Abstract
Background
Whether patient navigation improves outcomes in patients with comorbidities is unknown. Study aims were to determine the effect of comorbidities on time to diagnostic resolution following an abnormal cancer screening test, and to examine for patients with comorbidities, if patient navigation improves timeliness and likelihood of diagnostic resolution compared to patients without navigation.
Methods
A secondary analysis from the Patient Navigation Research Program sites that collected comorbidity data using the Charlson Comorbidity Index (CCI) was conducted. Participants were 6,349 patients with abnormal breast, cervical, colon or prostate cancer screening tests between 2007 and 2011. The intervention was patient navigation or usual care.
CCI data were highly skewed across projects and cancer sites and were categorized as 0, no comorbidities identified, CCI score of 0 (76% of cases); 1, CCI score of 1 (16% of cases); or 2, CCI score of ≥2 (8% of cases). A separate adjusted hazards ratio for each site and cancer type was obtained, and then pooled using meta-analysis random effects methodology.
Results
Having a CCI score of ≥2 delayed the time to diagnostic resolution following an abnormal cancer screening test compared with those with fewer than one comorbidity. Patient Navigation reduced delays in diagnostic resolution with the greatest benefit seen in those with a CCI score of ≥2.
Conclusions
Persons with a CCI score of ≥2 experienced significant delays in timely diagnostic care compared to patients without comorbidities. Patient navigation was effective in reducing delays in diagnostic resolution among those with CCI scores > 1.
Keywords: comorbidity, cancer screening, patient navigation
Background
A relationship between severity of comorbidities and outcomes after a cancer diagnosis has been found for several cancer sites, including breast, prostate and colon cancers. (1–4) One possible contributor to poor cancer outcomes in those with comorbidities is the delay in achieving diagnosis or treatment, owing to the need to manage and address other comorbid conditions. Concurrent comorbidities have the greatest impact on survival in cancers for which the overall mortality is lower, suggesting that comorbidities have a negative impact for those cancers where timely treatment may be of the greatest benefit. (5) Whether interventions that increase timely completion of screening, diagnostic evaluation, or treatment of cancer will improve outcomes in patients with comorbidities is unknown.
Patient Navigation (PN) has been shown to be effective in reducing delays and loss to follow up after an abnormal breast, cervical or colorectal cancer screening test in the Patient Navigation Research Program (PNRP). (6–12) PN focuses on identifying and reducing barriers to care that patients experience as they navigate the health care system. Nothing is known about whether patient navigation will improve the timeliness of diagnostic care following an abnormal cancer screening among patients with comorbidities. It is possible that individuals with comorbidities may have longer times to diagnostic resolution following an abnormal cancer screening test than those with no comorbidities and that patient navigation can support patients who require coordination of diagnostic testing with chronic disease management.
Objective
The purpose of our study was to analyze the effect of comorbidities, defined as the coexistence of chronic diseases, as measured by the Charlson Comorbidity Index (CCI), on time to diagnostic resolution following an abnormal cancer screening using data from PNRP. We examined whether patient navigation was effective in improving timeliness and likelihood of diagnostic resolution compared to participants who do not receive patient navigation. Two main hypotheses were explored: 1) Patients with increased severity in comorbidities will have longer times to diagnostic resolution (and smaller proportion resolved) than patients with no or less severe comorbidities, and 2) Navigation will significantly reduce time to diagnostic resolution (or increase percent resolved) among persons with more severe comorbidities and this effect will be greater than for patients with less severe or no comorbidity (interaction effect).
Design
We conducted a secondary analysis of data from four of the ten PNRP sites (Boston, Denver San Antonio and Tampa) that collected comorbidity data from PNRP participants, using the Charlson Comorbidity Index (CCI), a valid prognostic indicator for one year mortality, which measures and classifies comorbidities. (13, 14)
The PNRP was a cooperative effort of ten United States health care organizations serving primarily medically underserved populations, (6, 7) conducted between 2007–2011 and including over 10,500 participants. The PNRP investigated whether patient navigation reduced the time to diagnostic resolution following an abnormal screening for breast, cervical, colorectal and prostate cancers. Participants were censored at 365 days if they did not reach resolution. Each site allocated patients to either a usual care (control arm), or a patient navigation arm. The institutional review board of each organization approved the research.
