In the past decade, studies that provide insights into the mechanisms of action underlying ketamine’s rapid antidepressant effects have been key to identifying relevant targets for developing novel antidepressants with rapid and sustained effects1. In this context, glycogen synthase kinase 3 (GSK-3) appears to be a top candidate. GSK-3 has been extensively associated with mood disorders, particularly with regard to lithium’s effects on this target and the potential association with clinical improvement2–4. GSK-3 also plays a key role in relevant biological processes such as oxidative stress, neurogenesis, and inflammation3.
In an elegant preclinical study, Beurel and colleagues5 recently demonstrated an integrated functional effect between GSK-3 and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) in ketamine’s rapid antidepressant effects. Specifically, the study found that ketamine-induced GSK-3 inhibition upregulated AMPA GluA1 subunits and stabilized AMPA receptors at the cell surface. Ketamine also decreased post-synaptic density 95 (PSD-95) phosphorylation; PSD-95 is a known substrate for GSK-3 that directly regulates the number of AMPA receptors at the cell surface and, consequently, regulates synaptic strength6. Notably, the limiting effects at PSD-95 led to lower internalization of AMPA GluA1, which also had a central regulatory effect on AMPA receptor trafficking5. It is important to note that GSK-3 was previously shown to regulate AMPA receptor trafficking7 and is also associated with PSD-95 phosphorylation8. These recent findings by Beurel and colleagues underscore the relevance of GSK-3 and postsynaptic density proteins in ketamine’s rapid antidepressant effects.
Ketamine is a classic high-trapping N-methyl-D-aspartate (NMDA) receptor antagonist that displays slow ‘off-rate’ NMDA receptor dissociation9. This high-trapping ability was initially proposed as a key mechanism underlying its rapid antidepressant properties. Hypothetically, while trapped in the ion channel, ketamine would induce a prolonged receptor blockade, thereby dissociating glutamate from its binding site on the NMDA receptor. This, in turn, would exert rapid antidepressant effects in conjunction with its well-known adverse ‘dissociative state’ effects, even at lower concentrations10. However, recent studies have shown that other NMDA receptor antagonists—for instance, MK-0657 and AZD6765—do not appear to possess ketamine’s robust, rapid, and sustained antidepressant effects11, 12. As a result, the potential roles of alternative (non-NMDA) mechanisms underlying ketamine’s rapid antidepressant effects are being further explored.
The most compelling alternative model to explain ketamine’s rapid antidepressant effects beyond simple NMDA antagonism is based on ketamine’s ability to augment AMPA receptor activity in critical neuronal circuits, the so-called enhanced AMPA to NMDA throughput model13, 14 (see Fig. 1). Briefly, the AMPA throughput model posits that AMPA receptor activation is a key mediator of ketamine’s antidepressant effects. It builds on the observation that pre-treatment with the AMPA receptor antagonist NBQX attenuated ketamine-induced antidepressant-like effects and also upregulated hippocampal phosphorylated GluA1 AMPA receptors15. This model also suggests that NMDA receptor blockade increases synaptic glutamate release, thus preferentially favoring AMPA receptor activity in critical limbic circuits. Additional experiments have supported the notion that ketamine’s ability to induce rapid antidepressant effects is due to a rapid and transient increase in glutamate release in the prefrontal cortex associated with acute activation of AMPA receptors16.
Fig. 1.
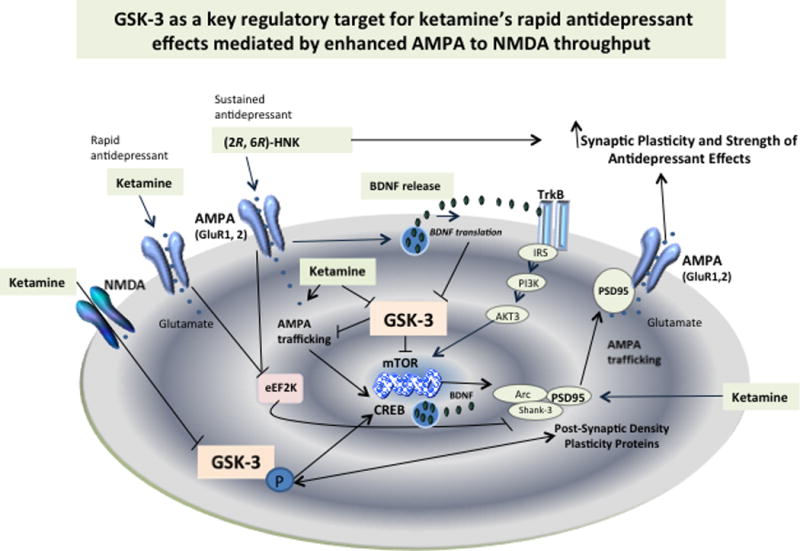
Enhanced AMPA to NMDA throughput is a key convergent model suggesting that augmentation of AMPA receptor signaling mediates the activation of synaptic plasticity and, consequently, the rapid antidepressant effects of the glutamatergic modulator ketamine. GSK-3 has been shown to critically regulate AMPA receptor activation and intracellular trafficking by limiting its activity and associated antidepressant efficacy. Increased GSK-3 phosphorylation by ketamine inactivates the protein and favors increases in mTOR, CREB, and PSD-95 expression. AMPA receptor trafficking is regulated by PSD-95, which also regulates GSK-3. Ketamine lowers phosphorylated PSD-95 on Thr-19, the GSK-3 target that promotes AMPA receptor internalization. Similar activation of synaptic strength and plasticity involving direct regulation at GSK-3 are expected to take place with the ketamine metabolite (2R, 6R)-HNK based on its direct ability to activate AMPA receptors and synaptogenesis in preclinical models.
