Abstract
Aim
The purpose of this paper is to report the analysis of the concept of biological embedding.
Background
Research that incorporates a life course perspective is becoming increasingly prominent in the health sciences. Biological embedding is a central concept in life course theory and may be important for nursing theories to enhance our understanding of health states in individuals and populations. Before the concept of biological embedding can be used in nursing theory and research, an analysis of the concept is required to advance it toward full maturity.
Design
Concept analysis.
Data Sources
PubMed, CINAHL and PsycINFO were searched for publications using the term ‘biological embedding’ or ‘biological programming’ and published through 2015.
Methods
An evaluation of the concept was first conducted to determine the concept’s level of maturity and was followed by a concept comparison, using the methods for concept evaluation and comparison described by Morse.
Results
A consistent definition of biological embedding – the process by which early life experience alters biological processes to affect adult health outcomes – was found throughout the literature. The concept has been used in several theories that describe the mechanisms through which biological embedding might occur and highlight its role in the development of health trajectories. Biological embedding is a partially mature concept, requiring concept comparison with an overlapping concept – biological programming – to more clearly establish the boundaries of biological embedding.
Conclusions
Biological embedding has significant potential for theory development and application in multiple academic disciplines, including nursing.
Keywords: nursing, concept analysis, biological embedding, life course theory, toxic stress
INTRODUCTION
Over the last two decades, there has been an increasing focus on the prevention of adult diseases with antecedents in early childhood. Based on life course theory, this focus is an area of intense research into the mechanisms by which childhood experiences establish health trajectories that predict adult disease as well as research into the potential interventions to modulate these effects. Coinciding with the increased research focus on the life course, discussions are emerging on how policy may be shifted to account for these new research findings (Shonkoff et al. 2009, Halfon et al. 2014). Such policy revisions might include changes in the way healthcare and research funds are distributed, the way that healthcare is reimbursed and the focus of clinicians in treating patients and preventing morbidity.
Biological embedding, a central concept in life course theory, is generally defined as the process by which early life experiences affect anatomy and biological processes in a manner that has an impact on long-term adult health outcomes (Shonkoff et al. 2009, Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Masten & Narayan 2012, McEwen 2012, McGowan 2012, Rutter 2012, Carroll et al. 2013, Clayton 2013, Kelly-Irving et al. 2013, Nelson 2013, Rutter 2013, Sasaki et al. 2013, Kundakovic & Champagne 2014, Barboza Solis et al. 2015, Bateson et al. 2015, Boyce & Kobor 2015, Cunliffe 2015, Demetriou et al. 2015, Provencal & Binder 2015, Stringhini et al. 2015, Tallon et al. 2015, Thomason et al. 2015). These environmental exposures and experiences may be psychological stressors (e.g. socio-economic disparities, childhood maltreatment, chaotic families, exposure to violence or disasters), or they may be cases of childhood deprivation (e.g. malnutrition, lack of developmentally-appropriate stimuli). Interestingly, in the literature, the concept is not commonly associated with positive experiences in early childhood. The concept, biological embedding, has important implications for health and illness throughout the life course, making it an attractive target for research among multiple academic disciplines, especially nursing. This concept has global relevance for nursing theory and research and may be particularly important in areas of the world confronting widespread poverty, civil unrest, terrorism, or natural disasters, as these stressors may have a significant impact on vulnerable children.
Background
Due to the increasing focus on health across the life course and the role that biological embedding may play in the development of adult disease, the theoretical and operational definitions of this concept must be clearly articulated through concept analysis. The goal of this paper is to advance the study of early life experience and health trajectories by establishing a common language, founded on a clearly-defined central concept. First, using the criteria specified by Morse et al. (1996b), the concept will be evaluated to determine its level of maturity and to allow for the selection of an appropriate method of concept analysis based on the concept’s maturity. Second, using the method of concept comparison, as described by Morse (1995), a concept analysis will be conducted. Based on this concept analysis, suggestions for advancing the concept toward greater maturity will be made and examples of how this concept may be incorporated into nursing scholarship will be provided.
Data Sources
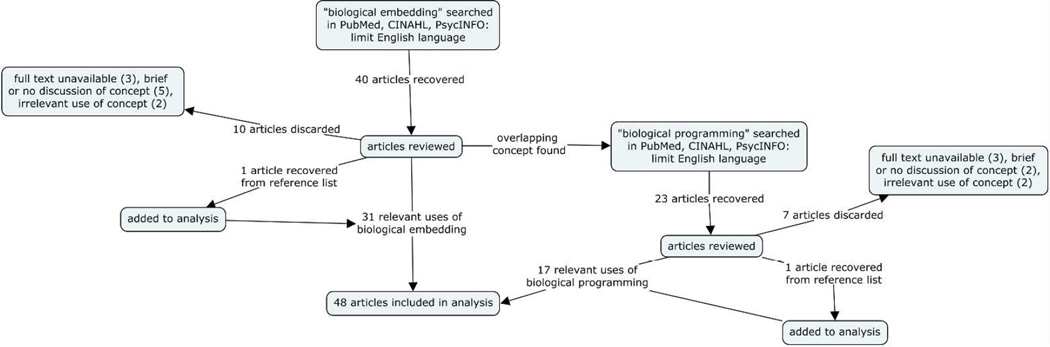
The research databases PubMed, CINAHL and PsycINFO were searched for the phrase ‘biological embedding’. Articles were included if they were published through 2015 and written in English language. Years of publication were not limited so that potential evolution of the term could be identified. Publications were excluded if they failed to mention the concept, provided too little discussion of the concept to determine its use, or were not available as full text. The original search resulted in 40 articles that were reviewed for relevance and discussion of the concept. During this review, an overlapping concept, ‘biological programming’ was discovered (Miller et al. 2011). To provide a comprehensive overview of the use of biological embedding, a second search of PubMed, CINAHL and PsycINFO was performed, using the phrase ‘biological programming’. This search resulted in 23 articles, which were similarly reviewed for relevance and discussion of the concept, based on the previously-described inclusion and exclusion criteria. Eleven articles were discarded from both searches for irrelevance or lack of discussion of either term. Six articles were discarded because the full text could not be obtained. Two additional articles were recovered from a reference list review. The remaining 48 articles provided a comprehensive evaluation and analysis of the concept. (Figure 1)
Figure 1. Reference database search results for the concept analysis.