Patient Navigators assisted patients in identifying barriers to care, developed strategies to address these barriers, and tracked participants through the steps in their medical evaluation. The patient navigators focused on supporting participants to timely diagnostic resolution. Navigation was initiated after a clinician informed the participant of the abnormal test result. Contacts between Patient Navigators and patients were face to face interactions, as well as telephone and mail contact. Patient Navigators worked with families, health care providers, and social service agencies to identify resources to address barriers to care. Patient navigators identified barriers from a pre-specified list of 21 barriers, and then coded activities they undertook to ameliorate these barriers. Examples of barriers to care and associated navigation activities included financial barriers and arranging financial support, language barriers and arranging for interpreter services, and lack of social support and linking to community resources. (15, 16) Patient navigators were specifically trained to support coordination and scheduling of needed appointments, including optimizing care of comorbid conditions.
Each site hired patient navigators with a minimum of a high school diploma. Navigators participated in annual national trainings and webinars in order to standardize the intervention and were assessed for national core competencies twice annually using a standardized checklist. (17)
Main Measures
Four PNRP sites collected comorbidity data for both treatment and control groups, yielding a total of 6,349 cases. Two sites (Boston and Denver) used the CCI macro code using ICD-9-CM. This program reads through the diagnosis codes of patient records and identifies whether the record belongs to one or more of different CCI groups. (18) Two sites (San Antonio and Tampa) collected comorbidity data from patient self-report and medical records. (11, 12) Cases that were missing a CCI score were excluded.
A prior diagnosis of leukemia, lymphoma and metastatic solid tumor were exclusion criteria for inclusion into the trial and therefore not included when calculating CCI score. Patients with all other comorbidities that make up the CCI score were included. Because CCI score data were highly skewed, a consistent finding across projects and across cancer screening sites, the weighted CCI scores were categorized as 0, CCI score of 0 or no comorbidities identified, (76% of cases); 1, CCI score of 1 (16% of cases); or 2, CCI score of 2 or greater (8% of cases).
Our primary measure of interest was time to diagnostic resolution, defined as the time from the date of the initial screening abnormality to the date when the final definitive diagnostic test or evaluation was completed. Participants without resolution of their screening abnormality were censored at 365 days. Based upon our prior work (7), our time to event analyses violated rules of proportionality across the one year timeframe. To address this, we divided our time frame and examined two timeframes to assess percent of patients achieving resolution – within 90 days as an indicator of early or timely resolution, and within 365 days as the end of the follow-up period for PNRP. Separate logistic regression analyses were performed for the 90 day and 365 day time periods using the same covariates as were used in the Cox regressions. The adjusted Odds-ratios (aOR) of achieving diagnostic resolution were obtained comparing patients with categorized CCI scores of 1 versus 0, and scores of 2+ versus 0. For each time period, a random-effects meta-analysis was performed separately for comorbidity scores of one and greater than one.
Analysis
Cox proportional hazards regression (Cox PH) was conducted for all PNRP centers/cancer site combinations to obtain estimates of the effect of comorbidity classification on time to diagnostic resolution after an abnormal finding on cancer screening. Comorbidity classification was the primary variable of interest and was included as an indicator variable in the analysis. Intervention and the interaction of comorbidity classification with intervention were included in the Cox PH regressions along with the primary patient-level covariates – race/ethnicity, insurance status, age, and marital status. (19)
We created a separate Cox PH regression for each site and each cancer screening type, for a total of 9 regressions. From each of the nine Cox PH regressions, an estimate of the effect of comorbidity on time to diagnostic resolution – an adjusted hazard rate ratio (aHR), was obtained. These nine estimates were then included in a meta-analysis to obtain a pooled estimate of the effect of comorbidities on time to resolution. An influence analysis (or sensitivity analysis) was performed, sequentially removing the effect of each project/cancer site estimate in turn, to determine if any observed pooled effect was unduly influenced by a single project/cancer site combination.
In addition to the measure of time to diagnostic resolution, logistic regression analyses were conducted on diagnostic resolution as a dichotomous variable. Two time points were chosen for analysis – percent resolved at 90 days and at 365 days, to capture a potential early effect of comorbidities on diagnostic resolution as well as a final follow-up time point of one year. The same covariates were included in the logistic regression analysis as in the Cox PH analyses. Similarly, the nine estimates (adjusted odds ratios, or aOR) of the effect of comorbidity on diagnostic resolution were included in a meta-analysis and subsequent influence analysis.
