L. Andersen and V. Planelles (submitted for publication) showed the specific phosphorylation of BRCA1 at Ser1423 in the presence of Vpr.
FIG. 3.
Vpr induces γ-H2AX and BRCA1 focus formation. (A and B) HeLa cells were transduced with lentivirus vector pHR-Vpr or pHR-GFP, mock transduced, or treated with 10 mM HU for 2 h. At 48 h after transduction, cells were stained with γ-H2AX- or BRCA1-specific antibodies and visualized for γ-H2AX (red) (A) or BRCA1 (red) (B) and GFP (green) localization by confocal microscopy. (C) γ-H2AX- and BRCA1-positive and -negative cells were visually counted. Results represent averages for three fields with approximately 50 cells per field. (D) Human primary thymocytes were infected with HIV-1NL4-3 (bottom panel) or mock infected (top panel) and, at 20 h postinfection, fixed and stained for γ-H2AX.
HIV-1 infection induces γ-H2AX foci in primary CD4+ thymocytes.
In previous work, it was established that the G2 arrest effect of Vpr is identical in many human cell lines tested and in primary lymphocytes (21, 41, 53). Therefore, HeLa cells, although not a target for HIV-1, constitute adequate cells in which to study the mechanism of G2 arrest by Vpr. Nonetheless, we wished to confirm our observations with primary human CD4+ cells infected with full-length HIV-1. Primary human CD4+ thymocytes were infected with full-length HIV-1NL4-3 (2) or mock infected. At 20 h after infection, cells were immunostained for γ-H2AX (Fig. 3D). HIV-1NL4-3 infection caused a staining pattern of distinct γ-H2AX nuclear foci that was not observed in mock-infected cells. These data indicate that full-length HIV-1 induces γ-H2AX focus formation in primary CD4+ cells.
ATR, but not ATM, is necessary for Vpr-induced G2 arrest.
ATR is primarily responsible for G2 checkpoint activation via Chk1 phosphorylation (25). However, it has been shown that ATM, which acts primarily on Chk2, can play a minor, more transient role in Chk1 phosphorylation (1). Although Bartz and colleagues demonstrated that ATM−/− cells were able to arrest in G2 in response to Vpr (8), a partial role for ATM would be formally possible. Specifically, two observations prompted us to reexamine the role of ATM. First, suppression of ATR or Chk1 by RNA interference is typically unable to completely relieve Vpr-induced G2 arrest (40). It is possible that residual ATR and/or Chk1 levels were responsible for the partial accumulation of cells in G2. Alternatively, the low level of G2 arrest in the context of ATR- and/or Chk1-specific inhibition could have been attributable to ATM. The second finding that prompted us to reexamine the role of ATM was that caffeine, an inhibitor of both ATR and ATM, was able to completely relieve Vpr-induced G2 arrest (40, 53).
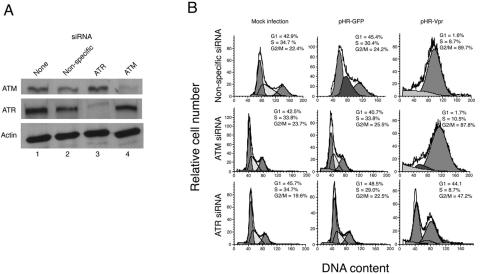
To test the potential contribution of ATM activity to Vpr-induced G2 arrest, we transfected siRNA directed at ATR and ATM in combination or separately and then performed infections as described above. As expected, pretreatment with ATR-specific siRNA produced a marked, although incomplete, alleviation of G2 arrest by Vpr (Fig. 4). Pretreatment with ATM-specific siRNA, which reduced ATM protein levels by 85% relative to those in mock-treated cells (Fig. 4A), produced no change in cell cycle arrest by Vpr compared with the results seen in cells transfected with nonspecific siRNA or no siRNA (Fig. 4B). In addition, simultaneous suppression of ATR and ATM did not produce any additional relief of G2 arrest (data not shown). Therefore, our results corroborate and extend previous observations that indicate that ATM is dispensable for Vpr-induced G2 checkpoint activation (8).
FIG. 4.