‘Biological embedding’ and ‘biological programming’ were searched separately to provide the literature base for this concept analysis.
RESULTS
Concept analysis is a difficult process without consideration of context. According to Risjord (2009), ‘for a scientific concept, the context of use is a theory or related group of theories’ (p. 687). Further, as suggested by Hupcey and Penrod (2005), ‘…concepts are assigned meaning through placement in the context of a theory…concepts cannot be analyzed irrespective of their theoretical frame’ (p. 199). Two theories provide the necessary framework for a targeted concept analysis of biological embedding. The first, introduced by Miller, et al. (2011), is a theory describing the process of biological embedding. The Biological Embedding of Childhood Adversity Model posits that early stress during critical or sensitive periods of development affects the functioning of immune cells, causing them to exhibit a pro-inflammatory phenotype and that the hormonal dysregulation and behavioral adaptations resulting from early childhood adversity further promote inflammation in a cyclic fashion. The second theory to include the concept of biological embedding was described by Shonkoff (2010). In Shonkoff’s Biodevelopmental Framework, biological embedding is one of two processes by which early life stress affects physiological functioning, resulting in an increased risk for adult disease (Shonkoff 2010). These theories provide the context for the following concept evaluation and analysis. It is important to note that in both of these theories, the antecedent of biological embedding is early experience that causes psychological stress.
Concept Evaluation
Few concept analysis methods prospectively consider the maturity of the concept to be analyzed, but concept maturity is an important characteristic that must be evaluated before choosing an approach to concept analysis. Morse et al. (1996b) describes a mature concept as one that ‘…is well-defined, has clearly described characteristics, delineated boundaries and documented preconditions and outcomes’ (p. 387). When concepts are mature, their use among different authors of the same discipline is consistent, allowing for the precise communication of ideas. Moreover, their relationship to other concepts in a theory are more readily understood. To determine the level of maturity of biological embedding, the definition of the concept, its attributes, boundaries, antecedents and consequences were evaluated using the method described by Morse et al. (1996b).
The theoretical definition of biological embedding – the process by which early life experiences affect biological processes in a manner that has an impact on long-term adult health outcomes – is clear and consistently used among most authors whose works were retrieved from the reference databases using the phrase ‘biological embedding.’ Only those authors describing irrelevant cases of biological embedding – epoxy resins for the embedding of biological material and the programming of biotechnology and bioinformatics – used different definitions of the term (Wilcox 1965, Burk 2002, Singh et al. 2009, Desai & Burra 2015). Evaluating biological embedding from this perspective reveals that it is a mature concept.
Defining attributes are those characteristics of a concept that are present in all instances of the concept (Morse et al. 1996). There are four defining attributes that are consistently present in discussions of biological embedding. These defining attributes are specifically delineated by Hertzman and Boyce (2010) and include the following: (1) early life experiences affect biological processes; (2) bio-developmental trajectories are established by the changes in biological processes, which differ among individuals with different early life experiences; (3) the effects on biological processes are relatively stable over time; and (4) the alterations in biological processes have long-term health consequences. When these attributes occur together, the process of biological embedding is present.
According to the first defining attribute of biological embedding, early experience must affect biological processes. Multiple studies have identified target processes that are particularly vulnerable to environmental-induced alterations. Changes in central nervous system, immune system and endocrine system functioning and alterations in gene expression have been described (Shonkoff et al. 2009, Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Masten & Narayan 2012, McEwen 2012, McGowan 2012, Rutter 2012, Clayton 2013, Kelly-Irving et al. 2013, Nelson 2013, Rutter 2013, Sasaki et al. 2013, Kundakovic & Champagne 2014, Betancourt et al. 2015, Cunliffe 2015, Demetriou et al. 2015, Provencal & Binder 2015, Stringhini et al. 2015, Tallon et al. 2015, Thomason et al. 2015). For example, one study found that the children who experienced maltreatment displayed a heightened inflammatory phenotype (Danese et al. 2011). Furthermore, children exposed to traumatic early events (i.e. victimization, violence, neglect) demonstrated alterations in neuronal circuitry between the prefrontal cortex and amygdala (Thomason et al. 2015). These empirical findings link early life experiences to changes in biological processes.
The second attribute – different experiences and environmental exposures among individuals result in different bio-developmental states – has been studied by comparing the health outcomes of individuals who were exposed to childhood stress with those who were not. Such studies have shown that adults who experienced maltreatment as children, for example, had poorer health outcomes than those who did not experience this adversity (Miller et al. 2011). Recent research has demonstrated an association between health outcomes and socio-economic gradients in early life (Hertzman 2012). In a study of otherwise healthy, term infants, Betancourt et al. (2015) found a linear relationship between the volume of cortical gray matter and maternal socioeconomic status (SES), suggesting that prenatal events are associated with alterations in anatomy. Additionally, studies of early life stress (i.e. competition) in animal models have demonstrated that those exposed to competition for survival had shortened telomeres that correlated with more impulsive decision-making in later life (Bateson et al. 2015). Thus, different bio-developmental states in adult life have been associated with the differences in the experiences of those who have been subjected to early life stress compared with those who have not.
