Abstract
Background
Previous studies have demonstrated that an individual’s race and ethnicity are important determinants of their areal bone mineral density (aBMD), assessed by dual-energy x-ray absorptiometry. However, there are few data assessing the impact of race on bone microarchitecture and strength estimates, particularly in older adolescent girls and young adults. We hypothesized that bone microarchitecture and strength estimates would be superior in Blacks compared to White and Asian American adolescent girls and young adults of similar age based on reports of higher aBMD in Blacks.
Methods
We assessed BMD using DXA, bone microarchitecture at the distal radius and distal tibia using high resolution peripheral quantitative computed tomography (HRpQCT) and estimated measures of bone strength using micro-finite element analysis (FEA) in 35 White, 15 Asian-American and 10 Black girls 14–21 years.
Results
After controlling for height, most DXA measures of aBMD and aBMD Z-scores were higher in Black girls compared with Whites and Asian-Americans. HRpQCT and FEA showed that at the distal radius, Blacks had greater cortical perimeter, cortical area, trabecular thickness, trabecular BMD, estimated failure load and stiffness than the other two groups. For the distal tibia, trabecular number and BMD were higher in Blacks than Asian-Americans.
Conclusions
Particularly at the distal radius, adolescent and young adult White and Asian-American girls have less favourable bone microarchitecture and lower bone strength than Blacks, possibly explaining the lower risk of fracture seen in Blacks.
Level of Evidence
Level II
Keywords: Race, microarchitecture, finite element analysis, failure load
INTRODUCTION
Osteoporosis affects women and men of all races and remains under-diagnosed and under-treated. Particularly, the lifetime risk of an osteoporosis related fracture is as high as 40% in White women (1, 2). In order to develop strategies to optimize bone health in women and prevent osteoporosis, it is essential to study determinants of low bone density in the general population. This is particularly important in the adolescent and young adult years, a critical time for bone mineral acquisition, and a time when effective treatment for vulnerable groups with risk factors for low bone mineral density (BMD) may optimize acquisition of peak bone mass and reduce future fracture risk (3).
There are many determinants of BMD in any individual, including genetic factors such as height, lean mass, and menarchal age which account for up to 70% of the variability in peak BMD, nutritional status, exercise activity, calcium and vitamin D intake, and hormonal changes. In addition, race appears to be an important determinant, as adolescent and adult Whites tend to have lower BMD than Blacks (4–9) and are variably reported to have higher, no different, or lower BMD than Asians or Asian Americans (3, 10–12). Moreover, race may be a determinant of bone strength and therefore fracture risk, and the incidence of hip fractures is reported to be higher in White versus Black and Asian women (13, 14). Of note, most studies thus far have relied on dual-energy x-ray absoptiometry (DXA) to measure areal bone mineral density (aBMD), which does not adjust for body or bone size, and does not differentiate between cortical and trabecular bone compartments.
With advances in imaging technology and availability of high resolution peripheral quantitative computed tomography (HR-pQCT), it is now possible to obtain measures of volumetric bone density and microarchitecture of cortical and trabecular bone at peripheral skeletal sites with minimal radiation exposure (15). These high resolution images can also be utilized to compute estimates of bone strength and assess the mechanical load distribution across the two compartments. HR-pQCT measurements have been used to show that adult women and men with a history of fractures have lower total and trabecular volumetric bone density (vBMD), cortical thickness, trabecular number and trabecular thickness, and higher trabecular separation compared to subjects with no history of fractures (16–20). Investigators are beginning to examine bone architecture (using peripheral QCT) (21, 22) and microarchitecture (using HRpQCT) (23–25) across racial groups, and have reported differences in Blacks versus Whites (21, 22, 25) and Asians versus Whites (23, 24). However, there are limited data regarding bone microarchitecture (assessed using HR-pQCT) and estimates of bone strength [assessed using micro-finite element analysis (μ-FEA)] in older adolescent girls, a time critical for bone accrual, across all three racial groups, namely Blacks, Asian Americans and Whites.
In this study, we sought to assess (i) whether bone microarchitecture, as assessed by HR-pQCT, and estimated bone strength, as assessed using μ-FEA, differ in White, Asian American, and Black adolescent girls, and (ii) determinants of differences in microarchitectural and estimated strength parameters. We hypothesized that bone microarchitecture and strength estimates would be superior in Black adolescent girls compared to White and Asian American adolescent girls.
MATERIALS AND METHODS
Subject Selection
We enrolled 60 adolescent girls and young women between the ages of 14–21 years old (35 self-identified as White, 15 as Asian-American and 10 as Black) between 2010–2014 from the control groups of two ongoing bone density studies in adolescent and young adult women. Subjects were recruited through medical clinics and advertising in local newspapers and colleges throughout greater Boston, MA. Inclusion criteria included a bone age of ≥ 14 years, a BMI between the 10th and 90th percentiles for age, and at least nine menses in the preceding year without a history of late menarche. Exclusion criteria included use of medications or underlying medical conditions that may affect bone metabolism. Twenty-six screened participants did not participate in the study: 19 did not meet weight criteria, four did not meet criteria for menses, and three lost interest in the study after the screen. To comply with ethical consideration denoted by the World Medical Association’s Declaration of Helsinki, the study was approved by the Institutional Review Board of Partners Health Care. Informed consent was obtained from subjects ≥ 18 years of age, and from parents of subjects who were <18 years old. Informed assent was obtained from subjects < 18 years of age.
Experimental Protocol
We obtained a complete history from our subjects, including self-identification of race. Subjects who reported belonging to more than one race were classified as Black or Asian if either parent was from these respective races. No participant identified as belonging to both Black and Asian races. Height was measured using a wall-mounted stadiometer as the average of three separate measurements. Weight was measured on an electronic scale. BMI was calculated as weight (in kilograms) divided by height (in meters) 2.
Bone age was assessed using the methods of Greulich and Pyle (26), and body composition, including fat mass, lean mass and percent body fat were assessed using dual-energy X-ray absorptiometry (DXA) (Hologic 4500A, Waltham, MA). Bone mineral density (BMD) of the spine, hip, and whole body was also assessed by DXA. In order to control for differences in height amongst groups, bone mineral apparent density (BMAD) of the lumbar spine was calculated using an established formula [bone mineral content of lumbar vertebrae 2–4/ (bone mineral area of lumbar vertebrae 2–4)1.5](27). Bone density Z-scores were not race adjusted.
