Abstract
Background
Veterans with posttraumatic stress disorder (PTSD) exhibit marked deficits in emotion regulation. Past research has demonstrated under-engagement of the prefrontal cortex during regulation of negative affect in those with PTSD, but has been unable to find evidence of impaired down-regulation of the amygdala. One possibility is that there exists variability in amygdala reactivity that cuts across diagnostic status and which can be characterized using a continuous measure of individual differences. In healthy/non-traumatized volunteers, individual variability in amygdala engagement during emotion processing and regulation has been shown to relate to habitual use of regulation strategies.
Methods
The current study examined whether self-reported use of cognitive reappraisal and expressive suppression regulation strategies correlated with brain activation during cognitive reappraisal in combat-exposed veterans with (n = 28) and without PTSD (combat-exposed controls, CEC; n = 20).
Results
Results showed that greater self-reported use of cognitive reappraisal was associated with less activation in the right amygdala during volitional attempts to attenuate negative affect using reappraisal, irrespective of PTSD diagnosis.
Conclusions
This finding is in line with prior work and extends evidence of an association between habitual use of regulation strategies and amygdala engagement during emotion regulation to a trauma-exposed sample of individuals both with and without PTSD. Furthermore, by providing evidence of individual differences in regulation-related amygdala response in a traumatized sample, this result may increase understanding of the neural mechanisms that support variability in symptom manifestation observed across individuals with PTSD.
Keywords: Functional MRI, Brain imaging/Neuroimaging, Trauma, PTSD/Posttraumatic Stress Disorder, Biological markers
1. Introduction
As many as 13% of veterans returning from Operations Enduring Freedom (OEF), Iraqi Freedom (OIF), and/or New Dawn (OND) have been diagnosed with PTSD1, making it one of the most common injuries suffered as a result of military deployment.2 Emotion dysregulation is considered a core deficit of PTSD,3–6 which is also characterized by a heterogeneous array of other difficulties, including reoccurrence of traumatic memories, avoidance of trauma-related stimuli, negative changes in cognition and mood, and alterations in arousal and reactivity.7 Work conducted in healthy individuals has shown that emotion regulation increases activation in the prefrontal cortex (PFC), and reduces activation in emotion-processing brain regions, such as the amygdala.8,9 Relative to traumatized non-PTSD controls, individuals with PTSD engage PFC less during the regulation of negative affect.10 Nonetheless, this work has failed to find evidence of group differences in reappraisal-related reductions in amygdala activity.10,11 Given evidence that amygdala engagement during emotion regulation may be related to habitual (i.e., everyday) use of emotion regulation strategies12–14, one possibility is that regulation-related change in amygdala activity in traumatized individuals can also be explained by a spectrum of individual differences in regulation use.
Cognitive reappraisal is an emotion regulation strategy in which individuals attempt to modulate the emotional salience of a stimulus by changing its meaning.15 Increased use of cognitive reappraisal has been linked to physical and psychological well-being16, and laboratory studies show that reappraisal reduces self-reported affective response to negative stimuli17,18, as well as peripheral markers of emotional arousal.18,19 Cognitive reappraisal also reduces amygdala activation20–23 and increases PFC activation,8,24 with decreases in amygdala responding inversely related to PFC engagement in some studies.25 In contrast to cognitive reappraisal, expressive suppression is an emotion regulation strategy in which individuals attempt to inhibit outward displays of emotional response (e.g., facial expression).15 Although suppression may have short-term benefits26, it does not diminish (and may even increase) physiological arousal15,27, can have negative social consequences28 and is linked to poor physical and psychological health.29 Further, decreases in amygdala activation are not typically observed during expressive suppression.26,30
Prior research that has investigated the neural correlates of reappraisal in individuals with PTSD has yielded mixed results. For instance, New and colleagues11 found that – regardless of PTSD diagnosis – traumatized participants as a whole showed reduced lateral PFC recruitment during reappraisal compared to non-traumatized controls.11 On the other hand, however, Rabinak and colleagues10 found that, compared to combat-exposed controls without PTSD, veterans with PTSD exhibited focal deficits in the dorsolateral prefrontal cortex (dlPFC) during cognitive reappraisal.10 Despite these differences in PFC engagement, neither study found evidence of trauma or PTSD-related differences in modulation of amygdala activity.10,11
This lack of group differences in amygdala activity during cognitive reappraisal is consistent with findings from a broader body of research that has examined PTSD-related aberrations in brain activation during emotion processing (e.g., using passive viewing instead of emotion regulation tasks). For instance, some studies have found that individuals with PTSD exhibit exaggerated amygdala response to negative images3,31–33, negative words34, and emotional faces.35,36 However, there is also evidence of hypo-engagement of the amygdala during the viewing of negative imagery in traumatized individuals with and without PTSD.3,37,38 Finally, several studies have failed to find evidence of PTSD-related differences in amygdala activation to negative stimuli.3, 39–43
An alternative approach towards conceptualizing amygdala response in those with and without PTSD is to consider the existence of significant within-group variability in amygdala reactivity that can be explained by individual difference measures. For instance, greater use of cognitive reappraisal in daily life has been linked to diminished amygdala responding during cognitive reappraisal (in individuals with remitted major depressive disorder MDD14), during the anticipation of negative stimuli (in MDD12) and during viewing of negative stimuli (in healthy individuals13). In addition, greater habitual use of cognitive reappraisal has been associated with increased PFC activation when inhibiting a behavioral response to sad faces (i.e., in the dlPFC44); during negative image processing (in the dlPFC, dorsomedial PFC (dmPFC) and orbitofrontal cortex (OFC)13 and during fear extinction recall (in the ventromedial PFC (vmPFC)45). Greater habitual use of expressive suppression, on the other hand, has been associated with reduced OFC activation during anticipation of negative stimuli46 and increased amygdala activation during response inhibition to sad faces.44 Therefore, prior work suggests that greater use of cognitive reappraisal is related to neural functioning that sub-serves successful down-regulation of negative affect.21 However, no study to date has examined whether such individual differences might also be evident in trauma-exposed individuals with and without PTSD, and whether a continuous measure of habitual emotion regulation might account for variability in amygdala responding that is not explained by diagnostic status alone.