The third defining attribute – changes in an individual’s biology are stable and long-term – has been demonstrated through research in the emerging field of epigenetics. This field of work has demonstrated that persistent and sometimes heritable, alterations in the human genome may be caused by changes in DNA methylation and chromatin structure among cells such as neurons and immune cells, which remain stable over time (Sasaki et al. 2013, Buschdorf & Meaney 2015). Adults with traumatic childhood experiences, including physical abuse and neglect, demonstrated altered patterns of DNA methylation in the hippocampus, compared with controls (Sasaki et al. 2013). Similarly, hypomethylation of pro-inflammatory genes were found among adults who experienced conditions of low SES over the life course (Stringhini et al. 2015).
Finally, the changes in biological processes caused by early life experiences must result in long-term health consequences. These are more than transient health effects (Rutter 2012). The health consequences associated with early life stress are numerous and include an increased risk for cardiovascular disease, premature mortality, psychopathology, obesity, accelerated aging, autoimmune disease and cancer (Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Masten & Narayan 2012, McEwen 2012, McGowan 2012, Nelson 2013, Rutter 2013, Sasaki et al. 2013, Kundakovic & Champagne 2014, Cunliffe 2015, Provencal & Binder 2015, Stringhini et al. 2015, Tallon et al. 2015).
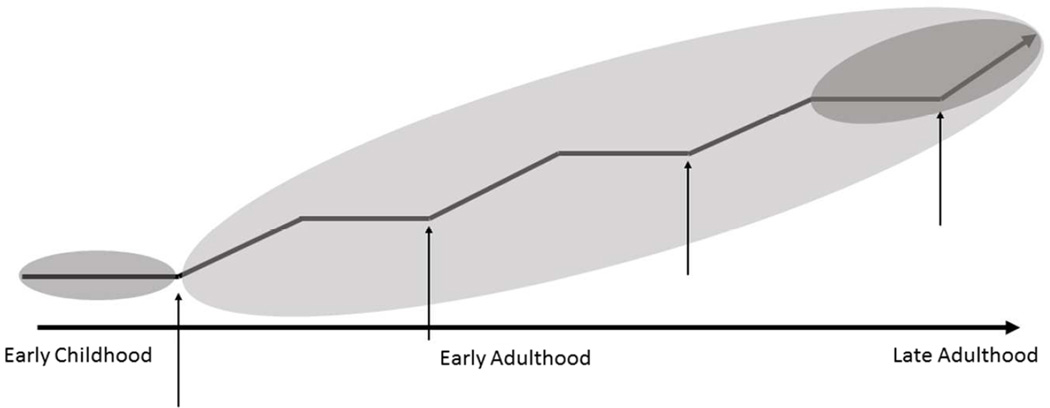
The boundaries of a concept help to determine what is included and excluded from a concept. While the theoretical definition and defining attributes were consistent in every use of biological embedding that was reviewed, there were subtle differences in the boundaries. The lack of clear boundaries resulted in the use of overlapping concepts in the literature, including ‘biological programming’. The reason for this significant overlap in concepts may be the result of how broadly ‘biological embedding’ has been defined. Hertzman (1999) describes biological embedding as including three mechanisms that affect long-term health outcomes – latency effects, pathway effects and cumulative effects (Figure 2). Latency effects are those that produce biological changes during sensitive periods of development and may not be recognized until many decades later. Pathway effects are those that establish a trajectory of health behaviors that have an impact on health over time. Cumulative effects are those that cause ‘wear and tear’ to the system. All included articles in the analysis of biological embedding included latency effects as a mechanism through which biological embedding occurs (Hertzman 1999, Shonkoff et al. 2009, Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Masten & Narayan 2012, McEwen 2012, McGowan 2012, Rutter 2012, Carroll et al. 2013, Clayton 2013, Hertzman 2013, Kelly-Irving et al. 2013, Nelson 2013, Rutter 2013, Sasaki et al. 2013, Kundakovic & Champagne 2014, Barboza Solis et al. 2015, Bateson et al. 2015, Boyce & Kobor 2015, Cunliffe 2015, Demetriou et al. 2015, Provencal & Binder 2015, Stringhini et al. 2015, Tallon et al. 2015, Thomason et al. 2015). A few authors included the pathway effects as a mechanism of biological embedding (Hertzman 1999, Hertzman & Boyce 2010, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Carroll et al. 2013, Kelly-Irving et al. 2013, Barboza Solis et al. 2015, Demetriou et al. 2015, Tallon et al. 2015). Some authors discuss cumulative effects as a mechanism of biological embedding; however, most refer to this as an entirely distinct concept – allostatic load (Hertzman 1999, Hertzman 2012, Barboza Solis et al. 2015). Allostatic load is a well-characterized and widely-used concept, consistently defined as wear-and-tear of the body’s systems that respond to stressful environmental challenges; allostatic load is the cumulative effect of sustained or repeated activation of the these systems (McEwen 2005). Shonkoff’s (2010) Biodevelopmental Framework, for example, includes the impact of cumulative effects but considers this concept distinct from biological embedding.
Figure 2. Latent, pathway and cumulative effects on a health trajectory.
Latent, pathway and cumulative effects have an effect on health trajectories. Latent effects (small grey circles) are the result of exposures in early life that affect health trajectories in later life. Pathway effects (thin arrows) may be the result of early exposures, but they result in behaviors that alter the health trajectory across the life course. Cumulative effects (large grey circle) result from the cumulative ‘wear and tear’ on the body’s systems over time that have an impact heath trajectories throughout the life course.
