Abstract
Introduction
Ozone (O3) has been linked to hypertensive disorders of pregnancy (HDP). However, inconsistent results have been reported, and no study has examined the critical exposure windows during pregnancy.
Materials and Methods
We used Florida birth vital statistics records to investigate the association between HDP and O3 exposure among 655,529 pregnancies with conception dates between 2005 and 2007. Individual O3 exposure was assessed at mothers’ home address at the time of delivery using the Hierarchical Bayesian space-time statistical model. We examined the association during three predefined exposure windows including trimester 1, trimester 2, and trimesters 1&2, as well as in each week of the first two trimesters using distributed lag models.
Results
Pregnancies with HDP had a higher mean exposure to O3 (39.07 in trimester 1, 39.02 in trimester 2, and 39.06 in trimesters 1&2, unit: ppb) than those without HDP (38.65 in trimester 1, 38.57 in trimester 2, and 38.61 in trimesters 1&2, unit: ppb). In the adjusted logistic regression model, increased odds of HDP were observed for each 5 ppb increase in O3 (ORTrimester1=1.04, 95% CI: 1.03, 1.06; ORTrimester2=1.03, 95% CI: 1.02, 1.04; ORTrimester1&2=1.07, 95% CI: 1.05, 1.08). In the distributed lag models, elevated odds of HDP were observed with increased O3 exposure during the 1st to 24th weeks of gestation, with higher odds during early pregnancy.
Conclusions
O3 exposure during pregnancy is related to increased odds of HDP, and early pregnancy appears to be a potentially critical window of exposure.
Keywords: Air pollution, Hypertensive disorders of pregnancy, Ozone, Windows of exposure
INTRODUCTION
Hypertensive disorders of pregnancy (HDP) are among the most common medical problems encountered during pregnancy, affecting up to 10% of all pregnancies (Duley, 2009; Miller and Carpenter, 2015). HDP is typically classified into four categories, including chronic hypertension, preeclampsia-eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension (National High Blood Pressure Education Program, 2000). HDP is characterized by high blood pressure, usually after 20 weeks of gestation, as a result of higher cardiovascular burden caused by significant changes in blood volume and other physiologic characteristics during pregnancy (Yoder et al., 2009). It is considered a risk factor for neonatal and maternal morbidity and mortality (Allen et al., 2004; Bauer and Cleary, 2009; Bellamy et al., 2007; Duley, 2009; Lo et al., 2013; Wang et al., 2012; Wu et al., 2009). In the United States, preeclampsia alone contributes to about 25% of all medically indicated preterm deliveries (Ananth and Vintzileos, 2006; Goldenberg et al., 2008; Romero et al., 2014). Despite serious consequences, the biological mechanisms underlying HDP remain to be determined. Known risk factors for HDP include maternal characteristics such as nulliparity, obesity, advanced maternal age, adolescent pregnancy, pre-pregnancy hypertension or diabetes mellitus; and pregnancy-related factors such as multiple gestation, placental abnormalities, weight gain, gestational diabetes mellitus (GDM), family history of pre-eclampsia as well as African American race (Wolf et al., 2004). To reduce the continuously increasing incidence of HDP (Wallis et al., 2008), a better understanding of modifiable risk factors for HDP is needed to guide intervention efforts.
Ambient air pollution has been linked to hypertension in the general population (Coogan et al., 2012; Dong et al., 2013; Foraster et al., 2014), and recent studies suggested a potential link between pregnancy air pollution exposure and HDP (Hu et al., 2014). Pregnant women experience higher cardiovascular burden given the fast and sudden change in blood volume (Ouzounian and Elkayam, 2012) as well as other physiologic characteristics, making them a potentially high-risk subpopulation. Furthermore, air pollution has been shown to have toxicological effects such as increasing circulating markers of oxidative stress, lipid peroxidation and inflammation (Ghio et al., 2012; Lee et al., 2011; Nagiah et al., 2015; Slama et al., 2008), all of which are associated with cardiovascular effects including hypertensive disorders of pregnancy (Fenzl et al., 2013; Sánchez-Aranguren et al., 2014; Zusterzeel et al., 2002). As a result, there are concerns that air pollution may also play a role in the development of HDP (Hu et al., 2014; Malmqvist et al., 2013).