Lastly, data were pooled across the four sites that collected comorbidity data and a subgroup analysis was performed on the pooled data to estimate the effect of patient navigation on time to diagnostic resolution for patients with and without comorbidities
Results
CCI scores in the PNRP dataset ranged from 0–11, out of a possible 25. The distribution of navigated and control patients by PNRP centers and within cancer sites was sufficient for the purposes of this analyses (Table 1). Of 6,349 study participants, 3,134 were assigned to the control, or usual care arm and 3,215 were assigned to the navigation arm. Almost half (48 percent) of the participants for this analyses were from Boston, with Tampa contributing 20 percent of the cases and Denver and San Antonio contributing 16 percent each. Overall, 95 percent of the study participants were female and the mean age was 43.6 years. Almost half (47 percent) of the study participants were Hispanic, 28 percent were White and 20 percent African American. Only 19 percent of study participants had private health insurance, 46 percent had public health coverage and 35 percent reported being uninsured. The majority of participants (62 percent) had an abnormal screening for breast cancer, 29 percent had an abnormal screening for cervical cancer, 7 percent had an abnormal screening for colorectal cancer and 2 percent had an abnormal screening for prostate cancer.
Table 1.
Demographic characteristics by patient navigation arm: Patient Navigation Research Program
| Overall (N=6349) | Control Arm (N=3134) | Navigation Arm (N=3215) | |
|---|---|---|---|
| Demographic Characteristics
| |||
| Female sex, no. (%) | 6013 (95%) | 2941 (94%) | 3072 (96%) |
| Mean age—yr, mean (SD) | 43.6 (14.5) | 45.7 (14.6) | 41.6 (14.2) |
| Race/ethnicity, no. (%) | |||
| White | 1744 (28%) | 978 (32%) | 766 (24%) |
| African American | 1279 (20%) | 693 (22%) | 586 (18%) |
| Hispanic | 2981 (47%) | 1302 (42%) | 1679 (52%) |
| Other | 291 (5%) | 110 (4%) | 181 (6%) |
| Health insurance, no. (%) | |||
| Uninsured | 2217 (35%) | 895 (29%) | 1322 (41%) |
| Public | 2885 (46%) | 1515 (49%) | 1370 (43%) |
| Private | 1224 (19%) | 714 (23%) | 510 (16%) |
| Marital Status, no. (%) | |||
| Currently married | 2120 (35%) | 1037 (35%) | 1083 (35%) |
| Previously married | 1167 (19%) | 605 (20%) | 562 (18%) |
| Never married | 2805 (46%) | 1337 (45%) | 1468 (47%) |
| Cancer type, no. (%) | |||
| Breast | 3906 (62%) | 2052 (65%) | 1854 (58%) |
| Cervical | 1871 (29%) | 771 (25%) | 1100 (34%) |
| Colorectal | 445 (7%) | 246 (8%) | 199 (6%) |
| Prostate | 127 (2%) | 65 (2%) | 62 (2%) |
| Sites, no. (%) | |||
| A | 3039 (48%) | 1543 (49%) | 1496 (47%) |
| D | 999 (16%) | 509 (16%) | 490 (15%) |
| F | 1030 (16%) | 399 (13%) | 631 (20%) |
| G | 1281 (20%) | 683 (22%) | 598 (19%) |
In order to confirm that CCI was not a proxy measure for number of barriers, we examined the relationship of CCI score (0, 1, >1) with number of unique barriers (categorized as 0, 1, 2–3, 4+): Chi2 (6) = 6.27, p=.394, and determined that these two variables were assessing different constructs.