Participants completed a written 4-day food and supplement diary to assess dietary intake (three weekdays and one weekend day), validated for use in young women (28, 29). Description of portion sizes and preparation methods were recorded and dietary intake data (from food and supplements) were analyzed by the Bionutrition Core of the Clinical Research Center of our institution using Nutrient Data System for Research software version 2008 developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Mineapolis, MN (30). In a comparison of various methods of dietary intake assessment, the 4-day food records had a higher correlation to estimates of food consumption than the 24 hour dietary recall and a detailed quantitative two month history. The analysis of the food record includes averages of total caloric intake, as well as intake of specific macronutrients and micronutrients. Supplement intake was also recorded and added to the corresponding nutrient totals from food intake. The food record was completed within two weeks of the study visit.
Areal Bone Mineral Density Assessment
DXA (Hologic QDR-Discovery A, Apex software version 13.3; Hologic Inc., Waltham, MA) was used to assess spine, total hip, femoral neck, and whole body areal BMD (aBMD), as well as body composition. The coefficients of variation for BMD, fat mass, and lean mass for this software are 0.8% to 1.1%, 2.1%, and 1.0%, respectively. The same scanner and software version were used for all participants.
Bone Microarchitecture
Trabecular and cortical bone density and microarchitecture at both the distal radius and tibia were assessed using HR-pQCT (XtremeCT, Scanco Medical AG, Basserdorf, Switzerland) in all participants. Scans were acquired at 82 μm isotropic voxel size. During scan acquisition, the non-dominant arm or leg of the patient (31) was immobilized in an anatomically formed carbon fibre shell. An antero-posterior scout view was used to manually place a reference line at the endplate of the radius or tibia. The first CT slice was acquired 9.5 mm proximal to the reference line of the distal radius and 22.5 mm proximal to the reference line of the distal tibia. At each skeletal site, 110 CT slices were acquired extending approximately 9 mm in the axial direction. The effective radiation dose was less than 5 μSv per measurement.
Using semi-automated software, total, trabecular and cortical volumetric bone density were determined as were multiple aspects of bone microarchitecture including total, cortical and trabecular cross-sectional area, cortical thickness and perimeter, trabecular thickness, trabecular number and trabecular separation. In addition, detailed cortical bone analysis was performed by a semi-automated segmentation technique as previously described (32–35) to determine cortical porosity, cortical pore volume, and cortical pore diameter. We also used the 3D HR-pQCT images to perform linear μ-FEA and calculate apparent biomechanical properties under uniaxial compression, as previously described (17, 19, 20, 35–37). We calculated estimates of stiffness and failure load, and the proportion of load carried by the cortical and trabecular compartments (%) at the distal and proximal ends of the region of interest. Short-term reproducibility, computed from repeat scans performed after repositioning on 19 healthy young subjects age 20 to 30 years recruited through a different protocol, ranged from 0.2% to 1.7% for density values and from 0.7% to 8.6% for microarchitecture variables, consistent with prior reports (16) .Short term reproducibility ranged from 2.1 to 4.8% for variables derived from μ-FEA.
Biochemical Analysis
25-hydroxyvitamin D [25(OH)D] was determined with a chemiluminescent immunoassay (DiaSorin, Stillwater, MN; sensitivity 4 ng/ml; intraassay coefficient of variation 2.9–5.5%).
Statistical Methods
JMP Statistical Discoveries (version 5.01; SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Data are reported as means ± standard deviation (SD). P values of less than 0.05 were considered significant. We first conducted an ANOVA or the Kruskal-Wallis test to assess whether a significant difference existed among the three groups (depending on whether or not the data were normally distributed). If a significant difference was noted on the overall ANOVA, the Tukey-Kramer analysis was performed to determine which groups were significantly different, while controlling for multiple comparisons. We similarly controlled for multiple comparisons when the Kruskal-Wallis test was employed by using the Steel-Dwass test. Pearson’s correlations were used to determine associations of height, BMI, lean mass, menarchal age and 25(OH)D status with bone microarchitectural parameters. Because height, lean mass and 25(OH)D levels are important determinants of BMD and microarchitectural parameters and differed among study groups, and because menarchal age is a very important determinant of bone density and microarchitecture (even though it did not differ across groups), we next used multivariate analysis to control first for (i) height alone (as height impacts bone size parameters), and then for (ii) height, lean mass, menarchal age and 25(OH)D levels, while assessing differences among groups for radial and tibial bone mircoarchitecture and estimated bone strength. We did not include BMI in this multivariate model because height and lean mass, which were included in the model, correlated strongly with BMI. We used stepwise regression analysis with race, height, lean mass, menarchal age and 25(OH)D levels entered into the model to determine the percent variability of specific microarchitectural and FEA parameters independently determined by combinations of these covariates. We had sufficient power to adjust for six variables in our analysis.
RESULTS
Subject Characteristics
No differences were noted among the groups for chronological age, bone age, age of menarche, weight, BMI and total fat mass (Table 1). Asian Americans were shorter than Whites and trended to be shorter than Blacks. Lean mass was lower, and percent trunk fat and trunk to extremity fat ratio higher in Asian Americans than the other two groups. Although reported calcium and vitamin D consumption did not differ among groups, 25(OH)D levels were highest in White girls, followed by Asian Americans, and lowest in Blacks.
Table 1.