ATM is not necessary for Vpr-mediated G2 arrest. (A) Immunoblot of total ATM (upper panel), ATR (middle panel), or actin (lower panel). Cells were mock transfected (lane 1) or transfected with nonspecific siRNA (lane 2), ATM-specific siRNA (lane 3), or ATR-specific siRNA (lane 4). (B) Cell cycle analysis of HeLa cells transfected as indicated and infected with lentivirus vector pHR-Vpr or pHR-GFP. Cell cycle distributions were analyzed at 48 h after infection. Left peaks represent diploid cells in G1. Right peaks represent tetraploid cells in G2/M.
DISCUSSION
Our studies have shown that Vpr activates ATR and its downstream signaling events in a manner thus far indistinguishable from that of activation by bona fide DNA damage (Fig. 5 shows a schematic diagram). Previously, various means of genetic analysis, including RNA interference, knockout cell lines, and dominant-negative constructs, were used to support this hypothesis (40). The present study extends these observations by providing important mechanistic details and illustrating the downstream signaling consequences.
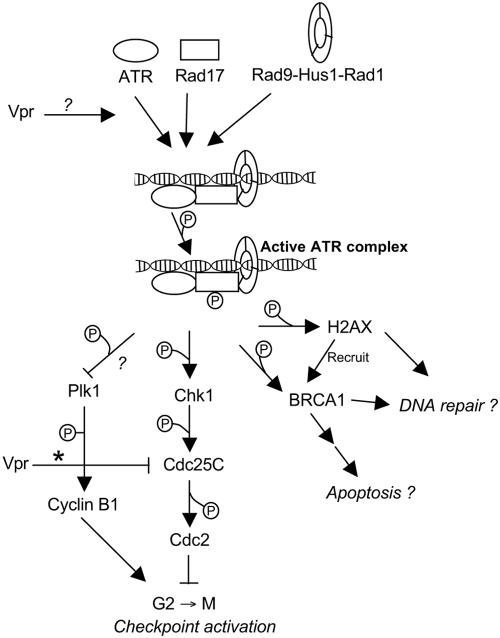
FIG. 5.
Schematic representation of the ATR pathway. ATR has eight known targets; only the ones with potential relevance to HIV-1 Vpr are represented. Question marks denote functional relationships that are expected but not confirmed. The asterisk denotes the inhibition of Cdc25C reported by Goh et al. (17).
Rad17 and Hus1 are required for signaling when ATR-dependent G2 arrest is induced in response to genotoxic stress. Upon recognition of genotoxic stress, Rad17 is phosphorylated and is the first target of ATR (54) (Fig. 5). This phosphorylation requires the participation of Hus1 (and the larger complex of which Hus1 is a part, 9-1-1) (54). Only after Rad17 is phosphorylated can ATR modify its next target, Chk1. By downregulating endogenous Rad17 protein levels via RNA interference, we have demonstrated that Rad17 function is also required for Vpr-induced G2 arrest. Additionally, we have shown that Hus1-deficient cells are refractory to the effects of Vpr on the cell cycle. We conclude that Rad17 and Hus1 are necessary components of the G2 checkpoint in response to Vpr expression.
Interestingly, Hus1 is known to be dispensable for ATR-mediated H2AX phosphorylation (46). Based on these findings, Ward and Chen (46) suggested that ATR activation may lead to two types of downstream events, which are Hus1 dependent and Hus1 independent. Hus1-dependent consequences of ATR activation (such as Chk1 phosphorylation) may specifically induce cell cycle arrest, while downstream events independent of Hus1 (such as H2AX phosphorylation) may recruit members of the DNA repair machinery, such as BRCA1, Nbs1, and Rad50 (15, 46).
The cyclin-dependent kinase inhibitor p21Waf1 was previously shown to be transcriptionally upregulated in a p53-dependent fashion in the context of Vpr expression (12). This observation led the authors to formulate the hypothesis that p21Waf1 may mediate Vpr-induced G2 arrest, although this hypothesis was not tested (12). Here we report that p21Waf1−/− mouse embryonic fibroblasts are able to activate the G2 checkpoint when transfected with Vpr (Fig. 2). This observation suggests that p21Waf1 does not play a major role in mediating G2 arrest by Vpr, although our experiments cannot exclude the possibility that p21 may make a minor contribution.
