Abstract
Polybrominated diphenyl ethers (PBDEs) are associated with impaired visual spatial abilities in toxicological studies, but no epidemiologic study has investigated PBDEs and visual spatial abilities in children. The Health Outcomes and Measures of the Environment Study, a prospective birth cohort (2003–2006, Cincinnati, OH), was used to examine prenatal and childhood PBDEs and visual spatial abilities in 199 children. PBDEs were measured at 16±3 weeks gestation and at 1, 2, 3, 5, and 8 years using gas chromatography/isotope dilution high-resolution mass spectrometry. We used the Virtual Morris Water Maze to measure visual spatial abilities at 8 years. In covariate-adjusted models, 10-fold increases in BDE-47, −99, and −100 at 5 years were associated with shorter completion times by 5.2 (95% Confidence Interval [CI] −9.3, −1.1), 4.5 (95% CI −8.1, −0.9), and 4.7 seconds (95% CI −9.0, −0.3), respectively. However, children with higher BDE-153 at 3 years had longer completion times (β=5.4 seconds, 95% CI −0.3, 11.1). Prenatal PBDEs were associated with improved visual spatial memory retention, with children spending a higher percentage of their search path in the correct quadrant. Child sex modified some associations between PBDEs and visual spatial learning. Longer path lengths were observed among males with increased BDE-47 at 2 and 3 years, while females had shorter paths. In conclusion, prenatal and postnatal BDE-28, −47, −99, and −100 at 5 and 8 years were associated with improved visual spatial abilities, whereas a pattern of impairments in visual spatial learning was noted with early childhood BDE-153 concentrations.
Keywords: Polybrominated diphenyl ether (PBDE), visual spatial learning, visual spatial memory retention, neurodevelopment, Virtual Morris Water Maze
1. Introduction
Polybrominated diphenyl ethers (PBDEs) were used extensively in a wide variety of commercial products, including building materials, electronics, polyurethane foams, and textiles, to retard, suppress, or inhibit combustion. PBDEs readily leach out from materials and have widespread environmental dispersion, bioaccumulating in virtually all abiotic and terrestrial compartments (Birnbaum and Staskal, 2004). Due to their high lipophilicity and long half-lives, accumulation of PBDEs in human tissue can last over 10 years depending on the congener (Geyer et al., 2004; Thuresson et al., 2006). BDE-47, −99, and −100 have half-lives up to 2–3 years, while BDE-153 has a half-life up to 12 years (Geyer et al., 2004). The phase-out of PBDEs started in 2004, but humans are continually exposed from older consumer products containing PBDEs. PBDE exposure begins during gestation from maternal transfer and continues postnatally via intake of breast milk and by direct routes of exposure, including inhalation and ingestion. PBDE concentrations have been reported to be highest among infants and toddlers due to breastfeeding, the frequency of hand-to-mouth behaviors, and the amount of time they spend in close proximity to the floor; levels in children and teenagers are several folds higher than those of adults (Jones-Otazo et al., 2005; Toms et al., 2008).
Prenatal and childhood PBDE exposure have been associated with decrements in cognitive function, impairments in executive function, increased attention deficit/hyperactivity disorder (ADHD) related behaviors and symptoms, and poorer motor coordination in children (Chao et al., 2011; Chen et al., 2014a; Eskenazi et al., 2013; Gascon et al., 2011; Herbstman et al., 2010; Hoffman et al., 2012; Roze et al., 2009; Sagiv et al., 2015; Shy et al., 2011; Vuong et al., 2016). The mechanism by which PBDEs exert their neurotoxic effects is unclear, but suspected mechanisms include disrupting thyroid hormone function (Costa and Giordano, 2007; Kodavanti et al., 2010; Szabo et al., 2009), altering the cholinergic systems (Dufault et al., 2005; Eriksson et al., 2002; Viberg et al., 2003a), causing oxidative stress (Belles et al., 2010; Cheng et al., 2009; Giordano et al., 2008; He et al., 2008), inducing cell apoptosis (Chen et al., 2014b; He et al., 2008; He et al., 2009b), impacting DNA methylation (Woods et al., 2012), and affecting neuronal proteins (e.g., CaMKII, GAP-43, synaptophysin, and tau) and N-methyl-d-aspartate (NMDA) receptors (Buratovic et al., 2014; Viberg et al., 2003b; Viberg et al., 2008; Yan et al., 2012).
Visual spatial abilities, including learning and memory retention, contribute to overall cognitive ability and are necessary for successful everyday functioning. Visual spatial cognition is highly complex, requiring the processing of images and surroundings while focusing on pertinent details, suppressing those that are irrelevant, and manipulating mental representations to govern actions and decisions. No studies have examined the impact of prenatal and childhood PBDE exposures on visual spatial abilities, despite several animal studies reporting impairments in learning and memory with prenatal and postnatal PBDE exposure (Buratovic et al., 2014; Chen et al., 2014b; Cheng et al., 2009; He et al., 2011; He et al., 2009a; Viberg et al., 2003a; Viberg et al., 2006; Woods et al., 2012; Yan et al., 2012). Further, several studies have found reduced visual spatial learning and memory retention in rats using the Morris Water Maze with PBDE exposure (Cheng et al., 2009; Eriksson et al., 2001; He et al., 2011; He et al., 2009a; Woods et al., 2012; Yan et al., 2012). Given that PBDE exposure coincides with critical periods of brain development, we examined the relations of prenatal and childhood PBDE exposure (measured at ages 1, 2, 3, 5, and 8 years) with visual spatial abilities in children at 8 years. We hypothesized that PBDE insults during gestation and early childhood (1–8 years) are associated with reduced visual spatial abilities.
2. Materials and methods
2.1 Study participants and design
We enrolled 468 women in the Health Outcomes and Measures of the Environment (HOME) Study between March 2003 and February 2006 (Braun et al., 2016). The HOME Study is a prospective pregnancy and birth cohort in the Greater Cincinnati Area (Ohio, USA) in which pregnant women 16±3 weeks of gestation were eligible to participate if they fulfilled the following criteria: 1) were at least 18 years of age; 2) lived in a house constructed prior to 1978 (a criterion related to the randomized trial examining lead and injury hazard reduction interventions); 3) intended to receive prenatal care and deliver in one of the nine collaborating obstetric practices and hospitals; 4) were HIV negative; and 5) did not take medications for seizures, thyroid disorders, or chemotherapy/radiation. Of the 390 women who remained to deliver live singleton infants, 199 mother-child pairs had at least one measure of PBDE concentration (prenatal and/or childhood) and an assessment of child visual spatial abilities at 8 years. The study protocol was approved by the Institutional Review Boards at the Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC).