Clinical Characteristics of Asian American, Black and White Adolescent Girls
| Asian American n = 15 | Black n = 10 | White n = 35 | P | P* | P** | |
|---|---|---|---|---|---|---|
| Age (years) | 18.5 ± 0.5 | 19.3 ± 0.6 | 18.6 ± 0.3 | 0.5 | ||
| Bone age (years) | 17.5 ± 0.3 | 17.8 ± 0.2 | 17.5 ± 0.2 | 0.7 | ||
| Menarchal age (years) | 12.1 ± 0.4 | 12.1 ± 0.3 | 12.8 ± 0.2 | 0.2 | ||
| Tanner stage (3,4, 5) | 2,0,13 | 0,2,8 | 1,3,31 | 0.2 | ||
| Height (cm) | 158.8 ± 2.3 | 165.4 ± 2.6 | 165.1 ± 1.1 | 0.02b | ||
| Weight (kg) | 55.1 ± 1.8 | 62.5 ± 3.7 | 61.0 ± 1.4 | 0.1 | ||
| BMI (kg/m2) | 21.8 ± 0.5 | 22.8 ± 0.9 | 22.3 ± 0.4 | 0.6 | ||
| Total fat mass (kg) | 16.2 ± 1.1 | 15.7 ± 1.9 | 15.9 ± 0.8 | 0.9 | ||
| Total lean mass (kg) | 38.8 ± 1.3 | 45.3 ± 2.4 | 44.0 ± 1.0 | 0.02bc | ||
| Percent Body Fat | 28.3 ± 1.2 | 24.4 ± 2.0 | 25.4 ± 0.8 | 0.07 | ||
| Caloric Intake (cals) | 1893± 128 | 1879 ± 205 | 1835 ± 121 | 0.9 | ||
| Vitamin D Intake (IU) | 164.5 ± 23.5 | 177.0 ± 35.8 | 197.9 ± 24.5 | 0.7 | ||
| Calcium Intake (mg/d) | 818 ± 78 | 719 ± 123 | 1053 ± 91 | 0.07 | ||
| 25(OH)D (ng/mL) | 22.5 ± 2.4 | 14.6 ± 2.0 | 31.6 ± 2.3 | 0.002a | ||
| Calcium (mg/dL) | 9.1 ± 0.1 | 9.0 ± 0.2 | 9.0 ± 0.1 | 0.6 | ||
| Spine BMD (g/cm2) | 0.943 ± 0.03 | 1.112 ± 0.04 | 0.992 ± 0.02 | 0.0007ac | 0.002ac | 0.07 |
| Spine BMD Z-score | −0.54 ± 0.25 | 1.1 ± 0.38 | −0.07 ± 0.15 | 0.0005ac | 0.002ac | 0.06 |
| Spine BMAD (g/cm3) | 0.13 ± 0.004 | 0.14 ± 0.004 | 0.13 ± 0.002 | 0.004ac | 0.002ac | 0.03c |
| Hip BMD (g/cm2) | 0.913 ± 0.03 | 1.065 ± 0.03 | 1.009 ± 0.02 | 0.001bc | 0.008bc | 0.03c |
| Hip BMD Z-score | −0.58 ± 0.27 | 0.77 ± 0.27 | 0.29 ± 0.17 | 0.003bc | 0.01c | 0.04c |
| FN BMD (g/cm2) | 0.784 ± 0.03 | 0.968 ± 0.03 | 0.869 ± 0.02 | 0.0002abc | 0.001ac | 0.002ac |
| FN BMD Z-score | −0.95 ± 0.31 | 0.75 ± 0.30 | −0.17 ± 0.16 | 0.0005ac | 0.002ac | 0.003ac |
| WB BMD (g/cm2) | 1.037 ± 0.02 | 1.158 ± 0.03 | 1.063 ± 0.01 | 0.004ac | 0.002ac | 0.06 |
| WB BMD Z-score | −0.97 ± 0.29 | 0.69 ± 0.39 | −0.62 ± 0.17 | 0.001ac | 0.002ac | 0.04ac |
White vs. Black p<0.05,
White vs. Asian p<0.05,
Black vs. Asian p<0.05;
Controlled for height;
Controlled for height, lean mass, menarchal age, 25(OH)D;
FN: femoral neck, WB: whole body
Areal Bone Mineral Density
Lumbar spine, femoral neck and whole body aBMD and corresponding Z-scores, as well as spine BMAD were higher in Blacks compared to Asian Americans and Whites (Table 1). Femoral neck BMD and BMD Z-scores were also higher in Whites than in Asian Americans. Hip BMD Z-scores were higher in Blacks and Whites compared with Asian Americans. After controlling for height, Blacks had higher BMD and BMD Z-scores at the spine, femoral neck and whole body than Asian Americans and Whites, and higher hip BMD Z-scores than Asian Americans. After controlling for height, lean mass, menarchal age and 25(OH)D levels, compared with Black participants, (i) Asian Americans had lower spine BMAD, total hip and femoral neck BMD and BMD Z-scores, and whole body BMD Z-scores, and (ii) Whites had lower femoral neck BMD and BMD Z-scores and lower whole body BMD Z-scores (Table 1).
Bone Microarchitecture and Estimated Strength Parameters
A. Distal Radius
Figure 1 shows scans from Black, Asian American and White participants. Table 2 shows HRpQCT and FEA data for the distal radius (non-weight bearing bone) in Black, Asian American, and White participants.
Figure 1.
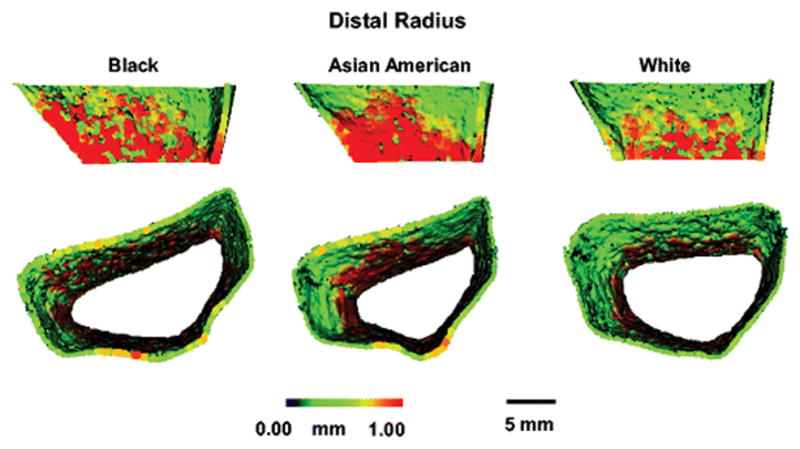
HRpQCT images of Black, Asian American and White girls at the distal radius, showing greater cortical thickness in the Black compared with the White girl, and greater cortical perimeter in the Black compared with the Asian American girl.
Table 2.