Ozone (O3) is the air pollutant of great concern to the state of Florida (Florida Department of Environmental Protection, 2012), and recent meta-analyses of existing studies on ozone and HDP suggest an overall positive association (Hu et al., 2014; Pedersen et al., 2014). However, results are still inconsistent among individual studies. Several important limitations exist from the current state of the literature. First, exposure assessment in many studies relied on sparsely located stationary air monitors (Mobasher et al., 2013; Olsson et al., 2013; van den Hooven et al., 2011; Vinikoor-Imler et al., 2012; Xu et al., 2013; Zhai et al., 2012). The poor spatial resolution resulting from this method limits the ability to adequately capture the spatial contrasts in O3 levels, leading to increased exposure misclassification bias. In addition, participants living far away from these monitors are routinely excluded from the analyses leading to potential selection bias. Other studies used traffic density and distance from major roads as a proxy for exposure to pollution, which may also introduce substantial exposure misclassification bias (van den Hooven et al., 2009). Second, critical exposure windows to O3 have not been established for HDP. Identification of such window is needed to improve the understanding of the underlying biological mechanisms between O3 and HDP and to help inform the design and implementation of targeted and effective preventive strategies.
To address these limitations, we used the data from the EPA and CDC’s National Environmental Public Health Tracking Network (U.S. EPA, 2014) to assess daily ambient O3 levels, and linked it to the Florida Vital Statistics Birth Record dataset to investigate the association between HDP and O3 among all eligible women residing in Florida with conception dates between January 1, 2005 and December 31, 2007. More importantly, we assessed potential critical pregnancy windows for O3 exposure using a distributed lag model to reduce the influences of autocorrelation and collinearity in weekly O3 exposure.
METHODS
Study sample
Birth record data were obtained from the Bureau of Vital Statistics, Office of Health Statistics and Assessment, Florida Department of Health (http://www.floridahealth.gov/certificates/certificates/, Jacksonville, Florida). The data included all registered live births in Florida between January 1, 2005 and December 31, 2008 (n=917,788). Women with residential addresses outside Florida (n=4,632) were excluded. Mother’s residential address at delivery was initially geocoded by Florida Department of Health using ArcGIS v10.1, and 864,247 records (94.6%) were successfully geocoded. We further geocoded the addresses that failed to be geocoded by DOH using the Google Maps API (Application Programming Interface) by “ggmap” package in R, and a total of 913,048 records (99.9%) were successfully geocoded. Women whose residential address could not be geocoded after the second phase were excluded (n=108). To avoid fixed cohort bias (Strand et al., 2011), women were included based on their conception date instead of delivery date. Conception date was back calculated using delivery date and gestational age which was mainly determined by ultrasound. When ultrasound data was not available, clinical examination or last menstrual period was used to estimate gestational age. Among the 913,048 women who delivered during 2005–2008, a total of 691,011 women had the conception date between January 1, 2005 and December 31, 2007. In addition, women were excluded if they had non-singleton deliveries (n=21,609) or pre-pregnancy hypertension (n=10,590). Women whose births had a birthweight <500 g or >5000 g (n=621), or with a gestational age <26 weeks (n=2,662) were also excluded, leaving a total of 655,529 women in the final analyses.
Outcome assessment
During the collection of Vital Statistics Birth Record data, medical history of each woman was recorded, and diagnoses of pre-pregnancy hypertension, gestational hypertension or preeclampsia, and eclampsia were abstracted for analyses. Gestational hypertension was determined as onset of hypertension after 20 weeks of pregnancy, and preeclampsia was defined as the new onset of hypertension and proteinuria after 20 weeks of gestation. Eclampsia was determined by the presence of preeclampsia in addition to the onset of convulsions. Similar to previous environmental studies on HDP (Hu et al., 2014), the restricted definition of HDP was used, which included gestational hypertension, preeclampsia, or eclampsia. We assessed them aggregately as HDP for two reasons: 1) we were not able to distinguish between gestational hypertension and preeclampsia since the Florida Vital Statistics Birth Record data aggregated them together; and 2) potential differences in disease coding and diagnosis may exist since increased gestational hypertension and reduced mild preeclampsia and eclampsia are compensated more (Savitz et al., 2015).
Ozone exposure assessment
O3 data were obtained from the EPA and CDC’s National Environmental Public Health Tracking Network (U.S. EPA, 2014), which were estimated using the hierarchical Bayesian space-time statistical model (HBM) during 2001–2008 with a daily temporal resolution and a spatial resolution of 12km×12km across the continental areas in the US (McMillan et al., 2010). The HBM approach combines the Air Quality System monitoring data with the Community Multiscale Air Quality modeled data, which includes emission, meteorology, and chemical modeling components, to predict air quality data for a specific time period and spatial scale (McMillan et al., 2010). Each woman’s geocoded residential address at the time of delivery was spatially linked to the corresponding grid of the HBM data. Exposures were calculated as daily concentrations averaged over each of the first two trimesters (trimester 1: 1–13 weeks and trimester 2: 14–26 weeks) and over both the first and second trimesters (1–26 weeks) determined by gestational age and delivery date of each woman. In addition, weekly average levels of O3 exposure during the first and second trimesters were calculated to assess the critical windows of exposure during pregnancy.