2.2 Assessment of prenatal and childhood PBDEs
Prenatal PBDEs were measured using maternal serum samples obtained at 16±3 weeks of gestation. Serum samples were collected from children at 1, 2, 3, 5, and 8 years and used to measure concentrations of PBDEs during childhood. All serum samples were stored at −80°C until analysis. Gas chromatography/isotope dilution high-resolution mass spectrometry was used to determine concentrations of BDE congeners −17, −28, −47, −66, −85, −99, −100, −153, −153, −183, and −209 (postnatal only) (Jones et al., 2012; Sjodin et al., 2004). The serum samples were extracted using automated liquid/liquid extraction with hexane/methyl tert-butyl ether after addition of internal standards and denaturation of serum proteins with hydrochloric acid (6M) and methanol (Jones et al., 2012; Sjodin et al., 2004). Each analytical batch of 24 unknowns included three quality control and method blank samples. PBDE values lower than the limit of detection (LOD), defined as 3 times the standard deviation (SD) of the method blanks or the lowest calibration standard point 0.5 pg/μL corresponding to 5 pg per sample (in the absence of detectable blanks), were substituted with LOD/√2 (Hornung and Reed, 1990). Percent detection for PBDEs by measurement timing are listed in Supplemental materials, Table S1. PBDE concentrations were lipid adjusted (ng/g lipid) based on measurements of triglycerides and total cholesterol using standard enzymatic methods (Phillips et al., 1989). The following congeners were included in our analysis of prenatal and postnatal PBDEs: BDE-28, −47, −99, −100, and −153. Of the HOME Study children who completed visual spatial assessments, 191, 76, 61, 61, 127, and 173 had PBDE concentrations available at 16±3 weeks gestation, 1 year, 2 years, 3 years, 5 years, and 8 years, respectively. The number of measurements and detection frequencies of PBDE congeners at each age category are given in Table S1 (see Supplemental material, Table S1). Numbers of children at 1–3 years were considerably lower, because only a subset of children had available serum to measure PBDEs.
2.3 Visual spatial abilities
Visual spatial learning and memory retention were assessed at 8 years of age using the Virtual Morris Water Maze (Astur et al., 1998), a computerized version of the Morris Water Maze test that is used to measure spatial learning and navigation in rodents (Morris, 1981). The Virtual Morris Water Maze has been demonstrated to be an effective assessment of spatial learning in humans (Astur et al., 1998). The virtual environment is comprised of a circular pool contained within a square floor plan. Four distal cues of equal size were positioned at each of the walls. The platform lay at the center of the northeast quadrant under the water surface. Children were informed that the platform would remain stationary throughout the trials and were instructed to locate the hidden platform as quickly and efficiently as possible. In order to familiarize the children with the virtual environment, they were given four practice trials in which the platform was visible but no visual landmarks were displayed on the walls before the test began. Children could freely navigate between the four quadrants and control the speed of movement by manipulating the joystick. Movement was limited to forward, left, and right to mirror the natural movement of rodents. After the practice trials, a series of four blocks of four trials were administered with the location of the hidden platform and visual landmarks fixed. Children were randomly placed in different locations (north, east, south, and west) by one side of the pool wall at the start of each block of trials. Once the platform was reached, an audible cue would sound and the platform would become visible. Children were given a two second interlude prior to the onset of the next trial. The Virtual Morris Water Maze measures time (seconds) and distance (pool units) traveled from the start location to the platform for each trial. An average of time and distance for each set of blocks was used to assess visual spatial learning, with shorter times and distance traversed indicating superior performance.
Lastly, a 30-second probe trial was administered within the same virtual environment, but the platform was removed. Exploration continued for the duration of the trial as crossing the platform did not terminate the trial. No indication was provided that the probe trial differed from the previous 16 trials. For the probe trial, time and proportion of distance traveled in the quadrant of the pool that previously contained the platform was used to assess children’s memory of the platform location, with higher times and distance percentages indicating better retention of spatial location.
2.4 Statistical analyses
We log10-transformed PBDE concentrations to approximate a normal distribution. The associations of log10-transformed prenatal and childhood PBDEs (1, 2, 3, 5, and 8 years) and visual spatial learning at 8 years were estimated using multiple informant models (Horton et al., 1999; Litman et al., 2007), which account for repeated measurements of PBDEs (prenatal and childhood) as well as the repeated measures of the Virtual Morris Water Maze (time and distance) by implementing a non-standard version of generalized estimating equations as described by Sanchez et al (2011). Each model included repeated measurements of BDE congeners or Σ5PBDEs in a long-formatted dataset (each observation was for one exposure window and one block performance measure [time, distance]). Beta coefficients and 95% confidence intervals (CIs) were estimated for each window of exposure from a single regression model. Because several interaction terms between PBDEs (continuous) and child age (categorical) were statistically significant (p<0.10), we present separate estimates for prenatal and childhood PBDEs (1, 2, 3, 5, and 8 years). Multiple informant models were also used to examine repeated measures of PBDEs and visual spatial memory retention at 8 years.
Effect modification by child sex was examined by including an interaction term between PBDEs (continuous), child sex (categorical), and child age (categorical), as well as 2-way interactions (PBDE*child age; PBDE*child sex; child age*child sex), with p<0.10 considered statistically significant. We assessed for linear trends using the median value of each tertile as a continuous variable in the previously described models (Greenland, 1995). In a sensitivity analysis, we additionally adjusted by the child’s reported experience playing three-dimensional computer games and history of motion sickness, because these measures could influence Virtual Morris Water Maze performance. Due to lower PBDE concentrations at 1–3 years (limited sample availability), we additionally ran a sensitivity analysis only including PBDE measurements at 16±3 weeks, 5 years, and 8 years to determine if findings differed.
Covariates included in the final models were selected based on bivariate analyses examining the relation with visual spatial abilities. We included maternal age at delivery, race/ethnicity, maternal education, family income at enrollment, maternal serum cotinine at 16±3 weeks gestation (continuous, ng/mL) marital status at enrollment, maternal IQ (continuous, assessed by Wechsler Abbreviated Scale of Intelligence) (Wechsler, 1999), child sex, and the Home Observation for Measurement of the Environment score at 1 year home visit in the adjusted models (Caldwell and Bradley, 1984). Maternal concentrations of serum polychlorinated biphenyl concentrations (ΣPCBs: −28, −74, −99, −105, −118, −146, −153, −156, −170, −180, −183, −187, −194, −199, and −206) and blood lead were not significantly correlated with PBDEs or visual spatial abilities and were not included in the final models (Table 1).
Table 1.