HRpQCT and FEA data for the distal radius (non-weight bearing bone) in Blacks, Asian Americans and Whites
| Radius | Black (n=10) | Asian (n=15) | White (n=35) | P | P* | P** |
|---|---|---|---|---|---|---|
| Size Parameters | ||||||
| Cortical Thickness (mm) | 0.97 ± 0.10 | 0.78 ± .06 | 0.73 ± 0.03 | 0.01a | 0.01ac | 0.02a |
| Cortical Perimeter (mm) | 74.5 ± 3.6 | 65.0 ± 1.5 | 68.7 ± 0.9 | 0.005c | 0.02ac | 0.06 |
| Cortical Area (mm2) | 59.4 ± 3.9 | 50.1 ± 3.7 | 49.4 ± 1.6 | 0.05a | 0.05a | 0.16 |
| % Cortical Area | 22.8 ± 1.8 | 21.5 ± 2.0 | 18.5 ± 0.8 | 0.07 | 0.10 | 0.15 |
| Microarchitecture Parameters | ||||||
| Cortical Pore Volume (mm3) † | 3.91± 0.71 | 2.95 ± 0.38 | 4.10 ± 0.42 | 0.23 | 0.63 | 0.26 |
| Cortical Porosity (%)† | 0.75 ± 0.11 | 0.68 ± 0.11 | 0.90 ± 0.10 | 0.37 | 0.54 | 0.22 |
| Cortical Pore Diameter (mm) † | 0.143 ± 0.004 | 0.138 ± 0.004 | 0.139 ± 0.001 | 0.40 | 0.57 | 0.94 |
| Trabecular Thickness (mm) | 0.082 ± .003 | 0.072 ± .004 | 0.072 ± .002 | 0.05a | 0.008ac | 0.15 |
| Trabecular Number (mm−1) | 2.07 ± 0.08 | 1.94 ± 0.07 | 2.03 ± 0.04 | 0.37 | 0.20 | 0.50 |
| Trabecular Separation (mm) † | 0.409 ± 0.016 | 0.456 ± 0.023 | 0.427 ± 0.011 | 0.44 | 0.14 | 0.38 |
| Density Parameters | ||||||
| Cortical Density (g/mm3) † | 841.9 ± 13.4 | 832.3 ± 23.1 | 819.0 ± 10.2 | 0.46 | 0.59 | 0.85 |
| Trabecular Density (g/mm3) | 202.1 ± 6.2 | 165.6 ± 8.2 | 176.6 ± 6.0 | 0.03c | 0.001ac | 0.04c |
| Total Density (g/mm3)† | 355.1 ± 14.5 | 325.6 ± 18.8 | 308.7 ± 8.9 | 0.08 | 0.04a | 0.17 |
| Estimated Strength Parameters | ||||||
| Stiffness (kN/m) | 90.8 ± 4.7 | 73.4 ± 4.1 | 78.3 ± 1.8 | 0.006ac | 0.01ac | 0.10 |
| Failure load (kN) | 4.59 ± 0.23 | 3.66 ± 0.19 | 3.98 ± 0.09 | 0.008ac | 0.005ac | 0.07 |
| Apparent modulus † | 2319 ± 119 | 2025 ± 124 | 1946 ± 55 | 0.02a | 0.01ac | 0.14 |
White vs. Black p<0.05,
White vs. Asian p<0.05,
Black vs. Asian p<0.05;
Controlled for height;
Controlled for height, lean mass, menarchal age and 25(OH)D;
Kruskal Wallis test
Size parameters
Cortical perimeter was higher in Black than Asian American and White participants, and cortical thickness and area were higher in Blacks than Whites. After controlling for height, Blacks had greater cortical thickness and perimeter than Asian Americans and Whites, and greater cortical area than Whites. After controlling for height, lean mass, menarchal age and 25(OH)D levels, cortical thickness remained higher in Blacks than Whites.
Density parameters
Trabecular vBMD was higher in Blacks than in Asian Americans, with differences persisting after multivariate adjustment. Also, after controlling for height, Blacks had higher trabecular and total vBMD than Whites.
Microarchitectural parameters
At the radius, trabecular thickness was higher in Black participants than in Whites, even after adjusting for height. In addition, after controlling for height, trabecular thickness was higher in Blacks than Asian Americans. These differences were lost after also controlling for menarchal age, lean mass and 25(OH)D levels. The races did not differ for trabecular number or separation, or cortical porosity.
Estimated strength parameters
Estimated stiffness and failure load of the distal radius was higher in Black compared with White and Asian American participants, and these differences persisted after controlling for height, and trended to remain significant after also controlling for lean mass, menarchal age, and 25(OH)D levels.
B. Distal Tibia
Table 3 shows HRpQCT and FEA data for the distal tibia (non-weight bearing bone) in Blacks, Asian Americans, and White girls and young women.
Table 3.
HRpQCT and FEA data for the distal tibia (weight bearing bone) in Blacks, Asian Americans and Whites
| Tibia | Black (n=10) | Asian (n=15) | White (n=35) | P | P* | P** |
|---|---|---|---|---|---|---|
| Size Parameters | ||||||
| Cortical Thickness (mm) | 1.33 ± 0.07 | 1.29 ± 0.05 | 1.23 ± 0.04 | 0.4 | 0.3 | 0.6 |
| Cortical Perimeter (mm) | 99.8 ± 3.0 | 93.9 ± 2.4 | 101.1 ± 1.3 | 0.02b | 0.5 | 0.4 |
| Cortical Area (mm2) | 131.9 ± 6.5 | 120.0 ± 4.6 | 123.5 ± 3.5 | 0.3 | 0.3 | 0.8 |
| % Cortical Area | 20.5 ± 1.4 | 21.1 ± 1.3 | 18.6 ± 0.7 | 0.2 | 0.3 | 0.6 |
| Microarchitecture Parameters | ||||||
| Cortical Pore Volume (mm3) † | 12.78 ± 2.59 | 11.80 ± 2.43 | 18.36 ± 1.94 | 0.02b | 0.2 | 0.3 |
| Cortical Porosity (%) † | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.8 ± 0.2 | 0.02b | 0.1 | 0.2 |
| Cortical Pore Diameter (mm) † | 0.143 ± 0.004 | 0.151 ± 0.005 | 0.152 ± 0.003 | 0.08 | 0.2 | 0.3 |
| Trabecular Thickness (mm) † | 0.087± 0.003 | 0.088 ± 0.004 | 0.084 ± 0.002 | 0.6 | 0.7 | 0.8 |
| Trabecular Number (mm−1) † | 2.09 ± 0.10 | 1.79 ± 0.08 | 2.00 ± 0.03 | 0.005b | 0.03c | 0.06 |
| Trabecular Separation (mm)† | 0.40 ± 0.02 | 0.49 ± 0.04 | 0.42 ± 0.01 | 0.007b | 0.09 | 0.06 |
| Density Parameters | ||||||
| Cortical Density (g/mm3) † | 896.1 ± 12.5 | 892.8 ± 11.5 | 874.9 ± 6.1 | 0.1 | 0.2 | 0.5 |
| Trabecular Density (g/mm3) † | 217.2 ± 5.3 | 185.3 ± 9.9 | 200.1 ± 5.3 | 0.05 | 0.03c | 0.3 |
| Total Density (g/mm3) | 358.8 ± 12.8 | 337.2 ± 14.7 | 326.7 ± 8.8 | 0.2 | 0.06 | 0.4 |
| Estimated Strength Parameters | ||||||
| Stiffness (kN/m) | 243.1± 11.8 | 209.2 ± 9.9 | 228.1 ± 5.9 | 0.07 | 0.2 | 0.6 |
| Failure load (kN) | 12.2 ± 0.6 | 10.5 ± 0.5 | 11.4 ± 0.3 | 0.05 | 0.2 | 0.6 |
| Apparent modulus | 2958 ± 402 | 2860 ± 136 | 2657 ± 78 | 0.1 | 0.08 | 0.3 |
White vs. Black p<0.05,
White vs. Asian p<0.05,
Black vs. Asian p<0.05;
Controlled for height;
Controlled for height, lean mass, menarchal age and 25(OH)D;
Kruskal Wallis test
Size parameters
In contrast to the radius, cortical perimeter, area and thickness were similar in Whites and Blacks at the distal tibia. Cortical perimeter was greater in Whites than Asian Americans, however, this difference was lost after controlling for covariates. Furthermore, cortical thickness and area did not differ between Whites and Asian Americans.