Covariates
Information on maternal characteristics such as age, race/ethnicity, education, marital status, pregnancy smoking status, pre-pregnancy body mass index (BMI), pre-pregnancy diabetes, parity, season and year of conception were obtained directly from the births records. Maternal age at delivery was categorized into six groups, with 5-year increments for women aged 20–40 years old as well as two additional groups for <20 and ≥40 years old. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, and others. In addition, dichotomous variables were used to indicate marital status and pregnancy smoking status. Maternal education was divided into three categories: <high school, high school or equivalent, and >high school. Pre-pregnancy BMI was categorized into four groups: underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30.0). Parity was categorized into three groups: no previous live birth, one previous live birth, and more than one previous live birth. Fine particulate matter (PM2.5) data was obtained from EPA and CDC’s National Environmental Public Health Tracking Network, and they were spatiotemporally linked to participants based on their geocoded residential addresses at the time of delivery. Season [warm (June–November) or cool (December–May)] and year (2005, 2006, or 2007) of conception were also treated as categorical variables.
Statistical analysis
Distribution of categorical covariates and continuous exposures between women with HDP and those without HDP were examined. First, we conducted logistic regression models to investigate the association between O3 exposure during the three predefined gestational windows (i.e. trimester 1, trimester 2, and trimesters 1&2) and odds of HDP. In addition to analyzing the level of O3 exposure as a continuous variable, we also analyzed it as a categorical (i.e. quartiles) variable to assess the potential nonlinear associations. Both an unadjusted model and an adjusted model controlling for maternal age, race/ethnicity, education, marital status, pregnancy smoking, pre-pregnancy BMI, season and year of conception were used. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained for each 5 ppb increase in average O3 concentration during each specific pregnancy window (Šrám et al., 2005). Second, we used constrained distributed lag models with the “dlnm” package in R to identify potential critical exposure windows. The associations between HDP and each of the 26 weekly O3 exposures was examined controlling for O3 exposures during other weeks. Similar to the study by Darrow et al. (2011), we assumed an underlying cubic structure, and we constrained the 26 weekly specific effect estimates to follow the shape of a natural cubic spline with a knot at lag 13 to reduce the influences of collinearity on the estimates. All statistical analyses were conducted using R 3.2.2.
Sensitivity analysis
Sensitivity analyses were conducted to test the robustness of our results. Firstly, to account for the correlations at census tract level, we further fitted mixed-effects models with random intercept for each census tract. Then we made comparisons between the results from the sensitivity analyses and our original results to check whether the potential correlations at census tract level have influenced the observed effects. Secondly, to assess the potential confounding effects of pre-pregnancy diabetes, parity, and PM2.5 exposure, we further fitted models additionally adjusting for these factors. Thirdly, to account for the fact that the same woman may be included in the analyses more than once during the study period, we restricted our analyses only on first time mothers and compared the results with the original findings.
RESULTS
Among the 655,529 women included in the analyses, 31,362 (4.8%) women had HDP. A total of 613,032 women including 29,286 HDP cases had complete data for all covariates. Table 1 shows the maternal characteristics by HDP status. Women with HDP were less likely than those without HDP to be between 25 to 34 years old, and more likely to belong to non-Hispanic Black racial/ethnic categories. Compared with women without HDP, HDP cases were less likely to be married and to have smoked during pregnancy. In addition, HDP cases had higher pre-pregnancy BMI than women without HDP.
Table 1.
Maternal characteristics by HDP status among women with conception date during 2005–2007 in Florida, USA.