Notably, the recent findings described by Beurel and colleagues5 suggest that a direct interaction between GSK-3 and AMPA receptors plays a central role in ketamine’s antidepressant effects. This association has been demonstrated in previous studies with ketamine. In addition to the recent findings showing that ketamine activates AMPA receptor signaling by inhibiting GSK-3 via a decrease in hippocampal PSD-95 phosphorylation, Beurel and colleagues had previously used GSK-3 knockin mice to demonstrate that cortical and hippocampal GSK-3 inhibition (based on its increased phosphorylation at serine 21 and 9) was required for ketamine’s rapid antidepressant-like effects17. In GSK-3 knockin mice, serine phosphorylation did not inhibit GSK-3; the latter was also associated with arousing depressive-like behaviors in rodents following diverse stressors18. In humans, ketamine was shown to inactivate GSK-3 activity (by lowering its phosphorylation) after a single bolus ketamine infusion in depressed patients19. In addition, lithium—which is a potent GSK-3 inhibitor—potentiated the antidepressant-like effects of ketamine in mice20. Furthermore, a polymorphism in the GSK-3 promoter gene was found to underlie the antidepressant response to total sleep deprivation in individuals with bipolar disorder21; sleep deprivation is an important model for studying the neurobiological basis of rapid antidepressant efficacy.
Additional evidence supports a role for AMPA receptor activity, particularly GluA1, in ketamine’s molecular actions. Specifically, low-dose ketamine significantly enhanced hippocampal AMPA—but not NMDA—receptor density in Wistar Kyoto rats22, a finding that may be associated with the potential role of AMPA in long-term antidepressant efficacy. Likewise, ketamine significantly upregulates AMPA receptor subunits23. In addition, subanesthetic doses of ketamine activate AMPA receptor-mediated prefrontal cortex synaptic transmission (AMPA-induced currents)24. Interestingly, potent AMPA positive modulators have recently been developed and tested in initial Phase 1 and 2 studies to overcome the lower bioavailability associated with previous studies; these newer agents have shown preliminary antidepressant efficacy in small samples25, 26, though larger studies are needed to definitively assess the antidepressant effects of these compounds. It should be noted that the antidepressant effects of the mood stabilizer lithium, a central GSK-3 inhibitor, have also been shown to be mediated via AMPA receptor signaling potentiation7, supporting a convergent mechanism.
Another promising area of study is the investigation of ketamine metabolites and enantiomers as tools to provide insight into potential central mechanistic models, with a convergent focus on the AMPA throughput model. Indeed, AMPA receptor throughput seems to be involved not only in ketamine’s rapid antidepressant effects but also in the acute and sustained effects of its metabolites27. Changes in the efficiency of the AMPA receptor are commonly used to evaluate synaptic plasticity, and low-frequency synaptic stimulation capable of increasing synaptic efficiency is mostly mediated by activation of AMPA receptors28. Reinforcing the concept of enhanced AMPA to NMDA throughput, a recent study demonstrated that the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) does not bind to or inhibit NMDA receptors; nevertheless, the metabolite independently and rapidly increased AMPA receptor-mediated hippocampal synaptic transmission and upregulation of GluA1 and GluA2 AMPA receptor subunits in synaptosomes27. (2R,6R)-HNK also induced antidepressant effects independently of NMDA receptor antagonism. Furthermore, the AMPA receptor antagonist NBQX also blocked the rapid and sustained antidepressant effects of (2R,6R)-HNK27.
Finally, further evidence supports enhanced AMPA to NMDA throughput related to ketamine’s effects. Several downstream targets of AMPA activate synaptic plasticity pathways, and these are known to be involved in ketamine’s rapid antidepressant effects. For instance, AMPA receptor activation targeting brain-derived neurotrophic factor (BDNF), eukaryotic elongation factor 2 (eEF2), and the mammalian target of rapamycin (mTOR) signaling pathways appear relevant to the rapid antidepressant effects of ketamine and related molecules in preclinical models. Ketamine enhanced BDNF signaling by activating both post-synaptic AMPA receptor and L-type voltage-dependent calcium channels, thus inducing calcium influx and activity-dependent BDNF exocytosis29. These effects also seem to be related to the stimulation of eEF2- and BDNF-dependent potentiation30. Activation of the mTOR signaling pathway—a central regulator of cell metabolism, growth, proliferation, and survival—through AMPA receptors has also been associated with ketamine’s rapid antidepressant-like effects31. In addition, this pathway has also been linked to reversal of stress- and/or depression-related deficits31. mTOR also regulates brain morphogenesis by mediating GSK-3 signaling32. However, it should be noted that even though mTOR has been implicated in ketamine’s rapid antidepressant effects, it was not involved in the early and sustained antidepressant effects of its (2R,6R)-HNK metabolite27.