Density parameters
Trabecular vBMD was higher in Blacks compared with Asians after controlling for height, but not when we also controlled for lean mass, menarchal age and 25(OH)D levels. Cortical and total volumetric density did not differ among groups.
Microarchitectural parameters
Trabecular number was lower in Asian Americans compared with Whites in the unadjusted model, and lower in Asian Americans than in Blacks after controlling for height, but not when we also controlled for lean mass, menarchal age and 25(OH)D levels. Trabecular separation was higher in Asian Americans compared with Whites in the unadjusted model, but not after controlling for covariates. Trabecular thickness did not differ among groups. Cortical porosity and pore volume were higher in Whites compared with Asian Americans, but these differences did not persist after controlling for covariates.
Estimated strength parameters
Estimated stiffness and failure load trended highest in Blacks. Differences were no longer apparent after controlling for height and other covariates.
Associations of HRpQCT and FEA measures with Covariates
Distal Radius
Menarchal age, height and lean mass correlated with several bone parameters (Table 4). We found no association of BMI or 25(OH) D levels with microarchitectural parameters, but a positive association was observed of BMI with strength estimates. In a stepwise regression model (with race, height, lean mass, menarchal age and 25(OH)D levels entered into the model), race and menarchal age accounted for 28.6%, 14.7%, and 22.8% of the variability in cortical thickness, area and percent cortical area. Race and lean mass explained 27.0% of the variability in cortical perimeter, and with height predicted 42.7% and 33.4% of the variability in trabecular vBMD and trabecular thickness. Race, menarchal age and lean mass explained 28.7% of the variability in stiffness, while menarchal age and lean mass explained 30.7% of the variability in failure load. Race was not a determinant of cortical porosity, pore volume, pore diameter, trabecular number and separation, and cortical and total vBMD. Menarchal age explained 11.1% of the variability in cortical vBMD, and with height explained 33.0% of the variability in total vBMD. Height alone predicted 9.0%, 7.6%, 11.0% and 17.6% of the variability in trabecular number and separation, and cortical pore volume and percent porosity respectively.
Table 4.
Distal Radius: Correlations of microarchitecture and FEA parameters with covariates
| Menarchal Age | Height | BMI | Lean mass | 25(OH) vitamin D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |||
| Size Parameters | ||||||||||
| Cortical Thickness (mm) | −0.36 | 0.005 | −0.18 | - | 0.09 | - | −0.05 | - | −0.15 | - |
| Cortical Perimeter (mm) | 0.13 | - | 0.43 | 0.0006 | 0.19 | - | 0.44 | 0.0005 | −0.09 | - |
| Cortical Area (mm2) | −0.28 | 0.03 | 0.06 | - | 0.17 | - | 0.18 | - | −0.03 | - |
| % Cortical Area | −0.39 | 0.002 | −0.30 | 0.02 | 0.05 | - | −0.17 | - | −0.11 | - |
| Microarchitecture Parameters | ||||||||||
| Cortical Pore Volume (mm3) | 0.08 | - | 0.29 | 0.02 | 0.20 | - | 0.26 | 0.04 | −0.01 | - |
| Cortical Porosity (%) | 0.15 | - | 0.26 | 0.05 | 0.18 | - | 0.21 | - | −0.01 | - |
| Cortical Pore Diameter (mm) | −0.20 | - | 0.05 | - | 0.06 | - | 0.07 | - | −0.17 | - |
| Trabecular Thickness (mm) | −0.41 | 0.001 | −0.30 | 0.02 | 0.18 | - | 0.007 | - | −0.10 | - |
| Trabecular Number (mm−1) | −0.05 | - | −0.10 | - | 0.12 | - | −0.05 | - | 0.12 | - |
| Trabecular Separation (mm) | 0.09 | - | 0.05 | - | −0.14 | - | −0.01 | - | −0.09 | - |
| Density Parameters | ||||||||||
| Cortical Density (g/mm3) | −0.32 | 0.01 | −0.23 | 0.08 | 0.03 | - | −0.10 | - | 0.005 | - |
| Trabecular Density (g/mm3) | −0.35 | 0.007 | −0.29 | 0.03 | 0.22 | 0.09 | −0.01 | - | 0.002 | - |
| Total Density (g/mm3) | −0.46 | 0.0003 | −0.37 | 0.004 | 0.14 | - | −0.14 | - | −0.08 | - |
| Estimated Strength Parameters | ||||||||||
| Stiffness (kN/m) | −0.30 | 0.02 | 0.21 | - | 0.27 | 0.04 | 0.40 | 0.002 | 0.09 | - |
| Failure load (kN) | −0.29 | 0.03 | 0.24 | 0.06 | 0.26 | 0.05 | 0.42 | 0.0008 | 0.06 | - |
Only p values of <0.1 are reported
Distal Tibia
Associations of menarchal age, height, BMI, lean mass and 25(OH)D levels with bone parameters are shown in Table 5. In a stepwise regression model (with race, height, lean mass, menarchal age, and 25(OH)D levels entered into the model), race was the sole predictor of trabecular number and separation, accounting for 11.8% and 13.5% of the variability in these parameters. Race and lean mass predicted 24.7% of the variability in cortical percent porosity. Height and lean mass predicted cortical thickness, perimeter and area, percent cortical area, trabecular and total vBMD, and estimated stiffness and failure load (accounting for 38.7%, 59.8%, 39.7%, 42.0%, 28.1%, 42.7%, 40.3% and 40.3% of the variability in these parameters respectively). Menarchal age was an independent determinant of trabecular thickness, lean mass of cortical pore volume, and height of cortical vBMD (14.0%, 19.4% and 24.6% of the variability explained).
Table 5.