Serum concentrations of prenatal Σ5PBDEs and at 8 years and the VMWM time and distance in block 4 by maternal and child characteristics, HOME Studya
| Maternal and Child Characteristics | Prenatal Σ5PBDEs | Σ5PBDEs (8y) | n | Time Mean (SD) | Distance Mean (SD) | ||
|---|---|---|---|---|---|---|---|
| n | GM (GSD) | n | GM (GSD) | ||||
| Maternal age, years | |||||||
| <25 | 39 | 49.4 (2.1) | 44 | 42.8 (2.2) | 50 | 48.6 (18.7) | 17.3 (7.5) |
| 25–34 | 96 | 39.4 (2.6) | 97 | 50.3 (2.2) | 117 | 45.5 (16.1) | 17.4 (7.9) |
| ≥35 | 26 | 30.3 (2.5) | 24 | 38.5 (2.4) | 31 | 45.2 (14.3) | 16.0 (9.3) |
| Race/ethnicityb,c | |||||||
| Non-Hispanic White | 99 | 32.3 (2.3) | 100 | 46.3 (2.2) | 124 | 43.6 (14.8) | 16.6 (8.1) |
| Non-Hispanic Black and Others | 62 | 56.1 (2.6) | 65 | 46.5 (2.3) | 74 | 50.6 (18.3) | 18.1 (7.8) |
| Educationb, d | |||||||
| High school or less | 42 | 57.1 (2.3) | 44 | 46.8 (2.1) | 49 | 49.0 (17.9) | 17.4 (5.8) |
| Some college/2 yr degree | 42 | 42.1 (2.1) | 47 | 50.3 (2.3) | 55 | 48.8 (17.5) | 19.3 (9.0) |
| Bachelor’s | 53 | 31.4 (2.8) | 44 | 43.0 (2.5) | 59 | 44.1 (15.5) | 16.6 (9.0) |
| Graduate or professional | 24 | 33.0 (2.6) | 30 | 44.8 (2.0) | 35 | 41.6 (13.3) | 14.6 (6.4) |
| Family Incomeb | |||||||
| <$40,000 | 63 | 51.7 (2.2) | 69 | 49.4 (2.2) | 78 | 49.3 (18.4) | 18.4 (6.7) |
| $40,000–$79,999 | 57 | 39.9 (2.7) | 51 | 46.1 (2.2) | 67 | 44.4 (15.6) | 15.8 (8.6) |
| ≥$80,000 | 41 | 26.8 (2.4) | 45 | 42.2 (2.2) | 53 | 43.9 (14.1) | 17.2 (8.9) |
| Maternal Depressionb | |||||||
| Minimal/mild | 143 | 37.2 (2.4) | 147 | 47.2 (2.2) | 180 | 46.9 (16.2) | 17.4 (8.0) |
| Moderate/severe | 17 | 72.1 (2.8) | 17 | 41.1 (2.7) | 17 | 40.0 (18.9) | 14.4 (7.7) |
| Home Observation for Measurement of the Environment scoreb | |||||||
| ≥40 | 92 | 33.7 (2.5) | 96 | 45.2 (2.2) | 119 | 45.3 (15.4) | 16.5 (8.2) |
| 35–39 | 33 | 51.9 (2.4) | 34 | 54.2 (2.5) | 37 | 42.6 (14.4) | 17.0 (6.6) |
| <35 | 26 | 50.3 (2.5) | 24 | 43.2 (1.9) | 29 | 51.7 (19.1) | 19.2 (8.4) |
| Marital statusb,c | |||||||
| Married/living with partner | 121 | 35.9 (2.5) | 118 | 46.8 (2.2) | 148 | 44.6 (15.6) | 16.8 (8.2) |
| Not married, living alone | 40 | 55.2 (2.3) | 47 | 45.3 (2.4) | 50 | 51.1 (18.1) | 18.2 (7.2) |
| Child Sexc | |||||||
| Male | 74 | 37.3 (2.2) | 78 | 47.8 (2.3) | 93 | 42.8 (16.4) | 16.1 (7.2) |
| Female | 88 | 41.8 (2.7) | 88 | 45.0 (2.1) | 106 | 49.0 (16.2) | 18.1 (8.6) |
| n | Pearson r | n | Pearson r | n | Pearson r | Pearson r | |
| Maternal IQb,c | |||||||
| (Mean±SD 105.9±15.4) | 153 | −0.21 | 157 | −0.07 | 187 | −0.18 | −0.14 |
| Maternal cotinineb | |||||||
| (GM±GSD 0.05±17.5 ng/mL) | 160 | 0.35 | 163 | −0.04 | 194 | 0.07 | 0.01 |
| Maternal Serum PCBse | |||||||
| (GM±GSD 45.3±1.8 ng/g lipid) | 153 | −0.06 | 146 | −0.05 | 176 | −0.12 | −0.07 |
| Maternal Blood Lead Levels | |||||||
| (GM±GSD 0.63±1.4 μg/dL) | 161 | 0.12 | 165 | −0.05 | 198 | 0.08 | 0.004 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; SD, standard deviation; Σ5PBDEs, sum of BDE-28, −47, −99, −100, and −153
Units: Σ5PBDEs (ng/g lipid); Cotinine (ng/mL); Time (sec); Lead (μg/dL); Distance (pool units)
Frequencies may not add to the total number of participants because of missing values. p < 0.05 for:
Prenatal ΣPBDEs;
Time;
Distance (two-sided p values using ANOVA or t-test)
Sum of congeners with detection frequencies >75% (congeners-28, 74, 99, 105, 118, 146, 153, 156, 170, 180, 183, 187, 194, 199, and 206)
3. Results
3.1 Participant characteristics
Prenatal Σ5PBDEs concentrations were lower among mothers who were non-Hispanic white, highly educated, of a higher income, minimally or mildly depressed, married or living with a partner, and whose Home Observation for Measurement of the Environment score was ≥40 (Table 1). Childhood Σ5PBDEs at 8 years did not significantly differ across participant characteristics. Children who were non-Hispanic white, male, and whose parents were married or living together had faster Virtual Morris Water Maze completion times. Total distance traveled was also lower among children of mothers who had higher education. Maternal IQ was correlated with faster completion time. Participants excluded (n=191) due to missing assessments of PBDEs and/or visual spatial abilities were more likely to be married or living with a partner than those included in the analyses (n=199), but did not differ with regard to other maternal sociodemographic characteristics, IQ, behavioral factors, or child sex (see Supplemental material, Table S2).