| Maternal Characteristics | HDP (n=31,362) n (%) | No HDP (n=624,167) n (%) | Total (n=655,529) n (%) | |
|---|---|---|---|---|
| Maternal age (years) | ||||
| <20 | 4,089 (13.0) | 67,388 (10.8) | 71,477 (10.9) | |
| 20–24 | 8,325 (26.5) | 164,444 (26.4) | 172,769 (26.4) | |
| 25–29 | 7,973 (25.4) | 170,781 (27.4) | 178,754 (27.3) | |
| 30–34 | 6,244 (19.9) | 134,271 (21.5) | 140,515 (21.4) | |
| 35–39 | 3,678 (11.7) | 71,101 (11.4) | 74,779 (11.4) | |
| ≥40 | 1,053 (3.4) | 16,156 (2.6) | 17,209 (2.6) | |
| Missing | 0 (0.0) | 26 (0.0) | 26 (0.0) | |
| Race/ethnicity | ||||
| Non-Hispanic White | 14,634 (46.7) | 279,996 (44.9) | 294,630 (45.0) | |
| Non-Hispanic Black | 7,426 (23.7) | 108,974 (17.5) | 116,400 (17.8) | |
| Hispanic | 1,924 (6.1) | 4,9681 (8.0) | 51,605 (7.9) | |
| Others | 7,378 (23.5) | 185,502 (29.7) | 192,880 (29.4) | |
| Missing | 0 (0.0) | 14 (0.0) | 14 (0.0) | |
| Maternal education | ||||
| <High school | 6,133 (19.6) | 128,415 (20.6) | 134,548 (20.5) | |
| High school or equivalent | 10,227 (32.6) | 194,102 (31.1) | 204,329 (31.2) | |
| >High school | 14,853 (47.4) | 298,307 (47.8) | 313,160 (47.8) | |
| Missing | 149 (0.5) | 3,343 (0.5) | 3,492 (0.5) | |
| Marital status | ||||
| Married | 16,159 (51.5) | 343,198 (55.0) | 359,357 (54.8) | |
| Not married | 15,199 (48.5) | 280,873 (45.0) | 296,072 (45.2) | |
| Missing | 4 (0.0) | 96 (0.0) | 100 (0.0) | |
| Smoking during pregnancy | ||||
| No | 28,916 (92.2) | 570,831 (91.5) | 599,747 (91.5) | |
| Yes | 2,419 (7.7) | 52,887 (8.5) | 55,306 (8.4) | |
| Missing | 27 (0.1) | 449 (0.1) | 476 (0.1) | |
| Pre-pregnancy BMI | ||||
| Underweight (<18.5) | 776 (2.5) | 31,191 (5.0) | 31,967 (4.9) | |
| Normal (18.5–24.9) | 10,589 (33.8) | 305,043 (48.9) | 315,632 (48.2) | |
| Overweight (25.0–29.9) | 7,942 (25.3) | 141,831 (22.7) | 149,773 (22.9) | |
| Obese (≥30.0) | 10,093 (32.2) | 108,143 (17.3) | 118,236 (18.0) | |
| Missing | 1,962 (6.3) | 37,959 (6.1) | 3,9921 (6.1) | |
| Season of conception | ||||
| Warm | 15,327 (48.9) | 306,634 (49.1) | 321,961 (49.1) | |
| Cool | 16,035 (51.1) | 317,533 (50.9) | 333,568 (50.9) | |
| Year of conception | ||||
| 2005 | 10,077 (32.1) | 205,628 (32.9) | 215,705 (32.9) | |
| 2006 | 10,611 (33.8) | 212,402 (34.0) | 223,013 (34.0) | |
| 2007 | 10,674 (34.0) | 206,137 (33.0) | 216,811 (33.1) |
Abbreviations: BMI, body mass index; HDP, hypertensive disorders of pregnancy.
Table 2 shows the distribution of exposures to O3 during the first two trimesters of pregnancy. Women with HDP had a higher exposure to O3 compared with those without HDP during the three pre-defined exposure windows (p<0.001 for each comparison).
Table 2.
O3 exposure by HDP status among women with conception date during 2005–2007 in Florida, USA (n=31,362 with HDP, n=624,167 without HDP, and total n= 655,529).
| Exposure | Trimester 1 | Trimester 2 | Trimesters 1 and 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statistics | HDP | No HDP | Total | HDP | No HDP | Total | HDP | No HDP | Total | |
| O3 (ppb) | ||||||||||
| Mean±SD | 39.07±6.64 | 38.63±6.76 | 38.65±6.76 | 39.02±6.66 | 38.57±6.69 | 38.59±6.69 | 39.06±5.20 | 38.61±5.33 | 38.63±5.33 | |
| Median | 38.39 | 37.89 | 37.91 | 38.29 | 37.81 | 37.84 | 38.84 | 38.39 | 38.42 | |
| IQR | 9.37 | 9.41 | 9.41 | 9.52 | 9.37 | 9.38 | 7.52 | 7.82 | 7.80 | |
Abbreviations: HDP, hypertensive disorders of pregnancy; IQR, interquartile range; O3, ozone; ppb, parts per billion; SD, standard deviation.