The antecedents, termed ‘preconditions’ by Morse et al. (1996) and consequences of biological embedding are clear and consistently described. The antecedents – conditions that must precede biological embedding – include environmental exposures and early life experiences. These experiences may be causes of psychological stress (i.e. socio-economic hardship, childhood abuse or neglect, or chaotic home environments) or environmental exposures (i.e. malnutrition, poor maternal nurturance and lack of developmentally-appropriate stimuli). The consequences of biological embedding have also been described. The broad, overarching consequence is adult disease, resulting from early childhood changes in biological processes. More immediate consequences of biological embedding include changes in brain structure, functioning of the HPA axis, immune system functioning and the epigenome (Shonkoff et al. 2009, Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Masten & Narayan 2012, McGowan 2012, Rutter 2012, Clayton 2013, Kelly-Irving et al. 2013, Nelson 2013, Rutter 2013, Sasaki et al. 2013, Kundakovic & Champagne 2014, Betancourt et al. 2015, Buschdorf & Meaney 2015, Cunliffe 2015, Demetriou et al. 2015, Provencal & Binder 2015, Stringhini et al. 2015, Tallon et al. 2015, Thomason et al. 2015).
Biological embedding appears to be a partially mature concept. It has a clear, consistently-used theoretical definition and defining attributes that describe the concept and distinguish it from others. The antecedents and consequences are also well-described. However, on close inspection, the boundaries of this concept are not fully established, resulting in significant overlap with biological programming. Until the boundaries are clarified, the development of an operational definition is not possible.
Concept Analysis
As a partially mature concept, biological embedding is well-defined with universally-accepted defining attributes; however, given the lack of stable boundaries and resultant overlapping concept (i.e., biological programming), a concept comparison is necessary to advance the concept towards an operational definition. Concept comparison is appropriate when there are competing concepts to explain a phenomenon (Morse 1995). In this case, biological embedding will be compared with biological programming to differentiate the uses, definitions and defining attributes of these concepts.
To perform a concept comparison, the researcher should review the literature for both concepts; separately ‘identify, describe, compare and contrast the attributes and rules of relation for each concept; and identify the limitations of each concept for explaining the phenomenon’ (Morse 1995, p. 40–41). A concept comparison will allow for the boundaries of each concept to be more clearly established.
As described above, the concepts ‘biological embedding’ and ‘biological programming’ were searched in the reference databases. Based on the previously described inclusion and exclusion criteria, only 17 articles were recovered for analysis from the search of biological programming. The scarcity of literature describing biological programming indicates that it is less widely applied in scholarly work than biological embedding. Analysis of the literature demonstrated multiple uses, defining attributes, antecedents and consequences and very broad boundaries for biological programming.
Biological programming is defined as the effects of early life events and development on lifelong developmental trajectories (Macintyre 1997, Wadsworth 1997a, Wadsworth 1997b, Eriksson & Forsen 2002, Blane et al. 2007, Rutter 2009, Sonuga-Barke et al. 2010, Pariante 2015). This broad definition is used in many ways by different authors. Many authors define biological programming as the establishment of the functioning parameters (i.e. the set-points) for biological systems resulting from early life experiences (Peck 1994, Macintyre 1997, Wadsworth 1997b, Wadsworth 1997a, Blane et al. 2007, Weeratunga et al. 2014, Buschdorf & Meaney 2015). Eriksson and Forsen (2002) describe the association between low birth weight and an increased risk for adult cardiovascular disease due to adaptation to metabolic demands in utero. One article used biological programming to describe biological characteristics inherent to a species (i.e. the drive to reproduce) (McCabe 2004). Some authors used biological programming in a manner synonymous with biological embedding, demonstrating a clear overlap of these concepts (Rutter & O’Connor 2004, McArthur et al. 2005, Rutter 2006, Schuler & Auger 2010, Infurna et al. 2015, Pariante 2015). Researchers using the concept biological programming discuss the evolution of developmental pathways that are both dependent on and reactive to early childhood experiences (Wadsworth 1997a, Rutter 2009).
Biological programming occurs through three mechanisms described in the literature – experience-expectant programming, experience-dependent programming and experience-adaptive programming (Rutter & O’Connor 2004, Rutter 2009, Sonuga-Barke et al. 2010). Experience-expectant programming occurs when normative experiences (i.e. exposures ‘expected’ and necessary during a particular phase of development), such as developmentally-appropriate visual cues and language exposures, lead to normal development of biological systems (Rutter & O’Connor 2004). Experience-dependent programming includes exposures throughout the life course, which may be positive or negative, that change an individual’s biology (Rutter 2009). Finally, experience-adaptive programming is developmental programming during a sensitive period resulting from adaptation to an environmental stimulus that conditions the organism for future responses to similar stimuli (Rutter & O’Connor 2004). Through these three mechanisms, biological programming defines both a normative and potentially pathological process, depending on the type of environmental exposure.
DISCUSSION
The literature provides evidence for a much broader definition of biological programming compared with biological embedding, resulting in wide-ranging uses of the concept. Biological programming may refer to the effect of developmentally appropriate experiences resulting in normal, healthy developmental trajectories. Conversely, biological embedding is typically limited to those experiences that adversely affect long-term health.