To address this gap in the literature, the current study examined whether habitual use of cognitive reappraisal and expressive suppression were related to individual variability in amygdala response during reappraisal in combat-exposed veterans with and without PTSD. Based on prior work,12–14 we hypothesized that greater habitual use of cognitive reappraisal would be associated with less amygdala activation during reappraisal in combat veterans with and without PTSD. Given prior reports that increased use of expressive suppression is positively associated with engagement of the amygdala during response inhibition to sad faces44, we tested the relationship between brain activation during cognitive reappraisal and daily suppression use as well. However, owing to the fact that we did not utilize a suppression/inhibition task, we did not have specific predictions regarding this relationship. Main effects of task and group differences (PTSD, CEC) in subjective negative affect ratings and BOLD activation during cognitive reappraisal can be found in prior published work.10
2. Materials and Methods
2.1. Participants
A total of 48 male OEF, OIF, and/or OND combat-exposed veterans with (n = 28) and without (Combat-exposed Controls, CEC; n = 20) PTSD were recruited at the Veterans Affairs (VA) Ann Arbor Healthcare System (Ann Arbor, MI). Inclusion criteria for all participants included discharge from active military service, aged 18–55 years, absence of head trauma that involved a loss of consciousness, free from psychoactive medication for at least four weeks prior to testing and negative urine drug screen at time of fMRI scanning, right-handedness, and ability to provide written consent. Exclusion criteria included the presence of a clinically-significant medical or neurologic illness, or life history of bipolar disorder, schizophrenia, or pervasive developmental disorder. Inclusion criteria for participants with PTSD were a primary diagnosis of PTSD, according to the Diagnostic and Statistical Manual of Mental disorders (DSM-IV) criteria and a score ≥ 40 on the Clinician-Administered PTSD Scale (CAPS47), reflecting moderate to severe PTSD symptoms47. Participants in the CEC group could not meet primary or subthreshold criteria for PTSD (i.e., they did not have any significant re-experiencing, avoidance, or hyperarousal symptoms) and could not have CAPS scores > 20. Exposure to combat was assessed using the Combat Exposure Scale (CES48), and all participants were required to meet a minimum level of combat-trauma related exposure (i.e., CES scores ≥ 1748) (see Table 1 for demographics and clinical composition of each group). All participants provided written informed consent for study participation and were monetarily compensated for their time as approved by the Institutional Review Boards of VA Ann Arbor and the University of Michigan Medical School.
Table 1.
Sample Demographics
| CEC (n = 20) |
PTSD (n = 28) |
|||
|---|---|---|---|---|
|
M (SD) |
M (SD) |
test statistic |
p |
|
| Age | 35.10 (9.70) | 29.86 (7.02) | 2.06 | 0.05 |
| Years of Education | 15.55 (1.73) | 13.25 (1.48) | 4.94 | < 0.001 |
| CES | 20.95 (5.22) | 25.32 (6.31) | 2.54 | 0.02 |
| ERQ | ||||
| Reappraisal | 29.30 (7.46) | 27.04 (6.82) | 1.09 | 0.28 |
| Suppression | 15.45 (5.69) | 18.96 (4.90) | 2.29 | 0.03 |
| CAPS | 5.20 (5.54) | 68.43 (12.98) | 23.01 | < 0.001 |
| Intrusive | 0.00 (0.00) | 2.93 (1.15) | 13.45 | < 0.001 |
| Avoidance | 0.25 (0.55) | 4.29 (1.01) | 17.73 | < 0.001 |
| Hyperarousal | 0.25 (0.44) | 4.36 (0.68) | 25.32 | < 0.001 |
|
n (%) |
n (%) |
|||
| Race (Caucasian) | 18 (90.00%) | 27 (96.43%) | 1.51 | 0.47 |
Note. CEC = combat exposed controls; PTSD = posttraumatic stress disorder; CES = Combat Exposure Scale; ERQ = Emotion Regulation Questionnaire; CAPS = Clinician Administered PTSD Scale. Group comparisons were performed using independent t-tests except for race, which was calculated using a Pearson chi-square.