In conclusion, the recent study by Beurel and colleagues5 supports the notion that GSK-3 plays a key role in the enhanced AMPA throughput model underlying ketamine’s rapid antidepressant efficacy. This convergent mechanism seems to involve AMPA receptor activation potentially mediated by GSK-3, with consequent glutamate release and resultant activation of downstream pathways related to ketamine’s rapid and sustained antidepressant effects. Furthermore, when taken in conjunction with the recent findings of Zanos and colleagues27, it appears that these late-phase effects may involve ketamine’s metabolites targeting at the eEF2 and BDNF pathways. Antidepressant effects would thus synergistically result from elevated glutamate levels, increased AMPA receptor insertion, and increased monoamines. Indeed, an evaluation of the potential role of GSK-3 in the sustained antidepressant effects of the (2R,6R)-HNK metabolite is expected to be performed soon. At present, the extant evidence definitively confirms that a single mechanism cannot be responsible for ketamine’s rapid antidepressant effects. Additional preclinical studies on enhanced AMPA throughput mechanisms that evaluate different downstream targets are needed, as are initial clinical studies exploring the therapeutic potential of ketamine metabolites.
Acknowledgments
We thank Ioline Henter, MA (NIMH) for providing invaluable editorial assistance.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857-10), by a NARSAD Independent Investigator Award to CAZ, and by a Brain and Behavior Mood Disorders Research Award to CAZ. CAZ is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. CAZ is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Abbreviations
- AKT3
protein kinase B3
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- BDNF
brain-derived neurotrophic factor
- CREB
cyclic adenosine monophosphate response element binding protein
- GSK-3
glycogen synthase kinase-3
- HNK
hydroxynorketamine
- IRS
insulin receptor substrate
- mTOR
mammalian target of rapamycin
- NMDA
N-methyl-D-aspartate
- PI3K
phosphoinositide-3 kinase
- PSD-95
post-synaptic density-95
- TrkB
tropomyosin receptor kinase B
Footnotes
Disclosures
RM-V has no conflicts of interest to disclose, financial or otherwise.
References
- 1.Machado-Vieira R, Henter ID, Zarate CA. New targets for rapid antidepressant action. Prog Neurobiol. 2015 Dec 23; doi: 10.1016/j.pneurobio.2015.12.001. pii: S0301-0082(15)30038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sousa RT, Streck EL, Zanetti MV, Ferreira GK, Diniz BS, Brunoni AR, et al. Lithium increases leukocyte mitochondrial complex I activity in bipolar disorder during depressive episodes. Psychopharmacology (Berl) 2015;232:245–50. doi: 10.1007/s00213-014-3655-6. [DOI] [PubMed] [Google Scholar]
- 3.Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado-Vieira R, Manji HK, Zarate CA. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beurel E, Grieco SF, Amadei C, Downey K, Jope RS. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord. 2016;18:473–480. doi: 10.1111/bdi.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Husseini A-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–63. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA. 2010;107:11573–8. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson CD, Kim MJ, Hsin H, Chen Y, Sheng M. Phosphorylation of threonine-19 of PSD-95 by GSK-3β is required for PSD-95 mobilization and long-term depression. J Neurosci. 2013;33:12122–35. doi: 10.1523/JNEUROSCI.0131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mealing GA, Lanthorn TH, Murray CL, Small DL, Morley P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-d-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther. 1999;288:204–10. [PubMed] [Google Scholar]
- 10.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–5. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Machado-Vieira R, Maeng S, Martinovitch K, Manji H, Zarate C. Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discovery Today: Disease Mechanisms. 2007;3:519–26. doi: 10.1016/j.ddstr.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA., Jr Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009;15:1595–611. doi: 10.2174/138161209788168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2016 Apr 12; doi: 10.1038/mp.2016.34. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beurel E, Song L, Jope R. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–74. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Zhou ZQ, Gao ZQ, Shi JY, Yang JJ. Acute increases in plasma Mammalian target of rapamycin, glycogen synthase kinase-3b, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psychiatry. 2013;73:e35–e6. doi: 10.1016/j.biopsych.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–77. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–6. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 22.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkholm C, Jardemark K, Schilstrom B, Svensson TH. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol. 2015;25:1842–7. doi: 10.1016/j.euroneuro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Dutta A, McKie S, Deakin JF. Ketamine and other potential glutamate antidepressants. Psychiatry Res. 2015;225:1–13. doi: 10.1016/j.psychres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, Zraket D, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2012;26:1525–39. doi: 10.1177/0269881112458728. [DOI] [PubMed] [Google Scholar]
- 27.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDA receptor inhibition-independent antidepressant actions of a ketamine metabolite. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA, et al. A pivotal role of GSK-3 in synaptic plasticity. Front Mol Neurosci. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu033. pii: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–6. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 31.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ka M, Condorelli G, Woodgett JR, Kim WY. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141:4076–86. doi: 10.1242/dev.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]