Distal Tibia: Correlations of microarchitecture and FEA parameters with covariates
| Menarchal Age | Height | BMI | Lean mass | 25(OH) vitamin D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Size Parameters | ||||||||||
| Cortical Thickness (mm) | −0.31 | 0.02 | −0.42 | 0.0009 | 0.39 | 0.002 | −0.02 | - | 0.02 | - |
| Cortical Perimeter (mm) | 0.40 | 0.001 | 0.78 | <.0001 | 0.31 | 0.02 | 0.74 | <0.0001 | 0.26 | 0.06 |
| Cortical Area (mm2) | −0.10 | - | −0.01 | - | 0.60 | <.0001 | 0.40 | 0.002 | 0.17 | - |
| % Cortical Area | −0.39 | 0.002 | −0.61 | <.0001 | 0.18 | - | −0.29 | 0.03 | −0.10 | - |
| Microarchitecture Parameters | ||||||||||
| Cortical Pore Volume (mm3) | 0.19 | - | 0.41 | 0.001 | 0.40 | 0.001 | 0.49 | <0.0001 | 0.24 | 0.09 |
| Cortical Porosity (%) | 0.22 | 0.08 | 0.47 | 0.0002 | 0.31 | 0.02 | 0.49 | <0.0001 | 0.21 | - |
| Cortical Pore Diameter (mm) | 0.10 | - | 0.10 | - | 0.08 | - | 0.11 | - | 0.10 | - |
| Trabecular Thickness (mm) | −0.39 | 0.002 | −0.38 | 0.003 | 0.03 | - | −0.08 | - | −0.02 | - |
| Trabecular Number (mm−1) | 0.16 | - | 0.32 | 0.01 | 0.26 | 0.05 | 0.35 | 0.006 | 0.19 | - |
| Trabecular Separation (mm) | −0.13 | - | −0.38 | 0.003 | −0.21 | - | −0.36 | 0.005 | −0.23 | - |
| Density Parameters | ||||||||||
| Cortical Density (g/mm3) | −0.31 | 0.01 | −0.48 | 0.0001 | 0.09 | - | −0.26 | 0.04 | −0.06 | - |
| Trabecular Density (g/mm3) | −0.24 | 0.06 | −0.05 | - | 0.23 | 0.08 | 0.24 | 0.07 | 0.12 | - |
| Total Density (g/mm3) | −0.40 | 0.002 | −0.47 | 0.0002 | 0.23 | 0.08 | −0.11 | - | −0.02 | - |
| Estimated Strength Parameters | ||||||||||
| Stiffness (kN/m) | −0.05 | - | 0.26 | 0.04 | 0.51 | <.0001 | 0.60 | <0.0001 | 0.32 | 0.02 |
| Failure load (kN) | −0.03 | - | 0.29 | 0.03 | 0.53 | <.0001 | 0.62 | <0.0001 | 0.29 | 0.04 |
Only p values of <0.1 are reported
Discussion
We report significant differences in areal and volumetric BMD and bone microarchitecture in Black, White and Asian American girls and young women 14–21 years old. Compared with Asian Americans and Whites, Blacks had higher measures of areal BMD at the spine, femoral neck and whole body, higher measures of trabecular vBMD and greater estimated stiffness and failure load at the distal radius, even after controlling for height. However, at the weight-bearing distal tibia, there were no differences among races for estimates of bone strength.
Measures of areal BMD at the spine, femoral neck and whole body were higher in Black participants compared with Whites and Asian Americans, as was spine BMAD, while total hip BMD was higher in Blacks and Whites compared with Asian Americans. Although areal BMD is susceptible to artifactual lowering in shorter individuals (27) and Asian Americans were overall the shortest group in this study, differences among groups persisted after controlling for height. Lean mass, an important positive determinant of bone density, was lower in Asian Americans than in Blacks and Whites. Menarchal age, an important inverse determinant of bone density (38–40), did not differ among groups (and is thus unlikely to explain differences across groups). 25(OH)D levels positively predict bone density in some studies (41), and were higher in Whites than Blacks. In this study, after controlling for height, lean mass, menarchal age and 25(OH)D, differences among groups for lumbar spine BMD and BMD Z-scores were lost (though groups continued to differ for lumbar spine BMAD, a height adjusted measure of BMD). However, even after controlling for these covariates, compared with Asian Americans, Blacks had higher spine BMAD, and total hip BMD and BMD Z-scores, and compared with Asian Americans and Whites, had higher femoral neck BMD and femoral neck and whole body BMD Z-scores. Our data are consistent with reports of higher BMD in Blacks than Whites (4–9, 42), although few studies have compared adolescent girls and young women of all three racial groups in a single study. Higher aBMD in Whites than Asian Americans at the hip and femoral neck is consistent with published reports in adults and adolescent girls (24, 43).
Although bone density can predict fracture risk, bone microarchitecture and FEA strength estimates are associated with fragility fractures independent of aBMD (17, 18, 44). Compared to non-fracture subjects, adult fracture subjects have lower cortical density (16, 18, 44), cortical thickness (45), trabecular density (16–18), thickness (17, 45) and number (16, 18), and higher trabecular separation (16–18). A few studies have used HR-pQCT and FEA to assess differences in Chinese American versus White women, and a few have utilized pQCT or histomorphometry to assess differences in Black versus White prepubertal children or late adolescent girls. However, studies in adolescent girls have not utilized HRpQCT or FEA to assess differences in microarchitecture and bone strength in White, Asian American and Black girls.
In our study, at the non-weight bearing distal radius Blacks had greater estimated bone strength (stiffness and failure load) measured using FEA compared with Whites and Asian Americans, and these differences persisted after controlling for height, and trended to remain significant after also controlling for lean mass, menarchal age, and 25(OH)D levels. Another study in adolescent girls using pQCT reported higher bone strength at the distal radius in Blacks than Whites, however, these differences were no longer evident after controlling for bone size (21). It should be noted that pQCT does not measure bone microarchitecture, and the previous study did not use FEA to estimate bone strength. A study using pQCT in children also reported greater bone strength at the radius in Blacks versus Whites, attributable to greater vBMD (22), as did a study using HRpQCT in postmenopausal women (25).
Our data suggest that Black girls and young women are at a lower risk of forearm fractures, or conversely, that Whites and Asian Americans are at a higher risk of such fractures. Although data are limited, there is some evidence that White girls are at a higher risk for fractures than Black girls, particularly at the upper limb (46). Specific microarchitectural parameters that may explain this include differences in bone size (as indicated by greater cortical perimeter), and in cortical and trabecular thickness, all of which were higher in Blacks compared with Whites and Asian Americans after controlling for height. Our data are consistent with histomorphometry studies in children that reveal greater cortical thickness in Blacks compared with Whites at the iliac crest (47), and with an HR-pQCT study in adults showing more favorable cortical parameters in postmenopausal African American women compared to Caucasians (25). Additionally, histomorphometric studies have reported higher trabecular thickness in Black compared with White adults (48). However, unlike studies in adults, we did not find thicker cortices (23, 24, 49) and trabeculae (23, 49) in Asian Americans compared with Whites. It is possible that unlike Whites, Asian Americans continue to increase their cortical and trabecular thickness through the adult years. However, given that most bone acquisition occurs in the perimenarchal years, it is more likely that Whites have relatively greater bone loss than Asian Americans in adulthood.