2.2. Materials
Diagnostic criteria were assessed by one of two trained clinicians: (1) a board-certified research psychiatrist (KLP) or (2) a licensed social worker (AEK) using the Structured Clinical Interview for DSM Disorders (SCID49). In addition, all participants completed the Emotion Regulation Questionnaire (ERQ50) prior to scanning. The ERQ is a 10-item self-report measure developed by Gross and John,50 which measures individual differences in the use of emotion regulation strategies - with a particular focus on use of cognitive reappraisal and expressive suppression (sample items include, “I control my emotions by changing the way I think about the situation I’m in” and “I keep my emotions to myself”). Responses are made on a 7-point Likert scale ranging from 1 (strongly disagree) to 7 (strongly agree). The ERQ yields two orthogonal subscales: a six-item cognitive reappraisal factor (Cronbach’s alpha = .79) and a four-item expressive suppression factor (Cronbach’s alpha = .73).50 Higher scores on a given subscale indicate a greater tendency to use that emotional regulation strategy.
2.3. fMRI acquisition and pre-processing
FMRI scanning was performed on a 3T GE Signa System (General Electric; Milwaukee, WI) using a standard radiofrequency coil at the University of Michigan Functional MRI Laboratory. Whole-brain functional images (i.e., blood oxygen level–dependent [BOLD]) were collected from 43 axial, 3-mm-thick slices using a T2*-sensitive gradient echo reverse spiral acquisition sequence (repetition time, 2,000 ms; echo time, 30 ms; 64 × 64 matrix; 220 mm field of view; flip angle, 90°), optimized to minimize susceptibility artifacts (signal loss) at the medial temporal lobe (including the amygdala).51 The first 4 volumes from each run were discarded to allow magnetization to reach equilibrium.
Functional images were processed and analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Trust Center for Neuroimaging, University College London, London, UK; www.fil.ion.ucl.ac.uk/spm). Images were temporally corrected to account for slice time acquisition differences and spatially realigned to the first image of the first run to correct for head movement; motion parameters were entered as regressors of no-interest to control for head movement during scanning. Images were normalized to a Montreal Neurological Institute (MNI) template using the echo-planar imaging (EPI) template, resampled to 2 mm3 voxels and smoothed with an 8 mm isotropic Gaussian kernel.
2.4. ERT
Participants completed a block-design Emotion Regulation Task (ERT) during fMRI scanning. The ERT is a variant of a commonly used emotion regulation task9,22 and has been used in prior studies of emotion regulation within our own laboratory.10,25,52 In brief, participants were shown 64 negative and 32 neutral images from the International Affective Picture System (IAPS53) across three conditions (Look, Maintain, Reappraise). In the Look condition, participants were instructed to simply view neutral images. In the Maintain condition, participants were asked to view negative images without attempting to change their affective experience in any way (i.e., to experience the picture as they normally would). In the Reappraise condition, participants were instructed to view negative images and to attempt to decrease their affective response to these images by employing cognitive reappraisal. Prior to task execution, participants were trained in the technique of cognitive reappraisal. To reduce negative affect evoked by the pictures, participants were taught to either (1) conceptualize the depicted scenario in a less negative way (e.g., women crying outside of a church could be attending a wedding instead of a funeral); or (2) objectify the content of the pictures (e.g., a woman with facial bruises could be an actor in a movie). Following training, participants were shown five images not used in the actual task and asked to practice using cognitive reappraisal while verbalizing their reappraisal strategies to a researcher, who provided feedback regarding use of these strategies. During task execution, participants were instructed to keep their eyes on the pictures at all times, which was confirmed by monitoring eye movement during scanning via an infrared camera mounted on the head-coil.
For each condition, there were two 20-s blocks per each of four runs (eight blocks in total), interspersed with 20-s blocks of a white fixation cross shown on a black background to enable the hemodynamic response to return to baseline. During baseline blocks, participants were instructed to “relax and clear your mind”. Within each condition block, a total of four images were presented, each for 5-s without inter-stimulus interval. Block order was pseudo-randomized over the course of four separate runs, each lasting five minutes. Prior to each block, an instruction screen (“Look”, “Maintain”, or “Reappraise”) was presented for 5-s. Following each block, participants viewed a screen that asked them to answer the question, “How negative to you feel?” Participants indicated their response on a 5-item Likert scale (1 = not at all; 5 = extremely) via a 5-button response using the dominant, right hand.
2.5. fMRI data analysis
At the first level, functional time series data were subjected to a general linear model, convolved with the canonical hemodynamic response function (HRF) and filtered with a 128 s high-pass filter. Box car regressors were used to model Look, Maintain, and Reappraise blocks with the main contrast of interest being Reappraise > Maintain representing regulated negative emotion processing. For completeness and to generate future hypotheses, we also examined and present results from the Maintain > Look contrast representing unregulated negative emotion processing. At the second level, one sample t-tests were used to regress ERQ Reappraisal and ERQ Suppression scores onto whole-brain activation for each contrast. Clusters of activation were identified using a whole-brain uncorrected voxel threshold of p < 0.001 with at least 20 contiguous voxels per cluster, which allowed us to compare current findings to studies from others that have used similar significance thresholds3,13 and which has been recommended as a balance between risk of Type I versus Type II error rates.54 Moreover, for activation clusters falling within the amygdala, we corrected for multiple comparisons using Small Volume Correction (SVC) using a bilateral anatomically-defined mask derived from MAsks for Region of INterest Analysis (MARINA55). The focus of this analysis centered on results within bilateral amygdala as a region-of-interest (ROI); however, to obviate bias and in order to generate hypotheses for future research, complete whole-brain results at p < 0.001 with an 20 extant cluster threshold for both contrasts (e.g., Reappraise > Maintain and Maintain > Look) are reported in Tables 1 and 2, respectively. For significant effects, mean beta values from clusters were extracted using the MarsBaR Toolbox56 to generate scatterplots for visual inspection, to clarify effects within each subgroup of our sample (i.e., PTSD, CEC) and generate Pearson product-moment correlations in Statistical Package for the Social Science (SPSS, Version 20.0).