We have shown that Vpr induces γ-H2AX and BRCA1 focus formation. Therefore, all four targets of ATR that we have tested (out of a total of eight described in the literature to date) (Table 1) play an active role in the response to Vpr expression. Because we can observe a very specific histone modification and directed recruitment of a known DNA repair protein in response to Vpr, we infer that Vpr-induced ATR stimulation occurs at distinct sites throughout chromatin. Whether this specificity is due to DNA sequence, chromatin modifications, or replication- and expression-dependent DNA and chromatin dynamics has yet to be determined. These findings also suggest that Vpr-induced signaling through ATR may have cellular effects other than G2 arrest, such as recruitment of DNA repair proteins and/or initiation of apoptotic signaling cascades (Andersen and Planelles, submitted).
We have also demonstrated that primary human CD4+ thymocytes, an in vivo target for HIV-1 (22, 23), display γ-H2AX foci when infected with full-length HIV-1. This observation suggests that our findings with HeLa cells can be extended to primary CD4+ cells, one of the target cell types of HIV-1. More importantly, our findings indicate that the host cell DNA damage response is activated in the context of an HIV-1 infection. Further corroboration of our findings in natural targets of HIV-1 (such as primary CD4+ lymphocytes and macrophages) will be needed in the near future. These experiments will be challenging, since human primary cells that are defective for genes in the ATR pathway are rare or nonexistent. An additional challenge will be to use RNA interference technology with primary cells in order to test the necessity of various mediators for activation of the G2 checkpoint by Vpr. Although transfection of primary cells with RNA duplexes is inefficient (data not shown), construction of lentivirus vectors expressing short hairpin RNAs (5, 6) should offer a viable alternative.
Future experiments will investigate the potential roles of additional ATR targets in the context of Vpr expression. The polo-like kinase (Plk1) has been described as a positive regulator of the G2/M transition. This effect is thought to be mediated by Plk1 kinase activity directed at cyclin B1 (13). Plk1 phosphorylation promotes nuclear accumulation of the cyclin B1-Cdc2 heterodimer, ultimately allowing progression into M phase (26). In instances of DNA damage, Plk1 kinase activity is inhibited to prevent advance into mitosis (Fig. 5) (42). It has been demonstrated that this inhibition is dependent on the kinase activity of ATR (13). Thus, inactivation of Cdc25C by Chk1 may not be the sole contributor to inducing G2 arrest, and concerted action by Plk1 may also be required. Future experiments will be aimed at finding the relative contributions of Cdc25C and Plk1 to Vpr-mediated G2 arrest.
The p53-binding protein, 53BP1, rapidly associates with nuclear foci containing γ-H2AX, ATR, and BRCA1 in response to genotoxic stress (7, 46). This organization into foci occurs in an ATR-dependent manner in response to replication stress (46, 48). If Vpr directly causes DNA lesions, stalls replication forks to cause double-stranded breaks, or somehow mimics DNA damage through DNA, chromatin, or protein-protein interactions, then one would expect 53BP1 to be activated by ATR.
The transcriptional activator E2F1 is another target of ATR. E2F1 is essential for promoting the G1/S transition and DNA replication. E2F1 is also involved in several stress response pathways, including apoptosis and DNA repair (reviewed in references 11 and 36). For example, E2F1 is implicated in p53-dependent apoptosis in response to DNA damage (19, 39). It has also been shown that E2F1 recruits the DNA repair proteins Nbs1 and Mre11 to origins of replication (30). Given these findings, it is possible that ATR-dependent E2F1 phosphorylation plays a role in the cellular response to Vpr.
The tumor suppressor p53 can be a target for ATR as well as for ATM, leading to the induction of cell cycle arrest and apoptosis in response to environmental insults, including DNA damage (reviewed in reference 24). Shostak et al. previously examined the role of p53 in mediating the effects of Vpr and found that p53 is dispensable for both checkpoint activation and apoptosis induction (41). However, it is possible that the activation of p53 by ATR may allow Vpr to modulate certain aspects of infected cells via the transcriptional effects of p53. For example, p53 is known to transcriptionally activate the p53-dependent ribonucleotide reductase, p53R2, thrombospondin-1, and aldehyde dehydrogenase-4, enzymes which participate in diverse processes, such as DNA repair, inhibition of angiogenesis, and the response to oxidative stress, respectively (for a review, see reference 31). Therefore, studies to further examine the role of p53 in Vpr-expressing cells will be compelling.