Suggestions for Further Concept Delineation
Based on this analysis of the literature, two defining attributes of biological embedding could be added to those already well-established to provide more distinct limits on the use of biological embedding and to better distinguish this concept from the overlapping concept of biological programming. First, in almost all cases of biological embedding, the environmental exposures are negative and abnormal for the organism or the experience results in psychological stress or a physiological stress response (Shonkoff et al. 2009, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Masten & Narayan 2012, McEwen 2012, McGowan 2012, Rutter 2012, Carroll et al. 2013, Clayton 2013, Kelly-Irving et al. 2013, Nelson 2013, Barboza Solis et al. 2015, Bateson et al. 2015, Boyce & Kobor 2015, Cunliffe 2015, Tallon et al. 2015, Thomason et al. 2015). A few authors included stressful early life experiences but also included environmental experiences that do not specifically result in stress and are not inherently negative (Hertzman 1999, Hertzman & Boyce 2010, Hertzman 2012, Sasaki et al. 2013, Kundakovic & Champagne 2014, Demetriou et al. 2015). In only one case, neither psychological stress nor negative environmental exposures were discussed as antecedents of biological embedding (Hertzman 2013). Moreover, both theories using the concept ‘biological embedding’ define the antecedent as a stressful early life experience (Shonkoff 2010, Miller et al. 2011). Because the context of a concept (i.e. the theories where it is used) is important for the concept’s overall meaning, the fact that stress is included as an antecedent in both theories should not be ignored.
Secondly, the presence of a critical or sensitive period of development should be considered to further delineate biological embedding from biological programming. As previously described, experience-dependent cases of biological programming do not require a sensitive period to affect biological systems (Rutter 2009). Conversely, almost all cases of biological embedding in the literature point to a sensitive period in development – one where the organism is particularly vulnerable to environmental influences – during which experiences exert changes in biological processes (Hertzman 1999, Shonkoff et al. 2009, Hertzman & Boyce 2010, Danese et al. 2011, Miller et al. 2011, Danese & McEwen 2012, Hertzman 2012, Masten & Narayan 2012, McEwen 2012, Carroll et al. 2013, Clayton 2013, Hertzman 2013, Kelly-Irving et al. 2013, Sasaki et al. 2013, Bateson et al. 2015, Boyce & Kobor 2015, Buschdorf & Meaney 2015, Cunliffe 2015, Demetriou et al. 2015, Provencal & Binder 2015, Thomason et al. 2015). During the sensitive or critical period – commonly defined as early childhood – the brain and body are undergoing rapid growth and maturation, allowing for significant plasticity (Sasaki et al. 2013, Boyce & Kobor 2015, Provencal & Binder 2015, Thomason et al. 2015).
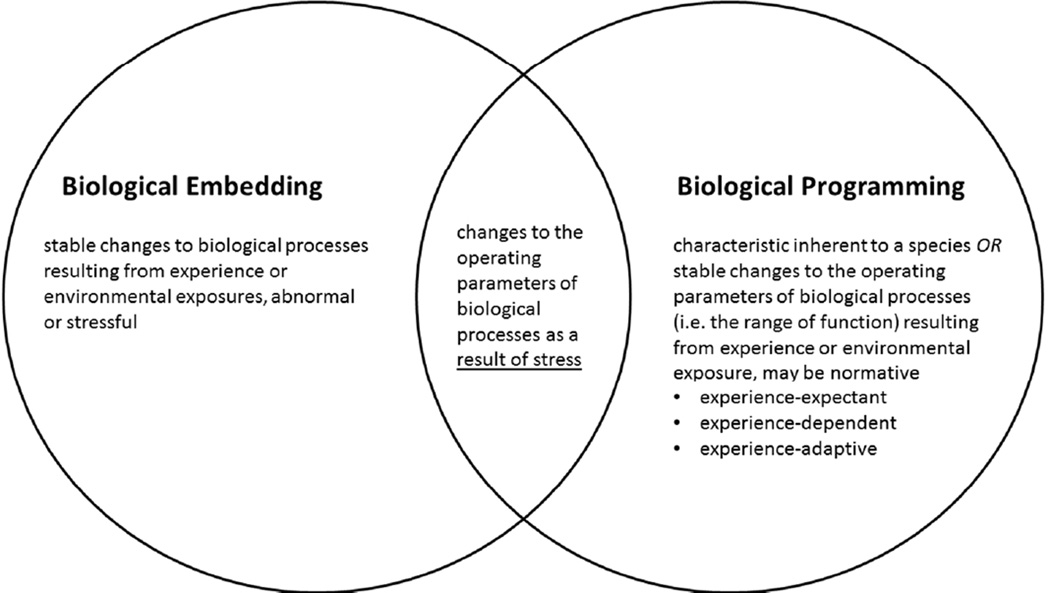
By adding these two defining attributes to biological embedding – attributes that are commonly included in discussions of biological embedding but not explicitly stated by Hertzman and Boyce (2010) – the concept becomes further distinguished from biological programming. There remains some overlap in the concepts, specifically in the description of how stressful life experiences might change the biological set-points, or operating parameters, of biological systems (Figure 3). However, biological programming appears to be a broader concept, including multiple mechanisms of both normal and abnormal development.
Figure 3. Relationship between biological embedding and biological programming.
Even with further delineation of biological embedding by the addition of 2 defining attributes, some overlap between biological embedding and the broader concept of biological programming exists. This overlap occurs when stressful experiences alter the operating parameters of biological processes.
Operationalizing the Concept
Concepts are useful to nursing science when they are measurable. Biological embedding is an example of an abstract concept, describing a complex process. Unlike some concrete concepts that can be perceived through the senses or directly observed, biological embedding can only be inferred from its consequences and, thus, indirectly measured. No single measurement will fully account for its existence or magnitude. Therefore, instruments to measure biological embedding must include the following: (1) a determination that toxic childhood stress is present; (2) a measurement of alterations in biological processes; and (3) a determination that the changes in biological processes have sequelae for adult health outcomes. Collectively, these measures will determine the presence of biological embedding.