3.2 Prenatal and childhood PBDE concentrations
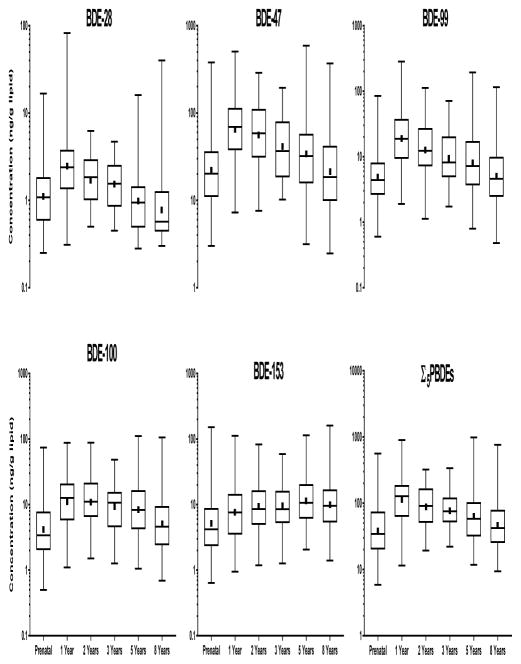
Serum concentrations of PBDEs among women in the HOME Study were generally comparable with pregnant women in the National Health and Nutrition Examination Survey (NHANES) 2003–2004 (Woodruff et al., 2011). The geometric mean of BDE-47 in the HOME Study was 21.6 ng/g lipid compared to 23.9 ng/g lipid among the NHANES pregnant women. Geometric mean PBDE concentrations at 1 year (except BDE-153) were about two-times higher than the prenatal levels in mothers at 16±3 weeks (Figure 1). Concentrations of PBDEs decreased and leveled off as children reached 8 years of age. In contrast, BDE-153 concentrations gradually increased from 16±3 weeks to 5 years.
Figure 1.
Serum concentrations of prenatal and postnatal polybrominated diphenyl ethers, HOME Study. Upper and lower lines of the body of the boxplot represent the 25th and 75th percentile, respectively. Median values are indicated by horizontal lines within boxes and solid squares represent geometric means.
We observed positive correlations between concentrations of BDE-28, 47, −99, −100, and −153 at 16±3 weeks gestation (rs=0.51–0.92, p<0.0001), 5 years (rs=0.44–0.95, p<0.0001), and 8 years (rs=0.38–0.96, p<0.0001) (see Supplemental material, Table S3). Most prenatal PBDEs were moderately correlated with concentrations at 5 years, but were not correlated with 8 year concentrations. For instance, prenatal BDE-47 was correlated with BDE-47 at 5 years (rs=0.26, p<0.01), but not at 8 years (rs=0.11, p>0.05). In contrast, prenatal BDE-153 remained significantly correlated with measurements at 5 (rs=0.34, p<0.001) and 8 years (rs=0.24, p<0.01).
3.3 PBDE concentrations and visual spatial learning
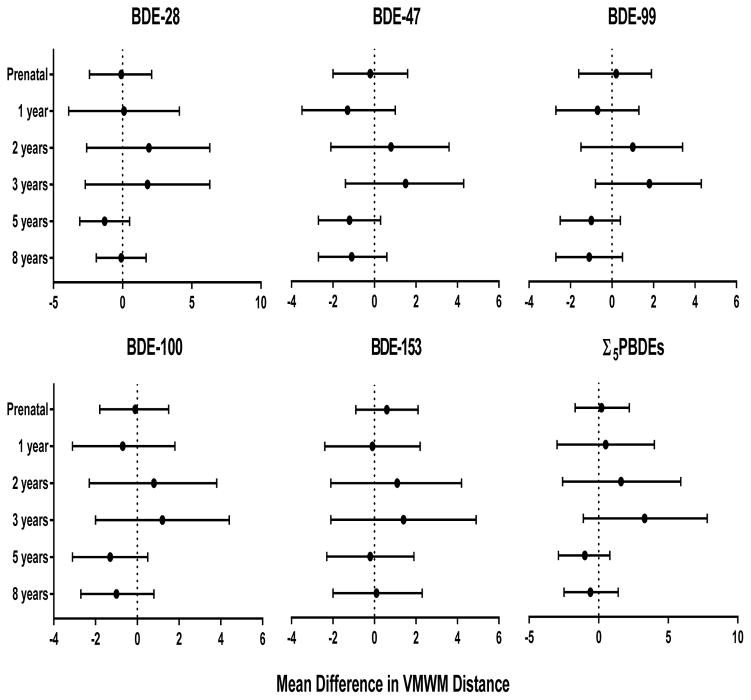
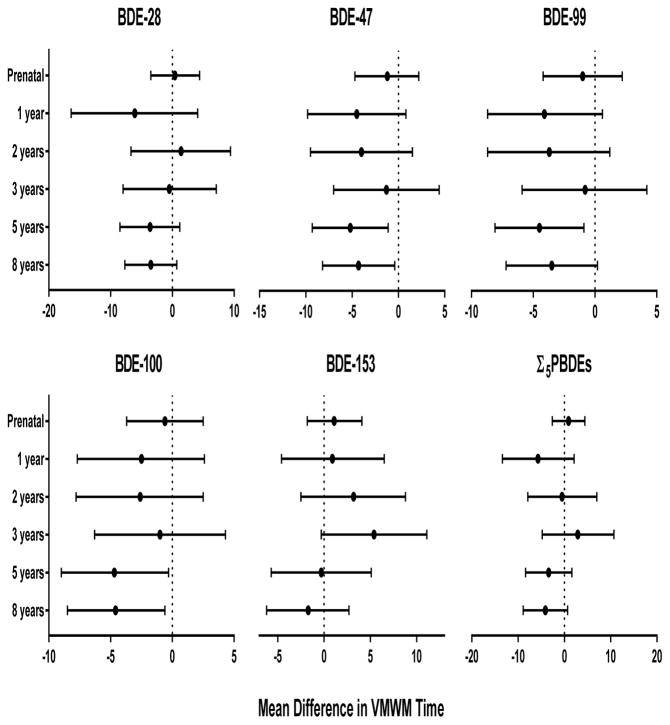
Children located the platform in significantly less distance (p=0.003) across blocks (see Supplemental Figure, Figure S1). However, overall estimates for all four blocks are presented, because interaction terms between PBDEs (continuous) and block (categorical) did not reach statistical significance (p<0.10). No statistically significant associations were observed between PBDE congeners or Σ5PBDEs and path length (Figure 2). Increased concentrations of BDE-153 during gestation and early childhood (1–3 years) were generally associated with slower Virtual Morris Water Maze completion time (Figure 3). Although none of these associations reached statistical significance, BDE-153 at 3 years was borderline significant with an increase in 5.4 seconds (95% CI −0.3, 11.1). In addition, significant linear trends between tertiles of BDE-153 at 2 years for distance and 3 years for both time and distance were observed (ptrend<0.05); children in the higher tertile of BDE-153 concentration had poorer performance (results not shown). In contrast, increasing concentrations of several PBDEs at 5 and 8 years were associated with better performance. A 10-fold increase in BDE-47, −99, and −100 at 5 years were associated with faster time to complete the Virtual Morris Water Maze by 5.2 (95% CI −9.3, −1.1), 4.5 (95% CI −8.1, −0.9), and 4.7 seconds (95% CI −9.0, −0.3), respectively. Similar findings were noted with BDE-47 and −100 at 8 years and Virtual Morris Water Maze completion time.