The precise mechanism of ATR activation in the context of HIV-1 Vpr remains unclear (Fig. 5). ATR activation is thought to be specific for DNA damage manifested as single-stranded DNA through either processed double-stranded breaks or stalled replication forks due to either replicational pausing or single-stranded breaks; in contrast, the ATM response is thought to be predominantly responsible for the immediate signaling of unprocessed double-stranded DNA breaks (1, 33). This pathway specificity suggests that Vpr activates the DNA damage-induced G2 checkpoint in a manner that resembles or causes the accumulation of single-stranded DNA. Therefore, there are several possible mechanisms by which Vpr may activate ATR. One possibility is that Vpr directly causes DNA lesions through intrinsic nuclease activity. This possibility seems unlikely, as Vpr shares no sequence homology or known structural motifs with any known nucleases. Another plausible explanation is that Vpr inappropriately recruits ATR or other DNA damage-sensing proteins to undamaged DNA through DNA-protein and protein-protein interactions, as discussed below. Alternatively, Vpr could interact with proteins or DNA in a manner that causes DNA damage. One possible mode of indirectly inducing DNA damage would be the recruitment of an endonuclease which would enzymatically induce single-stranded or double-stranded DNA breaks which, once processed into single-stranded DNA, would activate ATR.
Vpr may also interact with DNA or proteins present at sites of DNA replication in a manner that inhibits replication fork progression. It has been proposed that abnormally long, replication protein A-bound single-stranded DNA at stalled replication forks allows for ATR recruitment via an ATR-interacting protein (55). Additionally, if halted forks are not stabilized and resolved, then their eventual collapse can activate DNA damage sensors (1, 33). However, if this were a highly potent, nonspecific effect of Vpr, then one would expect a global inhibition of replication manifested as early S-phase arrest, instead of the conspicuous G2 arrest. Vpr could directly interact with DNA in a fashion that causes or resembles damaged DNA or stalled replication forks. It has been shown that the C-terminal alpha helix of Vpr binds DNA in vitro and that Vpr is detected in chromatin and nuclear matrix fractions in vivo (28, 52).
An alternative model suggests that Vpr may directly interact with ATR or other components of the checkpoint signaling pathway independent of DNA or chromatin localization. We have performed coprecipitation experiments for ATR and Vpr using conventional methods and have been unable to demonstrate any binding between these proteins (data not shown). In future experiments, we will use cross-linking agents to stabilize a potentially weak interaction or perhaps a complex one with multiple proteins bridging ATR and Vpr.
A recent study indicated that Vpr interacts directly with Cdc25C and inhibits Cdc25C phosphatase activity (17). Inhibition of Cdc25C then prevents activation of the cyclin B1-p34cdc2 complex. Although this finding does not explain why ATR, Rad17, Hus1, and Chk1 are required for Vpr-induced G2 arrest (Fig. 5), it is plausible that Vpr induces G2 arrest in a redundant manner, both by signaling DNA damage and by inhibiting downstream mediators of cell cycle progression, such as Cdc25C (17). It is also formally possible that Cdc25C inhibition has an unforeseen effect on the activation or expression of upstream proteins in the ATR signaling cascade. Therefore, further experiments will be needed in order to better explain the above seemingly conflicting observations.
The cytopathic effects of HIV-1 infection are thought to be multiple and related to the expression of several viral genes. Our studies so far have focused on the particular roles that Vpr plays, and we have performed these studies with lentivirus vectors that direct the expression of Vpr and a reporter gene. This approach has allowed us to dissociate deleterious effects of Vpr from those of other viral genes and to molecularly dissect the cellular pathways involved. In this context, we find Vpr to exert potent antiproliferative and proapoptotic effects. It is now compelling to investigate whether and how the effects that we report here occur in the context of infection with full-length, primary isolates of HIV-1. Specific questions that will need to be addressed are as follows. (i) Does the presence of other viral accessory genes in HIV-1 have an effect on the functions of Vpr? For example, nef was reported to be antiapoptotic (16, 50) and might counterbalance the effect of Vpr. (ii) What would be the result of downregulating Rad17 or BRCA1 in the context of HIV-1 infection? Since Rad17 is important for checkpoint activation and BRCA1 is potentially proapoptotic (Fig. 5), blocking the functions of these proteins might alleviate or eliminate HIV-1 Vpr-induced cytopathicity.