Table 3 shows the unadjusted and adjusted ORs from the logistic regression models. Positive associations between HDP and O3 exposure were consistently observed in all models and exposure windows with evidence of a dose response relationship. In the adjusted model, increased odds of HDP for each 5 ppb increase in O3 exposure were observed during all the three exposure windows (ORTrimester1=1.04, 95% CI: 1.03, 1.06; ORTrimester2=1.03, 95% CI: 1.02, 1.04; ORTrimester1&2=1.07, 95% CI: 1.05, 1.08). Consistent results were observed in the analyses when O3 exposure was categorized. Compared with women exposed to the lowest quartile of O3, women with O3 exposure in the other quartiles had higher odds of HDP. Figure 1 displays adjusted ORs of HDP for each 5 ppb increase in weekly exposure to O3 during the first two trimesters of pregnancy (first 26 weeks). Consistent with the previous logistic regression models, the results from the distributed lag models also showed positive associations between HDP and O3 exposure across the first 24 weeks of gestation, with higher odds of HDP observed in early pregnancy.
Table 3.
Association between O3 and HDP by pregnancy period of exposure among women with conception date during 2005–2007 in Florida, USA.
| O3 Exposure | Unadjusted Model | Adjusted Modela | ||
|---|---|---|---|---|
| n (HDP/Total) | OR (95% CI) | n (HDP/Total)b | OR (95% CI) | |
| Trimester 1 | ||||
| Continuous (each 5 ppb increase) | 31,362/655,529 | 1.05(1.04, 1.06) | 29,286/613,032 | 1.04(1.03, 1.06) |
| Quartile 1 (21.2 – 33.7 ppb) | 6,841/163,885 | Reference | 6,182/149,297 | Reference |
| Quartile 2 (33.8 – 37.9 ppb) | 8,000/163,883 | 1.18(1.14, 1.22) | 7,528/154,680 | 1.13(1.09, 1.17) |
| Quartile 3 (38.0 – 43.1 ppb) | 8,174/163,879 | 1.21(1.17, 1.25) | 7,706/153,717 | 1.18(1.14, 1.23) |
| Quartile 4 (43.2 – 57.4 ppb) | 8,347/163,882 | 1.23(1.19, 1.27) | 7,870/155,338 | 1.19(1.15, 1.25) |
| Trimester 2 | ||||
| Continuous (each 5 ppb increase) | 31,362/655,529 | 1.05(1.04, 1.06) | 29,286/613,032 | 1.03(1.02, 1.04) |
| Quartile 1 (21.2 – 33.6 ppb) | 6,996/163,891 | Reference | 6,288/148,506 | Reference |
| Quartile 2 (33.7 – 37.8 ppb) | 7,973/163,874 | 1.15(1.11, 1.19) | 7,520/154,514 | 1.09(1.06, 1.13) |
| Quartile 3 (37.9 – 43.0 ppb) | 7,915/163,883 | 1.14(1.10, 1.18) | 7,422/154,363 | 1.08(1.04, 1.12) |
| Quartile 4 (43.1 – 57.4 ppb) | 8,478/163,881 | 1.22(1.18, 1.26) | 8,056/155,649 | 1.14(1.10, 1.18) |
| Trimesters 1&2 | ||||
| Continuous (each 5 ppb increase) | 31,362/655,529 | 1.08(1.07, 1.09) | 29,286/613,032 | 1.07(1.05, 1.08) |
| Quartile 1 (23.2 – 34.8 ppb) | 6,748/163,883 | Reference | 6,036/148,205 | Reference |
| Quartile 2 (34.9 – 38.4 ppb) | 7,859/163,882 | 1.17(1.13, 1.21) | 7,369/153,480 | 1.13(1.09, 1.15) |
| Quartile 3 (38.5 – 42.6 ppb) | 8,333/163,883 | 1.25(1.21, 1.29) | 7,916/155,640 | 1.19(1.15, 1.17) |
| Quartile 4 (42.7 – 53.1 ppb) | 8,422/163,881 | 1.26(1.22, 1.30) | 7,965/155,707 | 1.20(1.23, 1.25) |
Abbreviations: CI, confidence interval; HDP, hypertensive disorders of pregnancy; O3, ozone; OR, odds ratio; ppb, parts per billion
Adjusted for maternal age, race, education, marital status, pregnancy smoking status, pre-pregnancy BMI, season of conception, and year of conception.
Women with complete data for all covariates.
Figure 1.