Figure 2.
Estimated mean differences and 95% confidence intervals in distance (pool units) for blocks 1–4 in the VMWM by a 10-fold increase in prenatal and postnatal PBDE concentrations, HOME Study. Adjusted by maternal age, race, education, income, maternal serum cotinine, marital status, maternal IQ, child sex, and Home Observation for Measurement of the Environment score. Lower distances indicate better performance on the VMWM maze.
Figure 3.
Estimated mean differences and 95% confidence intervals in time (seconds) for blocks 1–4 in the VMWM by a 10-fold increase in prenatal and postnatal PBDE concentrations, HOME Study. Adjusted by maternal age, race, education, income, maternal serum cotinine, marital status, maternal IQ, child sex, and Home Observation for Measurement of the Environment score. Lower times indicate better performance on the VMWM maze.
3.4 PBDE concentrations and visual spatial memory retention
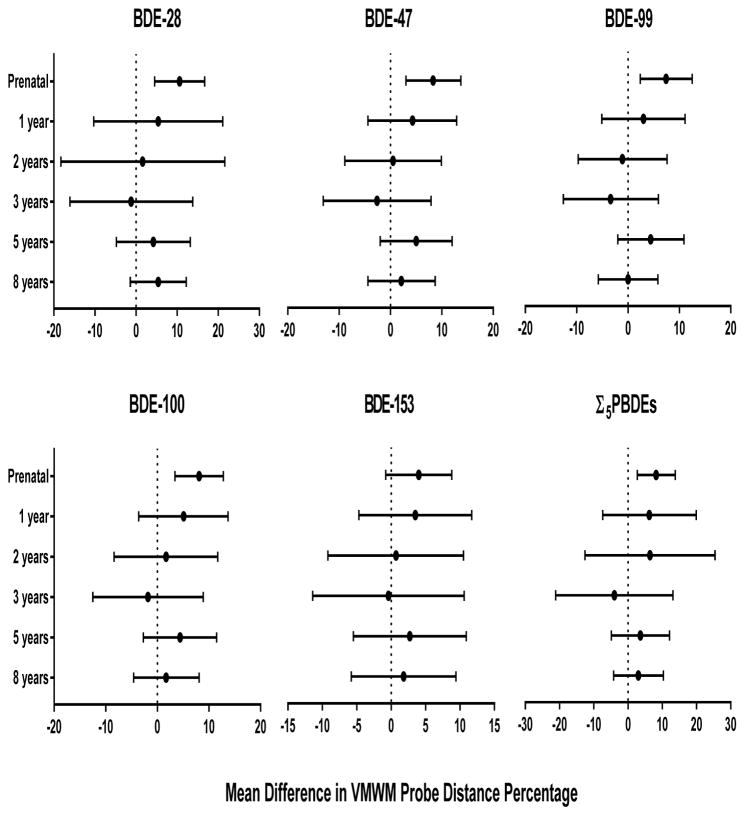
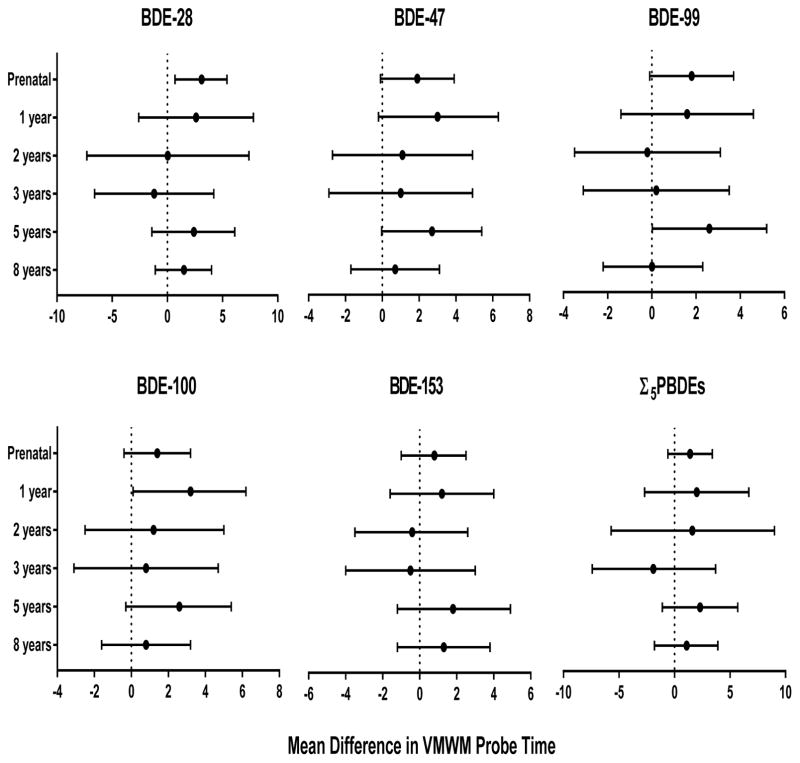
Prenatal PBDEs were associated with improved spatial retention as measured by children’s time and distance spent in the correct quadrant during the probe trial. Children who had higher prenatal PBDE concentrations spent a greater percentage of the total distance traveled searching in the vicinity of the pool that previously contained the platform, indicating better memory retention of the platform’s original location. Ten-fold increases in prenatal BDE-28, −47, −99, −100, and Σ5PBDEs were significantly associated with a 10.6 (95% CI 4.5, 16.7), 8.3 (95% CI 3.0, 13.7), 7.4 (95% CI 2.4, 12.5), 8.1 (95% CI 3.4, 12.8), and 8.2 percent (95% CI 2.7, 13.8) increase in the total distance, respectively (Figure 4). Prenatal BDE-28 was significantly associated with more time in the platform quadrant (β=3.1 seconds, 95% CI 0.7, 5.4). BDE-100 at 1 year and BDE-99 at 5 years were associated with improved spatial memory retention, increasing the time in the correct quadrant by approximately 3 seconds (Figure 5).
Figure 4.
Estimated mean differences and 95% confidence intervals in distance (%) spent in the correct quadrant during the probe trial by a 10-fold increase in prenatal and postnatal PBDE concentrations, HOME Study. Adjusted by maternal age, race, education, income, maternal serum cotinine, marital status, maternal IQ, child sex, and Home Observation for Measurement of the Environment score. Higher distance percentages indicate better performance on the VMWM probe trial.
Figure 5.