Table 2.
Whole-brain ERQ correlations for Reappraise > Maintain Contrast
| peak MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| ERQ Sub-Scale | Brain region | Laterality | Volume (mm3) |
Z-score | x | y | z |
| Positive Correlation | |||||||
| ERQ Reappraisal | No significant clusters | ||||||
| ERQ Suppression | No significant clusters | ||||||
| Negative Correlation | |||||||
| ERQ Reappraisal | Medulla | Midline | 368 | 4.24 | 0 | −38 | −46 |
| Amygdala | R | 824 | 3.94 | 28 | 2 | −22 | |
| Culmen | L | 288 | 3.77 | −10 | −42 | −20 | |
| Precentral gyrus | L | 1,016 | 3.71 | −46 | −16 | 28 | |
| L | 536 | 3.68 | −46 | −14 | 60 | ||
| Cuneus | L | 208 | 3.70 | −6 | −94 | 36 | |
| ERQ Suppression | No significant clusters | ||||||
Note. Display whole-brain threshold was p < 0.001, uncorrected with an extant threshold of 20 voxels. Bold italics reflect that the amygdala cluster finding was an a priori region-of-interest and significance reached pSVC < 0.05. Results control for age, years of education, and CES scores. ERQ = Emotion Regulation Questionnaire; CES = Combat-Exposure Scale; R = right; L = left.
Partial Pearson’s product-moment correlations were run in SPSS to test the association between ERQ scores (Reappraisal, Suppression) and subjective ratings of negative affect collected during the ERT, controlling for age, years of education, and CES scores. Correlations were run using difference scores reflecting affect ratings for Maintain minus Reappraise trials (to mimic the contrast comparison used for fMRI data analysis).
3. Results
3.1. ERQ distribution
Scores from both ERQ Reappraisal and Suppression sub-scales were normally distributed (Shapiro-Wilk’s test, p’s > 0.44). The average ERQ Reappraisal score was 27.98 ± 7.11 and the average ERQ Suppression score was 17.50 ± 5.47. Between PTSD (27.04 ± 6.82) and CEC (29.30 ± 7.46) groups, there was no difference in the reporting of reappraisal use (t(46) = 1.09, p > 0.28). However, compared to controls (15.45 ± 5.69), those with PTSD (18.96 ± 4.90) reported using suppression to a greater extent (t(46) = 2.29, p < .03).
3.2. ERQ correlation with subjective ratings
No correlation was found between ERQ Reappraisal and Suppression scores and subjective negative affect during Reappraise > Maintain trials (all p’s > 0.08).
3.3. Cognitive reappraisal and brain activation
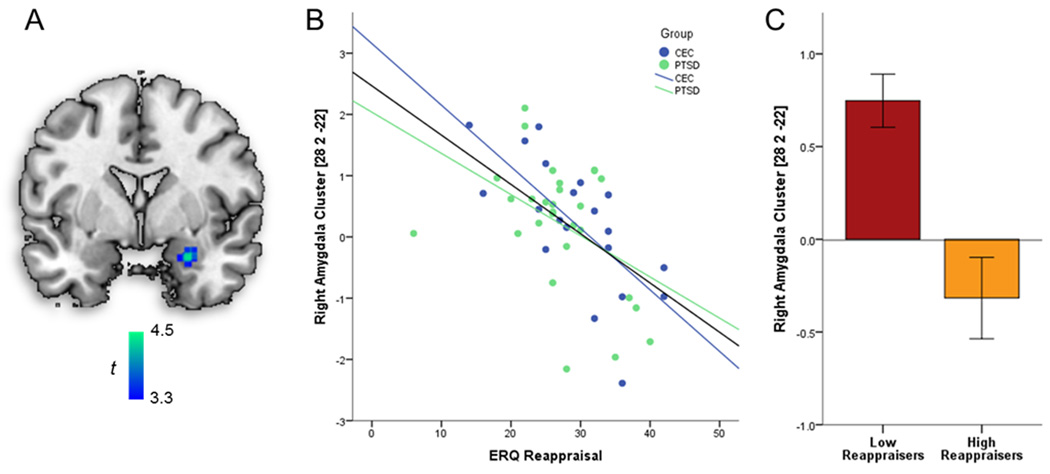
ERQ reappraisal scores correlated negatively with Reappraise > Maintain activation in the right amygdala (peak MNI coordinate: 28, 2, −22; 432 mm3; Z = 3.98; pSVC < 0.05). This correlation remained when adding covariates age, years of education, and CES scores as regressors of no-interest (peak MNI coordinate: 28, 2, −22; 440 mm3; Z = 3.94; pSVC < 0.05). To determine if this relationship was driven by one or both sub-groups (PTSD, CEC), beta values were extracted and used in subsequent partial Pearson product-moment correlations. Significant correlations were evident for veterans with (r(26) = − .44, p < 0.02) and without (r(18) = − .70, p < 0.01) PTSD. In order to assess whether the strength of these correlations differed significantly between PTSD and CEC groups, correlation coefficients were first converted into z-scores using Fisher r-to-z transformation. Then, factoring in sample sizes for each group, z-scores were compared using formula 2.8.5 from Cohen and Cohen.57,58 These correlations did not significantly differ between the groups (p < 0.21). Figure 1, Panel A depicts the spatial location of the negative correlation between ERQ Reappraisal scores and BOLD activation during Reappraise > Maintain. Figure 1, Panel B depicts the association between ERQ Reappraisal scores and activation in the right amygdala, such that high habitual use relates to negative beta values within the amygdala. Negative beta values indicate amygdala activation is higher during Maintain trials than in Reappraise trials. Figure 1, Panel C depicts mean activation in the right amygdala during Reappraise > Maintain for individuals high versus low in ERQ Reappraisal scores (participants were categorized using a median split for visualization purposes only). Again, negative beta values for individuals with higher Reappraisal scores indicate that amygdala activation was greater during Maintain than Reappraise, suggesting reduced amygdala response during Reappraise (e.g., when task instruction was to decrease affective response).