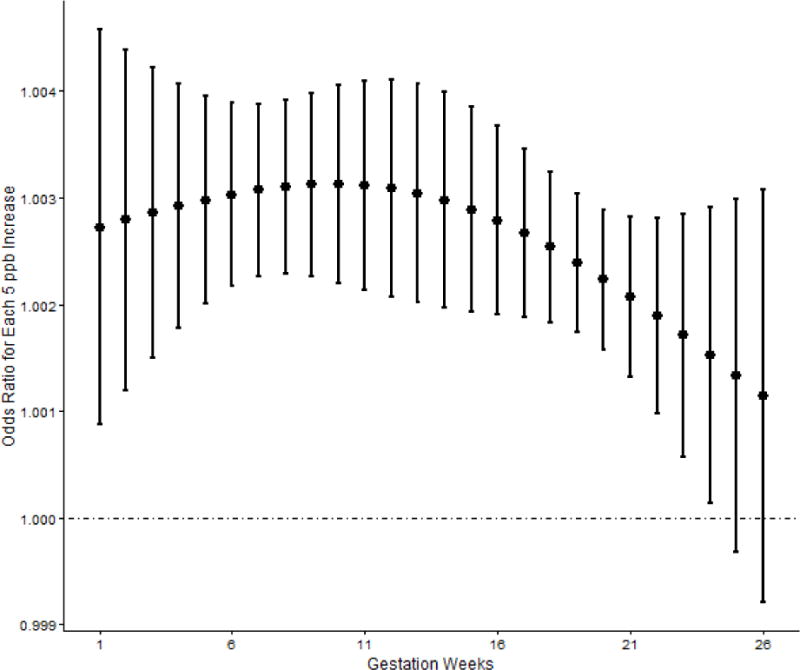
Adjusted Odds Ratios for HDP with Each 5 ppb Increase in Weekly O3 Exposure
Supplemental Table 1 shows the comparisons between the original results using fixed-effects model and the results of the sensitivity analyses using the mixed-effects model. Consistent results were observed in the mixed-effects model with intraclass correlation coefficients (ICCs) ranging from 0.059 to 0.063, indicating minimal clustering effects. Supplemental Table 2 shows the comparisons between the original analyses and the sensitivity analyses which additionally adjusted for pre-pregnancy diabetes, parity, and PM2.5 exposure. No significant difference was observed, suggesting these potential confounders had minimal impacts on the effect estimates. Supplemental Table 3 shows the adjusted ORs for PM2.5 exposure in the sensitivity analyses, and no significant association was observed. Supplemental Table 4 illustrates the comparisons between the original analyses on all participants and the sensitivity analyses restricted to first time mothers. The consistent results observed indicate a minimal impact by the potential inclusion of some women more than once in the study.
DISCUSSION
In this retrospective cohort study, we found a consistent pattern of elevated odds of HDP with increased exposure to O3 during the first two trimesters. Such associations persisted with adjustment for confounders including maternal age, race/ethnicity, education, marital status, pregnancy smoking, pre-pregnancy BMI, season and year of conception. The distributed lag models showed that early pregnancy was the most critical window for O3 exposure during pregnancy. The results of this study add to the emerging evidence linking O3 to HDP.
The observed association between HDP and exposure to O3 in early pregnancy is consistent with other studies. Michikawa et al. (2015) reported an elevated odds of HDP among women with O3 exposure during the first trimester in the highest quintile compared with those in the lowest quintile (OR=1.20, 95% CI: 1.01, 1.42), and similar effect estimates were observed in our study. Our previous meta-analyses on O3 and HDP found that exposure to O3 during the first trimester was associated with increased odds of HDP (OR=1.05, 95% CI: 1.02, 1.06 for each 5 ppb increase in O3), while no significant association was observed for O3 exposure during the second trimester (Hu et al., 2014). In this study, we observed a slightly larger effect size of O3 on HDP during the first trimester as well as a significant association during the second trimester. This is expected since previous studies assessed O3 exposure using data from stationary monitors, which may have been subject to non-differential exposure misclassification that biased the effect towards null.
The biological mechanisms underlying O3 and HDP have been suggested to be multifaceted. Evidence from animal and toxicological experiments shows that physiologic responses to environmental factors are associated with hypertension (Nagiah et al., 2015). Inhalation of air pollutants has been found to increase oxidative stress, lipid peroxidation and inflammation level among pregnant women (Ghio et al., 2012; Lee et al., 2011; Nagiah et al., 2015; Slama et al., 2008). These inflammatory responses have been known to increase the risk of hypertension (Rodrigo et al., 2011), and they may also lead to endothelial dysfunction, autonomic imbalance, and altered blood rheology (Bind et al., 2012; Brook et al., 2004; Huang et al., 2012; Nodari et al., 2006), all of which can increase the risk of hypertension. Blood pressure levels of women without HDP usually fall during the first trimester, and then gradually increase to the pre-pregnancy level after reaching the lowest point in mid-pregnancy (Ayala et al., 1997; Hermida et al., 2001). However, a different blood pressure pattern during pregnancy has been observed among women with HDP (Hermida et al., 2001). Instead of the fall during first trimester, HDP cases have stable blood pressure levels during the first half of pregnancy and then continuously increases until delivery. The differences in these blood pressure patterns suggest that HDP usually develops at an early stage of pregnancy, which are consistent with the elevated effect size observed in this study. Vasoconstriction has been regarded as one of the mechanisms underlying HDP, and previous studies found that vasoconstriction in HDP develops early in pregnancy (O’Brien, 1990; Orpana et al., 1996). Given O3’s ability to induce acute vasoconstriction observed in the general population (Brook et al., 2002), it is plausible that vasoconstriction may be the mechanism underlying the observed higher odds in early pregnancy period.