Time to Diagnostic Resolution
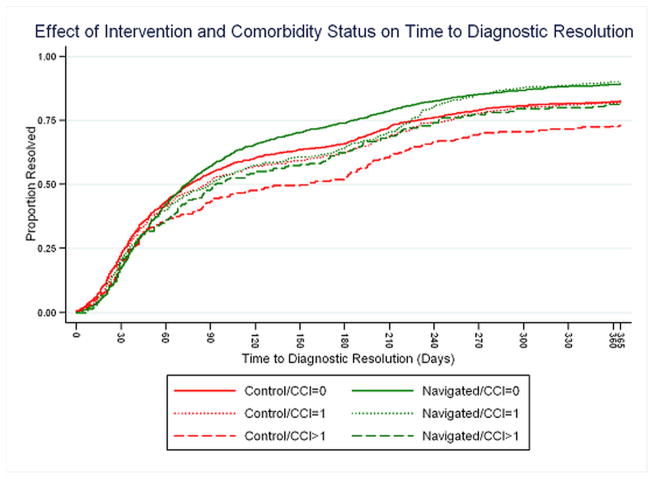
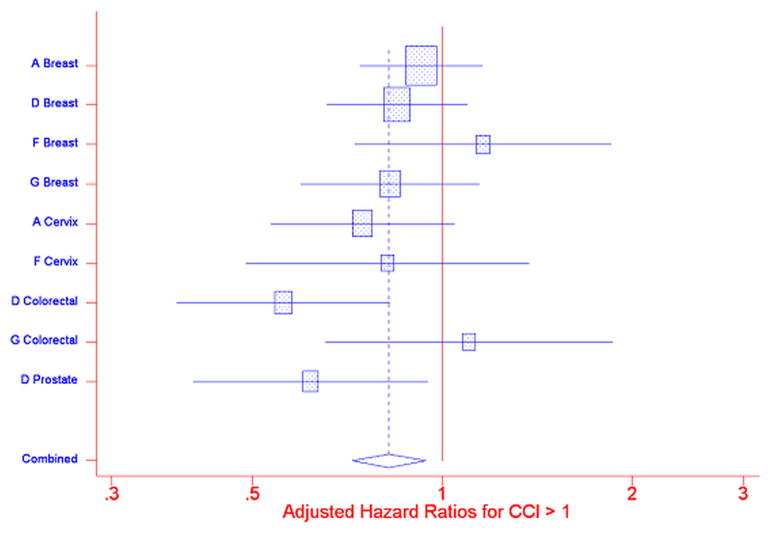
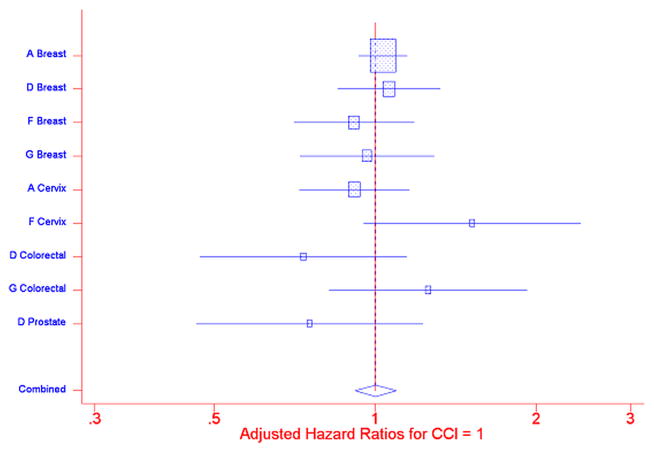
Individual Cox PH regressions were conducted for each of the PNRP/cancer site combinations; included in each analysis were Comorbidities scores, Intervention arm, interaction of Comorbidities score and Intervention arm, race/ethnicity, insurance status, age, marital status, and sex. The interaction of the Comorbidities score and Intervention arm was not statistically significant (Figure 1). Separate meta-analyses were performed for Comorbidity score = 1 and Comorbidity score >1, compared to Comorbidity score= 0, using a random effects model. (20) Figures 2: Meta-analysis (and Influence Analysis) for Adjusted Hazard Rate Ratios (aHRs) for CCI>1, and 3: Meta-analysis (and Influence Analysis) for Adjusted Hazard Rate Ratios (aHRs) for CCI=1 are forest plots by PNRP/cancer site, and include the combined result, to report the meta-analysis of the aHR of time to resolution of an abnormal screening result for each level of comorbidity, where an aHR less than one indicates a detrimental effect of the presence of comorbidities.
Figure 1.
Effect of Intervention and Comorbidity on Time to Diagnostic Resolution
Figure 2.
Meta-analysis (and Influence Analysis) for Adjusted Hazard Rate Ratios (aHRs) for CCI>1
Figure 3.