Figure 1.
(A) Location of the negative correlation between ERQ Reappraisal scores and activation in right amygdala during Reappraise > Maintain cluster around peak Montreal Neurological Institute (MNI) coordinates (26, 0, −18), controlling for age, years of education, and CES scores. Display threshold is p < 0.001 (whole-brain uncorrected). (B) Scatterplot of ERQ Reappraisal scores and contrast values extracted from the right amygdala cluster, organized by group (PTSD, CEC). (C) Activation in right amygdala cluster during Reappraise>Maintain by median-split of ERQ Reappraisal scores, representing both Low and High Reappraisers (median-split for illustrative purposes only; error bars reflect ± 1 standard error). ERQ = Emotion Regulation Questionnaire; PTSD = posttraumatic stress disorder; CEC = combat-exposed controls; CES = Combat Exposure Scale.
To determine if this effect was driven by a correlation specific to the Reappraise or Maintain condition, given that the contrast reflected a comparison between Reappraise > Maintain trials, subsequent one sample t-tests were run in SPM regressing ERQ Reappraisal scores onto activation within Reappraise > Look and Maintain > Look contrasts. No significant correlations were observed in the amygdala for either contrast (p-value’s > 0.001, uncorrected). Next, we used an analysis of covariance (ANCOVA) in SPM to determine whether a group difference would be evident in amygdala activation when controlling for ERQ Reappraisal scores. Reappraise > Maintain amygdala activation did not differ between the PTSD and CEC groups when controlling for ERQ Reappraisal scores (p-value > 0.001, uncorrected).
A complete list of regions that correlated with the ERQ are provided in Tables 1 and 2, listed separately for the Reappraise > Maintain and Maintain > Look contrasts, respectively. Correlations between ERQ Suppression scores and Reappraise > Maintain amygdala activation did not surpass our threshold for statistical significance.
4. Discussion
The current study examined the relationship between habitual use of emotion regulation strategies and amygdala response during cognitive reappraisal in trauma-exposed U.S. military veterans with and without PTSD. Results showed that habitual use of cognitive reappraisal correlated negatively with differential activation in the right amygdala during cognitive reappraisal, irrespective of PTSD diagnosis and when controlling for age, years of education, and severity of combat exposure.
The results observed here are in line with prior work showing that, among individuals with remitted MDD, greater everyday use of reappraisal was associated with less amygdala activation during cognitive reappraisal.14 More broadly, the results are also in line with findings showing that reappraisal use is related to amygdala responding during emotion processing (i.e., in healthy and depressed individuals viewing and anticipating negative imagery, respectively12,13). Here, findings suggest that individual differences in habitual use of cognitive reappraisal are predictive of amygdala activation during reappraisal in traumatized individuals, although controlling for variability in reappraisal use did not reveal group differences in amygdala engagement during reappraisal. Therefore, while we demonstrate that habitual use of reappraisal matters in predicting individual differences in neural response during regulation, more research is needed in order to isolate other factors that contribute to heterogeneity of amygdala response in this population. Additionally, it may be the case that traumatized individuals with versus without PTSD simply do not differ in amygdala engagement during cognitive reappraisal, even when controlling for individual differences that cut across diagnostic groups, although more research is needed in order to fully test this possibility. We note that while the correlation coefficients representing the strength of the relationship between daily reappraisal use and amygdala reduction during reappraisal appeared to differ between groups, we found that this difference was not significant. Nevertheless, this effect should be followed-up using larger samples sizes, which affords more power to detect group differences in this relationship should they exist.