The findings from our study have important implications for future health interventions. It is almost impossible to avoid air pollution exposure during one’s whole pregnancy period, but knowing the critical exposure windows is extremely helpful for targeted intervention. Future studies are needed to examine the potential use of emerging technologies to reduce risks of HDP, such as the wearable environment trackers and indoor air purifiers (Chen et al., 2015). Our study has several strengths. First, the sample size is large, and a high geocoded rate ensured high generalizability of findings to the state of Florida. Second, the HBM air pollution data covered the whole study area with a daily temporal resolution and a 12km×12km spatial resolution. This high temporal and spatial coverage allowed us to include all participants regardless of the distance to air monitors. Third, the use of the distributed lag models to assess critical exposure windows in addition to investigating the association in the predefined exposure windows is novel.
Several limitations need to be noted. First, the diagnosis dates of HDP were not available for analysis. Future studies with more detailed data on pregnancy outcomes and diagnoses time are needed to better understand the effects of O3 on different adverse pregnancy and birth outcomes by using more accurate exposure windows and comparing competing risks on outcomes. Second, instead of investigating gestational hypertension, preeclampsia, or eclampsia separately, we assessed them together as HDP because of the potential differences in disease coding and diagnosis. The variation in HDP coding has been suggested to be an issue since increased gestational hypertension and reduced mild preeclampsia and eclampsia are compensated more (Savitz et al., 2015). Third, it is possible that HDP may be underdiagnosed in the vital statistics records data. However, this misclassification is likely to be non-differential (Hu et al., 2015). Fourth, although we adjusted for PM2.5 in the sensitivity analyses, we were not able to include other pollutants such as NO2 and CO since they were not available in the EPA’s HBM dataset. Furthermore, we assessed O3 exposure based on women’s residential addresses at delivery, while information on residential history, daily mobility, and behavior patterns were not available, which may introduce misclassifications of exposure. Future studies with improved exposure assessments are needed to better quantify the effects of O3 on HDP.
CONCLUSION
In conclusion, using Florida birth vital statistics records, we found that exposure to O3 during pregnancy was associated with increased odds of HDP, with stronger association noted during early pregnancy periods.
Supplementary Material
Highlights.
O3 exposure during the first two trimesters is associated with HDP.
Such associations persisted with adjustment for confounders.
Distributed lag models show that early pregnancy is a critical window of exposure.
Acknowledgments
This work was supported by Grant Number K01ES019177 from the National Institute of Environmental Health Sciences (NIEHS), and was partially funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health. The data were provided by the Bureau of Vital Statistics, Florida Department of Health (DOH). All conclusions are the authors’ own and do not necessarily reflect the opinion of the NIEHS or the Florida DOH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors disclose that they have no actual or potential competing financial interests.