Meta-analysis (and Influence Analysis) for Adjusted Hazard Rate Ratios (aHRs) for CCI=1
Among patients without navigation, time to resolution of an abnormal cancer screening was similar for patients with a comorbidity score = 1 compared to patients with no reported comorbidities, combined aHR = 1.00 (95% CI 0.917, 1.095). For patients with a comorbidity score greater than one, however, the combined aHR was 0.82 (95% CI 0.721, 0.940) indicating that persons with more or more severe comorbidities obtained resolution of an abnormal finding on cancer screening at a slower rate than did patients with no comorbidities. The influence analysis indicated that the overall result was not unduly influenced by any one project/cancer type combination – the combined result remained statistically significant even with the removal of each project/cancer type combination.
We next conducted a meta-analysis to examine the impact of patient navigation on time to diagnostic resolution. The models included comorbidities as two dummy variables, and an interaction term for patient navigation and number of comorbidities. We then combined the effect size for each level of comorbidity across the nine cancer types and clinical sites. Persons with a CCI score >1 experienced significant delays in diagnostic resolution of an abnormal finding on cancer screening compared to those without comorbidities. None of the interaction terms were significant, indicating that navigation was not differentially effective depending on the level of comorbidities patients had.
Results of the analysis for the 90 day time period showed no differences in the odds of achieving diagnostic resolution for either patients with CCI scores of 1 or greater than 1, compared to patients with no comorbidities. However, at the end of one year, the results of the meta-analysis indicated that patients with CCI scores greater than 1 were less likely to achieve resolution of their abnormal finding than patients with no comorbidities – combined aOR = 0.722 (95% CI, 0.556, 0.938). An influence analysis of these data was performed and the results demonstrated that the removal of the Colorectal data resulted in a non significant combined result – aOR = 0.782 (95% CI, 0.593, 1.033).
Table 2 provides the results of the sub group analysis on the pooled data, using the control group with no comorbidity as the reference. In this adjusted analysis, patients with CCI scores greater than one had a significant delay (aHR=0.78, 95% CI 0.67, 0.91) compared to patients with no comorbidity. Those with navigation had more timely diagnostic resolution for each CCI score level. Time to diagnostic resolution for the navigated patients with no comorbidity was aHR = 1.11 (95% CI 1.04, 1.19). For navigated patients with a CCI score of one, the aHR was 1.16 (95% CI 1.04, 1.30), indicating that these groups reached diagnostic resolution more quickly than the control group with no comorbidities. For navigated patients with a CCI score of 2 or greater, the aHR was .99 (95%CI 0.86, 1.17), eliminating the delays in diagnostic care when compared to control patients with no comorbidities.
Table 2.
Adjusted hazard ratios for time to diagnostic resolution by patient navigation arm
Subgroup Analysis: National Patient Navigation Research Program
| Adjusted HR (95% C.I.) | P value | |
|---|---|---|
|
|
||
| Subgroup | ||
| Control/0 comorbidity | Ref. | |
| Control/1 comorbidity | 0.98 (0.88, 1.09) | 0.684 |
| Control/>1 comorbidity | 0.78 (0.67, 0.91) | 0.002 |
| Navigated/0 comorbidity | 1.11 (1.04, 1.19) | 0.002 |
| Navigated/1 comorbidity | 1.16 (1.04, 1.30) | 0.006 |
| Navigated/>1 comorbidity | 0.99 (0.86, 1.17) | 1.000 |
Model was adjusted for age, race/ethnicity, insurance, and marital status.
Discussion
The goal of our study was to explore the effect of comorbidities and patient navigation on time to diagnostic resolution following an abnormal cancer screening. Results of this study demonstrated that having more severe comorbidities delayed the time to diagnostic resolution compared to having either no or less severe comorbidities. Additionally, patients with more severe comorbidities (CCI score of 2 or greater) who did not achieve diagnostic resolution within 90 days were less likely to achieve diagnostic resolution within one year. Our second main finding was that patients with navigation had reduced times to diagnostic resolution at all levels of comorbidities, and that for patients with more severe comorbidities, navigation reduced disparities in timely care.