From a broader perspective, the finding observed here is consistent with a growing body of work that has documented heterogeneity in amygdala response to negative stimuli in PTSD.31–43 This heterogeneity may signal that not all individuals with PTSD are characterized by hyper-responsivity to negative stimuli59–67, in line with the notion that the disorder may consist of a number of distinct sub-types.68
Despite some evidence suggesting that individuals with PTSD show reduced PFC recruitment during reappraisal10, they do not seem to exhibit deficits in the modulation of amygdala response when compared to traumatized controls.10,11 Instead, greater experience with or an increased tendency to use reappraisal in everyday life may translate into improved ability to modulate amygdala responding, even for those exhibiting reduced recruitment of the PFC. Individual differences in reappraisal use may therefore signal alterations in the effectiveness of PFC engagement and/or the engagement of compensatory regions involved in the down-regulation of amygdala activity8, though this hypothesis remains to be tested in future work.
Contrary to prior studies that found a relationship between reappraisal use and PFC engagement during tasks of emotion processing13,44,45, we did not observe such a relationship during emotion regulation and within a traumatized sample. In previous work limited to a region-of-interest analysis focused on the dlPFC, Rabinak and colleagues10 examined the relationship between reappraisal use and dlPFC activation and reported no evidence of a correlation between everyday use of reappraisal and engagement of this region across the sample10, despite the fact that veterans with PTSD (as a group) under-engaged the dlPFC during cognitive reappraisal. Here, we extend these results to other regions of the PFC, finding that variability during cognitive reappraisal does not appear to map onto regulation use in traumatized individuals.
Additionally, we did not find evidence of a relationship between habitual use of expressive suppression and amygdala engagement in the present study, even though groups differed on daily use of expressive suppression. Prior work has found that expressive suppression use positively correlates with engagement of the amygdala during response inhibition to sad faces44, although other studies have failed to find an effect when changes in the amygdala were measured during cognitive reappraisal13 and negative emotion anticipation.12,46 Therefore, the relationship between suppression use and amygdala engagement may depend on task specificity, in that suppression use - which is an inhibitory emotion regulation strategy - may more closely relate to changes in amygdala responding when brain functioning is tested during suppression/inhibition. More research is needed on this topic as no study to-date has assessed whether suppression use is related to changes in amygdala engagement when individuals are asked to directly engage in this emotion regulation strategy.
We did not find evidence of a correlation between habitual reappraisal use and self-reported negative affect collected on-line during scanning. In line with these results, prior research also failed to find evidence of a correlation between reappraisal use and self-reported negative affect during fear conditioning45 or self-reported success during response inhibition to sad faces.44 Therefore, regular use of reappraisal seems to relate more to neural indices of affective processing during regulation (i.e., amygdala activity) than to an individual’s subjective experience of negative affect. Future work may wish to explore whether subjective report of regulation success (i.e., rather than report of negative affect) aligns more closely with habitual reappraisal use.
Beyond findings within a priori ROIs (e.g., amygdala), the present study was also able to demonstrate a negative relationship between daily use of reappraisal and engagement within the medulla, culmen, precentral gyrus, and cuneus during cognitive reappraisal (Table 2). Prior work demonstrates that medullary neurons are involved in the initiation of a stress response to emotional triggers while lesions of this region suppress amygdala responding in rodents.69 Therefore, the negative relationship to frequent reappraisal use and brain activation in the present study may be broadened to involve regions involved in stress responses that may, in turn, activate amygdala responding. Additionally, neuroimaging studies involving humans demonstrate increased engagement of the culmen70,71 and cuneus72 during the viewing of negative, compared to neutral, images. Engagement of the culmen occurs during predictive motor responses (e.g., when participants anticipate a future event that requires visuo-motor coordination73) and while no behavioral response was required in the present study, the initiation of subtle motor responses may occur during the viewing of negative imagery with these responses dampened during reappraisal.74 This line of thinking is supported by the fact that we also observed a negative correlation between reappraisal use and engagement of the left precentral gyrus75,76, a region involved in voluntary movement.77 Finally, while the cuneus is typically associated with visual processing72, activation also occurs during the experience of pain.78 Therefore, greater reappraisal use may also be associated with decreases in more than one aspect of a negative affect experience. Altogether, these results are in-line with the notion that frequent reappraisal use relates to diminished negative emotion responding, but demonstrate that this relationship is not specific to functioning of the amygdala.
Results of the current study should be viewed in light of several limitations. First, the sample was comprised of only male veterans and thus limits generalizability to civilians, non-combat traumas, and females, which may be relevant given prior evidence of sex differences in brain functioning during emotion processing79 (but see13). Second, it is difficult to know whether the results observed here are specific to traumatized individuals, given the lack of a non-traumatized control group. Third, the ERQ was used as a single predictor of brain functioning during emotion regulation. While prior work documents the validity of the ERQ as a measure of habitual use of emotion regulation50, future research might benefit from measuring additional dimensions of emotion regulation capacity apart from self-reported use. For instance, future work may wish to determine whether relationships between habitual use of cognitive reappraisal and brain activation change when more objective measures of regulation success are taken into account – perhaps via the inclusion of peripheral physiological markers of arousal response. Fourth, the current study did not utilize trauma-specific images; therefore, the relationship between regulation use and amygdala modulation when negative imagery is more central to an individual’s trauma remains to be tested.