Cortical porosity, trabecular number and separation did not differ among groups at the distal radius. In adult women, trabecular number has variably been reported to be lower (23, 24), no different (49) or higher (43) in Chinese Americans compared to Whites. Also, studies suggest lower total (23, 24, 49) and trabecular cross-sectional area in Asian American compared with Caucasian women (23). In our study of adolescent girls, although trends were similar, differences between Asian Americans and Whites were not significant for bone size at the radius.
Cortical vBMD at the distal radius did not differ among groups, however, trabecular vBMD was higher in Blacks than in Asian Americans, even after controlling for covariates, and was higher in Blacks compared with Whites and Asian Americans after controlling for height alone. In adult women, some studies have reported lower cortical vBMD in Whites compared with Chinese Americans (23, 24), and either no differences (23), or lower trabecular vBMD in Whites compared with Chinese Americans (49). We found no differences between Asian Americans and Whites for any microarchitecture or strength parameter at the distal radius, suggesting that racial differences between Asians and Caucasians are not attributable to differences in bone acquisition, but perhaps to differences in age-related bone loss in adulthood.
Interestingly, differences among groups were less marked at the weight-bearing distal tibia. Although White participants had greater cortical perimeter and trabecular area than Asians, these differences were not significant after controlling for covariates. Our data are similar to a study in adults that reported higher tibial total and trabecular cross-sectional area in Whites compared with Chinese Americans, and a lack of differences after controlling for height and weight (23). White girls and young women in our study had higher cortical porosity than Asian Americans; however, this difference was no longer significant after controlling for covariates. We found inverse associations of height with cortical porosity, which may be related to older age being associated greater cortical porosity, pore volume and diameter (50), and older age being associated with taller heights in adolescents. There were no differences among groups for tibial trabecular thickness, in contrast to reports of lower trabecular thickness in Whites compared with Chinese Americans (23, 24). However, trabecular number was lower and separation greater in Asians than Whites, consistent with a study that reported similar findings in Chinese American compared with White women (23, 24). We found lower trabecular vBMD in Asian Americans than Blacks after controlling for height (but not all covariates). Total and cortical vBMD did not differ across groups, in contrast to reports of higher total vBMD in Chinese Americans than Whites (23), and higher cortical vBMD in Black compared with White adolescents (21).
Differences among groups for cortical porosity, and trabecular number, spacing and density at the distal tibia did not translate to differences in estimated bone strength in our study. Studies in adult women have similarly reported no differences in Chinese Americans and Whites for bone stiffness at the distal tibia (24). In children and also older adolescent girls, however, bone strength at the tibia using pQCT was reported to be higher in Blacks than Whites (21, 22). Similarly, higher strength estimates have been reported at the tibia in African American compared with White post-menopausal women using HRpQCT (25). Of note, adult Asian American women have lower fracture risk than White women (51). It is possible that even in the absence of marked differences in bone parameters in adolescent Asian Americans compared with Whites, there may be differences in the rate and pattern of age-related bone loss in early adulthood that contributes to variations in skeletal fragility. Furthermore, persistence of lower weight and shorter height in Asian Americans could result in lower impact during falls and lower fractures rates than in heavier and taller women.
Limitations of this study include its cross-sectional nature and inability to correlate strength parameters with fracture rates, as the latter requires a much larger sample size. Furthermore, the study had a relatively modest sample size that may have limited our ability to detect differences between groups for some variables. Nonetheless, our data do provide important and novel information regarding differences between races for bone structure and strength in older adolescent girls and young women.
In conclusion, our preliminary data suggest that Blacks are at an advantage over their White and Asian American peers with more favorable bone microarchitecture that may contribute to lower fracture risk at the forearm. Our data also suggest that some of the racial differences in BMD and microarchitecture seen in adults are well established during the adolescent years. Mechanisms underlying these differences remain to be elucidated. Future studies are necessary that examine bone density, structure and strength parameters in adolescents and young adults across the various races using a larger number of participants in the different racial groups, as well as the determinants of these differences. Studies of these bone parameters are also necessary in adolescent boys and young adult men.
Acknowledgments
All authors have made substantial contributions to the manuscript. MM worked on study conception and design, data acquisition, analysis and interpretation, and drafted the manuscript. KEA, AF and MLB worked on data acquisition and interpretation and revised the manuscript critically for intellectual content. KP, AN, GG, LP worked on data acquisition, analysis and interpretation and reviewed the manuscript. MM and MLB accept responsibility for the integrity of data analysis. All authors have approved the final version of the submitted manuscript.
Sources of support: NIH Grants UL1 RR025758; R01 HD060827; R01 DK062249; NIH/NCRR 1 S10 RR023405
ABBREVIATIONS
- BMD
bone mineral density
- aBMD
areal BMD
- vBMD
volumetric BMD
- BMAD
bone mineral apparent density
- DXA
dual energy x-ray absorptiometr
- HRpQCT
high resolution peripheral quantitative computed tomography
- FEA
finite element analysis
- 25(OH)D
25-hydroxy vitamin D
Footnotes
Conflicts of Interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Disclosures: All authors state that they have no conflicts of interest.