Acknowledgments
We thank Robert Weiss at Cornell University for the generous gifts of Hus1−/− p21−/− mouse embryonic fibroblasts and Hus1+/+ p21−/− mouse embryonic fibroblasts, Wayne Green at the University of Utah Flow Cytometry Core Facility for expert assistance with flow cytometry, and Chris Rodesch at the University of Utah Cell Imaging Core Facility for help with confocal microscopy. Matt Mulvey, Jamie Sundsbak, and Danelle Eto provided invaluable help with immunofluorescence techniques.
This work was supported by National Institutes of Health research grants AI49057 and AI054188 to V.P.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akkina, R. K., R. M. Walton, M. L. Chen, Q. X. Li, V. Planelles, and I. S. Chen. 1996. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J. Virol. 70:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An, D. S., K. Morizono, Q. X. Li, S. H. Mao, S. Lu, and I. S. Chen. 1999. An inducible human immunodeficiency virus type 1 (HIV-1) vector which effectively suppresses HIV-1 replication. J. Virol. 73:7671-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An, D. S., Y. Xie, S. H. Mao, K. Morizono, S. K. Kung, and I. S. Chen. 2003. Efficient lentiviral vectors for short hairpin RNA delivery into human cells. Hum. Gene Ther. 14:1207-1212. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, J., A. Banerjea, V. Planelles, and R. Akkina. 2003. Potent suppression of HIV type 1 infection by a short hairpin anti-CXCR4 siRNA. AIDS Res. Hum. Retrovir. 19:699-706. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, L., C. Henderson, and Y. Adachi. 2001. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol. Cell. Biol. 21:1719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, E. J., and D. Baltimore. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 11.Cam, H., and B. D. Dynlacht. 2003. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cells 3:311-316. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury, I. H., X. F. Wang, N. R. Landau, M. L. Robb, V. R. Polonis, D. L. Birx, and J. H. Kim. 2003. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: a potential mechanism of G2/M cell cycle arrest. Virology 305:371-377. [DOI] [PubMed] [Google Scholar]
- 13.Deming, P. B., K. G. Flores, C. S. Downes, R. S. Paules, and W. K. Kaufmann. 2002. ATR enforces the topoisomerase II-dependent G2 checkpoint through inhibition of Plk1 kinase. J. Biol. Chem. 277:36832-36838. [DOI] [PubMed] [Google Scholar]
- 14.Emerman, M., and M. H. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 15.Furuta, T., H. Takemura, Z. Y. Liao, G. J. Aune, C. Redon, O. A. Sedelnikova, D. R. Pilch, E. P. Rogakou, A. Celeste, H. T. Chen, A. Nussenzweig, M. I. Aladjem, W. M. Bonner, and Y. Pommier. 2003. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278:20303-20312. [DOI] [PubMed] [Google Scholar]
- 16.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 17.Goh, W. C., N. Manel, and M. Emerman. 2004. The human immunodeficiency virus Vpr protein binds Cdc25C: implications for G2 arrest. Virology 318:337-349. [DOI] [PubMed] [Google Scholar]
- 18.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh, J. K., D. Yap, D. J. O'Connor, V. Fogal, L. Fallis, F. Chan, S. Zhong, and X. Lu. 2002. Novel function of the cyclin A binding site of E2F in regulating p53-induced apoptosis in response to DNA damage. Mol. Cell. Biol. 22:78-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. Jowett, L. Gao, L. M. Bloch, I. S. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitchen, S. G., and J. A. Zack. 1997. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J. Virol. 71:6928-6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koka, P. S., D. G. Brooks, A. Razai, C. M. Kitchen, and J. A. Zack. 2003. HIV type 1 infection alters cytokine mRNA expression in thymus. AIDS Res. Hum. Retrovir. 19:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, X., and R. L. Erikson. 2002. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. USA 99:8672-8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou, Z., and J. Chen. 2003. BRCA proteins and DNA damage checkpoints. Front. Biosci. 8:s718-s721. [DOI] [PubMed] [Google Scholar]