5. Conclusion
Despite these limitations, the current study extends prior research documenting a relationship between habitual use of reappraisal and amygdala engagement to a traumatized sample. Moreover, the results provide added evidence of variability of amygdala response in traumatized samples that can be explained by regulation use. This study also offers unique perspective on the neurobiological underpinnings of emotion dysregulation in PTSD. That is, by demonstrating heterogeneity in amygdala response during emotion regulation, we show that not all individuals with PTSD exhibit deficiency in the capacity for emotion regulation, when measured at the neural level. In this regard, this study may ultimately help increase understanding of the neural mechanisms that support variability in symptom manifestation observed across individuals with PTSD.
Table 3.
Whole-brain ERQ correlations for Maintain > Look Contrast
| peak MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| ERQ Sub-Scale | Brain region | Laterality | Volume (mm3) |
Z-score | x | y | z |
| Positive Correlation | |||||||
| ERQ Reappraisal | Middle frontal gyrus | R | 328 | 3.87 | 24 | 22 | 50 |
| Parahippocampal gyrus | R | 296 | 3.80 | 16 | −10 | −24 | |
| R | 304 | 3.55 | 20 | −26 | −12 | ||
| Angular gyrus | R | 736 | 3.76 | 40 | −68 | 38 | |
| Insula | L | 576 | 3.63 | −30 | −24 | 22 | |
| Precuneus | R | 504 | 3.46 | 20 | −56 | 48 | |
| Supramarginal gyrus | L | 208 | 3.45 | −42 | −54 | 32 | |
| ERQ Suppression | Superior temporal gyrus | L | 288 | 4.07 | −30 | 12 | −30 |
| L | 208 | 3.40 | −64 | −38 | 6 | ||
| Uncus | R | 768 | 3.96 | 16 | 4 | −26 | |
| Precuneus | R | 232 | 3.52 | 14 | −68 | 50 | |
| Middle temporal gyrus | R | 224 | 3.44 | 56 | −40 | −16 | |
| Negative Correlation | |||||||
| ERQ Reappraisal | No significant clusters | ||||||
| ERQ Suppression | vmPFC | L | 960 | 3.79 | −4 | 52 | 12 |
Note. Display whole-brain threshold threshold was p < .001, uncorrected with an extant threshold of 20 voxels. Results control for age, years of education, and CES scores. ERQ = Emotion Regulation Questionnaire; CES = combat-exposure scale; vmPFC = ventromedial prefrontal cortex; R = right; L = left.
Acknowledgments
Disclosures: This study was supported by funding from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (I01BX007080: KLP). JF is supported by the National Institute of Mental Health grant T32MH067631, AM is supported by the National Institute of Mental Health grant K23MH105553, CAB is supported the National Institute of Mental Health grant K01MH101123, and KLP is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development, and the Veterans Affairs Merit Review Program Award.
References
- 1.Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 2.Tanielian T, Jaycox L. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008. [Google Scholar]
- 3.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley B, DeFife JA, Guarnaccia C, et al. Emotion dysregulation and negative affect: association with psychiatric symptoms. J Clin Psychiatry. 2011;72:685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1074:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- 6.Seligowski AV, Lee DJ, Bardeen JR, Orcutt HK. Emotion regulation and posttraumatic stress symptoms: a meta-analysis. Cogn Behav Ther. 2015;44:87–102. doi: 10.1080/16506073.2014.980753. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 8.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 10.Rabinak CA, MacNamara A, Kennedy AE, et al. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 2014;31:851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New AS, Fan J, Murrough JW, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiat Res. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Drabant EM, McRae K, Manuck SB, et al. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 15.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 16.Cutuli D. Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00175. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denny BT, Ochsner KN. Behavioral effects of longitudinal training in cognitive reappraisal. Emotion. 2014;14:425–433. doi: 10.1037/a0035276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray RD, McRae K, Ochsner KN, Gross JJ. Cognitive reappraisal of negative affect: converging evidence from EMG and self-report. Emotion. 2010;10:587–592. doi: 10.1037/a0019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Hamann S. The effect of cognitive reappraisal on physiological reactivity and emotional memory. Int J Psychophysiol. 2012;83:348–356. doi: 10.1016/j.ijpsycho.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Eippert F, Veit R, Weiskopf N, et al. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks SJ, Eddy KT, Angstadt M, et al. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klucken T, Kruse O, Schweckendiek J, Stark R. Increased skin conductance responses and neural activity during fear conditioning are associated with a repressive coping style. Front Behav Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00132. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spokas M, Luterek JA, Heimberg RG. Social anxiety and emotional suppression: the mediating role of beliefs. J Behav Ther Exp Psychiatry. 2009;40:283–291. doi: 10.1016/j.jbtep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Musante L, Treiber FA. The relationship between anger-coping styles and lifestyle behaviors in teenagers. J Adolesc Health. 2000;27:63–68. doi: 10.1016/s1054-139x(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 30.Nelson BD, Fitzgerald DA, Klumpp H, et al. Prefrontal engagement by cognitive reappraisal of negative faces. Behav Brain Res. 2015;279:218–225. doi: 10.1016/j.bbr.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant RA, Kemp AH, Felmingham KL, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morey RA, Dolcos F, Petty CM, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin LM, Kosslyn SM, McNally RJ, et al. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 34.Protopopescu X, Pan H, Tuescher O, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 35.El Khoury-Malhame M, Reynaud E, Soriano A, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Felmingham K, Williams LM, Kemp AH, et al. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- 37.Britton JC, Phan KL, Taylor SF, et al. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Phan KL, Britton JC, Taylor SF, et al. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 39.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto H, Fukuda R, Okuaki T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 42.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 43.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Vanderhasselt M, Baeken C, Van Schuerbeek P, et al. Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: an event related fMRI study. Biological Psychology. 2013;92:433–439. doi: 10.1016/j.biopsycho.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Hermann A, Keck T, Stark R. Dispositional cognitive reappraisal modulates the neural correlates of fear acquisition and extinction. Neurobiology of Learning and Memory. 2014;113:115–124. doi: 10.1016/j.nlm.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Abler B, Hofer C, Walter H, et al. Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research: Neuroimaging. 2010;183:105–113. doi: 10.1016/j.pscychresns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 48.Keane TM, Fairbank JA, Caddell JM, et al. Clinical evaluation of a measure to assess combat exposure. J Consult Clin Psych. 1989;1:53–55. [Google Scholar]
- 49.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 50.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 51.Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)- weighted functional MRI. Magn Reson Med. 2000;44:525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phan KL, Fitzgerald DA, Nathan PJ, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- 54.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter B, Blecker C, Kirsch P, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses [abstract]. Presented at the 9th International Conference on Functional Mapping of the Human Brain; June 19–22, 2003; New York, NY. 2003. p. 2. Available on CD-Rom in NeuroImage. [Google Scholar]
- 56.Brett M, Andont J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain NeuroImage; 2002. p. 16. [Google Scholar]
- 57.Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- 58.Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] Available from http://quantpsy.org.
- 59.D’Andrea W, Pole N, DePierro J, et al. Heterogeneity of defensive responses after exposure to trauma: blunted autonomic reactivity in response to startling sounds. Int J Psychophysiol. 2013;90:80–89. doi: 10.1016/j.ijpsycho.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Felmingham KL, Falconer EM, Williams L, et al. Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One. 2014;9:e103653. doi: 10.1371/journal.pone.0103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kemp AH, Felmingham KL, Falconer E, et al. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: an fMRI study. Psychiatry Res. 2009;174:158–161. doi: 10.1016/j.pscychresns.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 62.MacNamara A, Post D, Kennedy AE, et al. Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biol Psychol. 2013;94:441–449. doi: 10.1016/j.biopsycho.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 63.McTeague LM, Lang PJ, Laplante MC, et al. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moser JS, Krompinger JW, Dietz J, Simons RF. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 65.Shepherd L, Wild J. Emotion regulation, physiological arousal and PTSD symptoms in trauma-exposed individuals. J Behav Ther Exp Psychiatry. 2014;45:360–367. doi: 10.1016/j.jbtep.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tso IF, Chiu PH, King-Casas BR, Deldin PJ. Alterations in affective processing of attack images following September 11, 2001. J Trauma Stress. 2011;24:538–545. doi: 10.1002/jts.20678. [DOI] [PubMed] [Google Scholar]
- 67.Zaba M, Kirmeier T, Ionescu IA, et al. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 2015;55:102–115. doi: 10.1016/j.psyneuen.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Lanius RA, Brand B, Vermetten E, et al. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- 69.Dayas CV, Buller KM, Day TA. Medullary neurons regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- 70.Belden AC, Luby JL, Pagliaccio D, et al. Neural activation associated with the cognitive emotion regulation of sadness in healthy children. Dev Cogn Neurosci. 2014;9:136–147. doi: 10.1016/j.dcn.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McRae K, Gross JJ, Weber J, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. SCAN. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buhle JT, Kober K, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periacqueductal gray. Soc Cogn Affect Neurosci. 2013;8:609–616. doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bares M, Lungu OV, Liu T, et al. The neural substrate of predictive motor timing in spinocerebellar ataxia. Cerebellum. 2011;10:233–244. doi: 10.1007/s12311-010-0237-y. [DOI] [PubMed] [Google Scholar]
- 74.Mobbs D, Hagen CC, Dalgleish T, et al. The ecology of human fear: survival optimization and the nervous system. Front Neurosci. 2015;9:55. doi: 10.3389/fnins.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allard ES, Kensinger EA. Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Front Psychol. 2014;5:296. doi: 10.3389/fpsyg.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pitskel NB, Bolling DZ, Kaiser MD, et al. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao SM, Binder JR, Bandettini PA, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- 78.Fulbright RK, Troche CJ, Skudlarski P, et al. Functional MR Imaging of regional brain activation associated with the affective experience of pain. Am J Roentgenol. 2001;177:1205–1210. doi: 10.2214/ajr.177.5.1771205. [DOI] [PubMed] [Google Scholar]
- 79.Andreano JM, Dickerson BC, Feldman Barrett L. Sex differences in the persistence of the amygdala response to negative material. SCAN. 2014;9:1388–1394. doi: 10.1093/scan/nst127. [DOI] [PMC free article] [PubMed] [Google Scholar]