References
- 1.Riggs BL, Melton LJ., 3rd The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995 Nov;17(5 Suppl):505S–11S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007 Jun;22(6):781–8. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 3.Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001 Jan-Feb;12(1):22–8. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 4.Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J. Demonstration that bone mass is greater in black than in white children. J Bone Miner Res. 1991 Jul;6(7):719–23. doi: 10.1002/jbmr.5650060709. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger B, Sidney S, Cummings SR, Libanati C, Bikle DD, Tekawa IS, et al. Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metab. 1997 Feb;82(2):429–34. doi: 10.1210/jcem.82.2.3732. [DOI] [PubMed] [Google Scholar]
- 6.McCormick DP, Ponder SW, Fawcett HD, Palmer JL. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. J Bone Miner Res. 1991 May;6(5):507–13. doi: 10.1002/jbmr.5650060513. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007 Dec;22( Suppl 2):V34–8. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, et al. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. The Journal of clinical endocrinology and metabolism. 2008 Oct;93(10):3907–14. doi: 10.1210/jc.2008-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thierry-Palmer M, Henderson VM, Hammali RE, Cephas S, Palacios C, Martin BR, et al. Black and white female adolescents lose vitamin D metabolites into urine. The American journal of the medical sciences. 2008 Apr;335(4):278–83. doi: 10.1097/MAJ.0b013e31815768db. [DOI] [PubMed] [Google Scholar]
- 10.Walker MD, McMahon DJ, Udesky J, Liu G, Bilezikian JP. Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res. 2009 Dec;24(12):1953–9. doi: 10.1359/JBMR.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu W, Qin M, Xu L, van Kuijk C, Meng X, Xing X, et al. Normal changes in spinal bone mineral density in a Chinese population: assessment by quantitative computed tomography and dual-energy X-ray absorptiometry. Osteoporos Int. 1999;9(2):179–87. doi: 10.1007/s001980050133. [DOI] [PubMed] [Google Scholar]
- 12.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, et al. Bone mineral density loss in relation to the final menstrual period in a multi-ethic cohort: Results from the study of women's health across the nation (SWAN) J Bone Miner Res. 2011 Oct 4; doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau EM, Lee JK, Suriwongpaisal P, Saw SM, Das De S, Khir A, et al. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS) Osteoporos Int. 2001;12(3):239–43. doi: 10.1007/s001980170135. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Zhou B, Prentice A, Wang X, Golden MH. Epidemiological study of hip fracture in Shenyang, People's Republic of China. Bone. 1999 Feb;24(2):151–5. doi: 10.1016/s8756-3282(98)00168-9. [DOI] [PubMed] [Google Scholar]
- 15.Amstrup AK, Jakobsen NF, Moser E, Sikjaer T, Mosekilde L, Rejnmark L. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. Journal of bone and mineral metabolism. 2015 Aug 21; doi: 10.1007/s00774-015-0708-9. [DOI] [PubMed] [Google Scholar]
- 16.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005 Dec;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 17.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008 Mar;23(3):392–9. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 18.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab. 2011 Jul;96(7):2041–8. doi: 10.1210/jc.2011-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilayphiou N, Boutroy S, Sornay-Rendu E, Van Rietbergen B, Munoz F, Delmas PD, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in postmenopausal women. Bone. 2010 Apr;46(4):1030–7. doi: 10.1016/j.bone.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Vilayphiou N, Boutroy S, Szulc P, van Rietbergen B, Munoz F, Delmas PD, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res. 2011 May;26(5):965–73. doi: 10.1002/jbmr.297. [DOI] [PubMed] [Google Scholar]
- 21.Pollock NK, Laing EM, Taylor RG, Baile CA, Hamrick MW, Hall DB, et al. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab. 2011 Jan;29(1):44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- 22.Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, et al. Ethnic differences in bone geometry and strength are apparent in childhood. Bone. 2009 May;44(5):970–5. doi: 10.1016/j.bone.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang XF, Wang Q, Ghasem-Zadeh A, Evans A, McLeod C, Iuliano-Burns S, et al. Differences in macro- and microarchitecture of the appendicular skeleton in young Chinese and white women. J Bone Miner Res. 2009 Dec;24(12):1946–52. doi: 10.1359/jbmr.090529. [DOI] [PubMed] [Google Scholar]
- 24.Walker MD, Liu XS, Stein E, Zhou B, Bezati E, McMahon DJ, et al. Differences in bone microarchitecture between postmenopausal Chinese-American and white women. J Bone Miner Res. 2011 Jul;26(7):1392–8. doi: 10.1002/jbmr.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putman MS, Yu EW, Lee H, Neer RM, Schindler E, Taylor AP, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013 Oct;28(10):2177–85. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford University Press; Stanford: 1959. [Google Scholar]
- 27.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991 Dec;73(6):1332–9. doi: 10.1210/jcem-73-6-1332. [Comparative Study Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 28.Sawaya AL, Tucker K, Tsay R, Willett W, Saltzman E, Dallal GE, et al. Evaluation of four methods for determining energy intake in young and older women: comparison with doubly labeled water measurements of total energy expenditure. The American journal of clinical nutrition. 1996 Apr;63(4):491–9. doi: 10.1093/ajcn/63.4.491. [DOI] [PubMed] [Google Scholar]
- 29.Taylor C, Lamparello B, Kruczek K, Anderson EJ, Hubbard J, Misra M. Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa. Journal of the American Dietetic Association. 2009 Mar;109(3):479–85. 85 e1–3. doi: 10.1016/j.jada.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. Journal of the American Dietetic Association. 1988 Oct;88(10):1268–71. [PubMed] [Google Scholar]
- 31.Adams JE, Engelke K, Zemel BS, Ward KA. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014 Apr-Jun;17(2):258–74. doi: 10.1016/j.jocd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010 Apr;25(4):882–90. doi: 10.1359/jbmr.091020. [Comparative Study Research Support, Non-U.S. Gov't Validation Studies] [DOI] [PubMed] [Google Scholar]
- 33.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007 Oct;41(4):505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010 Sep;47(3):519–28. doi: 10.1016/j.bone.2010.05.034. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010 May;25(5):983–93. doi: 10.1359/jbmr.091104. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008 Jun;42(6):1203–13. doi: 10.1016/j.bone.2008.01.017. [Evaluation Studies Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 37.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009 Jun;24(6):1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002 Jul;87(7):3162–8. doi: 10.1210/jcem.87.7.8637. [DOI] [PubMed] [Google Scholar]
- 39.Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012 Aug 2; doi: 10.1016/j.bone.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011 Oct;96(10):3123–33. doi: 10.1210/jc.2011-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr. 2002 Dec;76(6):1446–53. doi: 10.1093/ajcn/76.6.1446. [DOI] [PubMed] [Google Scholar]
- 42.Ackerman KE, Davis B, Jacoby L, Misra M. DXA surrogates for visceral fat are inversely associated with bone density measures in adolescent athletes with menstrual dysfunction. J Pediatr Endocrinol Metab. 2011;24(7–8):497–504. doi: 10.1515/jpem.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999 Dec;84(12):4702–12. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 44.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009 Nov;94(11):4351–60. doi: 10.1210/jc.2009-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007 Mar;22(3):425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 46.Thandrayen K, Norris SA, Pettifor JM. Fracture rates in urban South African children of different ethnic origins: the Birth to Twenty cohort. Osteoporos Int. 2009 Jan;20(1):47–52. doi: 10.1007/s00198-008-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnitzler CM, Mesquita JM, Pettifor JM. Cortical bone development in black and white South African children: iliac crest histomorphometry. Bone. 2009 Apr;44(4):603–11. doi: 10.1016/j.bone.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Schnitzler CM, Mesquita J. Bone marrow composition and bone microarchitecture and turnover in blacks and whites. J Bone Miner Res. 1998 Aug;13(8):1300–7. doi: 10.1359/jbmr.1998.13.8.1300. [DOI] [PubMed] [Google Scholar]
- 49.Liu XS, Walker MD, McMahon DJ, Udesky J, Liu G, Bilezikian JP, et al. Better skeletal microstructure confers greater mechanical advantages in Chinese-American women versus white women. J Bone Miner Res. 2011 Aug;26(8):1783–92. doi: 10.1002/jbmr.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, 3rd, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012 Mar;27(3):637–44. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005 Feb;20(2):185–94. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]