- 28.Lu, Y. L., P. Spearman, and L. Ratner. 1993. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 67:6542-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maser, R. S., O. K. Mirzoeva, J. Wells, H. Olivares, B. R. Williams, R. A. Zinkel, P. J. Farnham, and J. H. Petrini. 2001. Mre11 complex and DNA replication: linkage to E2F and sites of DNA synthesis. Mol. Cell. Biol. 21:6006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura, Y. 2004. Isolation of p53-target genes and their functional analysis. Cancer Sci. 95:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nghiem, P., P. K. Park, Y. Kim Ys, B. N. Desai, and S. L. Schreiber. 2002. ATR is not required for p53 activation but synergizes with p53 in the replication checkpoint. J. Biol. Chem. 15:15. [DOI] [PubMed] [Google Scholar]
- 33.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617-656. [DOI] [PubMed] [Google Scholar]
- 34.Pilch, D. R., O. A. Sedelnikova, C. Redon, A. Celeste, A. Nussenzweig, and W. M. Bonner. 2003. Characteristics of gamma-H2AX foci at DNA double-strand break sites. Biochem. Cell Biol. 81:123-129. [DOI] [PubMed] [Google Scholar]
- 35.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 16:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogakou, E. P., D. R. Pilch, A. H. Orr, V. S. Ivanova, and W. M. Bonner. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858-5868. [DOI] [PubMed] [Google Scholar]
- 38.Rogel, M. E., L. I. Wu, and M. Emerman. 1995. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogoff, H. A., M. T. Pickering, M. E. Debatis, S. Jones, and T. F. Kowalik. 2002. E2F1 induces phosphorylation of p53 that is coincident with p53 accumulation and apoptosis. Mol. Cell. Biol. 22:5308-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roshal, M., B. Kim, Y. Zhu, P. Nghiem, and V. Planelles. 2003. Activation of ATR-mediated DNA damage response by the HIV-1 viral protein R. J. Biol. Chem. 278:25879-25886. [DOI] [PubMed] [Google Scholar]
- 41.Shostak, L. D., J. Ludlow, J. Fisk, S. Pursell, B. J. Rimel, D. Nguyen, J. D. Rosenblatt, and V. Planelles. 1999. Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr. Exp. Cell Res. 251:156-165. [DOI] [PubMed] [Google Scholar]
- 42.Smits, V. A., R. Klompmaker, L. Arnaud, G. Rijksen, E. A. Nigg, and R. H. Medema. 2000. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2:672-676. [DOI] [PubMed] [Google Scholar]
- 43.Somasundaran, M., M. Sharkey, B. Brichacek, K. Luzuriaga, M. Emerman, J. L. Sullivan, and M. Stevenson. 2002. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc. Natl. Acad. Sci. USA 99:9503-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor, J. R., Jr., K. C. Kimbrell, R. Scoggins, M. Delaney, L. Wu, and D. Camerini. 2001. Expression and function of chemokine receptors on human thymocytes: implications for infection by human immunodeficiency virus type 1. J. Virol. 75:8752-8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 47.Ward, I. M., K. Minn, and J. Chen. 2004. UV-induced ATR activation requires replication stress. J. Biol. Chem. 279:9677-9680. [DOI] [PubMed] [Google Scholar]
- 48.Ward, I. M., K. Minn, K. G. Jorda, and J. Chen. 2003. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J. Biol. Chem. 278:19579-19582. [DOI] [PubMed] [Google Scholar]
- 49.Weiss, R. S., T. Enoch, and P. Leder. 2000. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 14:1886-1898. [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J., Z. P. Xu, Y. Huang, H. E. Hamrick, P. J. Duerksen-Hughes, and Y. N. Yu. 2004. ATM and ATR: sensing DNA damage. World J. Gastroenterol. 10:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, S., D. Pointer, G. Singer, Y. Feng, K. Park, and L. J. Zhao. 1998. Direct binding to nucleic acids by Vpr of human immunodeficiency virus type 1. Gene 212:157-166. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, Y., H. A. Gelbard, M. Roshal, S. Pursell, B. D. Jamieson, and V. Planelles. 2001. Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes. J. Virol. 75:3791-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou, L., D. Cortez, and S. J. Elledge. 2002. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]