Estimated mean differences and 95% confidence intervals in time (seconds) spent in the correct quadrant during the probe trial by a 10-fold increase in prenatal and postnatal PBDE concentrations, HOME Study. Adjusted by maternal age, race, education, income, maternal serum cotinine, marital status, maternal IQ, child sex, and Home Observation for Measurement of the Environment score. Higher times indicate better performance on the VMWM probe trial.
3.5 Child sex differences in visual spatial learning
Males performed significantly better than females with regard to shorter distances as trials progressed (see Supplemental material, Figure S2). We observed that child sex modified some associations between PBDEs and Virtual Morris Water Maze performance, with a consistent pattern for total completion distance. In general, prenatal and early childhood (up to 3 years of age) BDE-28, −47, −99, and −100 (pinteraction<0.10) were associated with longer distances in males and null or shorter distances in females (see Supplemental material, Figure S3). For example, childhood concentrations of BDE-47 at 2 and 3 years were associated with longer distances in males (β=3.6 pool units, 95% CI 0.3, 6.9 and β=4.1 pool units, 95% CI 0.9, 7.3), but shorter distances in females (β−1.7 pool units, 95% CI −5.4, 2.0 and β=−2.2 pool units, 95% CI −6.3, 2.0). Similar findings were observed between BDE-28 at 2 years and BDE-99 at 3 years and path length. In contrast, only one association with completion time showed significant effect measure modification by child sex (see Supplemental material, Figure S4). BDE-153 at 1 year was associated with shorter times in males and longer times in females.
The associations between prenatal and childhood PBDEs and visual spatial learning and memory retention did not change appreciably after additionally adjusting for history of motion sickness or playing video games (results not shown). Limiting analyses to PBDE concentrations at 16±3 weeks, 5 years, and 8 years did not change our overall conclusions (results not shown).
4. Discussion
The findings in our study do not support the hypothesis that prenatal and postnatal exposures to PBDEs are associated with impaired visual spatial abilities in children. Generally, we observed a pattern of increased Virtual Morris Water Maze completion time with increasing prenatal and early childhood (1–3 years of age) concentrations of BDE-153. A statistically significant linear trend was also observed between childhood BDE-153 (2 and 3 years) and poorer performance on the Virtual Morris Water Maze (both time and distance). There is no clear association between performance on the Virtual Morris Water Maze and cognitive function. However, impairments in visual spatial abilities, measured by other assessment tools, are related to poorer mathematical abilities and achievement, which have been associated with higher risk of unemployment and lower lifetime earnings (Dumontheil and Klingberg, 2012; Li and Geary, 2013; Sella et al., 2016; Slot et al., 2016; Wei et al., 2012). Deficits in visual spatial abilities can also affect quality of life and activities of daily living, including navigational ability, vestibular function, autobiographical memory, and social interactions in terms of recognizing social signals and interpersonal space (Bigelow et al., 2015; Janssen et al., 2015; Rodriguez et al., 2015). However, Penta-BDE congeners BDE-47, −99, and −100 at 5 and 8 years were associated with reduced Virtual Morris Water Maze completion time by 4–5 seconds. A similar pattern was observed with BDE-28 at 5 and 8 years. We also noted better visual spatial memory retention with higher prenatal BDE-28, −47, −99, −100 and Σ5PBDEs, with children spending a larger percentage of their total distance and time in the quadrant that previously held the platform.
It is unclear why our results differ with respect to the specific PBDE congeners and the timing of their measurement during gestation and childhood. It may be due to differing structural and chemical properties that influence the neurotoxic mechanisms of action and potency between the BDE congeners. BDE-47, −99, and −100 have estrogenic properties while BDE-153 is more antiandrogenic and antiestrogenic (Kojima et al., 2009). Co-pollutant confounding by the mixture of PBDEs may also be a factor that influenced results, but our sample size and the high correlation between PBDEs did not allow us to fully explore this. The timing of exposure could also influence our results. Concentrations of BDE-28, −47, −99, and −100 during gestation and later in childhood (5 and 8 years) were associated with improved visual spatial abilities while worse performance was generally noted with early childhood concentrations of BDE-153. Further, the body burden of PBDEs varied across time. Concentrations of BDE-28, −47, −99, and −100 peaked after birth and continued to decline as children aged to reach concentrations similar to that during gestation, while BDE-153 remained generally constant after the initial increase after delivery. BDE-153 concentrations at 8 years (9.8±2.2 ng/g lipid) was higher than concentrations during gestation (5.1±2.9 ng/g lipid).
Our results are not consistent with the findings of several animal studies that have reported deficits in visual spatial learning and memory retention with perinatal and childhood exposures to PBDEs. BDE-47 and −99 induced deficits in visual spatial learning and memory in rats, prolonging time and increasing distance traveled in the Morris Water Maze (Cheng et al., 2009; Eriksson et al., 2001; He et al., 2011; He et al., 2009a; Woods et al., 2012; Yan et al., 2012). However, the null associations in our study align with those of Driscoll et al. (2009), in which no relation was reported between chronic postnatal pentaBDE mixture DE-71 and visual discrimination learning. Further, no differences in visual spatial learning and memory were observed in studies in which BDE-99 was administered to adult rats (Belles et al., 2010; Daubie et al., 2011). Consistent with some of our findings showing improved Virtual Morris Water Maze performance with higher PBDE concentrations, Ta et al. (2011) reported significantly shorter swimming distances among female mice perinatally exposed to BDE-47 compared to female controls. Lastly, we observed a pattern of impairments in visual spatial learning and memory retention with childhood concentrations of BDE-153, although these associations were nonsignificant. Similar findings were reported with postnatal exposures to BDE-153, −183, −203, −206, and −209 and prenatal exposure to BDE-209 in rats and mice (Buratovic et al., 2014; Chen et al., 2014b; Viberg et al., 2003a; Viberg et al., 2006).
We did not consistently observe evidence that child sex modified some associations between PBDEs and the Virtual Morris Water Maze parameters. For BDE-28, −47, −99, and −100 during gestation and BDE-47, −99, and −100 at 1 and 3 years, increasing concentrations were associated with increased distance among males. However, we did not observe similar associations when we examined time to complete the Virtual Morris Water Maze. Previously, no significant sex differences were reported in mice perinatally exposed to BDE-47 exposure with visual spatial learning and memory (Ta et al., 2011). While prior epidemiologic studies have not examined prenatal and childhood PBDEs and their relation with visual spatial abilities, two studies have reported sexually dimorphic associations between PBDE exposure and other neurodevelopmental outcomes, with conflicting conclusions. Poorer behavior regulation was observed with increased prenatal BDE-153 concentrations in males, but not in females in this same cohort (Vuong et al., 2016). However, executive function deficits were noted among females with higher postnatal PBDEs in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort (Sagiv et al., 2015).