Toxic stress during childhood could be measured either through subjective patient interviews or objectively through biomarkers of chronic stress. Previous reports on the biological embedding of childhood stress have relied on child or parental reports of stressful exposures, such as abuse and neglect, parental separation and parental mental illness (Kelly-Irving et al. 2013, Barboza Solis et al. 2015, Thomason et al. 2015). Objective measures of SES (i.e. parental education and income) have also been used as a proxy for childhood stress (Carroll et al. 2013, Thomason et al. 2015). These measures may be useful in retrospective analyses of biological embedding; however, they may be subject to recall bias and do not account for variability in how patients perceive these stressors. In animal studies, researchers are able to directly manipulate the early environment to cause measurable stress among exposed animals (Burns et al. 2012, Chen et al. 2012, Bateson et al. 2015). Because direct manipulation of the childhood environment in human studies is neither possible nor ethical, these animal studies are useful in demonstrating the effects of early toxic stress. The results of these studies may be useful in determining the biological impact of stress on the organism, which may translate to future human studies. Finally, childhood stress could be measured objectively through biomarkers of chronic stress.
Secondly, instruments to measure biological embedding must include a measure of alterations in biological processes. Miller et al. (2011) suggest that the immune systems adopts a pro-inflammatory phenotype under conditions of childhood stress, which could be measured through inflammatory biomarkers, such as C-reactive protein (Danese et al. 2007). Changes in brain structure have also been used as markers of alterations in biological processes following childhood stress (Thomason et al. 2015). The study of epigenetic modifications to targeted genes, such as the glucocorticoid receptor, presents a potentially valuable mechanism for demonstrating changes to biological processes, if these modifications result in clinically meaningful changes to biological processes. Stringhini et al. (2015) found hypomethylation of pro-inflammatory genes among patients with low life course SES. Researchers conclude that epigenetic changes may permanently alter the functioning of the immune and nervous systems (Demetriou et al. 2015).
Finally, instruments to measure biological embedding must include a determination that the changes in biological processes have an impact on adult health outcomes. For example, changes in biological processes that have an impact on long-term mental health, cardiovascular and metabolic health and long-term morbidity may be used to demonstrate the presence of biological embedding in the presence of childhood stress.
Biological embedding will be a challenging concept to measure, as it must be measured longitudinally or retrospectively. Other abstract concepts, such as pain, may be measured in realtime and compared with a patient’s baseline. Conversely, biological embedding must be measured over the life course and can only be compared relative to populations of differentially exposed individuals. Retrospective studies may be subject to recall bias and unknown confounding variables that present throughout the life course.
Importance of the Concept for Nursing Theory, Research and Practice
Numerous opportunities for the application of the concept biological embedding have already been realized in sociology, psychology and biomedical research. Researchers have used the concept to describe the impact of early childhood psychosocial stress on adult health outcomes and have studied such health vulnerabilities across populations (Hertzman 2012). The effects of various forms of psychosocial stress, including childhood trauma and socioeconomic stress, have been described (Kelly-Irving et al. 2013, Nelson 2013). Furthermore, some of the specific epigenetic mechanisms through which these effects are mediated and the biomarkers that may be used to measure the effects have been elucidated (Danese et al. 2011, Hertzman 2012, Sasaki et al. 2013). Continued efforts in these areas could further explain the mechanisms through which biological embedding occurs and elucidate potential targets for therapeutic interventions to improve health trajectories.
While much attention has been given to the opportunities in biomedical and psychology research, the opportunities in nursing are abundant and, as of yet, underexplored. Shonkoff’s (2010) theory provides numerous propositions about the relationships between biological embedding, early stress and adult health outcomes that are opportune for empirical testing. Shonkoff (2010) theorizes that buffers may exist that modulate the effects of biological embedding, a theory that has been supported by research on the effects of nurturing environments (McEwen 2012, Carroll et al. 2013). The role of supportive caregiving is an optimal target for intervention research by nurse scientists. The biological embedding of early life experiences in the context of a family among hospitalized children has been considered (Tallon et al. 2015). Potential research questions could include (1) how do nurturing environments modulate the effects of early childhood stress? (2) do interventions that provide support for chaotic families reduce stress and improve long-term health outcomes among affected children? (3) how does nursing care affect the expression of biomarkers for stress among hospitalized children? Further, nurse scientists are perfectly suited to study the effects of other types of stress (i.e. physiologic stress) on long-term health outcomes, an area that has not yet been explored but is no less important than the psychosocial stress on which previous research has focused.
As our understanding of adult health outcomes is beginning to include consideration of childhood antecedents, the nursing theories used to guide practice and research should evolve to include a longitudinal, life course perspective. Adult health states cannot be fully understood without consideration of childhood experiences. Thus, middle range nursing theories that guide practice and research must include life course theory concepts such as biological embedding. Nursing theories that include biological embedding will allow nurses to explore clinical situations where the conditions for biological embedding are present in individuals and populations. Interventions, guided by theories that include biological embedding, can then be designed and tested to prevent the biological embedding of early life stress or to buffer its long-term effects.
Limitations
In conducting a search of the literature for a specific concept, it is possible that synonyms may be missed. However, to provide a consistent language to communicate scientific ideas, it is important to advocate for using the same language, including the naming of concepts. Nevertheless, it is possible that other terms used to describe the same process as biological embedding may have been excluded from this analysis.