Patients with more severe or multiple comorbidities have a number of reasons why diagnostic care would be delayed. One may relate to stabilization of other medical conditions before more advanced diagnostic testing, especially colonoscopy or biopsy, could be performed. This stabilization of care often requires additional coordination among specialists, added office visits, possibly other tests or procedures, all of which can add to time to final diagnostic testing, and which may be insensitive to patient navigation. However, this additional coordination of care can also result in new opportunities of missed appointments and other delays due to barriers to care that are addressable through patient navigation. A number of studies have shown that patients with comorbidities have delays in cancer screening, diagnosis and time to treatment (21, 22, 23, 24), with some studies showing that these delays are associated with upstaging of cancer by the time of surgical treatment. (25)
Our study results demonstrated that patient navigation had a positive effect on timely diagnostic care for patients with comorbidities reported. Navigation was effective in reducing delays in diagnostic resolution among those with more severe comorbidities (CCI score greater than one) who had the longest delays in care. Since patient navigation was developed to improve coordination of care, patient navigation may be particularly effective for patients with comorbidities. A patient navigator may be specifically able to coordinate care across multiple specialties, ensure that appropriate clinical information is available to providers and thus reduce delays and gaps in care.
This study has several strengths including a large, diverse population of participants and the availability of CCI data from four sites. This study was also able to demonstrate that comorbidities were not a proxy for other barriers to care, such as transportation or health insurance. Limitations of the study include heterogeneity in study design and analyses across PNRP sites, including several methods for collecting comorbidity data. To address this heterogeneity, we conducted separate aHR for each site and each cancer type, and then pooled these using meta-analysis random effects methodology with a sensitivity analysis to ensure no undue influence of findings from one site. Using this methodology (26) demonstrates that the effect of comorbidities on timely completion of care was seen broadly across most sites and screening tests, and not related to study design. Another limitation is that most of the data reflects care to women with breast or cervical cancer screening abnormalities, and may not generalize to men or other cancer screening.
Conclusions
As patient navigation is becoming the standard of care for diagnostic and therapeutic cancer management, there is a need for sites to determine which patients are at highest risks for delays, and which patients will benefit most from the patient navigation intervention. (27) These findings are important for providers to consider when ordering diagnostic tests after abnormal cancer screening for patients with multiple, chronic diseases. Patients with comorbidities are more likely to have delays in their diagnostic care. Therefore, additional resources, including patient navigation with tracking to completion of diagnostic resolution are indicated for this population in order to address and prevent additional delays in care.
Acknowledgments
Funders: Supported by the National Cancer Institute, National Institutes of Health (U01 CA116892, U01CA117281, U01CA116903, U01CA116937, U01CA116924, U01CA116885, U01CA116875, and U01CA116925); the American Cancer Society (#SIRSG-05-253-01 and #CRP-12-219-01-CPPB); and the Avon Foundation.
Contributors: The authors acknowledge the contributions of the following members of the Patient Navigation Research Program:
Patient Navigation Research Program Investigators:
-
Clinical Centers
Boston Medical Center and Boston University: Karen M Freund (principal investigator (PI)) and Tracy A Battaglia (co-PI).
Denver Health and Hospital Authority: Peter C Raich (PI) and Elizabeth M Whitley (co-PI).
George Washington University Cancer Institute: Steven R Patierno (PI), Lisa M Alexander, Paul H Levine, Heather A Young, Heather J Hoffman, and Nancy L LaVerda.
H. Lee Moffitt Cancer Center and Research Institute: Richard G Roetzheim (PI), Cathy Meade, and Kristen J Wells.
Northwest Portland Area Indian Health Board: Victoria Warren-Mears (PI).
Northwestern University Robert H. Lurie Comprehensive Cancer Center: Steven Rosen (PI) and Melissa Simon.
Ohio State University: Electra Paskett (PI). Douglas Post, Mira Katz
University of Illinois at Chicago and Access Community Health Center: Elizabeth Calhoun (PI) and Julie Darnell.
University of Rochester: Kevin Fiscella (PI) and Samantha Hendren.
University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center: Donald Dudley (PI), Kevin Hall, Anand Karnard, and Amelie Ramirez.
-
Program Office
National Cancer Institute, Center to Reduce Cancer Health Disparities: Martha Hare, Mollie Howerton, Ken Chu, Emmanuel Taylor, and Mary Ann Van Dyun
-
Evaluation Contractor
NOVA Research Company: Paul Young and Frederick Snyder
Footnotes
Authorship responsibility:
Substantial contributions to conception and design, or analysis and interpretation of data: Whitley, Raich, Freund and Snyder
Drafting the article or revising it critically for important intellectual content: Whitley, Freund, Paskett, Dudley, Patierno
Final review and approval of the version to be published: all authors
Agreement to be accountable for all aspects of the work: Whitley
Conflicts of Interest Summary: The authors report no conflicts of interest.