While some of our findings align with previous animal models, many of the conclusions were incongruent. Discrepancies between our findings and those in animal literature may be due to a number of factors. The Morris Water Maze is not directly comparable to the Virtual Morris Water Maze as the former relies on the rodents’ natural aversion to water. Water aversion may result in different motivations and strategies to complete the task in rodents compared to humans. Children completing the Virtual Morris Water Maze are not driven by a strong desire to find the platform to escape from the water and thus measures of spatial learning and memory retention may be influenced by other motivational factors, including interest and a desire to perform well. Further, the Virtual Morris Water Maze only requires children to utilize their visual perception and motor skills in a two-dimensional virtual reality while for rodents the experience is one that requires active involvement of all their senses in a real world setting. In addition, animal studies generally administer doses of PBDEs that are several orders of magnitude higher than those observed in the general population. Further, dosing windows varied across animal studies, spanning from perinatal to adulthood. Some of the null associations observed in our study may be due to concentrations of PBDEs that were below an as yet unknown threshold of neurotoxicity to visual spatial abilities. Further, most animal models examined only individual PBDEs, while humans are exposed to multiple PBDEs (and other potential neurotoxicants) simultaneously. Possible synergies between several environmental contaminants may have influenced the results in the present study. Finally, the processes and mechanism(s) of PBDE neurotoxicity may differ between species and PBDEs may impair visual spatial learning and memory in mice and rats, but not necessarily in humans.
Improvements in visual spatial abilities with increased concentrations of the Penta congeners BDE-28, −47, −99, and −100 during childhood could have been due to reverse causality. Children who are more proficient at video games may spend more time indoors, which could result in higher levels of exposure to PBDEs via dust inhalation since close physical contact would increase uptake of PBDEs from items, such as computer keyboards and foam furniture. While we additionally adjusted by video game playing in a sensitivity analysis and observed no change in our conclusions, the covariate only indicates whether a child played video games. We do not have information on the amount of time spent doing this activity. However, BDE-99, −47, and −100 had the highest loadings in dust sources in the HOME Study at 1, 2, and 3 years after BDE-209. The high loadings of these Penta-BDE congeners in the dust samples could partially explain why we observed an improvement in performance in the Virtual Morris Water Maze with these congeners while BDE-153 resulted in a pattern of decreased performance. These homes may have more electronic equipment, such as computers and gaming consoles, which could potentially influence PBDE concentrations and the child’s performance on the Virtual Morris Water Maze, because they may be more proficient in playing video games. Lastly, individuals with a higher socioeconomic status have been found to have lower PBDE concentrations than those of lower socioeconomic status in the HOME Study. Residual confounding may also be a concern as it is unclear whether everyday visual spatial activities differ between children from various socioeconomic backgrounds since the time that children spend outdoors versus indoors would be influenced by the environment (e.g., availability of electronics for gaming, neighborhood safety, green space in residential area).
Major strengths of the study include its longitudinal study design which enabled the examination of PBDE exposures during gestation and at multiple time points, from infancy to school-age. Secondly, concentrations of prenatal PBDEs in the HOME Study were comparable to those of US pregnant women in NHANES 2003–2004 (Woodruff et al., 2011). However, since PBDE concentrations are not available for children <12 years in NHANES, it is uncertain whether concentrations in the HOME Study are representative of US children. Previous studies of PBDE concentrations in children in California have reported levels that were higher than those in the HOME Study. The GM of BDE-47 was 47.1 ng/g lipid among CHAMACOS children at 7 years and 61.8 ng/g lipid among a subset of children 2–8 years in the Study of Use of Products and Exposure-Related Behavior compared to 21.2 ng/g lipid in our children at 8 years (Eskenazi et al., 2011; Wu et al., 2015). Childhood PBDE concentrations in the HOME Study may be more representative of the general US population, because serum concentrations in California are much higher than other states due to its stringent flammability standards (Rose et al., 2010; Zota et al., 2008). Third, the Virtual Morris Water Maze is a comparable assessment to the Morris Water Maze test that is used to measure visual spatial learning and memory in animal studies. However, the Virtual Morris Water Maze is not completely analogous to the Morris Water Maze as rodents are more motivated to locate the platform due to their natural aversion to water. Lastly, the HOME Study has data on a comprehensive list of possible confounders, including sociodemographics, maternal IQ, and a nurturing home environment, that were taken into account in our analysis. We were also able to adjust by children’s history of motion sickness and playing video games to determine whether conclusions differed.
The findings in our study are subject to several limitations. As with any longitudinal study, attrition is a concern. However, participants lost to follow-up only differed by marital status and were similar on all other sociodemographic characteristics, behavioral factors, and child sex to those that remained in the study, suggesting that selection bias may not be a major concern. Secondly, childhood assessments of PBDE concentrations during early childhood (1, 2, and 3 years) were limited to a subset of HOME Study children, which resulted in imprecise estimates. However, we used a multiple informant model approach to examine repeated measurements of PBDEs from gestation to school-age to increase power. In addition, assessment of visual spatial learning and memory retention at 8 years may not be a good predictor of visual spatial abilities later in life. Lastly, multiple analyses (totaling 24 primary statistical models) were performed to test the associations between PBDEs and visual spatial abilities. While 1 would be expected by chance alone, 13 models yielded statistically significant results. It is unlikely that the significant findings are due to chance.
5. Conclusions
Findings from this study are mixed as a pattern of impaired visual spatial learning was noted with increased concentrations of early childhood BDE-153, while improvements in visual spatial learning was observed with increased childhood concentrations of the Penta-BDE congeners BDE-47, −99, and −100 as well as BDE-28. Improved visual spatial memory retention was also observed with increased prenatal concentrations of BDE-28, −47, −99, and −100 as children spent more time and distance traveling in the correct quadrant. Results are suggestive of a sexually dimorphic relationship between PBDEs and visual spatial abilities, with male children performing more poorly on the Virtual Morris Water Maze. While the observed associations may have small effects at the individual level, at the population level the magnitude would be larger and thus be more meaningful. Additional epidemiologic studies are needed to examine prenatal and childhood PBDEs and visual spatial abilities in children as our study is the first to report on these associations, with mixed results.
Highlights.
The VMWM test was used to assess visual spatial abilities in children at 8 years
BDE-153 at 3 years was adversely associated with visual spatial learning
BDE-47, −99, and −100 at 5 years was associated with better visual spatial learning
Prenatal PBDEs were associated with improved visual spatial memory retention
Male children were observed to perform more poorly on the VMWM than females
Acknowledgments
Funding
This work was supported by grants from the US National Institute of Environmental Health Sciences and the Environmental Protection Agency (NIEHS P01 ES11261, R00 ES020346, R01 ES020349, R01 ES024381, R01 ES014575, T32ES010957, P30ES006096; EPA P01 R829389). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIEHS, EPA, or the Centers for Disease Control and Prevention (CDC).