CONCLUSION
Early stress can permanently alter biological processes during the sensitive periods of development, a phenomenon that had an impact on long-term adult health outcomes. This phenomenon is described as biological embedding, an important emerging concept for nursing science. Advancing this concept toward greater maturity requires the addition of 2 attributes, which help to distinguish it from biological programming: (1) biological embedding occurs during a critical or sensitive period of development and (2) biological embedding occurs when a stressful experience affects long-term adult health outcomes. To advance nursing science in a manner that is consistent with our current understanding of adult health, nursing theories must adopt a longitudinal approach that incorporates concepts from life course theory, such as biological embedding. Diseases that become apparent during adulthood, such as cardiovascular disease, diabetes and depression, often result from a developmental health trajectory established by the biological embedding of early life stress.
SUMMARY STATEMENT.
Why is this research needed?
To better describe the health trajectories of individuals and populations, nursing theory and research could be expanded to include concepts from life course theory.
Biological embedding is a central concept in life course theory that describes how early life events have an impact on long-term health outcomes.
Biological embedding is an emerging concept in need of concept analysis before it can be full incorporated into nursing theory.
What are the key findings?
Evaluation of the concept of biological embedding reveals that it is a partially mature concept with poorly defined boundaries that overlap with a separate concept, biological programming.
A concept comparison between biological embedding and biological programming reveals that while these concepts have overlapping boundaries, biological embedding refers to stressful early life experiences that result in pathologic health effects.
How should the findings be used to influence policy/practice/research/education?
By distinguishing biological embedding from biological programming, the concept of biological embedding is advanced toward greater maturity, making it amenable to inclusion in nursing theory and research.
Nursing theory and research that includes concepts from life course theory will have a greater impact on the long-term health of individuals and populations.
Acknowledgments
Special thanks to Dr. Deborah Steward, PhD, RN and Dr. Mary Beth Happ, PhD, RN, FAAN for their guidance through the writing and editing process.
Funding: This work was completed while the author was a trainee under NIH NINR 5T32NR014225-03, “Optimizing Health in Childhood: Interdisciplinary Training in Health Development”.
Footnotes
Conflict of Interest: No conflict of interest has been declared by the author.
Author Contributions:
- substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
- drafting the article or revising it critically for important intellectual content.
REFERENCES
- Barboza Solis C, Kelly-Irving M, Fantin R, Darnaudery M, Torrisani J, Lang T, Delpierre C. Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(7):E738–E746. doi: 10.1073/pnas.1417325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proceedings. Biological Sciences. 2015;282(1799):20142140. doi: 10.1098/rspb.2014.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt LM, Avants B, Farah MJ, Brodsky NL, Wu J, Ashtari M, Hurt H. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Develomental Science. 2015 doi: 10.1111/desc.12344. [DOI] [PubMed] [Google Scholar]
- Blane D, Netuveli G, Stone J. The development of life course epidemiology. Rev Epidemiologie et de Sante Publique. 2007;55(1):31–38. doi: 10.1016/j.respe.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Kobor MS. Development and the epigenome: the ‘synapse’ of gene-environment interplay. Developmental Science. 2015;18(1):1–23. doi: 10.1111/desc.12282. [DOI] [PubMed] [Google Scholar]
- Burk DL. Lex genetica: the law and ethics of programming biological code. Ethics and Information Technology. 2002;4:109–121. doi: 10.1023/a:1019996311122. [DOI] [PubMed] [Google Scholar]
- Burns JG, Svetec N, Rowe L, Mery F, Dolan MJ, Boyce WT, Sokolowski MB. Gene-environment interplay in Drosophila melanogaster: chronic food deprivation in early life affects adult exploratory and fitness traits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17239–17344. doi: 10.1073/pnas.1121265109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschdorf JP, Meaney MJ. Epigenetics/Programming in the HPA Axis. Comprehensive Physiology. 2015;6(1):87–110. doi: 10.1002/cphy.c140027. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(42):17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. Journal of Neuroendocrinology. 2012;24(7):1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The genomics of memory and learning in songbirds. Annual Review of Genomics and Human Genetics. 2013;14:45–65. doi: 10.1146/annurev-genom-090711-163809. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT. Experience-sensitive epigenetic mechanisms, developmental plasticity and the biological embedding of chronic disease risk. Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 2015;7(2):53–71. doi: 10.1002/wsbm.1291. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2011;16(3):244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou CA, van Veldhoven K, Relton C, Stringhini S, Kyriacou K, Vineis P. Biological embedding of early-life exposures and disease risk in humans: a role for DNA methylation. European Journal of Clinical Investigation. 2015;45(3):303–332. doi: 10.1111/eci.12406. [DOI] [PubMed] [Google Scholar]
- Desai S, Burra P. BioInt: an integrative biological object-oriented application framework and interpreter. International Journal of Bioinformatics Research and Applications. 2015;11(3):247–256. doi: 10.1504/ijbra.2015.069195. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ. Childhood growth and coronary heart disease in later life. Annals of Medicine. 2002;34(3):157–161. [PubMed] [Google Scholar]
- Halfon N, Wise PH, Forrest CB. The changing nature of children’s health development: new challenges require major policy solutions. Health Affairs (Project Hope) 2014;33(12):2116–2124. doi: 10.1377/hlthaff.2014.0944. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hertzman C. Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17160–17167. doi: 10.1073/pnas.1202203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C. Commentary on the symposium: biological embedding, life course development and the emergence of a new science. Annual Review of Public Health. 2013;34:1–5. doi: 10.1146/annurev-publhealth-031912-114500. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. 3p following 347. [DOI] [PubMed] [Google Scholar]