References
- Allen VM, et al. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Ayala DE, et al. Blood pressure variability during gestation in healthy and complicated pregnancies. Hypertension. 1997;30:611–618. doi: 10.1161/01.hyp.30.3.611. [DOI] [PubMed] [Google Scholar]
- Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Semin Perinatol. 2009;33:158–65. doi: 10.1053/j.semperi.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Bellamy L, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–40. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Chen R, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. Journal of the American College of Cardiology. 2015;65:2279–2287. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–72. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, et al. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environmental health perspectives. 2011;119:731. doi: 10.1289/ehp.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61:578–84. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Fenzl V, et al. Trace elements and oxidative stress in hypertensive disorders of pregnancy. Archives of gynecology and obstetrics. 2013;287:19–24. doi: 10.1007/s00404-012-2502-4. [DOI] [PubMed] [Google Scholar]
- Florida Department of Environmental Protection. Florida’s Ozone and Particulate Matter Air Quality Trends. 2012 [Google Scholar]
- Foraster M, et al. Association of long-term exposure to traffic-related air pollution with blood pressure and hypertension in an adult population-based cohort in Spain (the REGICOR study) Environ Health Perspect. 2014;122:404–11. doi: 10.1289/ehp.1306497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, et al. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida RC, et al. Predictable blood pressure variability in healthy and complicated pregnancies. Hypertension. 2001;38:736–741. doi: 10.1161/01.hyp.38.3.736. [DOI] [PubMed] [Google Scholar]
- Hu H, et al. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environmental health perspectives. 2015;123:853. doi: 10.1289/ehp.1408456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, et al. Ambient air pollution and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Atmospheric Environment. 2014;97:336–345. doi: 10.1016/j.atmosenv.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol. 2012;176:117–26. doi: 10.1093/aje/kwr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, et al. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22:524–31. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, et al. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25:124–32. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- Malmqvist E, et al. Gestational diabetes and preeclampsia in association with air pollution at levels below current air quality guidelines. Environmental health perspectives. 2013;121:488–493. doi: 10.1289/ehp.1205736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan N, et al. Combining numerical model output and particulate data using Bayesian space–time modeling. Environmetrics. 2010;21:48–65. [Google Scholar]
- Michikawa T, et al. A register-based study of the association between air pollutants and hypertensive disorders in pregnancy among the Japanese population. Environmental research. 2015;142:644–650. doi: 10.1016/j.envres.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Miller MA, Carpenter M. Medical Management of the Pregnant Patient. Springer; 2015. Hypertensive Disorders of Pregnancy; pp. 177–193. [Google Scholar]
- Mobasher Z, et al. Associations between ambient air pollution and Hypertensive Disorders of Pregnancy. Environ Res. 2013;123:9–16. doi: 10.1016/j.envres.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah S, et al. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum Exp Toxicol. 2015;34:838–47. doi: 10.1177/0960327114559992. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Vol. 183. American journal of obstetrics and gynecology; 2000. pp. s1–s22. [PubMed] [Google Scholar]
- Nodari S, et al. Endothelial damage due to air pollution. Heart Int. 2006;2:115. doi: 10.4081/hi.2006.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WF. Predicting preeclampsia. Obstetrics & Gynecology. 1990;75:445–452. [PubMed] [Google Scholar]
- Olsson D, et al. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpana AK, et al. The calcium-dependent nitric oxide production of human vascular endothelial cells in preeclampsia. American journal of obstetrics and gynecology. 1996;174:1056–1060. doi: 10.1016/s0002-9378(96)70350-2. [DOI] [PubMed] [Google Scholar]
- Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiology clinics. 2012;30:317–329. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Pedersen M, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, et al. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- Romero R, et al. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Aranguren LC, et al. Endothelial dysfunction and preeclampsia: role of oxidative stress. Frontiers in physiology. 2014;5:372. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, et al. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Hypertensive Disorders of Pregnancy in New York City. Epidemiology. 2015;26:748–757. doi: 10.1097/EDE.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–8. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šrám RJ, et al. Ambient air pollution and pregnancy outcomes: a review of the literature. Environmental health perspectives. 2005:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand LB, et al. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. doi: 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Air Quality Data for the CDC National Environmental Public Health Tracking Network. 2014 [Google Scholar]
- van den Hooven EH, et al. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: the generation R study. Hypertension. 2011;57:406–12. doi: 10.1161/HYPERTENSIONAHA.110.164087. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, et al. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environ Health. 2009;8:59. doi: 10.1186/1476-069X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, et al. The effects of exposure to particulate matter and neighbourhood deprivation on gestational hypertension. Paediatr Perinat Epidemiol. 2012;26:91–100. doi: 10.1111/j.1365-3016.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- Wallis AB, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- Wang IK, et al. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: a retrospective cohort study. Am J Med. 2012;125:251–7. doi: 10.1016/j.amjmed.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Wolf M, et al. Differential risk of hypertensive disorders of pregnancy among Hispanic women. Journal of the American Society of Nephrology. 2004;15:1330–1338. doi: 10.1097/01.asn.0000125615.35046.59. [DOI] [PubMed] [Google Scholar]
- Wu CS, et al. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201:269 e1–269 e10. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- Xu X, et al. Ambient air pollution and hypertensive disorder of pregnancy. J Epidemiol Community Health. 2013 doi: 10.1136/jech-2013-202902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder SR, et al. Hypertension in pregnancy and women of childbearing age. Am J Med. 2009;122:890–5. doi: 10.1016/j.amjmed.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Zhai D, et al. Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. Am J Obstet Gynecol. 2012;207:57 e1–9. doi: 10.1016/j.ajog.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Zusterzeel P, et al. Ethene and other biomarkers of oxidative stress in hypertensive disorders of pregnancy. Hypertension in pregnancy. 2002;21:39–49. doi: 10.1081/PRG-120002908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