References
- 1.Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: Significance for the prognostic classification of cancer. Cancer. 1996;77:834–842. [PubMed] [Google Scholar]
- 2.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 3.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of comorbidity on life expectancy among men with localized prostate cancer. J Urol. 1996;1(156):127–132. [PubMed] [Google Scholar]
- 4.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 5.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J of Clin Onc. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocols and measures. Cancer. 2008;113(12):3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund KM, Battaglia TA, Calhoun E, et al. The impact of patient navigation on timely cancer care: The Patient Navigation Research Program. J Natl Cancer Inst. 2014;106(6):dju15. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D. Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol, Biomarkers Prev. 2012;21(10):1629–1638. doi: 10.1158/1055-9965.EPI-12-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia TA, Bak SM, Heeren T, et al. Boston Patient Navigation Research Program: the impact of navigation on time to diagnostic resolution after abnormal screening. Cancer Epidemiol, Biomarkers Prev. 2012;21(10):1645–1654. doi: 10.1158/1055-9965.EPI-12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiscella K, Whitley E, Hendren S, et al. Patient navigation for breast and colorectal cancer treatment: A randomized trial. Cancer Epidemiol, Biomarkers Prev. 2012;21(10):1673–1681. doi: 10.1158/1055-9965.EPI-12-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley DJ, Drake J, Quinlan J, et al. Beneficial effects of a combined navigator/promatora approach for hispanic women diagnosed with breast abnormalities. Cancer Epidemiol, Biomarkers Prev. 2012;21(10):639–1644. doi: 10.1158/1055-9965.EPI-12-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells KJ, Lee J-H, Clacano ER, et al. A cluster randomized trial evaluating the efficacy of patient navigation in improving quality of diagnostic care for patients with breast or colorectal cancer abnormalities. Cancer Epidemiol, Biomarkers Prev. 2012;21(10):1664–1672. doi: 10.1158/1055-9965.EPI-12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 15.Katz M, Young GS, Reiter PL, et al. Barriers reported among patients with breast and cervical abnormalities in the Patient Navigation Research Program: Impact on timely care. Women’s Health Issues. 2014;24(1):e155–162. doi: 10.1016/j.whi.2013.10.010. doi:10:1016/j/whi.2013.10.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran A, Freund KM, Bak SM, et al. Multiple barriers delay care among women with abnormal cancer screening despite patient navigation. J of Women’s Health. 2015;24(1):30–36. doi: 10.1089/jwh.2014.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun EA, Whitley EM, Esparza A, et al. A national patient navigator training program. Health Promot Pract. 2010;11(2):205–215. doi: 10.1177/1524839908323521. [DOI] [PubMed] [Google Scholar]
- 18.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson Comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nova Research Company, Silver Springs Maryland.
- 20.DerSimonian R, Laird N. Meta-Analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Teppo H, Alho OP. Comorbidity and diagnostic delay in cancer of the larynx, tongue and pharynx. Oral Oncology. 2009;45(8):692–5. doi: 10.1016/j.oraloncology.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 22.King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459–69. doi: 10.1177/1062860614537676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731–41. doi: 10.1001/jamadermatol.2015.119. [DOI] [PubMed] [Google Scholar]
- 24.Hong CS, Atlas SJ, Ashburner JM, et al. Evaluating a model to predict primary care physician-defined complexity in a large academic primary care practice-based research network. J Gen Intern Med. 2015;30(12):1741–7. doi: 10.1007/s11606-015-3357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson P, Patel A, Garrett T, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage 1 non-small cell lung cancer. Ann Thorac Surg. 2015;99(6):1906–12. doi: 10.1016/j.athoracsur.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roetzheim RG, Freund KM, Corle DK, et al. Analysis of combined data from heterogeneous study designs: an applied example from the Patient Navigation Research Program. Clin Trials. 2012;9(2):176–87. doi: 10.1177/1740774511433284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Commission on Cancer. Cancer Program Standards: Ensuring Patient-Centered Care. American College of Surgeons; Chicago, IL: 2015. [Google Scholar]