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R00 ES020346, R01 ES020349, R01 ES024381, R01 ES014575, T32ES010957, P30ES006096; EPA P01 R829389). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIEHS or the Centers for Disease Control and Prevention (CDC). We acknowledge the CDC laboratory staff who participated in the analysis of the samples for environmental chemicals.
Abbreviations
- ADHD
Attention deficit/hyperactivity disorder
- CDC
Centers for Disease Control and Prevention
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- GM
Geometric mean
- HOME Study
Health Outcomes and Measures of the Environment Study
- LOD
Limit of detection
- NHANES
National Health and Nutrition Examination Survey
- NMDA
N-methyl-d-aspartate
- PBDEs
Polybrominated diphenyl ethers
- PCBs
Polychlorinated biphenyls
- SD
Standard deviation
Footnotes
The study protocol was approved by the Institutional Review Boards at the Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention.
Competing Financial Interests
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astur RS, et al. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Belles M, et al. Behavioral effects and oxidative status in brain regions of adult rats exposed to BDE-99. Toxicol Lett. 2010;194:1–7. doi: 10.1016/j.toxlet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Bigelow RT, et al. Association Between Visuospatial Ability and Vestibular Function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2015;63:1837–44. doi: 10.1111/jgs.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratovic S, et al. Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuroprotein analysis in adult male and female mice. Environ Toxicol Pharmacol. 2014;38:570–85. doi: 10.1016/j.etap.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. University of Arkansas; Little Rock, AR: 1984. [Google Scholar]
- Chao HR, et al. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res. 2011;70:596–600. doi: 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Chen A, et al. Prenatal Polybrominated Diphenyl Ether Exposures and Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ Health Perspect. 2014a;122:856–62. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, et al. Prenatal exposure to decabrominated diphenyl ether impairs learning ability by altering neural stem cell viability, apoptosis, and differentiation in rat hippocampus. Hum Exp Toxicol. 2014b doi: 10.1177/0960327113509661. [DOI] [PubMed] [Google Scholar]
- Cheng J, et al. Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere. 2009;75:963–8. doi: 10.1016/j.chemosphere.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubie S, et al. Neurobehavioral and physiological effects of low doses of polybrominated diphenyl ether (PBDE)-99 in male adult rats. Toxicol Lett. 2011;204:57–63. doi: 10.1016/j.toxlet.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, et al. Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels. Neurotoxicol Teratol. 2009;31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dufault C, et al. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 2005;88:172–80. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb Cortex. 2012;22:1078–85. doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Eriksson P, et al. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–8. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, et al. A brominated flame retardant, 2,2′,4,4′,5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67:98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. A comparison of PBDE serum concentrations in Mexican and Mexican-American children living in California. Environ Health Perspect. 2011;119:1442–8. doi: 10.1289/ehp.1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37:605–11. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004:66. [Google Scholar]
- Giordano G, et al. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–8. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6:450–4. doi: 10.1097/00001648-199507000-00025. [DOI] [PubMed] [Google Scholar]
- He P, et al. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29:124–9. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- He P, et al. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol Ind Health. 2011;27:279–88. doi: 10.1177/0748233710387002. [DOI] [PubMed] [Google Scholar]
- He P, et al. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. Neurotoxicology. 2009a;30:1088–95. doi: 10.1016/j.neuro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- He P, et al. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology. 2009b;30:10–5. doi: 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, et al. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect. 2012;120:1438–42. doi: 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Horton NJ, et al. Use of multiple informant data as a predictor in psychiatric epidemiology. Int J Methods Psychiatr Res. 1999;8:6–18. [Google Scholar]
- Janssen SM, et al. The relation between verbal and visuospatial memory and autobiographical memory. Conscious Cogn. 2015;31:12–23. doi: 10.1016/j.concog.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Jones-Otazo HA, et al. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39:5121–30. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Jones R, et al. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd. 2012;74:97–98. [Google Scholar]
- Kodavanti PR, et al. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Kojima H, et al. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–8. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Geary DC. Developmental gains in visuospatial memory predict gains in mathematics achievement. PLoS One. 2013;8:e70160. doi: 10.1371/journal.pone.0070160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman HJ, et al. Incorporating missingness for estimation of marginal regression models with multiple source predictors. Stat Med. 2007;26:1055–68. doi: 10.1002/sim.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. Spatial Localization Does Not Require the Presence of Local Cues. Learning and Motivation. 1981;12:239–260. [Google Scholar]
- Phillips DL, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, et al. Comparison of Visuospatial and Verbal Abilities in First Psychotic Episode of Schizophrenia Spectrum Disorder: Impact on Global Functioning and Quality of Life. Front Behav Neurosci. 2015;9:322. doi: 10.3389/fnbeh.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, et al. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol. 2010;44:2648–53. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, et al. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–8. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol. 2015;52:151–61. doi: 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BN, et al. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119:409–15. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sella F, et al. Basic and advanced numerical performances relate to mathematical expertise but are fully mediated by visuospatial skills. J Exp Psychol Learn Mem Cogn. 2016;42:1458–72. doi: 10.1037/xlm0000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy CG, et al. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87:643–8. doi: 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- Sjodin A, et al. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76:1921–7. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Slot EM, et al. Shared and Unique Risk Factors Underlying Mathematical Disability and Reading and Spelling Disability. Front Psychol. 2016;7:803. doi: 10.3389/fpsyg.2016.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo DT, et al. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TA, et al. Bioaccumulation and behavioral effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol Teratol. 2011;33:393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuresson K, et al. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–81. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LM, et al. Higher accumulation of polybrominated diphenyl ethers in infants than in adults. Environ Sci Technol. 2008;42:7510–5. doi: 10.1021/es800719v. [DOI] [PubMed] [Google Scholar]
- Viberg H, et al. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003a;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, et al. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003b;76:112–20. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Viberg H, et al. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92:211–8. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viberg H, et al. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–9. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Vuong AM, et al. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res. 2016;147:556–64. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wei W, et al. Cognitive correlates of performance in advanced mathematics. Br J Educ Psychol. 2012;82:157–81. doi: 10.1111/j.2044-8279.2011.02049.x. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, et al. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet. 2012;21:2399–411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, et al. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ Health. 2015;14:23. doi: 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, et al. Spatial learning and memory deficit of low level polybrominated diphenyl ethers-47 in male adult rat is modulated by intracellular glutamate receptors. J Toxicol Sci. 2012;37:223–33. doi: 10.2131/jts.37.223. [DOI] [PubMed] [Google Scholar]
- Zota AR, et al. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42:8158–64. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]