- Hupcey JE, Penrod J. Concept analysis: Examining the state of the science. Research and Theory for Nursing Practice. 2005;19(2):197–208. [PubMed] [Google Scholar]
- Infurna FJ, Rivers CT, Reich J, Zautra AJ. Childhood trauma and personal mastery: their influence on emotional reactivity to everyday events in a community sample of middle-aged adults. PLoS One. 2015;10(4):e0121840. doi: 10.1371/journal.pone.0121840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P, Lang T, Delpierre C. Adverse childhood experiences and premature all-cause mortality. European Journal of Epidemiology. 2013;28(9):721–734. doi: 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Early-Life Experience, Epigenetics and the Developing Brain. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S. The Black Report and beyond: what are the issues? Social Science & Medicine. 1997;44(6):723–745. doi: 10.1016/s0277-9536(96)00183-9. [DOI] [PubMed] [Google Scholar]
- Masten AS, Narayan AJ. Child development in the context of disaster, war and terrorism: pathways of risk and resilience. Annual Review of Psychology. 2012;63:227–257. doi: 10.1146/annurev-psych-120710-100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. Journal of Neuroendocrinology. 2005;17(8):475–482. doi: 10.1111/j.1365-2826.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- McCabe MP. Marriage: Every bride’s ambition satisfied? Sexual and Relationship Therapy. 2004;19(2):131–132. [Google Scholar]
- McEwen BS. Stressed or stressed out: what is the difference? Journal of Psychiatry & Neuroscience. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO. Epigenetic clues to the biological embedding of early life adversity. Biological Psychiatry. 2012;72(1):4–5. doi: 10.1016/j.biopsych.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse JM. Exploring the theoretical basis of nursing using advanced techniques of concept analysis. Advances in Nursing Science. 1995;17(3):31–46. doi: 10.1097/00012272-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Morse JM, Mitcham C, Hupcey JE, Tason MC. Criteria for concept evaluation. Journal of Advanced Nursing. 1996;24(2):385–390. doi: 10.1046/j.1365-2648.1996.18022.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Biological embedding of early life adversity. JAMA Pediatrics. 2013;167(12):1098–1100. doi: 10.1001/jamapediatrics.2013.3768. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Depression and antidepressants in pregnancy: Molecular and psychosocial mechanisms affecting offspring’s physical and mental health. Neuropsychopharmacology. 2015;40(1):246–247. doi: 10.1038/npp.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MN. The importance of childhood socio-economic group for adult health. Social Science & Medicine. 1994;39(4):553–562. doi: 10.1016/0277-9536(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Provencal N, Binder EB. The effects of early life stress on the epigenome: From the womb to adulthood and even before. Experimental Neurology. 2015;268:10–20. doi: 10.1016/j.expneurol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Risjord M. Rethinking concept analysis. Journal of Advanced Nursing. 2009;65(3):684–691. doi: 10.1111/j.1365-2648.2008.04903.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Implications of resilience concepts for scientific understanding. Annals of the New York Academy of Sciences. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- Rutter M. Understanding and testing risk mechanisms for mental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(1–2):44–52. doi: 10.1111/j.1469-7610.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Achievements and challenges in the biology of environmental effects. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17149–17153. doi: 10.1073/pnas.1121258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Developmental psychopathology: a paradigm shift or just a relabeling? Development and Psychopathology. 2013;25(4 Pt 2):1201–1213. doi: 10.1017/S0954579413000564. [DOI] [PubMed] [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sasaki A, de Vega WC, McGowan PO. Biological embedding in mental health: an epigenomic perspective. Biochemistry and Cell Biology. 2013;91(1):14–21. doi: 10.1139/bcb-2012-0070. [DOI] [PubMed] [Google Scholar]
- Schuler LA, Auger AP. Psychosocially influenced cancer: diverse early-life stress experiences and links to breast cancer. Cancer Prevention Research. 2010;3(11):1365–1370. doi: 10.1158/1940-6207.CAPR-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Singh G, Rice P, Mahajan RL, McIntosh JR. Fabrication and characterization of a carbon nanotube-based nanoknife. Nanotechnology. 2009;20(9):095701. doi: 10.1088/0957-4484/20/9/095701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Schlotz W, Rutter M. Physical growth and maturation following early severe institutional deprivation: Do they mediate specific psychopathological effects? Monographs of the Society for Research in Child Development. 2010;75(1):143–166. doi: 10.1111/j.1540-5834.2010.00554.x. [DOI] [PubMed] [Google Scholar]
- Stringhini S, Polidoro S, Sacerdote C, Kelly RS, van Veldhoven K, Agnoli C, Grioni S, Tumino R, Giurdanella MC, Panico S, Mattiello A, Palli D, Masala G, Gallo V, Castagne R, Paccaud F, Campanella G, Chadeau-Hyam M, Vineis P. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. International Journal of Epidemiology. 2015 doi: 10.1093/ije/dyv060. [DOI] [PubMed] [Google Scholar]
- Tallon MM, Kendall GE, Snider PD. Rethinking family-centred care for the child and family in hospital. Journal of Clinical Nursing. 2015;24(9–10):1426–1435. doi: 10.1111/jocn.12799. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR. Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME. Health inequalities in the life course perspective. Social Science & Medicine. 1997a;44(6):859–869. doi: 10.1016/s0277-9536(96)00187-6. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME. Changing social factors and their long-term implications for health. British Medical Bulletin. 1997b;53(1):198–209. doi: 10.1093/oxfordjournals.bmb.a011600. [DOI] [PubMed] [Google Scholar]
- Weeratunga P, Jayasinghe S, Perera Y, Jayasena G, Jayasinghe S. Per capita sugar consumption and prevalence of diabetes mellitus--global and regional associations. BMC Public Health. 2014;14:186. doi: 10.1186/1471-2458-14-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MA. Paraplex--a new biological embedding resin. Medical & Biological Illustration. 1965;15(4):230–233. [PubMed] [Google Scholar]